Abstract
Barberry fruit is consumed in different forms including dried fruit, juice, jam and marmalade in Iran. This fruit is also used as a food additive (flavoring and colorant) in soup and rice dishes. In present study, antioxidant activities of acetone, ethanol and water (infusion and decoction) extracts of barberry (Berberis vulgaris) fruit were investigated using 2,2-azinobis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and reducing power methods. Total phenolic contents of the extracts were also estimated using Folin-Ciocalteu assay. In ABTS assay, acetone and ethanol extracts showed the highest radical scavenging activity, while in DPPH and reducing power methods, acetone extract and decoction exhibited the strongest antioxidant activity. Meanwhile, the antioxidant potential of water extracts increased with increasing heating time (antioxidant activity of decoction was higher than that of infusion). The highest total phenolic content was found in the acetone extract (92.75 mg GAE per g). It was concluded that the acetone extract and decoction of barberry fruit can be used as an effective natural antioxidant in food industry.
Key Words: Antioxidant activity, Barberry, Extract, Fruit
Introduction
There is an increasing demand for replacing the synthetic preservatives with natural compounds in food products. This is due to consumer’s awareness about the toxic and carcinogenic effects of synthetic compounds.1 In this regard; natural antioxidants from plants and fruits are the most popular candidate among other substances. In recent years, herbal products such as various essential oils and extracts have been investigated for anti-inflammatory, antibacterial, antioxidant and radical scavenging activities.2-4
Barberry (Berberis vulgaris), belonging to Berberidaceae family, is native to Asia, Middle East and Europe. The barberry is a thorny shrub that grows to 1 to 3 m.5-7 Barberry fruits are deep red in color and about 10 mm in length. The plant is a long and red colored shrub. The roots and bark of plant contain alkaloids including berbamine, berberine and berberrubine. Besides, it has antioxidant, antitumor and antibacterial activities.8,9 Barberry is grown in many countries essentially for medical and ornamental purposes. In Iran, however, the seedless variety of this plant is cultivated and consumed as dried fruit, juice, jam, marmalade and food additive (soup, stews and rice dishes) that may be correlated to its unique, palatable and acceptable sour flavor and an exciting pink color.10
Barberry fruits contain vital nutritional components for human health. The plant contains various substances such as carbohydrates, organic acids, some vitamins, polyphenolic compounds, pectin, tannin, minerals, jatrorrhizine and palmatine.11 Furthermore, barberry fruits can be used for kidneys and urinary and gastrointestinal tracts disorders, liver diseases, bronchial discomforts and as a stimulant for the circulatory system.12 Also, it has been demonstrated that Berberis vulgaris root extract exhibits antimicrobial activity against some microorganisms such as Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans.13 To our knowledge, there is limited information about antioxidant properties of various extracts of barberry fruits.9,11,14-16 Therefore, the objective of this work was to evaluate the antioxidant activities of acetone, ethanol and water extracts of barberry fruit.
Materials and Methods
Fruit sample. The seedless barberry fruits (Berberis vulgaris L.) were purchased from a local market and identified at Faculty of Agriculture, Urmia University, Urmia, Iran.
Chemicals. Butylated hydroxyl toluene (BHT), 2,2-azinobis-3-ethylenzothiazoline-6-sulphonicacid (ABTS), potassium persulfate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), Ferric chloride, potassium ferricyanide and gallic acid were obtained from Sigma-Aldrich Chemie (Steinheim, Germany). Trichloroacetic acid, disodium hydrogen phosphate (Na2HPO4), sodium dihydrogen phosphate (NaH2PO4), Folin–Ciocalteu’s phenol reagent and sodium carbonate were procured from Merck (Darmstadt, Germany).
Preparation of the extracts. To prepare acetone and ethanol extracts, 40 g of ground barberry fruits were mixed with 400 mL acetone or 96.00% ethanol, respectively. The solutions were stirred at room temperature for overnight. The extracts were filtered through no.42 Whatman filter paper (Whatman, Pleasanton, USA) and concentrated under vacuum at 45.00 °C using a Heidolph rotary evaporator (Laborata 4003; Schwabach, Germany). Finally, the waxy concentrated extracts were then dried at desiccator and kept in dark at 4.00 ˚C until analysis.17
Preparation of infusion. To prepare infusion, 100 g of ground barberry fruits were refluxed with 1000 mL of distilled water at 100 ˚C for 10 min. The infusion was filtered through the filter paper and concentrated using the rotary evaporator. After drying, the sample was kept in dark at 4.00 ˚C until analysis.15
Preparation of decoction. Ground barberry fruits (100 g) were refluxed with 1000 mL of distilled water at 100 ˚C for 60 min. This extract is called a decoction. The next steps (filtering, concentrating, drying and storage) were similar to above-mentioned procedure. For antioxidant analysis, the dried samples were dissolved in their respective solvents.15
pH measurement. The pH values of the extracts were determined using a pH meter (pH-Meter E520, Metrohm Herisau, Switzerland) that was calibrated by buffer solutions at pH 4 and 7.
Determination of ABTS radical scavenging activity. The ABTS (7.00 mM) and potassium per sulfate (2.45 mM) was prepared, as described previously.18 These solutions were mixed together. After 16 hr, the solution was diluted in ethanol to get an absorbance of 0.70 ± 0.02 at 734 nm. Then, 2 mL of above solution was mixed with 200 μL of different concentrations of extracts (0.50, 1 and 2 mg mL-1) and after incubating for 1 min at room temperature, the absorbance was measured at 734 nm using a spectrophotometer (Novaspec II; Pharmacia LKB, Uppsala, Sweden). The ABTS radical scavenging activity (%) was calculated by the following equation:
ABTS radical scavenging activity (%) = (A blank − A sample )/ A blank ×100
where, Ablank is the absorbance of blank (containing all reagents except the test compound) and Asample is the absorbance of the test compound. The BHT (2 mg mL-1) was used as a reference compound.
Determination of DPPH radical scavenging activity. The ability of the extracts to scavenge DPPH radical was determined using the method of Blois with slight modification.19 A volume of 50 μL of the various concentrations of the extracts (0.50, 1and 2 mg mL-1) was added to 2 mL of methanol solution of DPPH (24 μg mL-1) and mixed. The mixture was stored in dark at room temperature for 60 min and the absorbance was measured at 517 nm using a spectrophotometer (model Novaspec II; Pharmacia LKB, Uppsala, Sweden). The DPPH radical scavenging activity (RSA) was calculated by the following equation:
DPPH radical scavenging activity (%) = (A blank − A sample )/ A blank ×100
where, Ablank is the absorbance of blank (containing all reagents except the test compound) and Asample is the absorbance of the test compound. The BHT (2 mg mL-1) was used as a positive control.
Determination of reducing power. The reducing power of the extracts was determined according to the method of Oyaizu.20 One milliliter of the extracts (0.50, 1 and 2 mg mL-1) was mixed with 2.50 mL of sodium phosphate buffer (0.20 M, pH 6.60) and 2.50 mL of potassium ferricyanide (1.00%). After incubation at 50 °C for 20 min, 2.50 mL of trichloroacetic acid (10.00%) was added to the mixture and centrifuged at 1,430 g for 10 min. Finally, the 2.50 mL of upper layer was mixed with 2.50 mL of distilled water and 0.50 mL of ferric chloride (0.10%). After 10 min, the absorbance was measured at 700 nm against blanks containing all reagents except the sample extracts. A higher absorbance value indicated a higher reducing power. The BHT was used as a positive control.
Determination of total phenolic contents. The total phenolic contents of the extracts were determined using the Folin-Ciocalteu reagent assay according to the method of Singleton and Rossi with gallic acid as a standard.21 Briefly, 500 μL of the extracts were mixed with 2.25 mL of distilled water and then 250 μL of Folin-Ciocalteu reagent was added. The mixture was vortexed for 1 min and allowed to react for 5 min. Then, 2 mL of Na2CO3 (7.50%) was added. After incubation at room temperature for 120 min, the absorbance of each mixture was measured at 760 nm. The same procedure was also applied to a standard solution of gallic acid and a standard curve was prepared. The total phenolic contents were expressed as mg of gallic acid equivalent per gram of the extract.
Statistical analysis. All tests were carried out in triplicate and data were presented as mean ± standard deviation. Statistical Analysis System (version 9.1; SAS Institute, Cary, USA) software was used for analysis of results and significant differences between mean values were determined by Tukey’s multiple-range test.
Results
pH of the extracts. Ethanol extracts had the highest pH value among others (pH = 4.80) that followed by acetone extract (pH = 4.40), infusion (pH = 3.60) and decoction (pH= 3.50). Differences between the pH values of the extracts were significant (p < 0.05) statistically. However, the difference between infusion and decoction was not significant (p > 0.05).
The ABTS radical scavenging activity. The ABTS radical is soluble in both aqueous and organic solvents. Thus, ABTS method evaluates the antioxidant activity of both hydrophilic and lipophilic compounds. In this method, blue-green color of ABTS solution changed to colorless by the process of hydrogen or electron donation. The ABTS radical scavenging activities of the extracts are shown in Table 1. Acetone extract showed the highest radical scavenging activity (p < 0.05). In contrast to DPPH and reducing power results, ethanol extract had significantly (p < 0.05) higher ABTS scavenging activity than decoction and infusion. There was no significant difference between ABTS scavenging activities of decoction and infusion.
Table 1.
The ABTS radical scavenging activities of the barberry fruit extracts
| Extract |
Concentration (mg mL
-1
)
|
||
|---|---|---|---|
| 0.50 | 1.00 | 2.00 | |
| Acetone extract | 87.92 ± 2.69a | 97.73 ± 1.28a | 97.61 ± 0.82a |
| Ethanol extract | 60.23 ± 1.41b | 89.71 ± 0.46b | 97.44 ± 0.35a |
| Infusion | 32.83 ± 0.46c | 69.82 ± 0.51c | 95.36 ± 0.25a |
| Decoction | 31.52 ± 0.53c | 69.96 ± 2.56c | 96.54 ± 1.16a |
| BHT | - | - | 98.65 ± 0.13a |
BHT = Butylated hydroxytoluene.
Values with different letters in each column are significantly different (p < 0.05).
The DPPH radical scavenging activity. The scavenging effects of the barberry fruit extracts at different concentrations on the DPPH radical are shown in Table 2. According to the results, radical scavenging activity of all extracts was dose-dependent. Acetone extract significantly (p < 0.05) showed the strongest DPPH radical scavenging activities. This activity was significantly (p < 0.05) followed by decoction, infusion and ethanol extract. In other words, ethanol extract showed the lowest scavenging activity.
Table 2.
The DPPH radical scavenging activity (%) of different extracts of barberry fruit
| Extract |
Concentration (mg mL
-1
)
|
||
|---|---|---|---|
| 0.50 | 1.00 | 2.00 | |
| Acetone extract | 28.75 ± 2.00a | 42.12 ± 0.86a | 68.40 ± 0.75b |
| Ethanol extract | 25.25 ± 0.40a | 30.15 ± 0.57c | 40.75 ± 0.10e |
| Infusion | 27.30 ± 0.34a | 36.75 ± 0.50b | 45.45 ± 2.30d |
| Decoction | 31.70 ± 1.50a | 41.61 ± 0.61a | 60.20 ± 0.80c |
| BHT | – | – | 78.65 ± 0.90a |
BHT = Butylated hydroxytoluene.
Values with different letters in each column are significantly different (p < 0.05).
Reducing power. The reducing capacity of various extracts is presented in Table 3. Similar to the results of DPPH assay, acetone extract significantly (p < 0.05) exhibited the highest reducing capacity that significantly (p < 0.05) followed by decoction, infusion and ethanol extract. Therefore, infusion and ethanol extract had the lowest reducing power when compared to other extracts. Similar to the ABTS and DPPH results, reducing capacity of the extracts increased in a dose-dependent manner.
Table 3.
Reducing power of different extracts of barberry fruit
| Extract |
Concentration (mg mL
-1
)
|
||
|---|---|---|---|
| 0.50 | 1.00 | 2.00 | |
| Acetone extract | 0.51 ± 0.04b | 0.98 ± 0.06b | 2.20 ± 0.16b |
| Ethanol extract | 0.18 ± 0.02e | 0.51 ± 0.03c | 1.15 ± 0.07d |
| Infusion | 0.28 ± 0.01d | 0.56 ± 0.02c | 1.05 ± 0.03d |
| Decoction | 0.42 ± 0.01c | 0.87 ± 0.06b | 1.73 ± 0.02c |
| BHT | 1.39 ± 0.01a | 2.36 ± 0.06a | 2.65 ± 0.02a |
BHT = Butylated hydroxytoluene.
abcde: Values with different letters in each column are significantly different (p < 0.05).
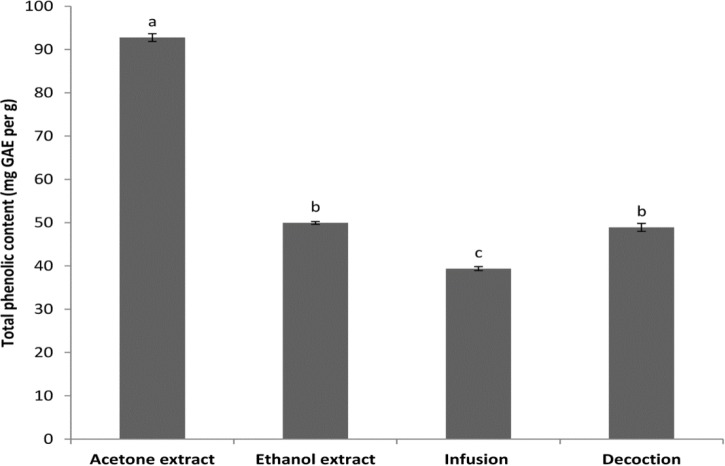
Total phenolic contents. Figure 1 shows total phenolic contents in different extracts of barberry fruit. The highest total phenolic contents were found in the acetone extract (92.75 mg GAE per g) that followed by ethanol extract (49.92 mg GAE per g), decoction (48.89 mg GAE per g) and infusion (39.37 mg GAE per g). There were significant differences (p < 0.05) between all of the extracts but difference between ethanol extract and decoction was not significant.
Fig. 1.
Total phenolic contents (mg GAE per g) of different extracts of barberry fruit. Different letters on the top of the bars indicate significant differences (p < 0.05).
Discussion
The pH of various extracts of barberry was ranged from 3.50 to 4.80. Decoction and ethanol extract had the lowest and the highest pH value, respectively. Our results were in agreement to findings of other researchers indicating that Berberis vulgaris fruit is an acidic crop that can be due to dominant organic acids such as malic, citric and tartaric acids.7,11
The DPPH method is one of the simplest methods to evaluate the antioxidant activity of plant extracts.22 It was reported that DPPH free radical scavenging activity of water extract of barberry (2 mg mL-1) was 74.08%.15 In present study, DPPH scavenging activity of decoction (2 mgmL-1) was 60.20%. This difference may be due to the extract drying method. In other words, they used freeze drier for drying the extracts, while a desiccator has been employed in the present study. The DPPH radical scavenging activity of water extract of barberry was more than its ethanol extract.14 In another study, antioxidant activities of the ethanol extracts of roots, twigs and leaves of common barberry (Berberis vulgaris L.) and Croatian barberry (Berberis croatica Horvat) have been determined.6 No significant differences were found between antioxidant activities of common and Croatian barberry, while antioxidant activity was related to the organ.
The reducing power of a compound is related to its electron transfer ability and may serve as a significant indicator of its potential antioxidant activity.20 In this assay, the yellow color of the test solution changes to green and blue depending on the reducing power of test substance. Greater absorbance at 700 nm indicates greater reducing power.23 Other studies have reported the strong reducing capacity of Berberidacea family fruits such as Berberis vulgaris and Berberis croatica.6,24 In a previous study, the reducing power of water extract of barberry has been reported 0.57 in concentration of 2 mg mL-1.15
In present study, the acetone extract of barberry fruit showed the highest total phenolic contents. Similarly, other researchers have reported that total phenolic contents of the water extract of barberry fruit were 33.06 mg GAE per g.15 In another study, the total phenolic contents of methanol and water extracts of barberry fruit were reported 280 and 100 mg GAE per g, respectively.14 These differences can be due to many factors such as geographic location, environmental and climate conditions, season of growth, soil type, storage and processing conditions that can influence the levels of phenolic compounds.22
In conclusion, according to the results of the present study, acetone extract and decoction of the barberry (Berberis vulgaris L.) fruit exhibited strong antioxidant potentials. Antioxidant activity of water extract of barberry fruit enhanced with increasing heating time (antioxidant activity of decoction was higher than that of infusion). The application of barberry in food models may be very valuable and desirable due to its health benefit and antioxidant potential as well as coloring and flavoring properties.
Acknowledgments
The authors would like to acknowledge the financial support of Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Giatrakou V, Savvaidis I. Bioactive packaging technologies with chitosan as a natural preservative agent for extended shelf-life food products. In: Arvanitoyannis I, editor. Modified atmosphere and active packaging technologies . 1st ed. Boca Raton, USA: Taylor & Francis ; 2012. pp. 685–730. [Google Scholar]
- 2.Chu YH, Chang CL, Hsu HF. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agr. 2000;80(5):561–566. [Google Scholar]
- 3.Koleva II, van Beek TA, Linssen JP, et al. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13(1):8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 4.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, et al. Antibacterial activity of different essential oils obtained from spices widely used in Mediterranean diet. Int J Food Sci Tech. 2008;43(3):526–531. [Google Scholar]
- 5.Akbulut M, Çalişir S, Marakoğlu T, et al. Some physicomechanical and nutritional properties of barberry (Berberis vulgaris L) fruits. J Food Process Eng. 2009;32(4):497–511. [Google Scholar]
- 6.Koncic MZ, Kremer D, Karlovic K, et al. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L and Berberis croatica Horvat. Food Chem Toxicol. 2010;48(8-9):2176–2180. doi: 10.1016/j.fct.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Gundogdu M. Determination of antioxidant capacities and biochemical compounds of Berberis vulgaris L. fruits. Adv Environ Biol. 2013;7(2):344–348. [Google Scholar]
- 8.Gundogdu M, Muradoglu F, Sensoy RG, et al. Determination of fruit chemical properties of Morus nigra L Morus alba L and Morus rubra L by HPLC. Sci Hortic. 2011;132:37–41. [Google Scholar]
- 9.Hanachi P, Golkho S. Using HPLC to determination the composition and antioxidant activity of Berberis vulgaris. Eur J Sci Res. 2009;29(1):47–54. [Google Scholar]
- 10.Sharifi A, Hassani B. Extraction methods and stability of color extracted from barberry pigments. Int J Agri Science. 2012;2(4):320–327. [Google Scholar]
- 11.Ozgen M, Saraçoglu O, Geçer EN. Antioxidant capacity and chemical properties of selected barberry (Berberis vulgaris L) fruits. Hortic Environ Biotechnol. 2012;53(6):447–451. [Google Scholar]
- 12.Blumenthal M, Busse W, Goldberg A, et al. Austin, USA: American Botanical Council; 1998. The Complete German Commission E Monographs—Therapeutic Guide to Herbal Medicines; pp. 122–123. [Google Scholar]
- 13.Kosalec I, Gregurek B, Kremer D, et al. Croatian barberry (Berberis croatica Horvat): A new source of berberine—analysis and antimicrobial activity. World J Microb Biot. 2009;25(1):145–150. [Google Scholar]
- 14.Motalleb G, Hanachi P, Kua S, et al. Evaluation of phenolic content and total antioxidant activity in Berberis vulgaris fruit extract. J Biol Sci. 2005;5(5):648–653. [Google Scholar]
- 15.Aliakbarlu J, Mohammadi S, Khalili S. A study on antioxidant potency and antibacterial activity of water extracts of some spices widely consumed in Iranian diet. J Food Biochem. 2014;38(2):159–166. [Google Scholar]
- 16.Hassanpour H, Alizadeh S. Evaluation of phenolic compound, antioxidant activities and antioxidant enzymes of barberry genotypes in Iran. Sci Hort. 2016;200:125–130. [Google Scholar]
- 17.Bursal E, Koksal E. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L) Food Res Int. 2011;44(7):2217–2221. [Google Scholar]
- 18.Ozgen M, Reese RN, Tulio AZ, et al. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2, 2'-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agric Food Chem. 2006;54(4):1151–1157. doi: 10.1021/jf051960d. [DOI] [PubMed] [Google Scholar]
- 19.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 20.Oyaizu M. Studies on products of browning reaction-antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44(6):307–315. [Google Scholar]
- 21.Singleton V, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 22.Katalinic V, Milos M, Kulisic T, et al. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94(4):550–557. [Google Scholar]
- 23.Gulcin I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids. 2007;32(3):431–438. doi: 10.1007/s00726-006-0379-x. [DOI] [PubMed] [Google Scholar]
- 24.Mohamadi M, Maskooki A, Mortazavi S. Evaluation of antioxidant properties of barberry fruits extracts using maceration and subcritical water extraction (SWE) Int J Nutr Food Eng. 2012;6(9):699–703. [Google Scholar]