Abstract
The objective of this study was to evaluate the pituitary gland dimensions due to age and weight using computed tomography (CT) in dogs and cats. The CT images of pituitary gland were assessed in 11 client-owned dogs (six males and five females; age range, 1 to 9 years) and 15 client-owned cats (eight males and seven females; age range, 1 to 14 years) with no evidence of pituitary diseases. The length, height, width and volume of the pituitary gland were measured in sagittal and transverse planes. Mean pituitary length, width, height and volume (± standard deviation: SD) were respectively 4.96 (± 0.69 mm), 3.62 (± 0.64 mm), 2.62 (± 0.05 mm) and 26.19 (± 7.99 mm3) in cats and were 7.00 (± 2.14 mm), 4.80 (± 1.20 mm), 3.80 (± 0.70 mm) and 77.53 (± 51.64 mm3) in dogs, respectively. Mean pituitary height-to-brain ratio (P:B ratio), (± SD) in cats and dogs was 0.28 (± 0.05) and 0.21 (± 0.03), respectively and mean percent of pituitary volume to brain volume (± SD) in cats and dogs was 0.10 (± 0.05) and 0.10 (± 0.07), respectively. There was no significant correlation between the size of pituitary gland and age, weight and body condition score (BCS) in dogs, however in cats, significant difference was found between height of pituitary gland and weight and BCS, pituitary width and weight and P:B ratio and BCS. These findings could be useful to identify abnormal pituitary gland enlargement in CT images. To be more accurate in the assessments, further studies are required.
Key Words: Cat, Computed tomography, Dog, Pituitary gland
Introduction
The pituitary gland is located in a bony recess (sella turcica) of basisphenoid bone at the base of the brain and has two distinct parts, the anterior lobe (adeno-hypophysis) and the posterior lobe (neurohypophysis).1,2 The anterior lobe has been termed as a master gland of body because of its important role in controlling of other hormones secretion.3 The adenohypophysis is made up of three distinct parts, the pars distalis, pars intermedia and pars tuberalis.4 The neurohypophysis (pars nervosa) makes a direct connection with hypothalamus via a stalk called infundibulum.3,4 Since pituitary gland secretes several hormones, its diseases and tumors can cause different abnormal conditions in animals. Specific diseases of the pituitary gland and their symptoms largely depend on the cause and affected area of this gland. The pituitary gland size of dogs has been measured with different modalities.5 One way to assess this gland is diagnostic imaging and in this case, computed tomography (CT) is remarkably utilizable. The CT has been used to examine normal and enlarged or abnormal pituitary glands in dogs.5 Also, it has been used to assess normal feline hypophysis.6 Pituitary gland dimensions are measured when pituitary adenomas are suspected or when trans-sphenoidal hypophysectomy is considered.5,7 The gland size is approximately 10.00 ×7.00 × 5.00 mm (length × width × height) in normal dogs and 5.20 × 3.10 mm (width × height) in normal cats.8
The pituitary gland was classified as enlarged if the gland was protruding above the suprasellar extension.8 In dogs, pituitary gland tumors first increase in height and then extend rostrally or caudally.5
The purpose of this research was a basic study of the relationship between pituitary gland dimensions and the age and weight in dogs and cats for this range of sample. It is useful to have further information about normal and enlarged hypophysis.
Materials and Methods
Animals. In this study, the CT images of dogs and cats brain both plain and with intravenous (IV) contrast were selected. The dogs were 1 to 9 years (mean 2.70 years) old weighing between 4.50 and 45.00 kg (mean 16.50 kg including six males and five females) and the cats were 1 to 14 years old (mean 4 years) and weighted between 1.50 and 5.00 kg (mean 3.00 kg including eight males and seven females) that had been referred to small animal hospital of University of Tehran.
Procedures. A chemistry panel and complete blood count (CBC) had been performed on all dogs prior to CT examination and there was no evidence of pituitary diseases in clinical signs, physical examination, clinical pathology and CT imaging findings. Animals were excluded if they had pituitary gland diseases related signs like polyuria, polydipsia, polyphagia, abdominal enlargement, endocrine alopecia, muscle wasting and hyperpigmentation and neurologic signs like seizures, visual deficits, ataxia, incoordination, facial hemiplegia, head tilt, somnolence, compulsive walking and depression or history of receiving regular steroid or insulin therapy.
Anesthesia was performed using ketamine (Alfasan, woerden, Netherlands) and acepromazine (Alfasan) in dogs (5.00 to 10.00 mg kg-1; IV and 0.05 to 0.10 mg kg-1; IV, respectively) and in cats (5.00 mg kg-1; IV and 0.10 to 0.20 mg kg-1; IM, respectively). During anesthesia continuous monitoring and observation were performed and then animals were transferred to the CT scan room.
All CT imaging was performed with third generation CT scanner (Somatom Spirit; Siemens, Munich, Germany). The animals were put in sternal recumbency and spongy radiolucent packs were used to avoid any movement during scans.
Pre-contrast scans of the skull (from the nostril to the end of the first cervical vertebra) were made with 110 to 130 kvp and 100 to 150 mA sec-1 (according to animal size and device traction), rotation time of 1.00 sec, slices thickness of 1 mm, pitch of 1 and interscan time of 9.50 sec without injecting contrast medium.8
Following 750 mg kg-1 IV administration of iodinated contrast medium (Iohexol; GE Healthcare, Oklahoma, USA) and on the previous radiation scanner settings, the post-contrast images were obtained.
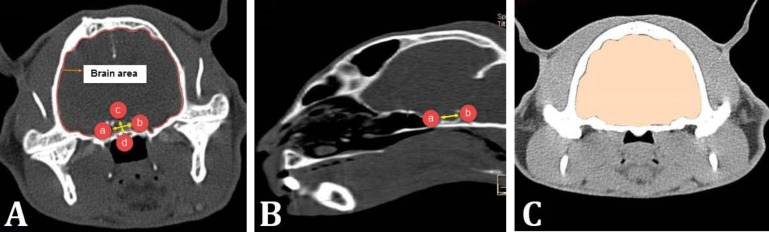
At the completion of the CT scan, the CT images were sent to the Syngo acquisition workstation and after their reconstruction by computers using installed software named Somaris/5.5 Syngo (Siemens Healthcare, Erlangen, Germany), the region of interest (ROI) was drawn around the pituitary gland. The dimensions of the pituitary gland include maximum length, height, width and brain area were drawn using ROI (Fig. 1). Also, pituitary gland volume, brain volume and percent of pituitary gland volume to brain volume were measured using volumetric software (manual method, slice thickness of 1.00 mm and upper Hansfield unit (HU) of 700 and lower HU of 0). Further, the pituitary height-to-brain ratio (P:B ratio) was obtained using the fallowing formula:8
Fig. 1.
Measurement of cat pituitary gland dimensions in transverse plane (A) and sagittal plane (B) and brain volume in transverse plane (C). A) a and b show pituitary width, c and d show pituitary height; B) a and b show pituitary length; C) Brain area was measured slice by slice (1 mm thickness) and brain volume was obtained by Syngo Volume CT software
P:B ratio = [Pituitary gland height (mm)×100 mm] / Brain area (mm 2 )
After recording the values, the correlations between pituitary gland and the brain measurements and age, weight and body condition score (BCS) of the animals were studied by SPSS software (version 22.0; IBM Corp., Armonk, USA). Independent-Samples t-test were used when data were classified into two groups, but if the data were classified into three groups or more one-way ANOVA tests were applied (p ˂ 0.05).
Results
Laboratory test results. All the dogs and cats had a normal clinical CBC and differential test analysis and no abnormality was found in dogs and cats. There was no history of hormonal abnormality in all samples, too.
Dogs. In all of the cases, the pituitary gland was almost oval- to spherical-shaped and had convex border in the sagittal plane and round shape in the transverse and dorsal planes. The mean pituitary gland length was 7.00 mm (± 2.14), the mean width was 4.80 mm (± 1.20) and the height was 3.80 mm (± 0.70). Measured range, mean and standard deviation for length, width, height, volume of pituitary gland, brain area, brain volume, P:B ratio and the percent of pituitary gland volume to brain volume in dogs are presented in Table 1.
Table 1.
Measurements of pituitary gland and brain in from CT images of in 11 dogs and 15 cats.
| Measurements |
Dogs
|
Cats
|
||||
|---|---|---|---|---|---|---|
| Range | Mean | SD | Range | Mean | SD | |
| Pituitary length (mm) | 4.50 - 9.80 | 7.00 | 2.14 | 3.50 – 5.90 | 4.98 | 0.69 |
| Pituitary width (mm) | 2.90 – 6.50 | 4.80 | 1.20 | 2.50 – 4.80 | 3.62 | 0.64 |
| Pituitary height (mm) | 2.60 – 5.10 | 3.80 | 0.70 | 2.10 – 3.50 | 2.62 | 0.34 |
| Pituitary volume (mm 3 ) | 20.40 – 166.40 | 77.53 | 51.64 | 12.60 – 40.00 | 26.19 | 7.99 |
| Brain area (mm 2 ) | 1498.00 – 1963.00 | 1729.55 | 153.27 | 818.00 – 1131.00 | 928.73 | 85.72 |
| Brain volume (mm 3 ) | 59580 – 92010 | 68832.73 | 8617.24 | 14840 – 29900 | 26420 | 3644.18 |
| P: B ratio | 0.15 – 0.27 | 0.21 | 0.03 | 0.19 – 0.31 | 0.28 | 0.05 |
| Pituitary volume to brain volume (%) | 0.03 – 0.23 | 0.10 | 0.07 | 0.03 – 0.26 | 0.10 | 0.05 |
P: B ratio: Pituitary height-to-brain ratio; SD: standard deviation.
There was no significant correlation between the size of pituitary gland and age, weight and BCS in dogs. No significant differences were found between the dimensions of pituitary gland in male and female dogs.
Following data analyses in all cats, significant difference was found between the height of pituitary gland and weight (Table 2) and BCS (Table 3). Also, significant correlation was found between the pituitary width and cat weights (Table 2) and between P: B ratio and BCS (Table 2). In a different manner to eliminate the impact of breed variations on the data survey, only the domestic short hair (DSH) cats were inserted in the statistical analysis and the result was much more accurate. In this case, significant difference was not found between pituitary gland measurements and DSH cats age. Pituitary height and P: B ratio in weight grouping (under 3.00 kg and up 3.00 kg), (Table 2) and BCS grouping from 1 to 5 (under 3.00 kg and up 3.00 kg), (Table 3) were significantly different (p ˂ 0.05).
Table 2.
All the significant differences between weight and pituitary measurements. Data are presented as mean
| Weight |
All cats
|
|
DSH cats
|
||
|---|---|---|---|---|---|
| Height (mm) * | Width (mm)# | Height (mm) † | P:B ratio ‡ | ||
| < 3.00 kg | 2.47 | 3.32 | 2.51 | 0.25 | |
| ≥ 3.00 kg | 2.85 | 4.06 | 3.00 | 0.32 | |
P:B ratio: Pituitary height-to-brain ratio; DSH: Domestic short hair.
Significant difference between height of pituitary gland and weight in all cats;
Significant correlation between the pituitary width and weight in all cats;
Significant difference between pituitary height and weight grouping in DSH cats (under 3 kg and up 3 kg);
Significant difference between P: B ratio and weight grouping in DSH cats (under 3 kg and up 3kg).
Table 3.
All the significant differences between body condition score and pituitary measurements. Data are presented as mean
| Weight |
All cats
|
|
DSH cats
|
||
|---|---|---|---|---|---|
| Height (mm) * | P: B ratio # | Height (mm) † | P: B ratio ‡ | ||
| < 3.00 kg | 2.34 | 0.23 | 2.40 | 0.24 | |
| ≥ 3.00 kg | 2.77 | 0.30 | 2.80 | 0.32 | |
P:B ratio: Pituitary height-to-brain ratio; DSH: Domestic short hair.
significant difference between the height of pituitary gland and BCS in all cats;
significant correlation between the P:B ratio and BCS in all cats;
significant difference between the pituitary height and BDS in DSH cats;
significant correlation between the P:B ratio and BCS in DSH cats.
Discussion
Because of the important function of pituitary gland in other glands and organs functions regulation, evaluation of disorders related to this part of the body is very valuable. One of the most common disorders of this gland is tumors such as adenoma and adenocarcinoma and the subsequent risk of pituitary-dependent hyperadrenocorticism. Trans-sphenoidal hypophysectomy is a highly efficient treatment for this disease.9 This treatment application and successful complete removal of pituitary gland require accurate information about the size and exact anatomical location of this gland; therefore, diagnostic imaging especially 2D planes CT scan and novel volumetric software method can be very helpful in this regard.10
In this study, pituitary gland dimensions and its volume in dogs were in agreement with previous studies. In our study, the mean pituitary gland length was 7.00 mm (± 2.14), the mean width was 4.80 mm (± 1.20), the mean height was 3.80 mm (± 0.70) and the mean brain area was 1729.55 mm2. In a study had been done in 2004, the height of pituitary gland was a little higher than our results, the brain area was a little lower and the P:B ratio was higher.11 In 2006, the results of another survey were very close to our results.5 The differences exist here may be due to the difference between breed and weights of samples. There was no significant difference between pituitary dimensions and age, weight and BCS of dogs confirming other studies done by CT scan and magnetic resonance imaging (MRI).12,13 Also, no significant difference was found regarding dimensions in male and female dogs, however significant differences have been found in humans.14-16 In order to describe this difference, differences and variation in the samples can be considered.
Evaluation of CT and MR images shows that the pituitary size in MRI is greater than it in CT scan.6,17 The results of this study regarding the range and dimensions of pituitary gland are in agreement with previous reports on the pituitary gland using CT scan and MRI.6,17 The minor differences could be due to the breed variation of the studied cats. Also, the mean pituitary volume was much closed to recent studies.17 In previous studies, there was no significant difference between the pituitary dimensions and age and body weight.6,17 However, in this study, the height and width of this gland in two groups of cats weighting 3.00 kg and over 3.00 kg had significant differences. This result can be interpreted that in weight groups, there is the possibility of differences in the size of the pituitary gland, which means that by increasing the weight or to some extent by increasing BCS, there is a possibility of pituitary size increasing and to prove this claim more samples are needed to be studied. The increase of pituitary gland height in human during puberty is well known and it is related to a hypersecretion of luteinizing hormone during this period.16 The growth of pituitary gland in human may be just a continuation of prenatal growth of the brain and may be related to puberty.15 There wasn’t any significant difference between the age and pituitary dimensions in this study and it is in agreement with previously published MR and CT scan evaluation in hypophysis.6,17 As a part of this paper, to remove the effect of breed variations on the measurement, only 10 DSH cats were selected and evaluated separately. The results obtained in this case seemed to be much more accurate and it indicated that race may have its impact on the dimensions. A P: B ratio was used to adjust body size in the referenced study.8 Different size of pituitary height in weight and BCS groupings could be greatly depended on the development of other tissues inside the skull including hypothalamus and brain and should also be noticed that in a particular race and age, weight gain is also affected by age.
Previously, significant difference was not found in the size of pituitary gland in male and female cats and it was corresponded with the results of our study in cats and dogs. 17 However, significant difference was found between men and women using MRI.15,16 A larger populations of non-neutered and neutered cats are needed to determine whether this is also true in cats or dogs. In the Tyson et al. study, the length of this gland was not measured to be compared with the present data.6
A limitation of Wallack et al. investigation was that some cats had signs relating to neurologic diseases and there were no assays on cats to support the contention that none had acromegaly or Cushing's disease.17 In our study, the onset of contrast enhancement and the index related to that are not discussed.
The higher P: B ratio in cats than dogs in this study might be due to the larger brain area in dogs rather than it in cats. In this study, the pituitary gland volume to brain volume ratio was presented, so that we have a better vision on pituitary dimensions and brain measurements and it could be a useful value to diagnose normal or abnormal enlarged pituitary gland.
The object size measured in CT images is highly dependent on the window level and the window width used to display images.5
The limitation of the present study is the lack of samples with less than one year old and it is suggested that use of samples under one year old would be more useful to understand the relationship between the pituitary gland and age or weight of animals.
To be more accurate in the assessments, it is better to select the samples just in one race as much as possible. None of the cats and dogs underwent necropsy confirmation of pituitary gland size and normalcy. The present study documented the normal range of pituitary gland size in dogs and cats especially in DSH cat breeds and can be used as guidelines along other studies to diagnose pituitary gland enlargement in CT images and it is much more useful in dogs and cats within this weight, BCS and age range. Findings in this paper can be useful to identify pituitary enlargement in CT images and in diagnosis or treatment process of hypophysis tumors.
Acknowledgements
The authors would like to thank Dr. Mirsepehr Pedram for technical support.
Conflict of interest
The authors do not have any potential conflicts of interest to declare.
References
- 1.Melmed S. The pituitary. 3rd ed. . London, UK: Academic Press; 2010. pp. 3–5. [Google Scholar]
- 2.Reece WO. Functional anatomy and physiology of domestic animals. 4th ed. . Ames, USA: John Wiley & Sons; 2009. pp. 374–376. [Google Scholar]
- 3.Evans HE, De Lahunta A. Miller's anatomy of the dog. 5th ed. Philadelphia, USA: Elsevier Health Sciences; 2013. pp. 810–814. [Google Scholar]
- 4.Sadler TW. Longman’s medical embryology. 9th ed. . Philadelphia, USA: Lippincott Williams & Wilkins; 2011. pp. 196–199. [Google Scholar]
- 5.Van der Vlugt-Meijer RH, Meij BP, Voorhout G. Intra-observer and interobserver agreement, reproducibility, and accuracy of computed tomographic measurements of pituitary gland dimensions in healthy dogs. Am J Vet Res. 2006;67(10):1750–1755. doi: 10.2460/ajvr.67.10.1750. [DOI] [PubMed] [Google Scholar]
- 6.Tyson R, Graham JP, Bermingham E, et al. Dynamic computed tomography of the normal feline hypophysis cerebri (Glandula pituitaria) Vet Radiol Ultrasound. 2005;46(1):33–38. doi: 10.1111/j.1740-8261.2005.00006.x. [DOI] [PubMed] [Google Scholar]
- 7.Vlugt‐Meijer RH, Meij BP, Ingh TS, et al. Dynamic computed tomography of the pituitary gland in dogs with pituitary‐dependent hyperadrenocorticism. J Vet Intern Med. 2003;17(6):773–780. doi: 10.1111/j.1939-1676.2003.tb02514.x. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz T, Saunders J. Veterinary computed tomography. 1st ed. . Chichester, UK: Wiley-Blackwell; 2011. pp. 97–203. [Google Scholar]
- 9.Meij BP. Hypophysectomy as a treatment for canine and feline Cushing's disease. Vet Clin North Am Small Anim Pract. 2001;31(5):1015–1041. doi: 10.1016/s0195-5616(01)50011-x. [DOI] [PubMed] [Google Scholar]
- 10.Hanson JM, Hoofd MM, Voorhout G, et al. Efficacy of transsphenoidal hypophysectomy in treatment of dogs with pituitary‐dependent hyperadrenocorticism. J Vet Intern Med. 2005;19(5):687–694. doi: 10.1892/0891-6640(2005)19[687:eothit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Van der Vlugt-Meijer RH, Meij BP, Voorhout G, et al. Dynamic computed tomographic evaluation of the pituitary gland in healthy dogs. Am J Vet Res. 2004;65(11):1518–1524. doi: 10.2460/ajvr.2004.65.1518. [DOI] [PubMed] [Google Scholar]
- 12.Auriemma E, Barthez PY, van der Vlugt-Meijer RH, et al. Computed tomography and low-field magnetic resonance imaging of the pituitary gland in dogs with pituitary-dependent hyperadrenocorticism: 11 cases (2001–2003) J Am Vet Med Assoc. 2009;235(4):409–414. doi: 10.2460/javma.235.4.409. [DOI] [PubMed] [Google Scholar]
- 13.Kippenes H, Gavin PR, Kraft SL, et al. Mensuration of the normal pituitary gland from magnetic resonance images in 96 dogs. Vet Radiol Ultrasound. 2001;42(2):130–133. doi: 10.1111/j.1740-8261.2001.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 14.Argyropoulou M, Perignon F, Brunelle F, et al. Height of normal pituitary gland as a function of age evaluated by magnetic resonance imaging in children. Pediatr Radiol. 1991;21(4):247–249. doi: 10.1007/BF02018614. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa K, Konishi Y, Matsuda T, et al. Development and aging of brain midline structures: Assessment with MR imaging. Radiology. 1989;172(1):171–177. doi: 10.1148/radiology.172.1.2740500. [DOI] [PubMed] [Google Scholar]
- 16.Yuh WT, Fisher DJ, Nguyen HD, et al. Sequential MR enhancement pattern in normal pituitary gland and in pituitary adenoma. AJNR Am J Neuroradiol. 1994;15(1):101–108. [PMC free article] [PubMed] [Google Scholar]
- 17.Wallack ST, Wisner ER, Feldman EC. Mensuration of the pituitary gland from magnetic resonance images in 17 cats. Vet Radiol Ultrasound. 2003;44(3):278–282. doi: 10.1111/j.1740-8261.2003.tb00455.x. [DOI] [PubMed] [Google Scholar]