Abstract
Zoonotic cutaneous leishmaniasis caused by Leishmania major is a most common type of vector-borne disease in Iran. The pentavalent antimonial drugs have been used in the treatment of cutaneous leishmaniasis for a long time, but drug resistance and some of serious side effects have been reported. Thus, discovery and development of new therapeutic candidates are needed. The CM11 peptide is one of these peptides that its anti-bacterial activity has been proven. This peptide is a short cecropin–melittin hybrid peptide obtained through a sequence combination approach. The aim of this study was to evaluate in vitro anti-leishmanial activity of CM11 peptide against amastigote forms of Leishmania major. In this study, amastigote forms of Iranian strain of L. major (MRHO/IR/75/ER) were cultured in the presence of different concentrations of meglumine antimoniate (Glucantime®) to find the most appropriate in vitro concentration of Glucantime® against L. major amastigotes. Then, the anti-leishmanial activities of various concentrations of CM11 peptide (8, 16, 32 and 64 µM) were evaluated for 24, 48 and 72 hr by DAPI staining. In addition, MTT assay was used to determine the cytotoxic effects of CM11 peptide on murine fibroblast cell line. The results showed that CM11 peptide has antimicrobial activity against Iranian isolate of L. major in the laboratory conditions. It seems that the CM11 peptide has significant potential to be used as a new anti-leishmanial agent.
Key Words: Amastigote, CM11 Peptide, Leishmania major
Introduction
Leishmaniasis is an infectious disease of humans and animals caused by different species of the genus Leishmania and transmitted by the bite of the female sand fly.1 Cutaneous leishmaniasis (CL) is one of the important health problems in tropical and subtropical countries around the world such as Iran.2 Iran is one of the 10 countries in the world with the most cases of CL.3 The CL has been observed clinically in two forms including rural type (wet wound) and urban type (dry wound). Rural cutaneous leishmaniasis caused by L. major is considered as zoonotic cutaneous leishmaniasis (ZCL). The ZCL is endemic in northeast, south and central parts of Iran and approximately 75.00% of reported CL in Iran are zoonotic type.4,5 The most common treatment is based on the administration of antimonial compounds including meglumine antimoniate (Glucantime®) and sodium stibogluconate (Pentostam®) which is known to be the first-line treatment in most parts of the world. However, in recent years, the effectiveness of these drugs has decreased by 20.00 to 50.00% and the emergence of resistant strains to these drugs is also one of the main problems of treatment.6-10 Therefore, achieving new anti-leishmanial drugs and therapeutic strategies is needed.
Antimicrobial peptides (AMPs) are important components of innate and adaptive immune system of humans and animals,11 which have been identified in various sources such as bacteria,12 plants,13 insects13,14 and mammals.15 The effects of various peptides including cecropin A and melittin (on the origin of insects) have been evaluated against L. donovani.16 Moreover, the effects of peptides such as histidin-5, skin polypeptide YY (with mammalian origin) were reported against L. donovani and L. major, respectively.17,18 According to potent antimicrobial activity of AMPs, in this study in vitro anti-leishmanial activity of a short cationic peptide (CM11) against amastigote forms of L. major was investigated. This peptide is an amphipathic hybrid peptide derived from 2-8 residues of cecropin A and 6-9 residues of melittin consisting of a highly basic N-terminal domain from cecropin A and a relatively hydrophobic C-terminal domain from melittin.19,20
Materials and Methods
Peptide synthesis. The CM11 peptide was synthesized by solid-phase synthesis method using standard protocols.21 The peptide was purified with reversed-phase semi-preparative high performance liquid chromatography on C18 tracer column. The purity of synthesized peptide was more than 95.00%.
Leishmania major culture. The Iranian standard strain of L. major promastigotes (MRHO/IR/75/ER) was prepared from the School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. Promastigotes were cultured in RPMI 1640 Glutamax (Gibco, San Francisco, USA) medium, 10.00% inactivated fetal bovine serum (FBS; Gibco), 100 mg mL-1 streptomycin and 100 IU mL-1 penicillin (Thermo Fisher Scientific Inc, Waltham, USA) at 25.00 ± 1.00 ˚C. Stationary-growth phases of promastigote culture were used to infect macrophage cell line RAW264.7.
Macrophage cell culture. Murine macrophage cell line RAW264.7 (ATCC number TIB-71) was provided from Iranian Biological Resource Center, Tehran, Iran. Adherent macrophages were cultured in RPMI 1640 medium containing 10.00% FBS and penicillin-streptomycin at 37 ˚C with 5.00% CO2. Macrophages were collected by scraping at almost 80.00% confluency.
Determination of effective concentration of Glucantime ® against L. major amastigote. Glucantime® (Rorer Rhone-Poulenc Specia, Paris, France) was received from the School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. Macrophage cell line RAW264.7 (2 × 104 cells per well) was cultured in RPMI medium containing 10.00% FBS in 8-chamber slide (SPL Life Sciences Co., Gyeonggi-do, Korea) and incubated at 37 ˚C with 5.00% CO2 for 6 hr. Then, adherent macrophages were infected with stationary phase promastigotes of L. major in a ratio of 10 parasites per macrophage and incubated at 37 ˚C for 18 hr. After 18 hr incubation, each well was washed by RPMI medium for three times to remove unphagocytosed parasites. Then, they were treated with Glucantime® solution at different concentrations (30.87, 61.75, 123.50 and 247.00 µM) for 24, 48 and 72 hr. No drug was added to the control wells. Finally, chamber slides were fixed in absolute methanol and stained with 10.00% Giemsa. The percentage of infected macrophages was evaluated by counting the number of amastigotes in each infected macrophage through examining 100 macrophages in comparison with control wells.22
Effect of CM11 peptide on L. major amastigote with DAPI staining. Briefly, RAW264.7 cells (2×104 cells per well) were seeded in 8-well chamber slide and incubated at 37 ˚C with 5.00% CO2 for 6 hr. Then, murine macro-phages were infected by promastigotes routinely at a ratio of 10:1 (parasites: macrophage) and incubated at 37 ˚C for 18 hr followed by three times washing with RPMI medium after 18 hr incubation period. The CM11 peptide solutions were used at concentrations of 8, 16, 32 and 64 µM. Infected macrophages without peptide were used as the negative control and Glucantime® in concentration of 123.50 μM mL-1 was used as a positive control. Macro-phages were fixed in methanol for 1 min and stained with 4', 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, USA). The slides were observed under ultraviolet light with the fluorescent microscope (BX50; Olympus Biotech, Hopkinton, USA). The number of amastigotes in 100 macrophages was counted. Moreover, the IC50 value was calculated using the GraphPad prism software (version 6.0; GraphPad software Inc., San Diego, USA).
Cytotoxic effect of CM11 peptide on fibroblast cells. The murine fibroblast cell line L929 (ATCC number CCL-1) a kind gift of National Institute of Genetic Engineering and Biotechnology, Tehran, Iran was used. The cytotoxic effect of CM11 peptide on fibroblast cells was performed by the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT; Sigma-Aldrich) metabolic activity assay for assessment of cell viability. Fibroblast cells (5 × 103 cells per well) were cultured in RPMI-1640 media with 10.00% FBS and kept in 5.00% CO2 incubator at 37 ˚C for 24 hr in a 96-well plate. The CM11 peptide solutions were used at different concentrations (8, 16, 32 and 64 µM) for 24, 48 and 72 hr. Fibroblast cells without peptide were used as negative control wells and Glucantime® (123.50 µM mL-1) was used as a positive control. The MTT (100 μL; 0.50 mg mL-1) was added in each well and then incubation was carried out at 37 ˚C for 4 hr. After that, the MTT solution was removed, isopropanol was added to dissolve the formazan crystals and the absorbance was determined using enzyme-linked immunosorbent assay reader (Awareness Technology, Ramsey, USA) at 570 nm filter.
Data analysis. All trials were performed in triplicate. Data were analyzed using the GraphPad Prism software to compare negative control and peptide-treated groups using ANOVA test. The p < 0.05 was considered statistically significant. Moreover, calculated IC50 value and created graph were provided using the GraphPad prism.
Results
Determination of effective concentration of Glucantime ® against L. major amastigote. In order to find the most appropriate concentration of Glucantime® with the highest effect and the lowest cytotoxicity, different concentrations of this drug were prepared and examined at 24, 48 and 72 hr after exposure in an in vitro assay. There was not a statistically significant difference between 30.87 and 61.75 μM mL-1 concentrations in comparison with the negative control after 24 and 48 hr exposure, however after 72 hr exposure, these concentrations of Glucantime® showed statistically significant difference (p < 0.05). The results indicated that 123.50 μM mL-1 of the Glucantime® was the best concentration for L. major amastigote growth inhibition at all times (Fig. 1).
Fig. 1.
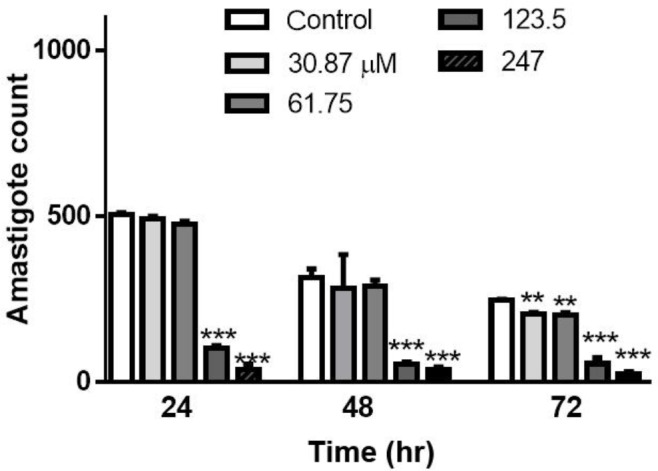
Distinguished effective concentrations with low toxicity of Glucantime® against Leishmania amastigote following in vitro assay after 24, 48 and 72 hr. Significance level of (**) is p ≤ 0.01 and (***) is p ≤ 0.001 and error bar displays the standard error of the mean
Anti-amastigote effect of the CM11 peptide. The effect of various concentrations of CM11 peptide against L. major amastigotes was evaluated with DAPI staining. All concentrations of CM11 peptide and Glucantime® as a positive control in a statistically significant manner decreased the number of amastigote in each infected macrophage compared to negative control (Fig. 2). The IC50 of CM11 peptide against amastigote forms was 9.58 μM after 48 hr. Statistical analysis demonstrated a dose-dependent anti-leishmanial activity of CM11 peptide.
Fig. 2.
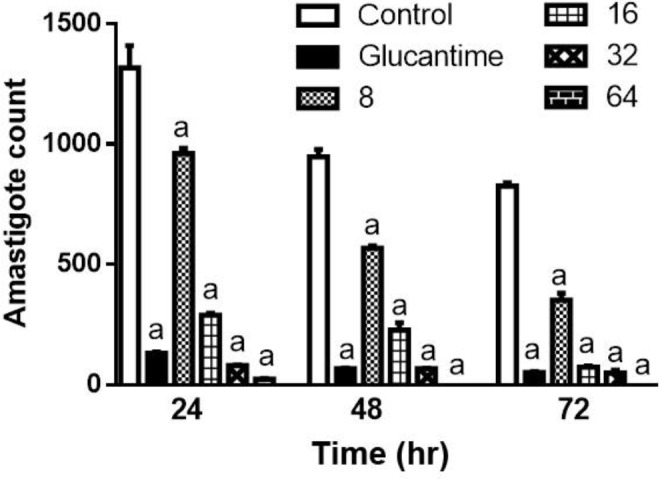
The Effect of CM11 peptide on Leishmania major amastigote following DAPI staining. Glucantime® was used as a control. Significance level of (a) is p ≤ 0.001. DAPI: 4', 6-diamidino-2-phenylindole
Cytotoxic effect of CM11 on murine fibroblast. The results of MTT assay at 24, 48 and 72 hr are shown in Figure 3. The 8 µM concentration of CM11 peptide did not show any cytotoxicity at all times, while fibroblast viability significantly decreased at 16, 32 and 64 µM concentrations. Also, Glucantime® indicated a higher toxic effect compared to the negative control group. The results showed that CM11 decreased fibroblasts viability with IC50 value of 36.57 µM after 48 hr.
Fig. 3.
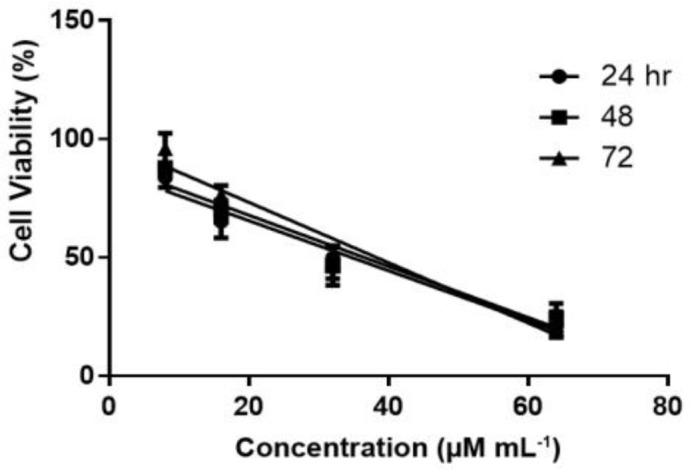
Cytotoxic activity of CM11 peptide on fibroblast cell line following MTT assay at 24, 48 and 72 hr. Inhibitory concentration at 50.00% (IC50) was assessed using GraphPad prism. MTT: 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
Discussion
According to the world health organization reports, leishmaniasis is one of the most important parasitic diseases and its treatment is the research priority.23,24 Leishmaniasis is still one of the health threats in Iran, therefore, control of this disease is very important. Thus, vaccine has been found for this disease and challenges to produce an effective vaccine are still ongoing.25,26 In this regard, the reservoirs elimination and pharmacological treatment are the most important methods for disease control and prevention. Sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime®) are used to treat leishmaniasis over the years and are still first-line drugs.27 However, in recent years, drug-resistant Leishmania has emerged in disease-endemic areas such as Iran.8,27 Hence, investigating a new group of anti-leishmanial compounds is essential. Accordingly, various types of anti-leishmanial agents such as plant extracts, nanomaterials and antimicrobial peptides have been investigated recently.
One of the most attractive anti-leishmanial agents are AMPs which have been recently evaluated for therapeutic strategies.28 It is noteworthy to mention that secondary infection by bacteria or fungi is one of the main problems in leishmaniasis treatment. Given the antibacterial and antifungal effects of AMPs, the use of these peptides can also be effective in bacteria and fungi contaminations removal from the lesion. However, the size of natural AMPs and some side effects such as toxicity on eukaryotic cells at high doses are a major problem. To overcome these problems, hybrid and short peptide analogs have been designed with more antimicrobial activity and lower cytotoxicity.29-31 One of these peptides is CM11, a short and amphipathic cationic peptide with antimicrobial effect against a range of pathogenic bacteria.20,32,33 Accordingly, in the present study, the effect of CM11 peptide against amastigote form of Leishmania was evaluated. For this purpose, first we determined the Glucantime® dosage as a positive control via in vitro assay. According to the results, 123.50 μM mL-1 of Glucantime® was the most appropriate concentration that inhibited the L. major amastigote forms growth in the culture medium at all times. Furthermore, DAPI staining and immunofluorescence microscopy showed that 8 μM concentration of the CM11 peptide was effective after 24 hr without toxicity on fibroblast cells. However, the IC50 value of peptide was 9.58 μM (13.50 µg mL-1) after 48 hr.
It is noteworthy to mention that all concentrations of the CM11 peptide and Glucantime® (123.50 μM mL-1) led to decrease in the number of amastigote forms in each infected macrophage compared to the negative control. Our findings indicated that CM11 peptide is effective against L. major in a dose-dependent manner.
Several studies have been recently performed to evaluate the anti-parasitic activity of different peptides against the Leishmania species. It should be noted that these studies have shown promastigotes susceptibility to AMPs more than amastigotes one; this can be attributed to the life cycle morphologies and biochemical differences of two stages of the protozoan parasite Leishmania and their location in the macrophage phagolysosome.16,34 Although many studies have reported the anti-leishmanial activity of peptides or other compounds, their evaluation methods have been different. Therefore, it's unlikely that their efficacy can be compared correctly. Nevertheless, it has been attempted to address some of these studies.
A study has suggested Nitroimidazolyl-1,3,4-thiadiazole as an anti-parasitic agent with an IC50 value of 9.35 ± 0.67 mM against the promastigote forms of L. major without cytotoxicity on host cells.35 However, in comparison with the CM11 peptide, the effective concentration of this compound is much higher.
Another study has shown that different concentrations of nano-silver can reduce the production of large L. major amastigotes. However, different concentrations of nano-silver did not remarkably reduce lesions and number of amastigotes in Balb/c mice.22 It has been previously indicated that treatment with 0.10% nano-silver for two weeks has considerable therapeutic potential on L. major (MRHO/IR/75/ER) in infected Balb/c mice, but compared to AMPs such as CM11 peptide, this compound is very toxic.36
Generally, the nature of antimicrobial peptides is different, therefore; each peptide can have different functions and effects such as antimicrobial activity or cytotoxicity. Based on many studies, these effects are dependent on hydrophobicity, amphipathicity, charge, stereochemistry, propensity of peptides to form barrels, target cell types and amino acids characteristics. On the other hand, sensitivity to AMPs and differences in eukaryotic cells viability are also dependent on membrane lipid compositions, cell membrane hydrophobicity and cells metabolic activity.20,32,37 Accordingly, it is also noteworthy to mention that the type of Leishmania parasitic stage will also be effective on the anti-leishmanial effect of CM11 peptide due to the cell membrane composition and structure.
Considering the use of anti-leishmanial drugs on skin, we examined the toxic effect of peptide on murine fibro-blasts. The MTT results indicated that CM11 peptide decreased fibroblasts viability with an IC50 value of 36.50 µM (51.60 µg mL-1) after 48 hr incubation which is much higher than anti-leishmanial concentration of the peptide. In a study by Moghaddam et al. it has been shown that the CM11 peptide can lead to death of 50.00% of some eukaryotic cells at 12.00 µM (16.00 µg mL-1) concentration after 48 hr, while macrophage viability was about 70.00%.33 Some differences in the findings can be influenced by the target pathogen type (bacteria or parasites) or host cells, research method and presence of stimulus and inhibitor.38
Also, a similar study has investigated the cytotoxic effect of BP100 peptide (one of the CM11 peptide analogues). According to the results, this peptide had cytotoxic effect in the range of 51.00 to 63.00 μM concentrations, but its evaluation in the mouse model revealed that the peptide up to a dose of 1000 to 2000 mg kg-1 of body weight is non-fatal for mice.30
By comparing the studies done in this field and the current study, it can be concluded that the CM11 peptide has significant potential to be applied for treatment of infections without harming living cells, however further researches on animal models are needed. This research was the first step in anti-leishmanial effect determination of CM11 peptide as a new drug candidate. The results showed that CM11 peptide has a significant anti-leishmanial activity in experimental conditions compared to the negative control group. Also, there was a significant difference between IC50 value of the CM11 peptide for amastigote forms (9.58 μM) and fibroblast cells (36.57 µM) after 48 hr exposure showing low in vitro cytotoxic effect of peptide at anti-amastigote concentration.
Acknowledgments
This study was approved and financially supported by a grant from Tehran University of Medical Sciences (Project No: 94-03-162-30313) and University of Tehran, Tehran, Iran (Project No. 30313). This study was a part of doctorate thesis of Mrs. Sara Khalili, a Ph.D student of Parasitology from Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran.
Conflict of interest
The authors declare there are no conflicts of interest.
References
- 1.Brito ME, Andrade MS, Dantas-Torres F, et al. Cutaneous leishmaniasis in northeastern Brazil: A critical appraisal of studies conducted in State of Pernambuco. Rev Soc Bras Med Trop. 2012;45(4):425–429. doi: 10.1590/s0037-86822012005000006. [DOI] [PubMed] [Google Scholar]
- 2.Aoun K, Bouratbine A. Cutaneous leishmaniasis in North Africa: A review. Parasite. 2014;21 doi: 10.1051/parasite/2014014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PloS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohebali M, Javadian E, Yaghoobi Ershadi M, et al. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. 2004;10(4-5):591–599. [PubMed] [Google Scholar]
- 5.Shirzadi M, Esfahani S, Mohebali M, et al. Epidemiological status of leishmaniasis in the Islamic Republic of Iran, 1983-2012. East Mediterr Health J. 2015;21(10):736–742. doi: 10.26719/2015.21.10.736. [DOI] [PubMed] [Google Scholar]
- 6.Murray HW, Berman JD, Davies CR, et al. Advances in leishmaniasis. Lancet. 2005;366(9496):1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 7.Mohebali M, Fotouhi A, Hooshmand B, et al. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007;103(1):33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Hadighi R, Mohebali M, Boucher P, et al. Un-responsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3(5):e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal MK, Rai S, Gupta S, et al. Characterization of natural antimony resistance in Leishmania donovani isolates. Am J TropMed Hyg. 2007;76(4):681–688. [PubMed] [Google Scholar]
- 10.Zarean M, Maraghi S, Hajjaran H, et al. Comparison of proteome profiling of two sensitive and resistant field Iranian isolates of Leishmania major to Glucantime® by 2-dimensional electrophoresis. Iran J Parasitol. 2015;10(1):19–29. [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 12.Cotter PD, Hill C, Ross RP. Bacteriocins: Developing innate immunity for food. Nat Rev Microbiol. 2005;3(10):777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 13.Thevissen K, Kristensen HH, Thomma BP, et al. Therapeutic potential of antifungal plant and insect defensins. Drug Discov Today . 2007;12(21-22):966–971. doi: 10.1016/j.drudis.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Slocinska M, Marciniak P, Rosinski G. Insects antiviral and anticancer peptides: New leads for the future? Protein Pept Lett. 2008;15(6):578–585. doi: 10.2174/092986608784966912. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Falla TJ. Host defense peptides for use as potential therapeutics. Curr Opin Investig Drugs. 2009;10(2):164–171. [PubMed] [Google Scholar]
- 16.Diaz-Achirica P, Ubach J, Guinea A, et al. The plasma membrane of Leishmania donovani promastigotes is the main target for CA (1–8) M (1–18), a synthetic cecropin A–melittin hybrid peptide. Biochem J. 1998;330(1):453–460. doi: 10.1042/bj3300453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luque-Ortega JR, Hof WV, Veerman EC, et al. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J. 2008;22(6):1817–1828. doi: 10.1096/fj.07-096081. [DOI] [PubMed] [Google Scholar]
- 18.Vouldoukis I, Shai Y, Nicolas P, et al. Broad spectrum antibiotic activity of skin-PYY. FEBS Lett. 1996;380(3):237–240. doi: 10.1016/0014-5793(96)00050-6. [DOI] [PubMed] [Google Scholar]
- 19.Ferre R, Badosa E, Feliu L, et al. Inhibition of plant-pathogenic bacteria by short synthetic cecropin A-melittin hybrid peptides. Appl Environ Microbiol. 2006;72(5):3302–3308. doi: 10.1128/AEM.72.5.3302-3308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammadi-Azad Z, Moravej H, Fasihi-Ramandi M, et al. In vitro synergistic effects of a short cationic peptide and clinically used antibiotics against drug-resistant isolates of Brucella melitensis. J Med Microbiol. 2017;66(7):919–926. doi: 10.1099/jmm.0.000524. [DOI] [PubMed] [Google Scholar]
- 21.Badosa E, Ferre R, Planas M, et al. A library of linear undecapeptides with bactericidal activity against phytopathogenic bacteria. Peptides. 2007;28(12):2276–2285. doi: 10.1016/j.peptides.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Mohebali M, Rezayat M, Gilani K, et al. Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): An in vitro and in vivo study. Daru J Pharm Sci. 2009;17(4):285–289. [Google Scholar]
- 23.Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27(5):305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Ouellette M, Papadopoulou B. Mechanisms of drug resistance in Leishmania. Parasitol Today. 1993;9(5):150–153. doi: 10.1016/0169-4758(93)90135-3. [DOI] [PubMed] [Google Scholar]
- 25.Handman E, Noormohammadi AH, Curtis JM, et al. Therapy of murine cutaneous leishmaniasis by DNA vaccination. Vaccine. 2000;18(26):3011–3017. doi: 10.1016/s0264-410x(00)00109-2. [DOI] [PubMed] [Google Scholar]
- 26.Stober CB, Lange UG, Roberts MT, et al. From genome to vaccines for leishmaniasis: Screening 100 novel vaccine candidates against murine Leishmania major infection. Vaccine. 2006;24(14):2602–2616. doi: 10.1016/j.vaccine.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Singh N, Kumar M, Singh RK. Leishmaniasis: Current status of available drugs and new potential drug targets. Asian Pac J Trop Med. 2012;5(6):485–497. doi: 10.1016/S1995-7645(12)60084-4. [DOI] [PubMed] [Google Scholar]
- 28.Cobb SL, Denny PW. Antimicrobial peptides for leishmaniasis. Curr Opin Investig Drugs. 2010;11(8):868–875. [PubMed] [Google Scholar]
- 29.Montesinos E. Antimicrobial peptides and plant disease control. FEMS Microbiol Lett. 2007;270(1):1–11. doi: 10.1111/j.1574-6968.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 30.Montesinos E, Bardaji E. Synthetic antimicrobial peptides as agricultural pesticides for plant-disease control. Chem Biodivers. 2008;5(7):1225–1237. doi: 10.1002/cbdv.200890111. [DOI] [PubMed] [Google Scholar]
- 31.Marcos JF, Munoz A, Perez-Paya E, et al. Identification and rational design of novel antimicrobial peptides for plant protection. Annu Rev Phytopathol. 2008;46:273–301. doi: 10.1146/annurev.phyto.121307.094843. [DOI] [PubMed] [Google Scholar]
- 32.Moghaddam MM, Abolhassani F, Babavalian H, et al. Comparison of in vitro antibacterial activities of two cationic peptides CM15 and CM11 against five pathogenic bacteria: Pseudomonas aeruginosa, Staphylococcus aureus, Vibrio cholerae, Acinetobacter baumannii, and Escherichia coli. Probiotics Antimicrob Proteins. 2012;4(2):133–139. doi: 10.1007/s12602-012-9098-7. [DOI] [PubMed] [Google Scholar]
- 33.Moghaddam MM, Aghamollaei H, Kooshki H, et al. The development of antimicrobial peptides as an approach to prevention of antibiotic resistance. Rev Med Microbiol. 2015;26(3):98–110. [Google Scholar]
- 34.Marr AK, Gooderham WJ, Hancock RE. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6(5):468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Poorrajab F, Ardestani SK, Emami S, et al. Nitroimidazolyl-1, 3, 4-thiadiazole-based anti-leishmanial agents: Synthesis and in vitro biological evaluation. Eur J Med Chem. 2009;44(4):1758–1762. doi: 10.1016/j.ejmech.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 36.Ghaffarifar F. Leishmania major: In vitro and in vivo anti-leishmanial effect of cantharidin. Exp Parasitol. 2010;126(2):126–129. doi: 10.1016/j.exppara.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Dolis D, Moreau C, Zachowski A, et al. Amino-phospholipid translocase and proteins involved in transmembrane phospholipid traffic. Biophys Chem. 1997;68(1-3):221–231. doi: 10.1016/s0301-4622(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 38.Rudenko SV, Wajdi KJM. Modulatory effect of peptide structure and equilibration conditions of cells on peptide-induced hemolysis. J VNKarazin Kharkiv Natl Univ Ser Biol. 2005;709(1-2):139–146. [Google Scholar]


