Abstract
Background
Malaria clinical outcomes vary by erythrocyte characteristics, including ABO blood group, but the effect of ABO blood group on asymptomatic, uncomplicated and placental Plasmodium falciparum (P. falciparum) infection remains unclear. We explored effects of ABO blood group on asymptomatic, uncomplicated and placental falciparum infection in the published literature.
Methods
A systematic review and meta-analysis was performed using the preferred reporting items for systematic reviews and meta-analyses guidelines. Articles in Pubmed, Embase, Web of Science, CINAHL and Cochrane Library published before February 04, 2017 were searched without restriction. Studies were included if they reported P. falciparum infection incidence or prevalence, stratified by ABO blood group.
Results
Of 1923 articles obtained from the five databases (Embase = 728, PubMed = 620, Web of Science = 549, CINAHL = 14, Cochrane Library = 12), 42 met criteria for systematic review and 37 for meta-analysis. Most studies (n = 30) were cross-sectional, seven were prospective cohort, and five were case-control studies. Meta-analysis showed similar odds of uncomplicated P. falciparum infection among individuals with blood group A (summary odds ratio [OR] 0.96, 15 studies), B (OR 0.89, 15 studies), AB (OR 0.85, 10 studies) and non-O (OR 0.95, 17 studies) as compared to those with blood group O. Meta-analysis of four cohort studies also showed similar risk of uncomplicated P. falciparum infection among individuals with blood group non-O and those with blood group O (summary relative risk [RR] 1.03). Meta-analysis of six studies showed similar odds of asymptomatic P. falciparum infection among individuals with blood group A (OR 1.05), B (OR 1.03), AB (OR 1.23), and non-O (OR 1.07) when compared to those with blood group O. However, odds of active placental P. falciparum infection was significantly lower in primiparous women with non-O blood groups (OR 0.46, 95% confidence interval [CI] 0.23 – 0.69, I2 0.0%, three studies), particularly in those with blood group A (OR 0.41, 95% CI 0.003 – 0.82, I2 1.4%, four studies) than those with blood group O.
Conclusions
This study suggests that ABO blood group may not affect susceptibility to asymptomatic and/or uncomplicated P. falciparum infection. However, blood group O primiparous women appear to be more susceptible to active placental P. falciparum infection.
Electronic supplementary material
The online version of this article (10.1186/s12879-019-3730-z) contains supplementary material, which is available to authorized users.
Keywords: ABO blood type, Asymptomatic malaria, Uncomplicated malaria, Placental malaria
Background
Malaria caused due to Plasmodium falciparum infection remains a major cause of death in tropical and subtropical countries [1, 2]. Individuals infected with P. falciparum may present with mild (e.g. fever, chills, headaches, nausea, malaise) or severe clinical symptoms (e.g. pulmonary edema, cerebral malaria, acute renal failure, severe anemia) [3, 4]. In malaria endemic areas, some individuals may have P. falciparum parasitemia, but not show symptoms suggestive of Plasmodium infection (asymptomatic parasitemia) [5, 6]. While severe P. falciparum infection may cause diverse organ dysfunction, uncomplicated P. falciparum infection usually causes mild health problems such as anemia and undernutrition [3, 4]. On the other hand, asymptomatic P. falciparum infection may not cause any significant health problems, although the parasite can persist in the blood for several months and produce gametocytes that can serve as a source of infection for the vector [5, 6]. Thus, asymptomatic infection contributes to the maintenance of malaria transmission in endemic regions [5, 6].
Various genetic variants or red blood cell polymorphisms have been identified that can make humans relatively more susceptible or resistant to P. falciparum infection and affect clinical outcomes of the disease [7, 8]. One of the genetic factor hypothesized to influence human susceptibility to clinical outcomes of malaria is ABO blood group [7–10]. Many studies have investigated the nature of interaction between ABO blood group and P. falciparum infection for decades (reviewed in [9, 10]). A recent meta-analysis study confirmed an increased severity of P. falciparum infection among individuals with blood group A, B and AB in comparison with those of blood group O [11]. However, the effect of ABO blood group on asymptomatic and uncomplicated P. falciparum infection remains uncertain. Some studies indicate that blood group O reduces, but blood groups A or B increase the odds of uncomplicated P. falciparum infection [12, 13]. Another study reported lower odds of uncomplicated P. falciparum infection in individuals with blood group A or B as compared to those with blood group O [14]. Still others reported lack of relationship between ABO blood group and uncomplicated P. falciparum infection [15, 16]. Findings on the relationship of ABO blood group and asymptomatic P. falciparum infection also remains heterogeneous [17–19].
In addition, it is hypothesized that ABO blood group could affect susceptibility to placental P. falciparum infection, which is common among pregnant women in malaria endemic regions particularly among primiparous women with low immunity [20, 21]. However, study findings vary. Studies among pregnant women in Gambia and Malawi showed that prevalence of active placental P. falciparum infection increased in primiparous women with blood group O, but decreased in multiparous women with blood group O as compared to those with non-O blood groups [22, 23]. However, the odds of past placental P. falciparum infection was found to be greater among pregnant women with blood group O than those with non-O blood group in both primiparous and multiparous women in Sudan [24]. On the other hand, a study in Gabon showed similar odds of active placental P. falciparum infection in both primiparous and multiparous women with different blood groups [25].
Understanding the effect of ABO blood group on clinical manifestations of P. falciparum infection may contribute to the understanding of malaria pathogenesis and clinical morbidity. This in turn will facilitate investigation of antimalarial treatments and vaccines. Loscertales et al (2007), and Cserti and Dzik (2007) reviewed literature on the relationship between ABO blood group and P. falciparum infection published before 2007 [9, 10]. Many studies that report data on the relationship of ABO blood groups and P. falciparum infection have been published since 2007. However, the relationship of ABO blood group with asymptomatic, uncomplicated and placental P. falciparum infection remains unclear. Published data show that ABO blood group affects progression to severe malaria after infection with P. falciparum. While blood type A delays clearance of parasitized red blood cells (pRBCs) by promoting rosetting and cytoadherence, blood group O increases clearance of pRBC by reducing rosetting and cytoadherence [26–29]. Thus, we hypothesized that the prevalence or odds of asymptomatic P. falciparum infection would be greater, but the odds of uncomplicated P. falciparum infection lower in individuals with blood group A, B and AB compared to those with blood group O. The objective of this study was to systematically summarize literature on relationships between ABO blood group and asymptomatic, uncomplicated and placental P. falciparum infection published before February 4, 2017.
Methods
The protocol for this review was registered in PROSPERO (ID = CRD42017068885), an international database of prospectively registered systematic reviews [30] and this report is accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for systematic reviews (Additional file 1: Table S1. PRISMA Checklist) [31].
Literature search strategies
Articles available in Pubmed, Embase, Web of Science, CINAHL and Cochrane Library were searched using the terms (“ABO blood type” OR “ABO blood group” OR “blood type” OR “blood group”) AND (Plasmodium OR malaria OR “Plasmodium falciparum” OR “Plasmodium vivax”) on February 04, 2017 (Additional file 2: Table S2. Literature search strategy). Language, date of inception, study design, age, gender and geography were not restricted. We examined references cited in reviews of malaria and ABO blood group for additional articles [9, 10]. Articles obtained from searches in the databases were combined in RefWorks. After the duplicates were removed, titles and abstracts of articles were screened, and full contents of eligible articles were reviewed. Two authors screened articles independently (AD and MG) using the eligibility criteria for the review. The degree of discrepancy in the screening and choice of articles between the two authors was very low and resolved by consensus.
Eligibility criteria
All published original studies of any design except case studies that assessed association between ABO blood group and asymptomatic, uncomplicated, or placental P. falciparum infection were included. Studies that did not confirm Plasmodium infection by PCR, microscopy, rapid diagnostic test, or histology were excluded. In addition, studies were excluded if the type of Plasmodium species assessed was not falciparum or was not clearly stated. Moreover, studies were excluded from meta-analysis when they lacked sufficient data to estimate the association between ABO blood group and asymptomatic, uncomplicated, or placental P. falciparum infection.
Outcome measures
Outcomes in this study were incidence or prevalence of asymptomatic, uncomplicated, or placental P. falciparum infection. Asymptomatic malaria refers to infections with Plasmodium parasite but without malaria-related symptoms such as fever, headache, nausea, chills, malaise, and sweating [5, 6]. Uncomplicated P. falciparum infection is accompanied by common malaria-related symptoms [3], but without severe malaria symptoms as defined by the World Health Organization [32]. Placental P. falciparum infection was defined as the presence of current (active) or past (passive) Plasmodium infection in the placenta as revealed by microscope or histology examination. Active placental P. falciparum infection refers to the presence of the parasite with or without parasite pigment [21]. Passive placental P. falciparum infection refers to the presence of parasite pigment without the parasite [21].
Data collection
For each study, we extracted data on the study area, study year, sample size, study design, prevalence of P. falciparum infection, and method of malaria diagnosis among individuals with different blood groups. We also extracted data on the measures of association (odds ratio (OR) or relative risk (RR)) between the ABO blood group and asymptomatic, uncomplicated, or placental P. falciparum infection among individuals. Two authors extracted data independently (AD and MG). The degree of discrepancy in data extraction was minimal between the two authors. When there was discrepancy, it was resolved by consensus. When studies did not report adjusted OR or RR of P. falciparum infection, these values were calculated using raw data on the prevalence of P. falciparum infection among individuals with blood groups A, B, AB and O.
Quality and bias assessment
We assessed quality of each study using six characteristics; selection bias, study design, confounder, blinding, data collection methods, withdrawal, and drop-outs following the scales suggested by the Effective Public Health Practice Project guidelines [33]. Each study was grouped as low, moderate, or high quality with respect to each of the six characteristics and an overall study quality was then determined based on the quality of all six characteristics. An overall quality of a study was grouped as high when the study has no weak rating with respect to each of the six characteristics, and moderate when the study has one weak rating in one of the six characteristics. An overall quality was graded as low when two or more weak ratings recorded out of the six characteristics.
Data analysis
Meta-analyses were performed using Stata version 11 [34]. The summary ORs or RRs of P. falciparum infection and the corresponding 95% confidence interval (CI) values across different blood groups were estimated using Der Simonian and Laird method following a random effects model (Moran’s I2 ≥ 30%) or using the inverse variance method following a fixed effects model (Moran’s I2 < 30%) [35]. Publication bias was assessed using funnel plots and Egger’s asymmetry test (bias if p < 0.1) [36, 37]. The magnitude of heterogeneity across the studies was determined using Moran’s I2 and statistical significance was tested using Cochrane chi-square test based on the inverse-variance fixed-effect model [38]. Sub-group analysis of the studies, which assessed the relationship between ABO blood group and placental P. falciparum infection, was performed after grouping studies based on the type of placental P. falciparum infection (active versus (vs) passive) and parity (primiparous vs multiparous). Meta-regression was conducted to estimate association of study-specific ORs of P. falciparum infection by study regions (Africa, Asia, South America), age of the study participants (children, adult, all ages combined), and study design (cross-sectional, case control, cohort).
Results
Characteristics of the included studies
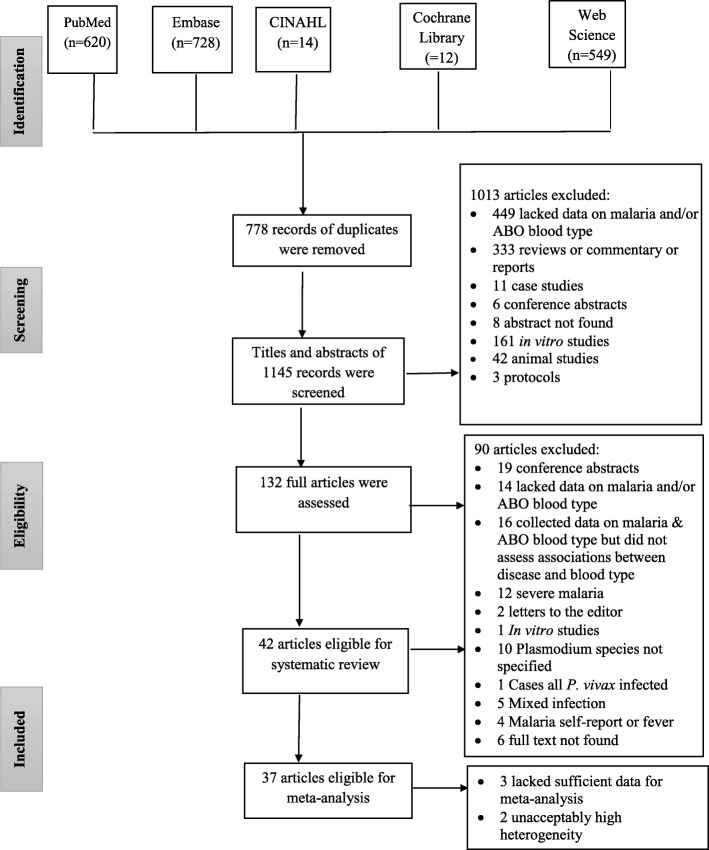
A total of 1923 articles were obtained after searching literature from five databases: Embase (n = 728), PubMed (n = 620), Web of science (n = 549), CINAHL (n = 14) and Cochrane Library (n = 12). Of the 1, 923 articles, 778 were found to be duplicates. After screening the titles and abstracts of the remaining 1145 articles, 132 were found eligible for full-text review. Ninety articles were excluded after full-text review based on inclusion/exclusion criteria. A total of 42 articles were included in this systematic review; 37 of them were also included in the meta-analysis (Fig. 1) [12–19, 22–25, 39–68]. Most (n = 30) studies were cross-sectional, seven studies were prospective cohort, and five were case-control in design. The 42 studies also differ in the age of the study population groups, 14 studies involved individuals of all age groups, 12 studies involved adolescents or adults, eight studies involved children and the remaining eight studies were conducted in pregnant women. Majority (n = 27) of the studies used microscope for the diagnosis of malaria. However, some studies, used both microscope and PCR (n = 6), histology (n = 4), rapid diagnostic (n = 1) or serology (n = 1) tests for the diagnosis of malaria. Still some studies used only PCR (n = 1) or histology technique (n = 2) for malaria diagnosis. (Additional file 3: Table S3. Characteristics of the studies).
Fig. 1.
PRISMA flow diagram showing the number of articles retrieved, screened, excluded and included at each stage of the search
ABO blood group and uncomplicated P. falciparum infection
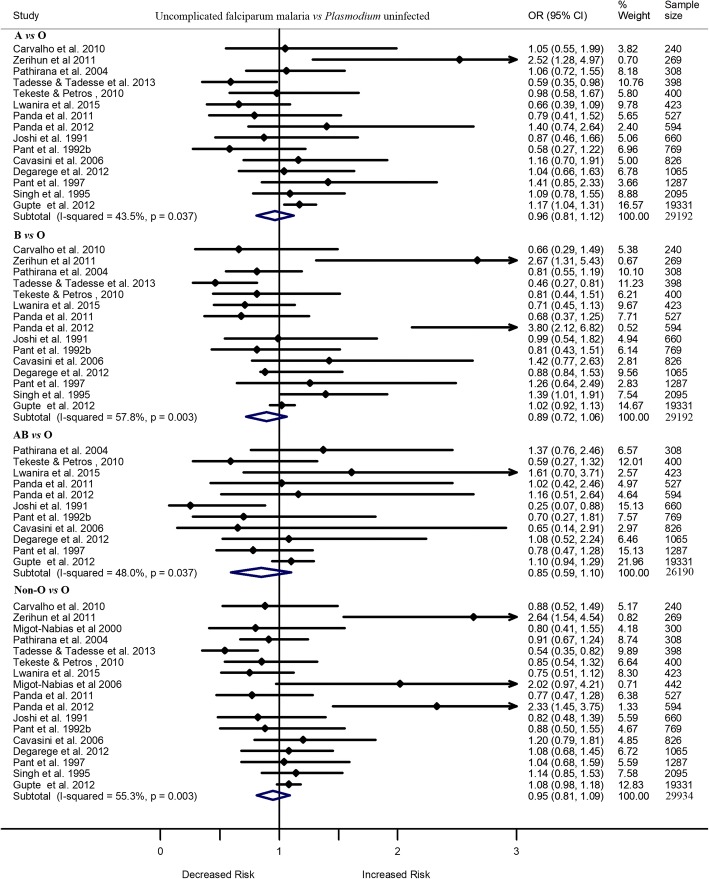
Of 42 included studies, 20 compared the odds of uncomplicated P. falciparum infection vs the odds of being uninfected with Plasmodium, and two studies compared the odds of uncomplicated P. falciparum infection vs the odds of developing asymptomatic P. falciparum infection among individuals with different blood group. Of the 20 studies, six reported significantly greater odds of uncomplicated P. falciparum infection, as compared to the odds of being not infected with the parasite, among individuals with blood group A [49, 66], B [12, 13, 64], AB [59], or non-O [12, 13] than those with blood group O. However, one study reported significantly lower odds of uncomplicated P. falciparum infection, as compared to being uninfected with Plasmodium, among individuals with blood group A, B, or non-O than those with blood group O [14]. The other 13 studies showed comparable odds of uncomplicated P. falciparum infection (vs uninfected with Plasmodium) among individuals with blood group A, B, AB, or non-O (A/B/AB) and those with blood group O. Meta-analysis of these studies showed that the odds of uncomplicated P. falciparum infection, as compared to the odds of being not infected with the parasite, did not differ significantly by blood group. All ORs were nonsignificant. They included: A vs O (OR 0.96, 95%CI 0.81–1.12, I2 43.5%, 15 studies), B vs O (OR 0.89, 95%CI 0.72 –1.06, I2 57.8%, 15 studies), AB vs O (OR 0.85, 95%CI 0.59 –1.10, I2 48.0%, 10 studies), or non-O vs O (OR 0.95, 95%CI 0.81–1.09, I2 55.3%, 17 studies) (Fig. 2).
Fig. 2.
Forest plot showing the odds of uncomplicated P. falciparum infection in individuals with blood group A, B, AB or non-O vs those with blood group O
Meta-analysis of two studies that compared the odds of uncomplicated P. falciparum infection vs asymptomatic P. falciparum infection also showed similar odds of uncomplicated P. falciparum infection among individuals with blood group A, B, or non-O and those of blood group O, but the odds of uncomplicated P. falciparum infection was lower in individuals with blood group AB than those of blood group O (OR 0.64, 95%CI 0.39 – 0.88, I2 0.0%) [43, 56].
Out of the 20 studies, four were cohort studies [53–55, 64]. Meta-analysis of these four cohort studies showed similar risk of uncomplicated P. falciparum infection (vs uninfected with Plasmodium) among individuals with non-O blood groups and those with blood group O (RR 1.03, 95%CI 0.84 –1.22, I2 57.3%) [53–55, 64]. Another study with a cohort design also reported similar risk of uncomplicated P. falciparum infection among children with blood group A, B, or AB as compared to those with blood group O, but this study was excluded from the meta-analysis due to lack of sufficient data [48]. Two other studies that reported data on the odds of uncomplicated P. falciparum infection among children by blood group were also not included in the meta-analysis because of wide 95% CI estimates (small number of cases), which introduced heterogeneity to the meta-analysis [59, 66].
ABO blood group and asymptomatic P. falciparum infection
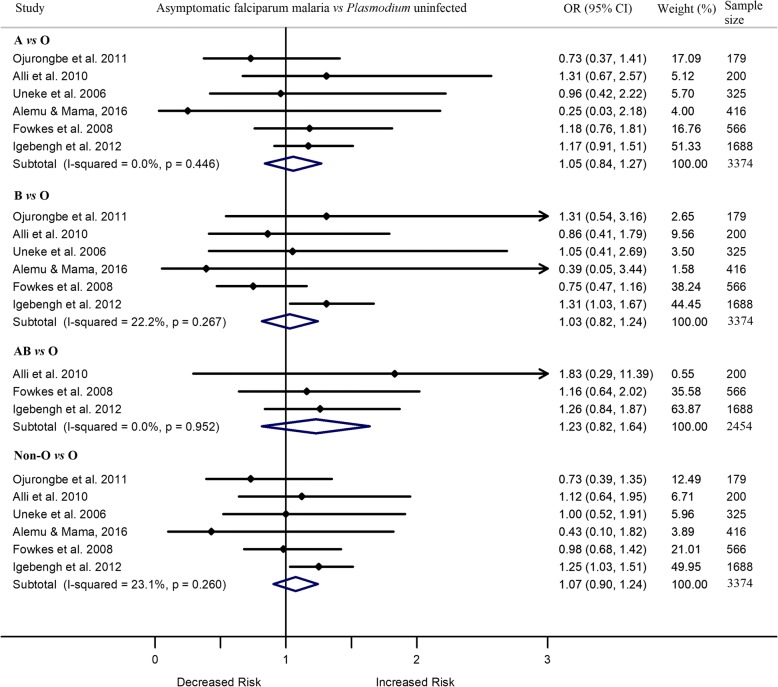
Out of 42 included studies, type of P. falciparum infection was assessed as asymptomatic in seven cross-sectional studies. Out of the seven, two studies in Nigeria reported contradictory results [18, 19]. Igebengh et al. 2012 [18] reported higher odds of asymptomatic P. falciparum infection in adults with blood group B or non-O than those with blood group O. However, Jeremiah et al. 2012 [19] reported lower odds of asymptomatic P. falciparum infection in children with blood group A, B, or non-O than those with blood group O. The remaining five studies showed similar odds of asymptomatic P. falciparum infection in children and adults with blood groups A, B, or AB and those with blood group O. Meta-analysis of the seven studies showed that individuals with asymptomatic infection had similar odds of having blood group A (OR 0.86, 95%CI 0.52 –1.20, I2 63.4%), B (OR 0.84, 95%CI 0.44 –1.24, I2 72.3%), AB (OR 1.21, 95%CI 0.82 –1.61, I2 0.0%), or non-O (OR 0.85, 95%CI 0.51–1.19, I2 78.5%) vs blood group O. The level of heterogeneity significantly decreased but the odds of asymptomatic P. falciparum infection remained similar between the comparison blood groups after removing one unique study [19], conducted in children. In these comparisons, ORs varied from 1.03 to 1.23 (A vs O [OR 1.05, 95%CI 0.84 –1.27, I2 0.0%], B vs O [OR 1.03, 95%CI 0.82 –1.24, I2 22.2%], AB vs O [OR 1.23, 95%CI 0.82 –1.64, I2 0.0%] and all non-O vs O [OR 1.07, 95%CI 0.90 –1.24, I2 23.1%]) (Fig. 3).
Fig. 3.
Forest plot showing the odds of asymptomatic P. falciparum infection in individuals with blood group A, B, AB or non-O vs those with blood group O
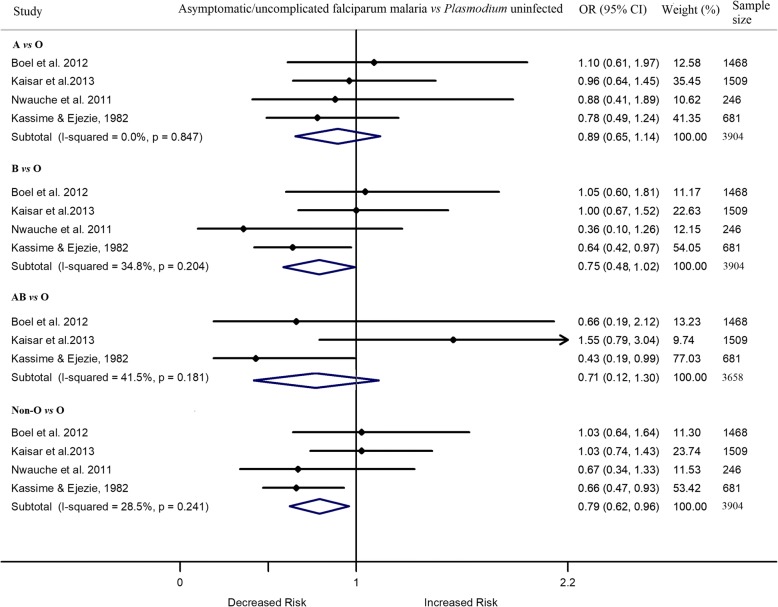
Five studies assessed the relationship between asymptomatic and/or uncomplicated P. falciparum infection and ABO blood group without distinction between cases being asymptomatic or uncomplicated falciparum malaria [45, 51–53, 63]. Meta-analysis of the four studies showed a similar likelihood of asymptomatic/uncomplicated P. falciparum infection between individuals with blood group A, B, or AB and those with blood group O [45, 51–53] (Fig. 4). One study was excluded from the meta-analysis due to lack of sufficient data [63].
Fig. 4.
Forest plot showing the odds of asymptomatic/uncomplicated P. falciparum infection in individuals with blood group A, B, AB or non-O vs those with blood group O
ABO blood group and placental P. falciparum infection
Of the 42 studies, eight compared the prevalence of placental P. falciparum infection among women by blood group. When compared to blood group O, odds of active placental falciparum infection in blood group non-O was greater in primiparous women but lower in multiparous ones in Ghana [44]. In contrast, in a Malawi study, the odds of active placental P. falciparum infection was greater among non-O blood group than blood group O in multiaparous women, but lower among non-O blood group than blood group O in primiparous women [23]. In a Nigerian study, the odds of active placental P. falciparum infection was lower among pregnant women with non-O blood group than those with blood group O [67], but this difference was not significant when data were analyzed after stratifying based on parity. The odds of passive placental P. falciparum infection in a study in Gambia was greater among pregnant women with non-O blood group than those with blood group O [22]. However, four studies showed no significant difference in the odds of placental falciparum infection among pregnant women with blood groups A, B, or AB compared to those with non-O blood group [24, 25, 39, 42]. Meta-analysis based on the eight studies showed similar odds of placental P. falciparum infection as compared to those without placental P. falciparum infection among pregnant women with non-O blood group and those with blood group O (Table 1).
Table 1.
Meta-analysis of the studies that compare the odds of placental P. falciparum infection among individuals with blood group A, B, AB or non-O vs those with blood type O
| Odds of Placental malaria (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Parity | Placental malaria Type | Study | Sample size | A vs O | B vs O | AB vs O | Non-O vs O |
| Primiparae or Multiparae | Active or passive vs none | Adam et al. 2007 | 293 | 0.8 (0.44, 1.44) | 0.55 (0.27, 1.1) | 0.57 (0.21, 1.55) | 0.67 (0.41, 1.1) |
| Loscertales & Brabin, 2006 | 198 | 1.35 (0.63, 2.91) | 1.17 (0.59, 2.29) | 1.58 (0.28, 9.01) | 1.26 (0.71, 2.22) | ||
| Senga et al. 2007 | 647 | 1.32 (0.89, 1.96) | 1.22 (0.83, 1.79) | 3.68 (1.39, 9.74) | 1.35 (0.99, 1.85) | ||
| Bedu-Addo et al. 2014 | 827 | 1.16 (0.81, 1.66) | 1.62 (1.09, 2.39) | 1.77 (0.8, 3.91) | 1.38 (1.04, 1.85) | ||
| Adegnika et al. 2011 | 378 | 1.11 (0.39, 3.19) | 2.38 (0.91, 6.23) | 2.00 (0.23, 17.13) | 1.64 (0.75, 3.59) | ||
| Ukaga et al. 2007 | 586 | 0.72 (0.46, 1.13) | 0.68 (0.39, 1.18) | 0.21 (0.07, 0.62) | 0.61 (0.42, 0.89) | ||
| Adam et al. 2009 | 236 | – | – | – | 1.25 (0.91, 2.5) | ||
| Alim et al. 2015 | 126 | – | – | – | 1.11 (0.4, 3.33) | ||
| summary OR (95% CI) | 0.97 (0.75, 1.19) I2 = 8.2%, p = 0.007 | 1.02 (0.64, 1.40) I2 = 58.0%, p = 0.036 | 0.59 (0.02, 1.14) I2 = 32.9%, p = 0.189 |
1.01 (0.73, 1.29) I2 = 58.8%, p = 0.017 | |||
| Primiparae | Active vs uninfected | Adam et al. 2007 | 293 | 2/31 vs 0/51 | 0/24 vs 0/51 | 1/8 vs 0/51 | 3/50 vs 0/51 |
| Loscertales & Brabin, 2006 | 198 | 0.55 (0.15, 1.99) | 0.33 (0.10, 1.08) | – | 0.41 (0.16, 1.08) | ||
| Senga et al. 2007 | 647 | 0.31 (0.10, 0.97) | 0.79 (0.30, 2.06) | 0.93 (0.06, 15.62) | 0.54 (0.24, 1.21) | ||
| Bedu-Addo et al. 2014 | 827 | 2.59 (1.03, 6.50) | 1.56 (0.84, 2.91) | 4.53 (0.52, 39.74) | 1.90 (1.10, 3.28) | ||
| Adegnika et al. 2011 | 378 | 4.8 (0.73, 31.48) | 1.85 (0.16, 2.2) | - | 3.2 (0.55, 18.56) | ||
| summary OR (95% CI) | 0.41 (0.003, 0.82) I2 = 1.4%, p = 0.385 | 1.04 (0.3, 1.78) I2 = 68.9%, p = 0.022 |
1.42 (−5.81, 8.65) I2 = 0.0%, p = 0.738 |
0.75 (0.17, 1.34) I2 = 53.8%, p = 0.090 | |||
| Passive vs uninfected | Adam et al. 2007 | 293 | 0.46 (0.15, 1.41) | 0.31 (0.08, 1.20) | 0.87 (0.15, 5.00) | 0.44 (0.18, 1.08) | |
| Loscertales & Brabin, 2006 | 198 | 2.13 (0.42, 10.73) | 1.28 (0.28, 5.93) | – | 2.00 (0.55, 7.18) | ||
| Senga et al. 2007 | 647 | 1.28 (0.58, 2.81) | 1.24 (0.54, 2.83) | 2.48 (0.26, 23.38) | 1.32 (0.67, 2.52) | ||
| Bedu-Addo et al. 2014 | 827 | – | – | – | 2.23 (1.17, 4.26) | ||
| summary OR (95% CI) | 0.68 (0.13, 1.22) I2 = 0.0%, p = 0.390 | 0.59 (0.03 1.21) I2 = 14.4%, p = 0.331 | 0.94 (0.44, 3.31) I2 = 0.0%, p = 0.789 | 0.82 (0.08, 1.57) I2 = 42.5%, p = 0.176 | |||
| Active vs passive | Loscertales & Brabin, 2006 | 198 | 0.26 (0.05, 1.26) | 0.63 (0.27, 1.49) | 0.38 (0.04, 3.53) | 0.42 (0.2, 0.86) | |
| Senga et al. 2007 | 647 | 0.24 (0.08, 0.70) | 0.69 (0.31, 1.53) | 0.49 (0.05, 4.34) | 0.46 (0.23, 0.92) | ||
| Bedu-Addo et al. 2014 | 827 | – | – | – | 1.44 (0.56, 3.69) | ||
| summary OR (95% CI) | 0.24 (0.03, 0.52), I2 = 0.0%, p = 0.954 |
0.66 (0.23, 1.09), I2 = 0.0%, p = 0.892 | 0.42 (0.01, 1.78), I2 = 0.0%, p = 0.938 |
0.46 (0.23, 0.69) I2 = 0.0%, p = 0.458 |
|||
| Multiparae | Active vs uninfected | Adam et al. 2007 | 293 | 3.59 (0.66, 19. 69) | 2 (0.26, 15.12) | – | 2.37 (0.47, 11.91) |
| Loscertales & Brabin, 2006 | 198 | 1.55 (0.45, 5.34) | 2.07 (0.72, 5.93) | 2.07 (0.26, 16.27)) | 1.87 (0.77, 4.56) | ||
| Senga et al. 2007 | 647 | 1.78 (0.95, 3.35) | 1.54 (0.84, 2.86) | 5.27 (1.44, 19.32) | 1.78 (1.07, 2.96) | ||
| Bedu-Addo et al. 2014 | 827 | 0.21 (0.11, 0.40) | 0.36 (0.19, 0.66) | 0.36 (0.12, 1.07) | 0.29 (0.17, 0.48) | ||
| Adegnika et al. 2011 | 378 | 0.51 (0.11, 2.37) | 2.59 (0.91, 7.37) | 2.44 (0.27, 21.98) | 1.36 (0.55, 3.34) | ||
| summary OR (95% CI) | 0.76 (0.02, 1.54) I2 = 51.7%, p = 0.082 | 1.11 (0.15, 2.07) I2 = 52.2%, p = 0.079 | 0.38 (0.09, 0.86) I2 = 0.0%, p = 0.515 | 1.20 (0.22, 2.19) I2 = 72.1%, p = 0.006 | |||
| Passive vs uninfected | Adam et al. 2007 | 293 | 0.56 (0.19, 1.66) | 0.99 (0.37, 2.62) | 0.47 (0.10, 1.94) | 0.70 (0.32, 1.53) | |
| Loscertales & Brabin, 2006 | 198 | 3.22 (0.94, 11.05) | 2.93 (0.94, 9.13) | – | 2.76 (1.04, 7.34) | ||
| Senga et al. 2007 | 647 | 1.22 (0.58, 2.58) | 1.18 (0.58, 2.39) | 6.32 (1.71, 23.41) | 1.38 (0.78, 2.45) | ||
| summary OR (95% CI) | 0.82 (0.24, 1.41) I2 = 0.0%, p = 0.375 | 1.16 (0.46, 1.85) I2 = 0.0%, p = 0.668 | 1.01 (0.001, 2.77) I2 = 16.1%, p = 0.304 |
1.06 (0.38, 1.74) I2 = 31.8%, p = 0.231 | |||
| Active vs passive | Loscertales & Brabin, 2006 | 198 | 1.46 (0.6, 3.54) | 0.71 (0.21, 2.34) | – | 0.68 (0.24, 1.95) | |
| Senga et al. 2007 | 647 | 1.46 (0.6, 3.54) | 1.31 (0.56, 3.08) | – | 1.29 (0.64, 2.59) | ||
| Adam et al. 2007 | 293 | 5.18 (0.76, 21.87) | 2.9 (0.36, 26.39) | – | 1.21 (0.16, 9.23) | ||
| summary OR (95% CI) | 1.49 (0.46, 2.53) I2 = 0.0%, p = 0.790 | 0.97 (0.16, 1.78) I2 = 0.0%, p = 0.743 | – | 0.95 (0.31, 1.59) I2 = 0.0%, p = 0.650 | |||
Summary OR estimated using a meta-analysis technique applying fixed (I2 < 30%) or random (I2 ≥ 30%) effect model. Statistical significance of the heterogeneity of the studies was tested using the Cochran’s Q test at α = 5%
Subgroup analysis based on parity and nature of placental P. falciparum infection showed lower odds of active placental P. falciparum infection (compared to absence of placental P. falciparum infection) among women with blood group A than those with blood group O in primiparous women (OR 0.41, 95%CI 0.003 – 0.82, I2 1.4%, four studies). The difference in odds of active placental P. falciparum infection between blood group A (OR 0.24, 95%CI 0.03 – 0.52, I2 0.0%; 2 studies) or non-O blood group (OR 0.46, 95%CI 0.23 – 0.69, I2 0.0%, three studies) and blood group O in primiparous women remained significant even when the comparison groups were women with passive placental P. falciparum infection. However, the difference in the odds of passive placental P. falciparum infection (as compared to without placental P. falciparum infection) among women with blood group O and those with blood group A, B, or AB were not significant in both primiparous and multiparous women (Table 1).
Publication bias and source of heterogeneity
The funnel plots based on the odds ratio of uncomplicated P. falciparum infection (vs uninfected) and the corresponding standard errors among individuals with blood group A vs O, B vs O, AB vs O and non-O vs O were symmetrical (Additional file 4: Figure S1. Funnel plots). The Egger’s test for the asymmetry was not significant for all the comparisons blood groups; A vs O (p = 0.234), B vs O (p = 0.932), AB vs O (p = 0.162) and non-O vs O (p = 0.846). Similarly, the funnel plots that showed the odds ratio of asymptomatic P. falciparum infection and the corresponding standard errors among individuals with blood group A vs O, B vs O and AB vs O were symmetrical. The corresponding Egger’s tests were non-significant for all the comparison blood groups except for blood group non-O vs O (Additional file 5: Figure S2. Funnel plots). The Egger’s test for asymmetry of funnel plots that showed the OR of placental P. falciparum infection among pregnant women with blood group A vs O (p = 0.911), B vs O (p = 0.666), AB vs O (p = 0.917) and non-O vs O (p = 0.991) were all nonsignificant (Additional file 6: Figure S3. Funnel plots).
The OR of uncomplicated P. falciparum among individuals with blood group A, B, AB or non-O vs those with blood group O did not vary by age of study participants, study region, or design of the study. Similarly, OR of asymptomatic infection among individuals with blood group A, B, AB or non-O vs those with blood group O did not vary with age of study participants, study region or and study design (Additional file 7: Table S4. Meta regression test values).
Quality of the studies
Most studies reported high quality data-collection methods and control for confounders. Many studies followed procedures of moderate quality for recruitment of study participants. However, most studies had low quality study design (cross-sectional). Overall quality of the studies using six characteristics---- selection bias, study design, confounder, blinding, data collection methods, withdrawal and dropouts showed that nine studies were of strong quality, six studies were moderate quality and 27 studies were low quality (Table 2).
Table 2.
Assessment of the quality of all studies included in the review
| Study no. | Author, Year [References] | Selection bias | Study design | Confounders | Blinding | Data collection methods | Withdrawals and drop-outs | Final rating |
|---|---|---|---|---|---|---|---|---|
| 1 | Adam et al. 2009 [39] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 2 | Adam et al. 2007 [24] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 3 | Adegnika et al. 2011 [25] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 4 | Alemu and Mama, 2016 [40] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 5 | AlIi et al. 2010 [41] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 6 | Alim et al. 2015 [42] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 7 | Amodu et al. 2012 [43] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 8 | Bedu-Addo et al. 2014 [44] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 9 | Boel et al. 2012 [45] | 1 | 1 | 2 | 2 | 1 | 2 | 1 |
| 10 | Carvalho et al. 2010 [15] | 1 | 2 | 2 | 2 | 1 | 2 | 1 |
| 11 | Cavasini et al. 2006 [46] | 1 | 2 | 2 | 2 | 1 | 2 | 1 |
| 12 | Degarege et al. 2012 [16] | 2 | 3 | 1 | 1 | 2 | 2 | 1 |
| 13 | Fowkes et al. 2008 [47] | 2 | 3 | 3 | 2 | 2 | NA | 3 |
| 14 | Giha et al. 2000 [48] | 2 | 1 | 3 | 2 | 1 | 2 | 2 |
| 15 | Gupte et al. 2012 [49] | 2 | 2 | 3 | 2 | 2 | 2 | 2 |
| 16 | Igbeneghu et al. 2012 [18] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 17 | Jeremiah et al. 2012 [19] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 18 | Joshi et al. 1987 [50] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 19 | Kaisar et al. 2013 [51] | 1 | 3 | 2 | 2 | 1 | NA | 2 |
| 20 | Kassime & Ejezie, 1982 [52] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 21 | Loscertales & Brabin, 2006 [22] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 22 | Lwanira et al. 2015 [53] | 1 | 1 | 1 | 2 | 2 | 1 | 1 |
| 23 | Migot-Nabias et al. 2000 [54] | 2 | 1 | 1 | 2 | 1 | 2 | 1 |
| 24 | Migot-Nabias et al. 2006 [55] | 2 | 1 | 1 | 2 | 1 | 2 | 1 |
| 25 | Missinou et al. 2003 [56] | 2 | 1 | 2 | 2 | 1 | 2 | 1 |
| 26 | Nwauche et al. 2011 [57] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 27 | Ojurongbe et al. 2011 [17] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 28 | Panda et al. 2012 [12] | 2 | 3 | 2 | 2 | 1 | NA | 2 |
| 29 | Panda et al. 2011 [58] | 2 | 3 | 2 | 2 | 1 | NA | 2 |
| 30 | Pant et al. 1992a [59] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 31 | Pant et al. 1992b [60] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 32 | Pant et al. 1997 [61] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 33 | Pathirana et al. 2004 [62] | 2 | 3 | 3 | 1 | 1 | NA | 3 |
| 34 | Rabha et al. 2012 [63] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 35 | Senga et al. 2007 [23] | 2 | 3 | 3 | 2 | 2 | NA | 3 |
| 36 | Singh et al. 1995 [64] | 2 | 1 | 2 | 2 | 1 | 2 | 1 |
| 37 | Tadesse & Tadesse, 2013 [14] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 38 | Tekeste & Petros, 2010 [65] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 39 | Thakur & Verma, 1992 [66] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 40 | Ukaga et al. 2007 [67] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 41 | Uneke et al. 2006 [68] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 42 | Zerihun et al. 2011 [13] | 2 | 3 | 3 | 2 | 1 | NA | 3 |
1 = strong; 2 = moderate; 3 = weak; NA = not applicable
Discussion
The current meta-analysis showed lack of association of ABO blood group with prevalence or incidence of asymptomatic and/or uncomplicated P. falciparum infection. This suggests that the ABO blood group may not affect susceptibility to asymptomatic and/or uncomplicated P. falciparum infection. A meta-analysis by Taylor et al. (2012) also confirmed lack of effect of different human red blood cell polymorphism (e.g. hemoglobin S, hemoglobin C, α and β thalassemia) on susceptibility to asymptomatic and uncomplicated malaria [69]. Indeed, there is no substantial research evidence that confirms any influence of ABO blood group on human contact with mosquitoes. Two older (and one more recent) studies reported potential biting preference of Anopheles gambae [70, 71] and Aedes albopictus [72] mosquitos for blood group O [70–72]. However, most findings suggest that ABO blood group affects pathogenesis of malaria after the parasite enters the human body [9–11, 28]. Thus, effect of the ABO blood group on uncomplicated P. falciparum infection would be better investigated with comparison groups consisting of asymptomatic falciparum malaria cases rather than who are uninfected (or healthy normal) with Plasmodium. Only two studies included in this review compared odds of uncomplicated P. falciparum infection to asymptomatic P. falciparum infection by blood groups [43, 56].
On the other hand, the odds of active placental P. falciparum infection was lower in primiparous women with non-O blood group particularly in those with blood group A, than in those with blood group O. This suggests that primiparous women with blood group O may be at increased risk and that of blood group A could be protective against active placental P. falciparum infection. A meta-analysis of three studies by Adegnika et al. (2011) also showed increased odds of active placental P. falciparum infection in primiparous women with blood group O than non-O blood group [25]. Maternal blood group may affect placental P. falciparum infection in a counterintuitive way. It may be that mothers with blood group A (if they are A1) are more likely to sequester their infected red cells in the maternal circulation (due to more cytoadhesion) leaving less infected red cells to passively reach the placenta [26]. Whether those P. falciparum erythrocytes membrane protein (PfEMP-1) positive red cells that do reach the placenta remain in the placenta or not would seem to be affected by placental gene expression. Indeed, studies showed association between peripheral parasitaemia and placental malaria infection during pregnancy [73, 74]. Maternal blood group may also affect maternofetal antibody transfer efficiency. Transfer efficiency of IgG1 and IgG3 against placental P. falciparum infection from mother to the fetus might be reduced in primiparous women with blood group O than those with blood group A. A recent study confirmed association of placental P. falciparum infection with reduced transfer efficiency of IgG1 and IgG3 from primiparous mother to the fetus, but this association was not seen in multiparous women [75]. Moreover, the increased occurrence of active placental P. falciparum infection among primiparous women with blood group O might be related to other genetic factors linked to ABO gene at chromosome 9 (9q34.2; especially to O alleles) that could act as permissive factors to active placental P. falciparum infection. Ordi et al (1998) reported increased placental lesions, characterized by accumulation of inflammatory infiltrate in the intervillous space, among primiparous women with placental malaria [76].
This study has implication for research. The reasons for the finding of increased odds of active placental P. falciparum infection among individuals with blood group O than those with blood group non-O is unclear. Additional studies are essential to understanding of mechanisms by which maternal O blood group increases the risk of active placental P. falciparum infection, while blood group A protects against it. Future studies may characterize and evaluate the nature of antigens on erythrocyte membranes of different blood types which would increase or decrease the risk of active placental P. falciparum infection.
This is the first systematic review and meta-analysis to assess the relationship of ABO blood group with asymptomatic and uncomplicated P. falciparum infection. In addition, although a previous study estimated the relationship between ABO blood group and placental P. falciparum infection [23] using four studies, the current study summarizes that relationship using eight studies. Moreover, there was no publication bias among the studies which compared the odds of asymptomatic, uncomplicated P. falciparum infection among individuals with blood group A, B, AB or Non-O and those with blood group O. However, this review has some limitations. There was a moderate level of heterogeneity in some of the meta-analyses performed. Variations in the age of the study participants, and study regions and designs did not significantly contribute to the increased heterogeneity. In addition, studies included in this review compared the odds of uncomplicated and/or asymptomatic P. falciparum infection with the odds of being uninfected with Plasmodium. Thus, it is impossible to confirm fully if the ABO blood group can affect progression from asymptomatic to uncomplicated P. falciparum infection. Future studies may compare the odds of uncomplicated P. falciparum infection with the odds of having asymptomatic P. falciparum infection among individuals with different blood groups. Moreover, limitations in the original studies could have affected the current summary estimates. For example, some studies that investigated relationship of ABO blood group with placental P. falciparum infection involved small sample size or few cases, thus had very wide confidence interval for the effect measure estimates and low statistical power to reject false associations. In addition, most studies included in the review did not control for confounders (e.g. socioeconomic factors, nutrition, infection, thalassemias, and haemoglobin variants including HbS, HbC and HbE) while they evaluated the relationship of ABO blood group with P. falciparum infection [77–82].
Conclusions
This review suggests that primiparous women with blood group O appear to be more susceptible to active placental P. falciparum infection than those with non-O blood group. However, ABO blood group may not influence susceptibility to asymptomatic and uncomplicated P. falciparum infection. Future studies need to investigate the mechanisms by which blood group A reduces risk of active placental falciparum infection in primiparous women.
Additional files
Table S1. PRISMA checklist. (DOC 65 kb)
Table S2. Literature search strategy (DOCX 13 kb)
Table S3. Characteristics of the studies included in this review (DOCX 42 kb)
Figure S1. Funnel plot. Odds ratio against standard error of odds ratio for studies, which compared the odds of uncomplicated Plasmodium falciparum infection vs Plasmodium uninfected among individuals with blood group A vs O, B vs O, AB vs O and Non-O vs O. (DOCX 279 kb)
Figure S2. Funnel plot. Odds ratio against standard error of odds ratio for studies, which compared the odds of asymptomatic Plasmodium falciparum infection vs Plasmodium uninfected among individuals with blood group A vs O, B vs O, AB vs O and Non-O vs O. (DOCX 244 kb)
Figure S3. Funnel plot. Odds ratio against standard error of odds ratio for studies, which compared the odds of placental Plasmodium falciparum infection vs Plasmodium uninfected among individuals with blood group A vs O, B vs O, AB vs O and Non-O vs O. (DOCX 205 kb)
Table S4. Sources of heterogeneity assessment based on meta-regression analyses. (DOCX 16 kb)
Acknowledgements
We thank all the authors of the studies included in this review.
Funding
This study did not get financial support from external sources.
Availability of data and materials
All data analyzed in this study are included in this article and its additional files.
Abbreviations
- CI
Confidence interval
- OR
Odds ratio
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- PROSPERO
International prospective register of systematic reviews
- RR
Relative risk
Authors’ contributions
AD conceived the idea and developed the protocol. AD and MTG searched and screened the literature and extracted the data. AD and MTG resolved discrepancies. AD analysed the data, interpreted the result, and drafted the manuscript. CMB, MW, LCM and PM reviewed and significantly contributed for the improvement of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abraham Degarege, Email: ameng002@fiu.edu.
Merhawi T. Gebrezgi, Email: mgebr003@fiu.edu
Consuelo M. Beck-Sague, Email: cbecksag@fiu.edu
Mats Wahlgren, Email: Mats.Wahlgren@ki.se.
Luiz Carlos de Mattos, Email: luiz.carlos@famerp.br.
Purnima Madhivanan, Email: pmadhiva@fiu.edu.
References
- 1.Snow RW. Global malaria eradication and the importance of Plasmodium falciparum epidemiology in Africa. BMC Med. 2015;13:23. doi: 10.1186/s12916-014-0254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, et al. Malaria: Global progress 2000–2015 and future challenges. Infect Dis Poverty. 2016;5(1):61. doi: 10.1186/s40249-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Disv. 2012;4(1):e2012026. doi: 10.4084/mjhid.2012.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maitland K. Management of severe paediatric malaria in resource-limited settings. BMC Med. 2015;13:42. doi: 10.1186/s12916-014-0263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect. 2013;11(6):623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 6.Laishram DD, Sutton PL, Nanda N, Sharma VL, Sobti RC, Carlton JM, et al. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J. 2012;11:29. doi: 10.1186/1475-2875-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driss A, Hibbert JM, Wilson NO, Iqbal SA, Adamkiewicz TV, Stiles JK. Genetic polymorphisms linked to susceptibility to malaria. Malar J. 2011;10:271. doi: 10.1186/1475-2875-10-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahlgren M, Goel S, Akhouri RR. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat Rev Microbiol. 2017;15(8):479–491. doi: 10.1038/nrmicro.2017.47. [DOI] [PubMed] [Google Scholar]
- 9.Cserti CM, Dzik WH. The ABO blood group system and Plasmodium falciparum malaria. Blood. 2007;110(7):2250–2258. doi: 10.1182/blood-2007-03-077602. [DOI] [PubMed] [Google Scholar]
- 10.Loscertales MP, Owens S, O'Donnell J, Bunn J, Bosch-Capblanch X, Brabin BJ. ABO blood group phenotypes and Plasmodium falciparum malaria: unlocking a pivotal mechanism. Adv Parasitol. 2007;65:1–50. doi: 10.1016/S0065-308X(07)65001-5. [DOI] [PubMed] [Google Scholar]
- 11.Degarege A, Gebrezgi MT, Ibaneza G, Wahlgren M, Madhivanan P. Effect of the ABO blood group on susceptibility to severe malaria: a systematic review and meta-analysis. Blood Rev. 2018;33:53–62. doi: 10.1016/j.blre.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Panda AK, Panda M, Tripathy R, Pattanaik SS, Ravindran B, Das BK. Complement receptor 1 variants confer protection from severe malaria in Odisha, India. PLoS One. 2012;7(11):e49420. doi: 10.1371/journal.pone.0049420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zerihun T, Degarege A, Erko B. Association of ABO blood group and Plasmodium falciparum malaria in Dore Bafeno area, Southern Ethiopia. Asian Pac J Trop Biomed. 2011;1(4):289–294. doi: 10.1016/S2221-1691(11)60045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tadesse H, Tadesse K. Assessing the association of severe malaria infection and ABO blood groups in northwestern Ethiopia. J Vector Borne Dis. 2013;50(4):292–296. [PubMed] [Google Scholar]
- 15.Carvalho DB, de Mattos LC, Souza-Neiras WC, Bonini-Domingos CR, Cósimo AB, Storti-Melo LM, et al. Frequency of ABO blood group system polymorphisms in Plasmodium falciparum malaria patients and blood donors from the Brazilian Amazon region. Genet Mol Res. 2010;9(3):1443–1449. doi: 10.4238/vol9-3gmr803. [DOI] [PubMed] [Google Scholar]
- 16.Degarege A, Medhin G, Animut A, Legess M, Erko B. Association of ABO blood group and P. Falciparum malaria related outcomes: a cross-sectional study in Ethiopia. Acta Trop. 2012;123(3):164–169. doi: 10.1016/j.actatropica.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Ojurongbe O, Tijani BD, Fawole AA, Adeyeba OA, Kun JF. Prevalence of dihydrofolate reductase gene mutations in Plasmodium falciparum isolate from pregnant women in Nigeria. Infect Dis Rep. 2011;3(2):e16. doi: 10.4081/idr.2011.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igbeneghu C, Odaibo GN, Olaleye DO, Odaibo AB. Malaria infection and ABO blood grouping in Iwo community, southwestern Nigeria. Res J Med Sci. 2012;6(5):247–250. [Google Scholar]
- 19.Jeremiah ZA, Jeremiah TA, Emelike FO. Frequencies of some human genetic markers and their association with Plasmodium falciparum malaria in the Niger delta. Nigeria J Vector Borne Dis. 2010;47(1):11–16. [PubMed] [Google Scholar]
- 20.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 22.Loscertales MP, Brabin BJ. ABO phenotypes and malaria related outcomes in mothers and babies in the Gambia: a role for histo-blood groups in placental malaria? Malar J. 2006;5:72. doi: 10.1186/1475-2875-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senga E, Loscertales MP, Makwakwa KE, et al. ABO blood group phenotypes influence parity specific immunity to Plasmodium falciparum malaria in Malawian women. Malar J. 2007;6:102. doi: 10.1186/1475-2875-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam I, Babiker S, Mohmmed AA, Salih MM, Prins MH, Zaki ZM. ABO blood group system and placental malaria in an area of unstable malaria transmission in eastern Sudan. Malar J. 2007;6:110. doi: 10.1186/1475-2875-6-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adegnika AA, Luty AJ, Grobusch MP, Ramharter M, Yazdanbakhsh M, Kremsner PG et al. ABO blood group and the risk of placental malaria in sub-Saharan Africa. Malar J. 2011;10:101-2875-10-101. [DOI] [PMC free article] [PubMed]
- 26.Cserti-Gazdewich CM, Mayr WR, Dzik WH. Plasmodium falciparum malaria and the immunogenetics of ABO, HLA, and CD36 (platelet glycoprotein IV) Vox Sang. 2011;100(1):99–111. doi: 10.1111/j.1423-0410.2010.01429.x. [DOI] [PubMed] [Google Scholar]
- 27.Moll K, Palmkvist M, Ch'ng J, Kiwuwa MS, Wahlgren M. Evasion of immunity to Plasmodium falciparum: rosettes of blood group a impair recognition of PfEMP1. PLoS One. 2015;10(12):e0145120. doi: 10.1371/journal.pone.0145120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe JA, Handel IG, Thera MA, Deans AM, Lyke KE, Koné A, et al. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc Natl Acad Sci U S A. 2007;104(44):17471–17476. doi: 10.1073/pnas.0705390104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goel S, Palmkvist M, Moll K, Joannin N, Lara P, Akhouri RR, et al. RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nat Med. 2015;21:314–317. doi: 10.1038/nm.3812. [DOI] [PubMed] [Google Scholar]
- 30.Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, Stewart L. PROSPERO at one year: an evaluation of its utility. Syst Rev. 2013;2:4. doi: 10.1186/2046-4053-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF, PRISMA-IPD development group Preferred reporting items for systematic review and Meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 32.WHO Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:1–90. [Google Scholar]
- 33.Effective Public Health Practice Project. Quality assessment tool for quantitative studies. Hamilton, ON: Effective public health practice project. Available from: https://merst.ca/ephpp. Accessed 29 June 2018.
- 34.Stata Corp. Stata statistical software: release 11. College Station: StataCorp LP.
- 35.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol. 2005;15(6):235–243. doi: 10.2188/jea.15.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14:951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 39.Adam I, Adamt GK, Mohmmed AA, Salih MM, Ibrahuim SA, Ryan CA. Placental malaria and lack of prenatal care in an area of unstable malaria transmission in eastern Sudan. J Parasitol. 2009;95(3):751–752. doi: 10.1645/GE-1912.1. [DOI] [PubMed] [Google Scholar]
- 40.Alemu G, Mama M. Assessing ABO/Rh blood group frequency and association with asymptomatic malaria among blood donors attending Arba Minch blood bank, South Ethiopia. Malar Res Treat. 2016;2016:8043768. doi: 10.1155/2016/8043768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali JA, Okonko IO, Abraham OA, Kolade AF, Ogunjobi PN, Salako AO, et al. A survey of blood parasites (plasmodium, microfilaria, HIV, HBsAG, HCV antibodies) in prospective Nigerian blood donors. Res J Med Sci. 2010;4(4):255–275. [Google Scholar]
- 42.Alim A, Bilal NE, Abass A, Elhassan EM, Mohmmed AA, Adam I. Complement activation, placental malaria infection, and birth weight in areas characterized by unstable malaria transmission in Central Sudan. Diagn Pathol. 2015;10:49. doi: 10.1186/s13000-015-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amodu OK, Olaniyan SA, Adeyemo AA, Troye-Blomberg M, Olumese PE, Omotade OO. Association of the sickle cell trait and the ABO blood group with clinical severity of malaria in Southwest Nigeria. Acta Trop. 2012;123(2):72–77. doi: 10.1016/j.actatropica.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Bedu-Addo G, Gai PP, Meese S, Eggelte TA, Thangaraj K, Mockenhaupt FP. Reduced prevalence of placental malaria in primiparae with blood group O. Malar J. 2014;13:289–2875–13-289. [DOI] [PMC free article] [PubMed]
- 45.Boel ME, Rijken MJ, Pimanpanarak M, Keereecharoen NL, Proux S, Nosten F, et al. No association of phenotypic ABO blood group and malaria during pregnancy. Am J Trop Med Hyg. 2012;87(3):447–449. doi: 10.4269/ajtmh.2012.12-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavasini CE, De Mattos LC, Alves RT, Couto AA, Calvosa VS, Domingos CR, et al. Frequencies of ABO, MNSs, and duffy phenotypes among blood donors and malaria patients from four brazilian amazon areas. Hum Biol. 2006;78(2):215–219. doi: 10.1353/hub.2006.0034. [DOI] [PubMed] [Google Scholar]
- 47.Fowkes FJ, Michon P, Pilling L, et al. Host erythrocyte polymorphisms and exposure to Plasmodium falciparum in Papua new guinea. Malar J. 2008;7:1–2875-7-1. [DOI] [PMC free article] [PubMed]
- 48.Giha HA, Rosthoj S, Dodoo D, Ripley RM, Tavul L, Imrie HJ, et al. The epidemiology of febrile malaria episodes in an area of unstable and seasonal transmission. Trans R Soc Trop Med Hyg. 2000;94(6):645–651. doi: 10.1016/S0035-9203(00)90218-9. [DOI] [PubMed] [Google Scholar]
- 49.Gupte SC, Patel AG, Patel TG. Association of ABO groups in malaria infection of variable severity. J Vector Borne Dis. 2012;49(2):78–81. [PubMed] [Google Scholar]
- 50.Joshi H, Raghavendra K, Subbarao SK, Sharma VP. Genetic markers in malaria patients of Delhi. Indian J Malariol. 1987;24(1):33–38. [PubMed] [Google Scholar]
- 51.Kaisar MM, Supali T, Wiria AE, Hamid F, Wammes LJ, Sartono E, et al. Epidemiology of plasmodium infections in Flores island, Indonesia using real-time PCR. Malar J. 2013;12:169–2875–12-169. [DOI] [PMC free article] [PubMed]
- 52.Kassim OO, Ejezie GC. ABO blood groups in malaria and schistosomiasis haematobium. Acta Trop. 1982;39(2):179–184. [PubMed] [Google Scholar]
- 53.Lwanira CN, Mukasa MK, Swedberg G, Kironde F. Frequency of RANTES gene polymorphisms and their association with incidence of malaria: A longitudinal study on children in Iganga district, Uganda. Malar J. 2015;14:341–015–0875-0. [DOI] [PMC free article] [PubMed]
- 54.Migot-Nabias F, Mombo LE, Luty AJ, Dubois B, Nabias R, Bisseye C, et al. Human genetic factors related to susceptibility to mild malaria in Gabon. Genes Immun. 2000;1(7):435–441. doi: 10.1038/sj.gene.6363703. [DOI] [PubMed] [Google Scholar]
- 55.Migot-Nabias F, Pelleau S, Watier L, Guitard J, Toly C, De Araujo C, et al. Red blood cell polymorphisms in relation to Plasmodium falciparum asymptomatic parasite densities and morbidity in Senegal. Microbes Infect. 2006;8(9–10):2352–2358. doi: 10.1016/j.micinf.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 56.Missinou MA, Lell B, Kremsner PG. Uncommon asymptomatic Plasmodium falciparum infections in Gabonese children. Clin Infect Dis. 2003;36(9):1198–1202. doi: 10.1086/374555. [DOI] [PubMed] [Google Scholar]
- 57.Nwauche CA, Chijioke-Nwauche IN, Sutherland C, Oguike MC, Ebong OO. The interaction between malaria parasites and blood groups in port Harcourt, Nigeria. Am J Trop Med Hyg. 2011;85(6):358. [Google Scholar]
- 58.Panda AK, Panda SK, Sahu AN, Tripathy R, Ravindran B, Das BK. Association of ABO blood group with severe falciparum malaria in adults: Case control study and meta-analysis. Malar J. 2011;10:309–2875–10-309. [DOI] [PMC free article] [PubMed]
- 59.Pant CS, Gupta DK, Bhatt RM, Gautam AS, Sharma RC. An epidemiological study of G-6-PD deficiency, sickle cell haemoglobin, and ABO blood groups in relation to malaria incidence in Muslim and Christian communities of Kheda, Gujarat, (India) J Commun Dis. 1992;24(4):199–205. [PubMed] [Google Scholar]
- 60.Pant CS, Gupta DK, Sharma RC, Gautam AS, Bhatt RM. Frequency of ABO blood groups, sickle-cell haemoglobin, G-6-PD deficiency and their relation with malaria in scheduled castes and scheduled tribes of Kheda district, Gujarat. Indian J Malariol. 1992;29(4):235–239. [PubMed] [Google Scholar]
- 61.Pant CS, Srivastava HC. Distribution of three genetic markers and malaria in other backward castes of Kheda district, Gujarat. Indian J Malariol. 1997;34(1):42–46. [PubMed] [Google Scholar]
- 62.Pathirana SL, Alles HK, Bandara S, Phone-Kyaw M, Perera MK, Wickremasinghe AR, et al. ABO-blood-group types and protection against severe, Plasmodium falciparum malaria. Ann Trop Med Parasitol. 2005;99(2):119–124. doi: 10.1179/136485905X19946. [DOI] [PubMed] [Google Scholar]
- 63.Rabha B, Goswami D, Dhiman S, Das NG, Talukdar PK, Nath MJ, et al. A cross sectional investigation of malaria epidemiology among seven tea estates in Assam, India. J Parasit Dis. 2012;36(1):1–6. doi: 10.1007/s12639-011-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh N, Shukla MM, Uniyal VP, Sharma VP. ABO blood groups among malaria cases from district Mandla, Madhya Pradesh. Indian J Malariol. 1995;32(2):59–63. [PubMed] [Google Scholar]
- 65.Tekeste Z, Petros B. The ABO blood group and Plasmodium falciparum malaria in awash, Metehara and Ziway areas, Ethiopia. Malar J. 2010;9:280–287. doi: 10.1186/1475-2875-9-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thakur A, Verma IC. Malaria and ABO blood groups. Indian J Malariol. 1992;29(4):241–244. [PubMed] [Google Scholar]
- 67.Ukaga CN, Nwoke BE, Udujih OS, Udujih OG, Ohaeri AA, Anosike JC, et al. Placental malaria in Owerri, Imo state, South-Eastern Nigeria. Tanzan Health Res Bull. 2007;9(3):180–185. doi: 10.4314/thrb.v9i3.14326. [DOI] [PubMed] [Google Scholar]
- 68.Uneke CJ, Ogbu O, Nwojiji V. Potential risk of induced malaria by blood transfusion in South-Eastern Nigeria. Mcgill J Med. 2006;9(1):8–13. [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(6):457–468. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood CS, Harrison GA, Dore C, Weiner J. S Selective feeding of Anopheles gambiae according to ABO blood group status. Nature. 1972;239:165. doi: 10.1038/239165a0. [DOI] [PubMed] [Google Scholar]
- 71.Wood CS. New evidence for a late introduction of malaria into the New World. Curr Anthropol. 1975;16:93–104. doi: 10.1086/201519. [DOI] [Google Scholar]
- 72.Shirai Y, Funada H, Seki T, Morohashi M, Kamimura K. Landing preference of Aedes albopictus (Diptera: Culicidae) on human skin among ABO blood groups, secretors or nonsecretors, and ABH antigens. J Med Entomol. 2004;41(4):796–799. doi: 10.1603/0022-2585-41.4.796. [DOI] [PubMed] [Google Scholar]
- 73.Kapisi J, Kakuru A, Jagannathan P, Muhindo MK, Natureeba P, Awori P. Relationships between infection with Plasmodium falciparum during pregnancy, measures of placental malaria, and adverse birth outcomes. Malar J. 2017;16(1):400. doi: 10.1186/s12936-017-2040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Beaudrap P, Turyakira E, White LJ, Nabasumba C, Tumwebaze B, Muehlenbachs A, et al. Impact of malaria during pregnancy on pregnancy outcomes in a Ugandan prospective cohort with intensive malaria screening and prompt treatment. Malar J. 2013;12:139. doi: 10.1186/1475-2875-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McLean ARD, Stanisic D, McGready R, Chotivanich K, Clapham C, Baiwog F. P. falciparum infection and maternofetal antibody transfer in malaria-endemic settings of varying transmission. PLoS One. 2017;12(10):e0186577. doi: 10.1371/journal.pone.0186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–1011. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 77.Degarege A, Degarege D, Veledar E, Erko B, Nacher M, Beck-Sague CM, et al. Plasmodium falciparum Infection Status among Children with Schistosoma in Sub-Saharan Africa: A Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2016;10(12):e0005193. doi: 10.1371/journal.pntd.0005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Degarege A, Veledar E, Degarege D, Erko B, Nacher M, Madhivanan P. Plasmodium falciparum and soil-transmitted helminth co-infections among children in sub-Saharan Africa: a systematic review and meta-analysis. Parasit Vectors. 2016;9(1):344. doi: 10.1186/s13071-016-1594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noor AM, Gething PW, Alegana VA, Patil AP, Hay SI, Muchiri E, et al. The risks of malaria infection in Kenya in 2009. BMC Infect Dis. 2009;9:180. doi: 10.1186/1471-2334-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Degarege A, Legesse M, Medhin G, Animut A, Erko B. Malaria and related outcomes in patients with intestinal helminths: a cross-sectional study. BMC Infect Dis. 2012;12:291. doi: 10.1186/1471-2334-12-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Unger HW, Ashorn P, Cates JE, Dewey KG, Rogerson SJ. Undernutrition and malaria in pregnancy - a dangerous dyad? BMC Med. 2016;14(1):142. doi: 10.1186/s12916-016-0695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Degarege A, Fennie K, Degarege D, Chennupati S, Madhivanan P. Improving socioeconomic status may reduce the burden of malaria in sub Saharan Africa: a systematic review and meta-analysis. Plos One. 2019. 10.1371/journal.pone.021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PRISMA checklist. (DOC 65 kb)
Table S2. Literature search strategy (DOCX 13 kb)
Table S3. Characteristics of the studies included in this review (DOCX 42 kb)
Figure S1. Funnel plot. Odds ratio against standard error of odds ratio for studies, which compared the odds of uncomplicated Plasmodium falciparum infection vs Plasmodium uninfected among individuals with blood group A vs O, B vs O, AB vs O and Non-O vs O. (DOCX 279 kb)
Figure S2. Funnel plot. Odds ratio against standard error of odds ratio for studies, which compared the odds of asymptomatic Plasmodium falciparum infection vs Plasmodium uninfected among individuals with blood group A vs O, B vs O, AB vs O and Non-O vs O. (DOCX 244 kb)
Figure S3. Funnel plot. Odds ratio against standard error of odds ratio for studies, which compared the odds of placental Plasmodium falciparum infection vs Plasmodium uninfected among individuals with blood group A vs O, B vs O, AB vs O and Non-O vs O. (DOCX 205 kb)
Table S4. Sources of heterogeneity assessment based on meta-regression analyses. (DOCX 16 kb)
Data Availability Statement
All data analyzed in this study are included in this article and its additional files.