Abstract
Anti-beta-2-glycoprotein I antibodies (anti-β2GPI) which are the main antiphospholipid antibodies that characterize the autoimmune “antiphospholipid syndrome” are pathogenic and are contributing to thrombosis. We aimed to evaluate the presence and the diagnostic importance of these antibodies in children with different rheumatologic diseases with or without thrombosis risk. A total of 100 children with different rheumatologic diseases evaluated retrospectively. The mean anti-β2GPI IgG (p = 0.108), IgA (p = 0.547), and IgM (p = 0.807) levels showed no statistically significant difference between different diagnosis groups. But anti-β2GPI IgA and IgM levels were higher in SLE patient group. The mean anti-β2GPI IgG (p = 0.375), IgA (p = 0.811), and IgM (p = 0.276) levels were not also showed difference between disease groups with/without predisposition to thrombosis even though concentrations were higher in thrombosis group. In children with rheumatological complaints, anti-β2GPI antibody measurements should not be the first diagnostic criteria if vasculitis is not thought as the primary defect underlying the clinical symptoms.
Keywords: Anti-β2GPI, Autoimmunity, Rheumatological disorder, Thrombosis
Introduction
Beta-2-glycoprotein I (β2GPI), also called apolipoprotein H, is a 50 kDa β2 globulin which is associated in vivo with lipoprotein, platelets and phospholipids [1] and is composed of five domains which cause structural heterogeneity [2]. It is known as an inhibitor of the contact activation of the intrinsic coagulation pathway [3] and is predominantly synthesized in hepatocytes, the lesser extent in endothelial cells and trophoblasts and circulates in blood at variable levels [4].
From 1990 onwards, the interest in β2GPI increased significantly, when this protein was identified as the most important antigen in an autoimmune disease called antiphospholipid syndrome (APS) [5], which occurs due to the autoimmune production of antibodies against phospholipid, a cell membrane substance and is a disorder of coagulation causing thrombosis in vessels, as well as pregnancy-related complications such as miscarriage, preterm delivery or severe preeclampsia [6]. Antiphospholipid antibodies are mainly targeted against complexes composed of negatively charged phospholipids (cardiolipin) and plasma proteins (β2GPI, prothrombin, protein C, protein S) and are first reported in patients affected by systemic lupus erythematosus (SLE) and subsequently in association with other collagen vascular diseases, infectious conditions and certain drugs. β2GPI is more specific than other molecules in APS [5]. β2GPI dependent anticardiolipin antibodies are correlated with thrombosis and are only found in the case of autoimmune diseases; β2GPI independent anticardiolipin antibodies are often found in connection with infectious diseases as syphilis, borreliosis, hepatitis, tuberculosis [7]. Anti-β2GPI antibodies recognize specific epitopes on human β2GPI and are expressed only when β2GPI interacts with lipid membranes or when absorbed to other surfaces. Detection of these antibodies provides a serological aid for the differentiation of autoimmune diseases from infections and also enables the discrimination between primary and secondary APS which are characterized by the same hematological immune responses [8, 9]. In secondary APS, they occur during the course of the disease as secondary reactions, most frequently in connection with rheumatic diseases, connective tissue diseases such as SLE [10] as well as related autoimmune diseases.
Strongly positive results for anti-β2GPI IgG and IgM antibodies (> 40 U/mL for IgG and/or IgM) are diagnostic criterion for APS and lesser levels of anti-β2GPI antibodies and the IgA isotype may occur in patients with clinical signs of APS, but the results are not considered diagnostic [6]. Anti-β2GPI IgA concentrations higher than 15 U/mL with negative IgG and IgM isotype results are not diagnostic. After International Congress on Antiphospholipid Antibodies in 2010, an update for the guideline on anticardiolipin and anti-β2GPI testing was published in [11]. This guideline concludes that the evidence for an association between anti-β2GPI and APS is strongest for the IgG isotype and a confirmatory test following a positive anti-phospholipid test, has to be repeated to avoid the detection of transient antibodies as usually occur in infectious diseases [6]. Recently, domain-specific studies are more preferred and a subgroup of anti-β2GPI IgG which is directed to domain 1 of the molecule is shown strongly associated with thrombosis [12].
Low concentrations of natural β2GPI IgG autoantibodies are normally found in healthy individuals [13] and levels increase with age. However, these antibodies may cause leading to differentiation APS. The triggering mechanism and pathophysiology Show uncertainty. Autoantibodies increase the risk for blood clotting especially other conditions that favor clotting are present such as prolonged inactivity, surgery, pregnancy, hypertension, obesity, smoking, atherosclerosis, the use of estrogens, and an associated systemic autoimmune disease (mainly SLE or SLE—like diseases).
Many rheumatologic diseases share similar clinical symptoms and this makes it hard to distinguish them from one to another. In this case, the diagnosis depends on specific laboratory data as the main presence of antibodies against self-proteins. In this study, we aimed to examine the diagnostic value of anti-β2GPI antibodies besides other inflammatory parameters and its relationship with thrombosis risk in children with different rheumatologic diseases.
Patients and Methods
A total of 100 patients (37 boys, 63 girls) who were followed up for rheumatological findings in Outpatient Clinics of Pediatric Rheumatology, Ege University Faculty of Medicine, Izmir, Turkey during 2016 were evaluated retrospectively for identifying the diagnostic performance of anti-β2GPI antibodies. Written informed consents were obtained from parents and the study was performed after approval of ethical committee in Ege University Faculty of Medicine. All patients’ data were evaluated retrospectively and an evaluation sheet was used to summarize the patients’ demographic information including name, gender, date of birth and clinical and laboratory data. The patient group was classified into different rheumatologic diagnosis groups according to clinical and laboratory findings and also evaluated as two disease groups with [Behcet disease, Henoch Schonlein vasculitis (HSV) and SLE] or without [juvenile idiopathic arthritis (JIA), familial Mediterranean fever (FMF) and undifferentiated connective tissue disease (UCTD)] predisposition to thrombosis.
Serum complement 3 (C3), complement 4 (C4), C—reactive protein (CRP), serum amyloid A (SAA) concentrations were analyzed quantitatively by a nephelometer (Dade Behring BNII, Siemens, Germany).
Serum anti-β2GPI IgG, IgM, and IgA levels were measured by a commercially available ELISA kit (Euroimmun, Lubeck, Germany). This test kit contains microtiter strips which each well is coated with β2GPI. In the first reaction step, diluted patient samples are incubated with the wells. In case of positive samples, specific IgG, IgA or IgM antibodies bind to the antigens. To detect the bound antibodies, a second incubation is carried out using an enzyme—labeled anti-human IgG-enzyme conjugate catalyzing a color reaction. The upper limit of the normal range recommended by the manufacturer is 20 relative units (RU)/mL for anti-β2GPI IgG, IgA, and IgM.
Autoimmunity was also evaluated with the presence of antinuclear antibody (ANA) in serum which was determined by immunofluorescence on mosaic Hep-2-10/liver monkey cell (Euroimmun, Lubeck, Germany) and ANA titers of 1:100 were taken as cut-off value.
All statistical analyses were performed by using SPSS Windows Version 16.0, SPSS Inc., Chicago, IL. One sample Kolmogorov–Smirnov test was used to check the Gaussian distribution of all variables. Student t test, Mann–Whitney U or Kruskal–Wallis test and Pearson or Spearman correlation coefficient were used in keeping with data normality distribution. A two-sided p value less than 0.05 was considered as statistically significant.
Results
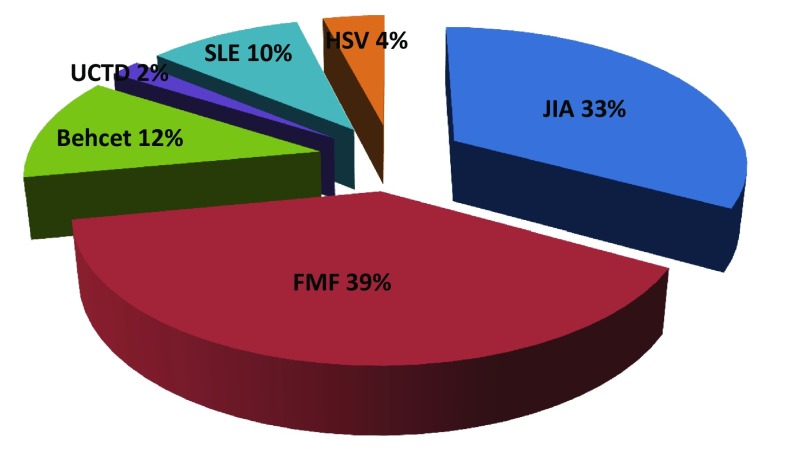
A total of 100 patients (37 boys, 63 girls) with a mean age of 12.2 ± 4.57 years were evaluated retrospectively. The frequencies of disease diagnosis groups in relation to clinical and laboratory findings were as follows (Fig. 1); FMF (39%), JIA (33%), Behcet disease (12%), HSV (4%), SLE (10%) and UCTD (2%). Diseases with a predisposition to thrombosis (Behcet, HSV, SLE) were seen in 26% of patients and the rest were patients with FMF, JIA or UCTD. Autoimmunity was also evaluated in patients and the frequency of positive ANA testing was 52%.
Fig. 1.
The frequencies (%) of different diagnosis groups in study population
The mean complement C3 concentration was 129.9 ± 35.3 mg/dL (Table 1) and the values were lower than cut off in three patients (1 FMF, 2 SLE) and were higher in five patients (4 JIA, 1 FMF). Mean complement C4 concentration was 24.4 ± 11.7 mg/dL (Table 1) and values were lower than cut off in seven patients (2 FMF, 2 JIA, 2 SLE, 1 HSV) and were higher in two patients (1 JIA, 1 FMF). Mean C3 (p = 0.027) and C4 (p = 0.002) concentrations were significantly higher in patients with leukocytosis.
Table 1.
Demographical and general laboratory data for all patients
| Mean ± SD | Min–max | Reference range | |
|---|---|---|---|
| Age (years) | 12.2 ± 4.57 | 2–20 | – |
| WBC (cells/mm3) | 7807 ± 2282 | 2790–14,200 | 4600–10,200 |
| Complement 3 (mg/dL) | 129.9 ± 35.3 | 30–195 | 90–180 |
| Complement 4 (mg/dL) | 24.4 ± 11.7 | 6–67 | 10–40 |
| C reactive protein (mg/dL) | 0.54 ± 1.46 | 0–11 | 0–0.5 |
| Serum amyloid A (mg/dL) | 43.6 ± 84.2 | 3–472 | 0–6.79 |
| Erythrocyte sedimentation rate (mm/h) | 15.8 ± 13.2 | 2–70 | 0–20 |
| Anti-β-2-glycoprotein I IgG (RU/mL) | 4.85 ± 7.61 | 2–72 | < 20 |
| Anti-β-2-glycoprotein I IgA (RU/mL) | 10.2 ± 13.7 | 2–125 | < 20 |
| Anti-β-2-glycoprotein I IgM (RU/mL) | 14.3 ± 37.7 | 1–265 | < 20 |
SD standard deviation
The mean CRP and SAA concentrations were 0.54 ± 1.46 and 43.6 ± 84.2 mg/dL (Table 1) and values were higher than reference range in 25 and 47% of patients, respectively.
The mean anti-β2GPI IgG, IgA, and IgM antibody levels were 4.85 ± 7.61, 10.2 ± 13.7 and 14.3 ± 37.7 RU/mL, consecuitively (Table 1). Autoantibody concentrations were higher than cut off in 4% of patients for anti-β2GPI IgG (1 FMF, 1 JIA, 2 SLE), 12% for anti-β2GPI IgM (4 FMF, 4 JIA, 1 HSV, 3 SLE) and 9% for anti-β2GPI IgA (3 FMF, 3 JIA, 3 SLE). The mean autoantibodies, especially IgA and IgM isotypes were higher in SLE group. None of them showed statistically significant difference between diagnosis groups (p = 0.108, p = 0.547, p = 0.807, for IgG, IgA and IgM respectively).
None of the mean C3 (p = 0.164), C4 (p = 0.366), CRP (p = 0.378), SAA (p = 0.156), ESR (p = 0.844), anti-β2GPI IgG (p = 0.456), IgA (p = 0.109) and IgM (p = 0.064) levels showed any significant difference in relation to gender.
Anti-β2GPI IgG concentrations positively associated with anti-β2GPI IgA values (p < 0.001, r = 0.401) and showed significant difference between patient groups with normal and high anti-β2GPI IgA (p = 0.010). And also IgG isotype concentrations were significantly different between patients with normal and high C3 concentrations (p = 0.042). Mean complement C3 (p = 0.113) and C4 (p = 0.55) concentrations were lower and anti-β2GPI IgM concentrations were higher in patients with high anti-β2GPI IgG (p = 0.017).
None of the acute phase reactants (CRP, SAA, ESR) showed a significant difference between patient groups with normal and high anti-β2GPI IgG, IgA, or IgM values.
The mean anti-β2GPI IgG (p = 0.375), IgA (p = 0.811) and IgM (p = 0.276) levels were not statistically different between disease groups with/without predisposition to thrombosis even though concentrations were higher in thrombosis group (Table 2). Anti-β2GPI autoantibody positivity did not show any relation to the presence of thrombosis. However, the mean C4 concentration was statistically significant different (p = 0.002) and was lower in thrombosis group (Table 2).
Table 2.
Comparison of laboratory data of patients with disease accompanied with a predisposition to thrombosis or not
| Disease with a predisposition to thrombosis | p value | ||
|---|---|---|---|
| (+) (n:26) | (−) (n:74) | ||
| Age (years) | 13.3 ± 4.10 | 11.8 ± 4.69 | 0.135 |
| WBC (cells/mm3) | 7566 ± 2515 | 7887 ± 2212 | 0.519 |
| Complement 3 (mg/dL) | 119.0 ± 36.2 | 137.5 ± 32.1 | 0.063 |
| Complement 4 (mg/dL) | 18.9 ± 7.34 | 28.7 ± 12.9 | 0.002* |
| C reactive protein (mg/dL) | 0.42 ± 1.24 | 0.58 ± 1.53 | 0.543 |
| Serum amyloid A (mg/dL) | 33.3 ± 65.6 | 46.7 ± 89.3 | 0.771 |
| Erythrocyte sedimentation rate (mm/h) | 12.9 ± 8.86 | 16.7 ± 14.3 | 0.326 |
| Anti-β-2 glycoprotein I IgG (RU/mL) | 7.00 ± 13.6 | 4.09 ± 3.48 | 0.375 |
| Anti-β-2 glycoprotein I IgA (RU/mL) | 13.4 ± 24.5 | 9.20 ± 7.60 | 0.811 |
| Anti-β-2 glycoprotein I IgM (RU/mL) | 14.6 ± 38.5 | 14.2 ± 37.7 | 0.276 |
Student-test, Mann–Whitney U test
* p < 0.05
Anti-β2GPI IgG values were higher (p = 0.020) in patients with positive ANA, but lower than cut off. However, none of C3 (p = 0.133), C4 (p = 0.191), SAA (p = 0.741), CRP (p = 0.517), anti-β2GPI IgA (p = 0.229) and anti-β2GPI IgM (p = 0.832) concentrations showed any difference in relation to autoimmunity.
Discussion
The presence of anti-β2GPI antibodies in plasma has a physiologic relevance and also play different roles in innate immunity. The triggering mechanism is not known but these autoantibodies deteriorate into pathologic risk factors when their residence time in the circulation becomes indefinite [14]. Many experimental analyses have revealed that several cell types, change their phenotype toward a more prothrombotic and proinflammatory state in the presence of these autoantibodies [14]. In particular in children, antiphospholipid antibodies can readily be detected although they do not show any clinical signs of APS [13]. In a study carried out by Avcin et al. [15] the prevalence of anti-β2GPI in 61 healthy children was 6.6%. High frequency of infections in childhood may be the causative factor in healthy children.
The precise role of anti-β2GPI isotypes is still incompletely resolved and this can often lead to clinical uncertainty when interpreting the significance of a positive anti-β2GPI result. As it is well known that anticardiolipin antibodies can be seen in many conditions other than APS, positive results have to be evaluated in a wide spectrum [14]. Medications, infections and other illnesses have been reported in association with antiphospholipid antibodies which are often transient [14, 16–18]. Anti-β2GPI antibodies are found in 6–8% of patients with HIV, syphilis, and malaria and in 89 and 30% respectively of patients with leprosy and hepatitis C [19]. An increased prevalence of anti-β2GPI IgA has been reported in a variety of disorders such as autoimmune hepatitis, coeliac disease, metabolic syndrome, and haemodialysed patients with end-stage renal failure [20–23]. β2GPI has also been identified in atherosclerotic plaques as a general consequence of autoimmune diseases [24].
Autoantibody formation in secondary APS is mainly related to secondary reactions most frequently in connection with rheumatic diseases, connective tissue diseases as well as related autoimmune diseases [10]. The frequency of antiphospholipid antibodies is reported to be 20–50% in patients with SLE [25–27], which is slightly higher than that seen in those with systemic scleroderma [28], Sjögren’s syndrome [29], and/or rheumatoid arthritis [30]. Avcin et al. [31] speculated that APS might be the forerunner in 30% of the SLE cases.
In this study, we evaluated the positivity of anti-β2GPI in patients with different rheumatologic diseases. At least one of the anti-β2GPI IgG, IgA or IgM autoantibodies was positive in nineteen patients. When evaluated individually, anti-β2GPI IgG values were higher than cut off in 4% of all patients, while anti-β2GPI IgM values were higher in 12% and anti-β2GPI IgA values were higher in 9%. Mean anti-β2GPI levels, especially IgA and IgM levels were higher in SLE group as expected.
International Consensus guideline on anticardiolipin and anti-β2GPI testing suggest an association between anti-β2GPI and APS which is strongest for the IgG isotype (11). In our study group, neither anti-β2GPI IgG (p = 0.108) nor anti-β2GPI IgA (p = 0.547) and anti-β2GPI Ig M (p = 0.807) values showed statistically significant different between different diagnosis groups.
It is well known that β2GPI dependent anticardiolipin antibodies are correlated with thrombosis and are only found in the case of autoimmune diseases [7]. The frequency of diseases with a predisposition to thrombosis was 26% in the study group and at least one of the anti-β2GPI autoantibodies was positive in five patients. Mehrani et al. [32] emphasized that anti-β2GPI IgM did not associate with arterial or venous thrombosis in contrast to IgG and IgA anti-β2GPI isotypes. In the study group, anti-β2GPI IgG, IgA or IgM levels did not show any difference between disease groups with and without predisposition to thrombosis even though concentrations were higher in thrombosis group (Table 2). So, anti-β2GPI IgG, IgA or IgM positivity did not show any relation to the presence of thrombosis.
Anti-β2GPI IgA positivity is accepted as lesser diagnostic than IgG and IgM isotypes. But, Sweiss et al. [33] showed that isolated IgA anti-β2GPI positivity was associated with an increased risk of thromboembolic events, especially within a background of systemic SLE. In our study group, IgA anti- β2GPI positivity was not isolated but was accompanied with high anti-β2GPI IgG levels in two SLE patients.
Reduced anti-β2GPI levels are seen in pregnant women and in patients with stroke and myocardial infarction [34, 35]. In literature, there is no data about decreases in anti-β2GPI in children and none of our patients showed low concentrations in this group.
The acute phase reactants, CRP, SAA and ESR values were higher in a group of patients, respectively; but they showed no statistically significant difference between different diagnosis groups. These nonspecific tests also showed no significant difference between patient groups with normal and high anti-β2GPI values.
The mean complement levels were low for the patient group with thrombosis risk (Table 2). β2GPI is suggested as a binding site for the complement C3 [36], thus mediates for C3 degradation by factor H and this may be the cause of complement impairment.
The frequency of positive autoimmunity testing was 52% in the whole study group. All anti-β2GPI isotypes were high in positive ANA group but only anti-β2GPI IgG levels showed statistically significant difference (p = 0.020) in relation to autoimmunity. The positive autoimmunity testing frequency increased from 50% for non-thrombotic to 55% for thrombotic risk group. Anti-β2GPI IgG, IgA, and IgM levels were slightly higher in thrombosis group having no relation to ANA positivity.
In summary, anti-β2GPI antibodies are thought to be mainly associated with APS disease activity, and also directly involved in the pathogenesis of thrombosis. By using the data in this study, it could be concluded that if vasculitis is not the primary defect underlying the clinical symptoms of rheumatic disease in pediatric age group, anti-β2GPI antibody measurement should not be the first investigation for diagnosis. In the near future, specific domain specific anti-β2GPI measurements seem to be preferred in many routine laboratories. However, clinicians must be aware of thrombotic risks, especially if anti-β2GPI antibody positivity is accompanied with positive autoimmune findings.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not—for—profit sectors.
Compliance with ethical standards
Conflict of interest
The Authors declared that they have no conflict of interests.
References
- 1.Groot PG, Meijers JC. β(2)-Glycoprotein I: evolution, structure, and function. J Thromb Haemost. 2011;9(7):1275–1284. doi: 10.1111/j.1538-7836.2011.04327.x. [DOI] [PubMed] [Google Scholar]
- 2.Nalli C, Piantoni S, Andreoli L, Motta M, Tincani A. Antiphospholipid syndrome and antiphospholipid antibodies in children: the two sides of the coin. Isr Med Assoc J. 2012;14(5):310–312. [PubMed] [Google Scholar]
- 3.Schousboe I. β2-glycoprotein I: a plasma inhibitor of the contact activation of the intrinsic blood coagulation pathway. Blood. 1985;66(5):1086–1091. [PubMed] [Google Scholar]
- 4.Rioche M, Masseyeff R. Synthesis of plasma beta 2 glycoprotein I by human hepatoma cells in tissue culture. Biomedicine. 1974;21:420–423. [PubMed] [Google Scholar]
- 5.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyakis S, Lockshin MD, Atsumi M, Branch DW, Brey RL, Cervera R. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 7.Chamley LW, McKay EJ, Pattison NS. Cofactor dependent and cofactor independent anticardiolipin antibodies. Thromb Res. 1991;61(3):291–299. doi: 10.1016/0049-3848(91)90106-7. [DOI] [PubMed] [Google Scholar]
- 8.Asherson RA, Khamashta MA, Ordi-Ros J, Derksen RH, Machin SJ, Barquinero J, et al. The “primary” antiphospholipid syndrome; major clinical and serological features. Medicine. 1989;68:366–374. doi: 10.1097/00005792-198911000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Alarcon-Segovia D, Deleze M, Oria CV, Sanchez-Guerrero J, Gomez-Pacheco L. Antiphospholipid antibodies and the anticardiolipin syndrome in SLE. A prospective analysis of 500 consecutive patients. Medicine. 1989;68:353–365. doi: 10.1097/00005792-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Avcin T, Silverman ED. Antiphospholipid antibodies in pediatric systemic lupus erythematosus and the antiphospholipid syndrome. Lupus. 2007;16:627–633. doi: 10.1177/0961203307079036. [DOI] [PubMed] [Google Scholar]
- 11.Lakos G, Favaloro EJ, Harris EN, Meroni PL, Tincani A, Wong RC, et al. International consensus guidelines on anticardiolipin and anti-beta2-glycoprotein I testing: report from the 13th international congress on antiphospholipid antibodies. Arthritis Rheum. 2012;64:1–10. doi: 10.1002/art.33349. [DOI] [PubMed] [Google Scholar]
- 12.de Laat B, Pengo V, Pabinger I, Musial J, Voskuyl AE, Bultink IE, et al. The association between circulating antibodies against domain I of beta2-glycoprotein I and thrombosis: an international multicenter study. J Thromb Haemost. 2009;7:1767–1773. doi: 10.1111/j.1538-7836.2009.03588.x. [DOI] [PubMed] [Google Scholar]
- 13.Shi W, Krilis SA, Chong BH, Gordon S, Chesterman CN. Prevalence of lupus anticoagulant and anticardiolipin antibodies in a healthy population. Aust N Z J Med. 1990;20(3):231–236. doi: 10.1111/j.1445-5994.1990.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 14.Groot PG, Urbanus RT. The significance of autoantibodies against b2-glycoprotein I. Blood. 2012;120:266–274. doi: 10.1182/blood-2012-03-378646. [DOI] [PubMed] [Google Scholar]
- 15.Avcin T, Ambrozic A, Kuhar M, Kveder T, Rozman B. Anticardiolipin and anti-beta(2) glycoprotein I antibodies in sera of 61 apparently healthy children at regular preventive visits. Rheumatology. 2001;40(5):565–573. doi: 10.1093/rheumatology/40.5.565. [DOI] [PubMed] [Google Scholar]
- 16.Asherson RA, Cervera R. Antiphospholipid antibodies, and infections. Ann Rheum Dis. 2003;62:388–393. doi: 10.1136/ard.62.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sene D, Piette JC, Cacoub P. Antiphospholipid antibodies, antiphospholipid syndrome, and infections. Autoimmun Rev. 2008;7(4):272–277. doi: 10.1016/j.autrev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, et al. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest. 2002;109(6):797–804. doi: 10.1172/JCI0212337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loizou S, Singh S, Wypkema E, Asherson RA. Anticardiolipin, anti-beta(2)-glycoprotein I and antiprothrombin antibodies in black south African patients with infectious disease. Ann Rheum Dis. 2003;62:1106–1111. doi: 10.1136/ard.62.11.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabeta S, Norman GL, Gatselis N, Liaskos C, Papamichalis PA, Garagounis A, et al. IgA anti- B2GPI antibodies in patients with autoimmune liver diseases. J Clin Immunol. 2008;28:501–511. doi: 10.1007/s10875-008-9211-6. [DOI] [PubMed] [Google Scholar]
- 21.Mankai A, Achour A, Thabet Y, Manoubia W, Sakly W, Ghedira I. Anticardiolipin and anti-beta 2-glycoprotein I antibodies in celiac disease. Pathol Biol. 2012;60:291–295. doi: 10.1016/j.patbio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Borges RB, Bodanese LC, Muhlen CA, Repetto G, Viehe M, Norman GL, et al. Anti-beta2-glycoprotein I autoantibodies and metabolic syndrome. Arq Bras Cardiol. 2011;96:272–276. doi: 10.1590/S0066-782X2011005000021. [DOI] [PubMed] [Google Scholar]
- 23.Serrano A, Garcia F, Serrano M, Ramirez E, Alfaro FJ, Lora D, et al. IgA antibodies against beta2 glycoprotein I in hemodialysis patients are an independent risk factor for mortality. Kidney Int. 2012;81:1239–1244. doi: 10.1038/ki.2011.477. [DOI] [PubMed] [Google Scholar]
- 24.Petri M. The lupus anticoagulant is a risk factor for myocardial infarction (but not atherosclerosis): Hopkins Lupus Cohort. Thromb Res. 2004;114:593–595. doi: 10.1016/j.thromres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Tubach F, Hayem G, Marchand JL, Weber M, Palazzo E, de Bandt M, et al. IgG anti-beta-2-glycoprotein I antibodies in adult patients with systemic lupus erythematosus: prevalence and diagnostic value for the antiphospholipid syndrome. J Rheumatol. 2000;27:1437–1443. [PubMed] [Google Scholar]
- 26.Long AA, Ginsberg JS, Brill-Edwards P, Johnston M, Turner C, Denburg JA, et al. The relationship of antiphospholipid antibodies to thromboembolic disease in systemic lupus erythematosus: a cross-sectional study. Thromb Haemost. 1991;66:520–524. doi: 10.1055/s-0038-1646452. [DOI] [PubMed] [Google Scholar]
- 27.Sebastiani GD, Passiu G, Galeazzi M, Porzio F, Carcassi U. Prevalence and clinical associations of anticardiolipin antibodies in systemic lupus erythematosus: a prospective study. Clin Rheumatol. 1991;10:289–293. doi: 10.1007/BF02208692. [DOI] [PubMed] [Google Scholar]
- 28.Gupta R, Thabah MM, Gupta S, Shankar S, Kumar A. Clinical significance of antiphospholipid antibodies in Indian scleroderma patients. Rheumatol Int. 2009;30:277–279. doi: 10.1007/s00296-009-1107-0. [DOI] [PubMed] [Google Scholar]
- 29.Pasoto SG, Chakkour HP, Natalino RR, Viana VS, Bueno C, Lianza AC, et al. Lupus anticoagulant: a marker for stroke and venous thrombosis in primary Sjögren’s syndrome. Clin Rheumatol. 2012;31:1331–1338. doi: 10.1007/s10067-012-2019-z. [DOI] [PubMed] [Google Scholar]
- 30.Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8(2):100–108. doi: 10.1007/s11926-006-0049-8. [DOI] [PubMed] [Google Scholar]
- 31.Avcin T, Cimaz R, Silverman ED, Cervera R, Gattorno M, Garay S, et al. Pediatric antiphospholipid syndrome: clinical and immunologic features of 121 patients in an international registry. Pediatrics. 2008;122:e1100–e1107. doi: 10.1542/peds.2008-1209. [DOI] [PubMed] [Google Scholar]
- 32.Mehrani T, Petri M. IgM anti-beta2 glycoprotein I is protective against lupus nephritis and renal damage in systemic lupus erythematosus. J Rheumatol. 2011;38:450–453. doi: 10.3899/jrheum.100650. [DOI] [PubMed] [Google Scholar]
- 33.Sweiss NJ, Bo R, Kapadia R, Manst D, Mahmood F, Adhikari T, et al. IgA anti-beta2-glycoprotein I autoantibodies are associated with an increased risk of thromboembolic events in patients with systemic lupus erythematosus. PLoS ONE. 2010;5(8):e12280. doi: 10.1371/journal.pone.0012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Laat B, de Groot PG, Derksen RH, Urbanus RT, Mertens K, Rosendaal FR, et al. Association between beta2-glycoprotein I plasma levels and the risk of myocardial infarction in older men. Blood. 2009;114:3656–3661. doi: 10.1182/blood-2009-03-212910. [DOI] [PubMed] [Google Scholar]
- 35.Gropp K, Weber N, Reuter M, Micklisch S, Kopka I, Hallstrom T, et al. Beta(2)-glycoprotein I, the major target in antiphospholipid syndrome, is a special human complement regulator. Blood. 2011;118:2774–2783. doi: 10.1182/blood-2011-02-339564. [DOI] [PubMed] [Google Scholar]
- 36.Lin F, Murphy R, White B, Kelly J, Feighery C, Doyle R, et al. Circulating levels of b2-glycoprotein I in thrombotic disorders and in inflammation. Lupus. 2006;15:87–93. doi: 10.1191/0961203306lu2270oa. [DOI] [PubMed] [Google Scholar]