Abstract
The aims of this study were to explore whether a phenolic acid and flavonoid fraction (named PAFF) isolated from Lolium multiflorum Lam. protects against d-galactosamine (GalN)-induced liver damages in mice and to investigate the associated mechanisms. ICR mice received oral administration with various concentrations (50, 100, and 200 mg/kg body weight) of PAFF once per 2 days for seven times before intraperitoneal injection with 800 mg/kg GalN. After a day of GalN challenge, blood and tissue samples were analyzed by biochemical, histopathological, real time RT-PCR, and Western blot methods. GalN challenge induced severe damage to hepatocytes with hepatocellular vacuolization and necrosis. GalN treatment increased serum ALT, ALP, AST, and LDH levels and hepatic MDA levels and stimulated mRNA and protein expressions of Nrf2 and HO-1 in the liver. GalN treatment also diminished the levels of GSH and the activities of CAT, SOD, and GPx in the liver. However, combined treatment with PAFF inhibited GalN-mediated increases in the histological damages and the levels of serum enzymes and hepatic MDA, restored the activities of hepatic antioxidant enzymes up to those in the control values, and augmented the GalN-stimulated expression of Nrf2 and HO-1 in the liver. Furthermore, PAFF treatment alone increased the cellular SOD activity and the expression of Nrf2 and HO-1 in the liver. Our results suggest that PAFF may protect against GalN-induced liver damage by decreasing oxidative stress and increasing cellular antioxidant activities through an activation of Nrf2/HO-1-dependent pathway.
Keywords: Italian ryegrass, d-Galactosamine, Hepatic damage, Nrf2, Bioactive compounds
Introduction
d-Galactosamine (GalN), a selective hepatotoxin, induces liver damage resembling viral hepatitis [1]. GalN causes apoptotic and necrotic cell death in the liver by inducing oxidative stress. Numerous reports highlight that GalN-induced liver damage is attenuated by use of naturally occurring antioxidants and/or free radical scavengers, such as dietary Spirulina platensis [2], pentoxifyline and caffeic acid phenethyl ester [3], catechin [4], biochanin A [5], kaempferol and p-coumaric acid [6], and quercetin [7]. These reports suggest that phenol acids and flavonodic compounds are the attractive bioactive components that protect against acute liver damages.
Lolium multiflorum Lam. (Italian ryegrass; IRG) is presently cultivated around the world for various purposes. IRG contains highly active and nutritional compounds [8]. We previously found that IRG includes various types of fatty acids capable of stimulating adipogenic differentiation [9]. A chloroform-soluble extract from the IRG showed greater antioxidative and anti-inflammatory potentials than those from sorghum, barley, and alfalfa [10]. These potentials of the IRG extract were closely associated with its higher contents of phenolic acids and flavonoidic compounds compared with those of other dietary plants [10]. We subsequently separated total 16 fractions from the IRG chloroform extract according to the methods described previously [11, 12] and found that 7th fraction, named PAFF, showed greater antioxidant and anti-inflammatory activities than other fractions. We recently found that catechin, caffeic acid, ferulic acid, p-coumaric acid, myricetin, quercetin, and kaempferol are the primary components in the PAFF through high performance liquid chromatography (HPLC) analysis [13]. We also demonstrated in vitro and in vivo antioxidant, anti-inflammatory, and anti-septic potentials of the PAFF, thereby highlighting its clinical usefulness in suppressing oxidative injury and inflammatory disorders [13]. Taken as a whole, our previous results with other reports [2–7] lead us to postulate that the PAFF is to be potentially a bioactive fraction capable of protecting GalN-induced liver injury.
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a redox-sensing transcription factor that regulates the gene expression of antioxidant proteins that protect against oxidative damage [14]. Heme oxygenase-1 (HO-1) is also an antioxidative enzyme that plays an important role in defense against oxidative stress [15]. Activation of Nrf2 stimulates the induction of many cytoprotective proteins including the HO-1. Actually, the induction of HO-1 has been shown to protect against various pathologic disorders including sepsis, hypertension, and acute tissue injuries [16]. These reports suggest that activating Nrf2/HO-1-mediated signaling may contribute to a protection on GalN-induced liver injury.
In this study, we investigated whether PAFF protects against GalN-induced liver damages in mice and the associated mechanisms. To this end, male ICR mice received oral administration with various concentrations of PAFF once per 2 days for seven times before injection of GalN solution. After a day of the GalN challenge, blood and tissue samples were collected and processed for biochemical and histopathological analyses. The effects of PAFF on mRNA and protein expressions of Nrf2 and HO-1 in the liver of GalN-treated mice were also examined by real time reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analyses.
Materials and Methods
Chemicals and Laboratory Materials
Unless otherwise specified, all chemicals and laboratory materials were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA) and Falcon Labware (Becton- Dickinson, Franklin Lakes, NJ, USA), respectively.
Preparation of PAFF and Identification of Primary Constituents
The aerial parts of IRG were collected in September 2011 from the Grassland and Forages Research Center at the National Institute of Animal Science (NIAS) (Cheonan, South Korea). The IRG sample was authenticated by a taxonomist at NIAS, harvested at the flowering stage without the root, and ensiled. Procedures for collection, dryness, and pulverization of IRG silages were described in previous reports [10–12]. The methods to isolate PAFF from the IRG chloroform-soluble extract and to identify the components of PAFF by HPLC were described elsewhere [13]. In brief, IRG chloroform-soluble extract was separated into 16 subfractions by chromatography using a Sephadex LH-20 column and then the 7th fraction was evaporated and lyophilized to yield the powdered PAFF. HPLC revealed that the PAFF contained catechin, caffeic acid, ferulic acid, syringic aldehyde, myricetin, propyl gallate, quercetin, and kaempferol as primary components.
Mice
Male ICR mice (six weeks old, n = 60) were supplied by Orient Co. (Seoul, South Korea) and randomly divided into six groups (n = 10/group). Mice were caged separately (n = 5/cage) and then equilibrated for 7 days before administration with PAFF. Mice were housed in automatically controlled conditions with a 12–h light/dark cycle, 22 ± 1°C, and 45–55% relative humidity. All mice had free access to standard rodent pellet food and water ad libitum. The consumption of food and water and behavior of the animals were monitored every 12 h per day during the experimental periods.
PAFF Administration and GalN Challenge
PAFF and GalN were dissolved in dimethyl sulfoxide (DMSO, 99% purity) and phosphate buffered saline (PBS), respectively. The DMSO stock solution was mixed with PBS before oral treatment, where the final concentration of DMSO administered did not exceed 10%. Group 1 mice (control group) received only oral administration with vehicle solution (300 μl PBS containing 10% DMSO) once per 2 days for seven times. Group 2 mice (GalN group) were orally administrated with the vehicle solution for the same periods and received 200 μl PBS containing 800 mg/kg GalN intraperitoneally just after the last oral administration of vehicle solution. Mice in groups 3, 4, and 5 received oral administration with 300 μl PBS containing the different concentrations (50, 100, and 200 mg/kg body weight) of PAFF for the same periods followed by intraperitoneal injection of GalN solution. Group 6 mice received oral administration with 300 μl PBS containing 200 mg/kg of PAFF alone for the same periods.
Preparation of Blood and Tissue Samples
Animals were weighed on the first and last days of oral administration with vehicle or PAFF solution. After a day of GalN challenge, whole blood of mice was collected into serum separation tubes (BD Bioscience, San Jose, CA, USA) by cardiac puncture using a syringe. Blood samples were centrifuged at 10,000g for 10 min at 4 °C and the supernatants were used as serum samples to determine enzyme activities specific to alkaline phosphate (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH). After blood collection, liver tissues of mice were isolated, weighed, and rinsed with ice cold isotonic saline followed by dissection into three pieces. To analyze mRNA and protein levels of Nrf2 and HO-1, a piece of the liver samples is quickly immersed into liquid nitrogen and then stored at – 80 °C. Another liver samples are processed for histopathological analysis. In addition, accurately weighed liver samples were homogenized in 50 mM ice-cold KH2PO4 solution (1:5 w/v) or in 200 μl of cold assay buffer provided by BioAssay Systems (Hayward, CA, USA) using a homogenizer (PRO Scientific Inc., Oxford, CT, USA). After centrifugation at 10,000g for 10 min at 4 °C, the supernatants were used to analyze the levels of malondialdehyde (MDA) and the activities of primary antioxidative defense systems such as glutathione (GSH), glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT).
MDA Determination
The effects of PAFF on GalN-mediated lipid peroxidation in the liver of mice were determined by measuring intracellular levels of MDA, an end product of lipid peroxidation. Briefly, liver tissue homogenates (100 μl per each sample) were mixed with 50 μl of 8% sodium dodecyl sulfate (SDS) followed by incubation for 10 min at room temperature. The mixtures were resuspended in a buffer containing 375 μl of 20% acetic acid (pH 3.5) and 375 μl of 0.6% thiobarbituric acid and further incubated at 80 °C for 60 min. Thereafter, 250 μl of distilled water and 1.25 ml of a butanol:pyridine mixture (15:1, v/v) were added to the mixtures before centrifugation at 1,000 g for 5 min. MDA levels in the supernatants were measured at 532 nm using 1,1,3,3-tetraethoxypropane as a standard.
Determination of Intracellular Antioxidant Activities
Concentrations of GSH and SOD (EC 1.15.1.1) in liver tissues were measured using the assay kits (#K264-100 for GSH and#K335-100 for SOD) provided by BioVision Research Products (Milpitas, CA, USA). The activities of CAT (EC 1.11.1.6) and GPx (EC 1.11.1.9) were determined using Amplex® Red Catalase Assay Kit (A22180, Molecular Probes, Inc., Eugene, OR, USA) and GPx assay kit (EGPX-100; BioAssay Systems), respectively. All assays were carried out according to the manufacturer’s instructions provided.
Measurement of Serum Enzyme Activities
The activities of serum enzymes such as ALP (EC 3.1.3.1), ALT (E.C 2.6.1.2), AST (EC 2.6.1.1), and LDH (EC 1.1.1.27) were measured using their specific assay kits provided by Bayer (ADVIA 1650, Bayer, Japan) according to the manufacturer’s instructions.
Histopathology
Briefly, liver tissue samples were fixed in 4% paraformaldehyde solution for 24 h, dehydrated, and then embedded in paraffin followed by dissection at a thickness of 5.0 µm. The sections were mounted on glass slides and stained with hematoxylin and eosin (H & E) before observation under a light microscope (EL-Einsatz 451888, Carl Zeiss, Ostalbkreis, Germany).
Real Time RT-PCR
Total RNA was extracted with Trizol reagent (Invitrogen Corp., Carlsband, CA, USA) according to the manufacturer’s instructions. RNA samples (1 µg per reaction) from a total of individual group were used for preparation of cDNA for RT-PCR using AmpiGene™ cDNA Synthesis Kit (Applied Biosystems, Foster City, CA, USA). cDNA synthesized was quantified using AmpiGene™ qPCR Green Mix Hi-ROX and primers in ABI StepOnePlus sequence detection system (Applied Biosystems) with following thermo-cycle programming: predenaturation at 95°C for 2 min followed by 40 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 30 s, and extension at 65°C for 30 s. Oligonucleotide primers specific to Nrf2 and HO-1 were designed using primer express 3.0 (Applied Biosystems) and the PCR primer sequences used are as follows: 5′-cttggcctcagtgattctgaagtg-3′ and 5′-cctgagatggtgacaagggttgta-3′ for Nrf2 and 5′-aagaggctaagaccgccttc-3′ and 5′-gcataaattcccactgccac-3′ for HO-1. All PCR reactions were performed at least in triplicate and the expression levels were normalized to the GAPDH signal in the same reaction.
Western Blot Analysis
Equal amounts of protein samples were separated by 12% SDS–polyacrylamide gel electrophoresis and blotted polyvinylidene fluoride membranes. The blots were probed with primary antibodies specific to HO-1, Nrf2, or α-tubulin and incubated with horseradish peroxidase-conjugated anti-IgG in a blocking buffer for 1 h. The blots were developed with enhanced chemiluminescence (GE Healthcare, Buckinghamshire, UK) and immunoreactive bands were visualized by exposure to X-ray film (Eastman-Kodak, Rochester, NY, USA). The band intensities were quantified with ImageJ densitometry software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Unless otherwise specified, all of the data are expressed as the mean ± standard deviation (SD) of three independent experiments. One-way ANOVA using SPSS ver. 12.0 software was used for multiple comparisons. Values of p < 0.05 were considered statistically significant.
Results
Effects of PAFF on Liver and Spleen Weights in GalN-Treated Mice
All animals survived the experimental periods until sacrifice. During the experimental periods, there were no detectable alterations in the general states of the mice. There were also no significant differences in the weight of spleen tissue among the experimental groups (Table 1). In contrast, liver weight in the mice treated with GalN was significantly (p < 0.001) higher than that in the control mice. GalN-mediated increase in the liver weight was not seen in the mice that received oral administration with 100 or 200 mg/kg PAFF in combination with GalN.
Table 1.
Liver and spleen weights of mice
| Parameters groups | Tissue weights (g) | |
|---|---|---|
| Liver | Spleen | |
| Group 1 (Control) | 1.20 ± 0.11 | 0.11 ± 0.01 |
| Group 2 (GalN) | 2.42 ± 0.54*** | 0.13 ± 0.03 |
| Group 3 (GalN + 50 mg/kg PAFF) | 2.01 ± 0.34*** | 0.12 ± 0.01 |
| Group 4 (GalN + 100 mg/kg PAFF) | 1.70 ± 0.30## | 0.11 ± 0.01 |
| Group 5 (GalN + 200 mg/kg PAFF) | 1.29 ± 0.18### | 0.11 ± 0.01 |
| Group 6 (200 mg/kg PAFF) | 1.19 ± 0.11 | 0.12 ± 0.01 |
Each value represents the mean ± SD from eight animals in each group (n = 5)
***p < 0.001, compared with the DMSO control (group 1)
##p < 0.01 and ###p < 0.001, compared with the GalN treatment alone (group 2)
Preventive Effect of PAFF on GalN-Induced Hepatocellular Damage
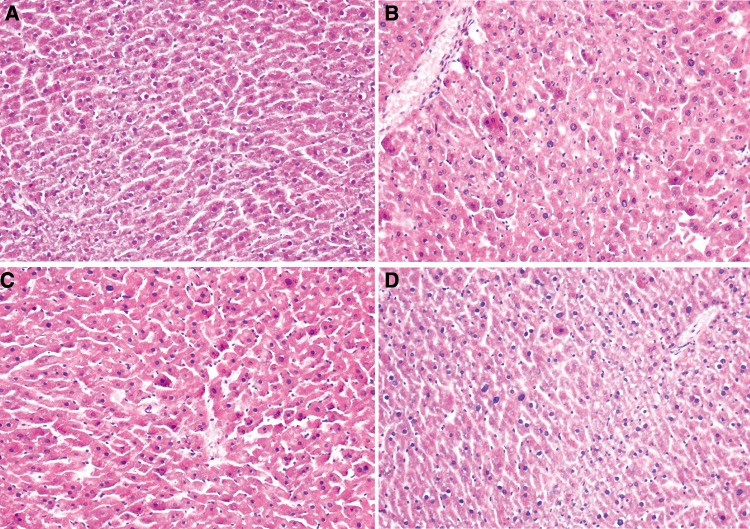
The control group showed normal histological structures of hepatic lobules, whereas group treated with GalN showed severe damage to hepatocytes with hepatocellular vacuolization and necrosis (Fig. 1). Oral supplementation with PAFF at the concentrations of 100 and 200 mg/kg revealed a dose-dependent improvement in hepatocytes as compared to that of the mice treated with GalN only.
Fig. 1.
H and E staining of liver tissues of mice treated with GalN and/or PAFF. The panels show the histological structures of untreated control a GalN control b, and experimental groups treated with 100 c or 200 mg/kg body weight d of PAFF before GalN challenge
Ameliorating Effects of PAFF on GalN-Mediated Increases in the Serum Enzyme Activities
The levels of serum enzymes such as ALT, ALP, AST and LDH in the mice treated with GalN and/or PAFF are summarized in Table 2. No significant differences were found in these serum enzymes between groups 1 (the control only with PBS treatment) and 6 (PAFF treatment alone), although the values of ALT, ALP, and AST, but not of LDH, in the group 6 were lower than those of the control group. GalN challenge significantly increased the levels of all serum enzymes tested, such that the serum levels of ALT, ALP, AST, and LDH were increased by 18–, 2.12–, 4.8–, and 2.8–fold, respectively, in GalN-treated mice compared with the untreated control values. Combined treatment with more than 50 mg/kg PAFF inhibited significantly GalN-mediated releases of the serum enzymes. Specifically, the serum levels of ALT, ALP, and LDH in the group 5 treated with GalN + 200 mg/kg PAFF were statistically similar to those in the untreated control mice.
Table 2.
Effects of PAFF on serum enzyme activities in GalN-treated mice
| Enzymes (U/L) | Group 1 (Control) | Group 2 (GalN) | Group 3 (GalN + 50 mg/kg PAFF) | Group 4 (GalN + 100 mg/kg PAFF) | Group 5 (GalN + 200 mg/kg PAFF) | Group 6 (200 mg/kg PAFF) |
|---|---|---|---|---|---|---|
| ALT | 11 ± 4 | 198 ± 31*** | 171 ± 25*** | 111 ± 21***, ### | 32 ± 15### | 10 ± 2 |
| ALP | 38 ± 7 | 81 ± 11*** | 64 ± 22** | 49 ± 15## | 43 ± 8### | 36 ± 9 |
| AST | 26 ± 5 | 125 ± 26*** | 102 ± 16*** | 86 ± 19***, ## | 64 ± 22**, ### | 23 ± 5 |
| LDH | 370 ± 68 | 1037 ± 205*** | 918 ± 136*** | 603 ± 202### | 502 ± 167### | 318 ± 65 |
Values are expressed as the mean ± SD for eight animals in each group (n = 5)
**p < 0.01 and ***p < 0.001, compared with group 1
##p < 0.01 and ###p < 0.001, compared with group 2
Beneficial Effects of PAFF on Antioxidant Enzyme Activities and MDA Levels in the Liver of GalN-Treated Mice
Table 3 shows the effects of PAFF on GalN-mediated changes of primary antioxidant status and lipid peroxidation in the liver. Significant decreases (p < 0.001) in the level of GSH and in the activities of CAT, SOD, and GPx were observed in the liver tissue of GalN-treated mice, compared with the untreated control values. These decreases were inhibited by oral administration with PAFF, where the mice treated with GalN + 200 mg/kg PAFF (group 5) showed similar levels of GSH, CAT, and SOD to those of the untreated control mice. In contrast, the level of MDA in the liver of GalN-treated mice was augmented up to 4.67–fold, compared with the control group. Oral administration with PAFF suppressed GalN-mediated MDA increases, in which PAFF treatment even at a dose of 50 mg/kg showed a significant reduction (p < 0.01) in the MDA level. Specifically, PAFF treatment alone significantly (p < 0.05) increased the activity of SOD in the liver, compared with the untreated control mice.
Table 3.
Effects of PAFF on antioxidant defense systems and MDA content in liver tissues of GalN-treated mice
| Factors | Group 1 (Control) | Group 2 (GalN) | Group 3 (GalN + 50 mg/kg PAFF) | Group 4 (GalN + 100 mg/kg PAFF) | Group 5 (GalN + 200 mg/kg PAFF) | Group 6 (200 mg/kg PAFF) |
|---|---|---|---|---|---|---|
| GSH | 2.8 ± 0.3 | 1.1 ± 0.2*** | 1.3 ± 0.3*** | 1.8 ± 0.5***, ## | 2.5 ± 0.3### | 3.2 ± 0.3 |
| CAT | 61 ± 6.0 | 34 ± 7.2*** | 41 ± 8.9** | 52 ± 7.9# | 56 ± 12.0## | 66 ± 7.8 |
| SOD | 4.7 ± 0.4 | 1.2 ± 0.4*** | 1.7 ± 0.5*** | 2.8 ± 0.6***, ### | 3.9 ± 0.7### | 6.2 ± 0.5* |
| GPx MDA |
34.6 ± 6.6 1.2 ± 0.3 |
9.4 ± 3.1*** 5.6 ± 0.9*** |
14.7 ± 6.4*** 4.0 ± 1.1***, ## |
19.7 ± 6.4***, ##
2.7 ± 0.5**, ### |
23.0 ± 6.0*, ##
1.9 ± 0.6### |
35.7 ± 6.7 0.9 ± 0.3 |
Values are expressed as the mean ± SD for eight animals in each group (n = 5)
*p < 0.05, **p < 0.01, and ***p < 0.001, compared with group 1
#p < 0.05,##p < 0.01, and ###p < 0.001, compared with group 2
Units: GSH: mg/g tissue weight; CAT: U/mg tissue weight; SOD: U/mg protein; MDA: nmol/mg tissue weight; GPX: U/mg protein
Augmentation Effects of PAFF on Nrf2 and HO-1 Expression at mRNA and Protein Levels in GalN-Treated Mice
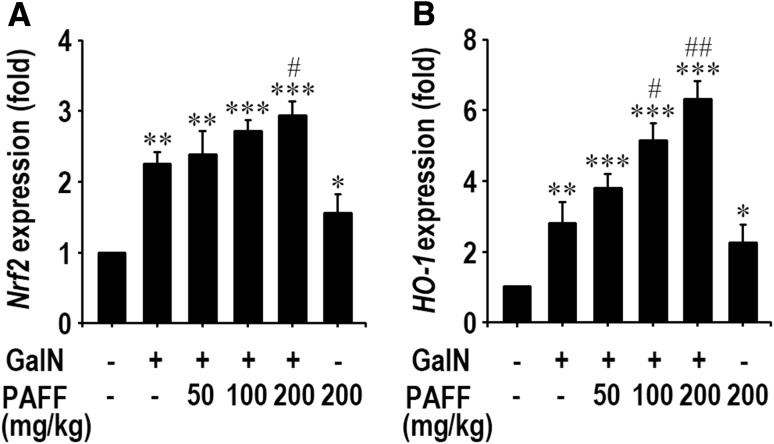
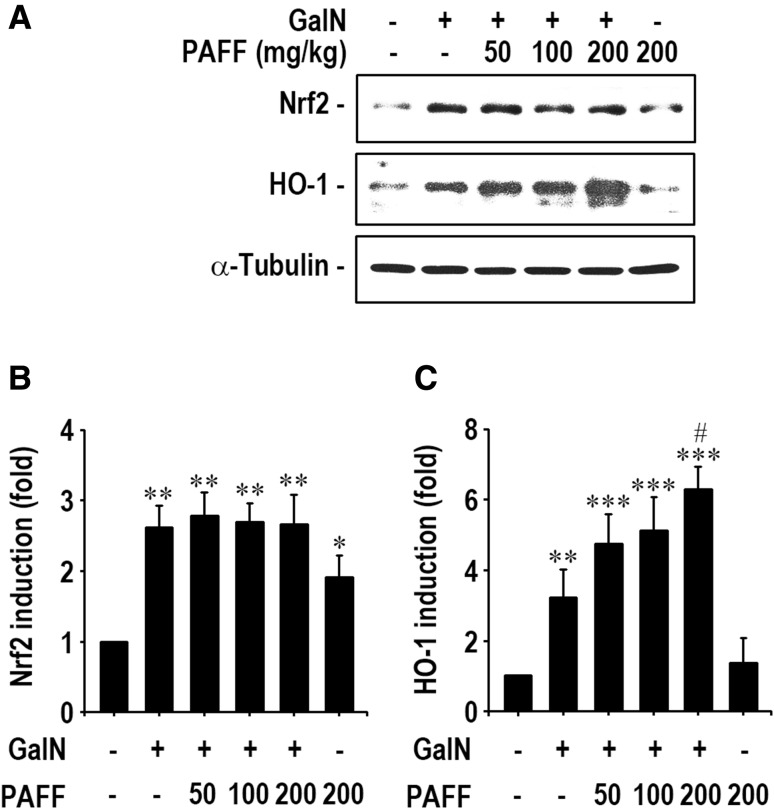
In order to understand a possible mechanism by which PAFF inhibits GalN-mediated liver damages, we determined mRNA and protein levels of Nrf2 and HO-1 in liver tissues by real time RT-PCR and immunoblotting analyses. As shown in Fig. 2, mRNA expressions of Nrf2 and HO-1 were significantly (p < 0.01) up-regulated after GalN treatment, compared with those of the untreated control mice. Oral supplementation with PAFF augmented GalN-mediated mRNA expression of Nrf2 (Fig. 2a). Such augmentation by the fraction was shown to be further prominent, when HO-1 mRNA levels were determined (Fig. 2b). PAFF treatment alone (200 mg/kg body weight) also increased significantly (p < 0.05) the mRNA expression of Nrf2 and HO-1 in the liver. In addition, GalN treatment alone increased significantly (p < 0.01) the induction of Nrf2 and HO-1 proteins in the liver (Fig. 3a), whereas the level of HO-1, but not of Nrf2 protein, in the liver of GalN-treated mice, was augmented significantly (p < 0.05) according to the administration with 200 mg/kg IRG-AG7 (Fig. 3b and c).
Fig. 2.
Effects of PAFF on mRNA expression of Nrf2 and HO-1 in the liver of GalN-treated mice. Total mRNAs from the liver samples of mice were analyzed by real time RT-PCR using oligonucleotide primers specific to Nrf2 a and HO-1 b. GAPDH was used as an internal control. The results are expressed as mean ± SD (n = 5). *p < 0.05, **p < 0.01, and ***p < 0.001 versus the untreated control value. #p < 0.05 and ##p < 0.01 versus the GalN treatment alone
Fig. 3.
Effect of PAFF on the induction of Nrf2 and HO-1 proteins in the liver of GalN-treated mice. Whole proteins were separated from the liver samples of mice and the protein levels of Nrf2 a and HO-1 b were analyzed by Western blotting. The results are expressed as mean ± SD (n = 5). *p < 0.05, **p < 0.01, and ***p < 0.001 versus the untreated control value. #p < 0.05 versus the GalN treatment alone
Discussion
GalN is one of the most commonly used hepatitis-inducing agents in animals and oxidative stress is considered to be related to the GalN-induced liver injury. This suggests that antioxidant supplementation may protect against GalN-mediated hepatocellular damages. Numerous studies have shown chemopreventive potentials of flavonoids on oxidative liver damages by suggesting beneficial effects on health via their intake from fruit, vegetables, and beverages [4–7]. Naturally occurring antioxidative compounds also exert various pharmacological and medicinal activities [17] and improve primary antioxidant defense systems [18]. We previously found that PAFF has antioxidant potentials in cell-free and cell-mediated assay systems which generate reactive oxygen species (ROS) and indentified the presence of several phenolic acids and flavonoidic components as primary compounds [11–13]. We also detected that the PAFF exerts greater anti-inflammatory activity in lipopolysaccharide-stimulated macrophages than that of single compound such as caffeic acid, ferulic acid, myricetin, or kaempferol at the same concentrations [13]. Taken as a whole, all the present results support our hypothesis that PAFF may protect against GalN-mediated liver injury and this is due to its potentials to prevent oxidative stress and to increase antioxidant enzyme activities.
ALT and AST are the important class of aminotransferases linking amino acid and carbohydrate metabolism [19]. Alteration in ALP activity may affect the permeability of membranes as well as the transport of metabolites. It is known that increased LDH activity in serum is to be a key event occurred in liver injury [20]. When the liver tissue is injured, all these enzymes can be released from the liver into the bloodstream [20, 21]. Therefore, these enzymes are to be the critical diagnostic indicators for determining liver damage and their activities in serum might be relatively elevated according to the status of hepatocellular damages. This strongly suggests that the PAFF-induced protection on GalN-mediated liver injury is tightly accompanied by the reduced serum activities of these diagnostic enzymes. In parallel with the previous reports [4–7], our current findings demonstrated that GalN challenge increased significantly the activities of all serum enzymes examined, compared with those of the control group, whereas these increases were markedly diminished by supplementation with the phenolic and flavonoidic compound fraction, PAFF.
GSH, a nonenzymatic free radical scavenger, protects tissues from oxidative stress by directly interacting with ROS or as a cofactor for enzymatic detoxification [22, 23]. SOD converts superoxide radicals to H2O2 that is removed by the reaction with CAT and/or GSH [24]. GPx also protects against oxidative damage by converting H2O2 to water [25]. Accumulated findings have demonstrated that hepatic GSH levels and SOD, CAT, and GPx activities were reduced in GalN-treated animals and this reduction was prevented significantly by supplementation with antioxidative extracts or single compounds [4–7]. There were also reports that lipid peroxidation is proportionally increased by elevated generation of ROS with an attendant decrease in primary antioxidant status [26]. Increased lipid peroxidation is believed to be one of the main markers of ROS-mediated damage due to GalN administration [4–7, 27]. Consequently, our results support that PAFF prevents hepatic damage by activating antioxidant defense systems as well as by inhibiting lipid peroxidation in GalN-treated mice.
This study further determined mRNA and protein expressions of Nrf2 and HO-1 in the liver, because the activation of Nrf2/HO-1 signaling pathway contributes to a protection on oxidative stress by enhancing an intracellular antioxidant defense system. Indeed, up-regulation of HO-1 induction is considered to be one of the mechanisms involved in the protection of GalN-mediated hepatotoxicity [7, 28]. Many studies also showed that single compounds or plant extracts having antioxidant activity up-regulate the expression of Nrf2 [5, 29, 30]. In this regard, we speculated that PAFF activated Nrf2/HO-1-mediated signaling and eventually enhanced a protective mechanism on GalN-induced liver injury. Our results support at least in part such speculation by showing the PAFF-induced augmentation in Nrf2 and HO-1 expressions in the liver of GalN-treated mice. These augmenting effects of PAFF appeared to be partially associated with its chemical property to stimulate Nrf2 expression. Thus, it is likely that the increased SOD activity in the liver of mice administered with PAFF alone was due to the Nrf2 expression. However, it is important to note that unlikely the mRNA expression, the level of Nrf2, but not of HO-1 protein, was not augmented in the liver of mice administered in combination with PAFF and GalN, compared with that of GalN treatment alone. We postulate that nuclear translocation of Nrf2 from the cytoplasm will be an important event in activating the expression of HO-1, as well as in stimulating antioxidant defense mechanisms [7]. More detail experiments to elucidate the exact roles of Nrf2 in the PAFF-induced protection on GalN-mediated liver damages might be necessary.
In conclusion, this study highlights that PAFF has a protective effect on GalN-induced liver damage by decreasing oxidative stress and increasing cellular antioxidant activities. Regarding the mechanisms, we suggest the PAFF-mediated activation of Nrf2/HO-1 signal transduction pathways. Collectively, PAFF is to be a potential fraction capable of preventing oxidative hepatic damages and activating antioxidant defense systems.
Acknowledgement
This work was supported by a grant from the Rural Development Administration, Ministry of Agriculture and Forestry, South Korea (Grant No.: PJ010903032015).
Conflict of interest
Young-Ok Son, Jung-Min Hwang, Ki-Choon Choi and Jeong-Chae Lee declares that they have no conflict of interest.
Human and Animal Rights
This study was carried out in strict accordance with the recommendations in the Guide for the Animal Care and Use of the Chonbuk National University. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The protocol in this study was approved by the University Committee on Ethics in the Care and Use of Laboratory Animals (Permit No. CBU 2012–0039).
Contributor Information
Ki-Choon Choi, Phone: +82415806752, Email: choiwh@korea.kr.
Jeong-Chae Lee, Phone: +82632704049, Email: leejc88@jbnu.ac.kr.
References
- 1.Wang Y, Wan Y, Ye G, Wang P, Xue X, Wu G, et al. Hepatoprotective effects of AdipoRon against d-galactosamine-induced liver injury in mice. Eur J Pharm Sci. 2016;93:123–131. doi: 10.1016/j.ejps.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Ren DF, Wang JZ, Sanada H, Egashira Y. Protection by dietary Spirulina platensis against d-galactosamine- and acetaminophen-induced liver injuries. Br J Nutr. 2010;103(11):1573–1576. doi: 10.1017/S0007114509993758. [DOI] [PubMed] [Google Scholar]
- 3.Taslidere E, Vardi N, Esrefoglu M, Ates B, Taskapan C, Yologlu S. The effects of pentoxifylline and caffeic acid phenethyl ester in the treatment of d-galactosamine-induced acute hepatitis in rats. Hum Exp Toxicol. 2016;35:353–365. doi: 10.1177/0960327115586820. [DOI] [PubMed] [Google Scholar]
- 4.Vasanth Raj P, Nitesh K, Sagar Gang S, Hitesh Jagani V, Raghu Chandrashekhar H, Venkata Rao J, et al. Protective role of catechin on d-galactosamine induced hepatotoxicity through a p53 dependent pathway. Indian J Clin Biochem. 2010;25(4):349–356. doi: 10.1007/s12291-010-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Wang T, Liu X, Cai L, Qi J, Zhang P, et al. Biochanin A protects lipopolysaccharide/d-galactosamine-induced acute liver injury in mice by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Int Immunopharmacol. 2016;38:324–331. doi: 10.1016/j.intimp.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda H, Ninomiya K, Shimoda H, Yoshikawa M. Hepatoprotective principles from the flowers of Tilia argentea (linden): structure requirements of tiliroside and mechanisms of action. Bioorg Med Chem. 2002;10(3):707–712. doi: 10.1016/S0968-0896(01)00321-2. [DOI] [PubMed] [Google Scholar]
- 7.Lekić N, Canová NK, Hořínek A, Farghali H. The involvement of heme oxygenase 1 but not nitric oxide synthase 2 in a hepatoprotective action of quercetin in lipopolysaccharide-induced hepatotoxicity of d-galactosamine sensitized rats. Fitoterapia. 2013;87:20–26. doi: 10.1016/j.fitote.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Ilavenil S, Arasu MV, Lee JC, Kim DH, Vijayakumar M, Lee KD, et al. Positive regulations of adipogenesis by Italian ryegrass [Lolium multiflorum] in 3T3-L1 cells. BMC Biotechnol. 2014;14:54. doi: 10.1186/1472-6750-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valan Arasu M, Ilavenil S, Kim DH, Gun Roh S, Lee JC, Choi KC. In vitro and in vivo enhancement of adipogenesis by Italian ryegrass (Lolium multiflorum) in 3T3-L1 cells and mice. PLoS ONE. 2014;9(1):e85297. doi: 10.1371/journal.pone.0085297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang JM, Choi KC, Bang SJ, Son YO, Kim BT, Kim DH, et al. Anti-oxidant and anti-inflammatory properties of methanol extracts from various crops. Food Sci Biotechnol. 2013;22(1):265–272. doi: 10.1007/s10068-013-0076-y. [DOI] [Google Scholar]
- 11.Choi KC, Hwang JM, Bang SJ, Kim BT, Kim DH, Chae M, et al. Chloroform extract of alfalfa (Medicago sativa) inhibits lipopolysaccharide-induced inflammation by downregulating ERK/NF-κB signaling and cytokine production. J Med Food. 2013;16(5):410–420. doi: 10.1089/jmf.2012.2679. [DOI] [PubMed] [Google Scholar]
- 12.Choi KC, Hwang JM, Bang SJ, Son YO, Kim BT, Kim DH, et al. Methanol extract of the aerial parts of barley (Hordeum vulgare) suppresses lipopolysaccharide-induced inflammatory responses in vitro and in vivo. Pharm Biol. 2013;51(8):1066–1076. doi: 10.3109/13880209.2013.768274. [DOI] [PubMed] [Google Scholar]
- 13.Choi KC, Son YO, Hwang JM, Kim BT, Chae M, Lee JC. Antioxiant, anti-inflammatory and anti-septic potential of phenolic acids and flavonoid fractions isolated from Lolium multiflorum. Pharm Biol. 2017;55(1):611–619. doi: 10.1080/13880209.2016.1266673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Pi J, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 2010;244(1):84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleszczyński K, Zillikens D, Fischer TW. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK) J Pineal Res. 2016;61(2):187–197. doi: 10.1111/jpi.12338. [DOI] [PubMed] [Google Scholar]
- 16.Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens. 2014;23(1):17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firuzi O, Miri R, Tavakkoli M, Saso L. Antioxidant therapy: current status and future prospects. Curr Med Chem. 2011;18(25):3871–3888. doi: 10.2174/092986711803414368. [DOI] [PubMed] [Google Scholar]
- 18.Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006;5(1):47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Tan KK, Bang SL, Vijayan A, Chiu MT. Hepatic enzymes have a role in the diagnosis of hepatic injury after blunt abdominal trauma. Injury. 2009;40(9):978–983. doi: 10.1016/j.injury.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Preetha SP, Kanniappan M, Selvakumar E, Nagaraj M, Varalakshmi P. Lupeol ameliorates aflatoxin B1-induced peroxidative hepatic damage in rats. Comp Biochem Physiol C: Toxicol Pharmacol. 2006;143(3):333–339. doi: 10.1016/j.cbpc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Yener Z, Celik I, Ilhan F, Bal R. Effects of Urtica dioica L. seed on lipid peroxidation, antioxidants and liver pathology in aflatoxin-induced tissue injury in rats. Food Chem Toxicol. 2009;47(2):418–424. doi: 10.1016/j.fct.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Larsson P, Busk L, Tjälve H. Hepatic and extrahepatic bioactivation and GSH conjugation of aflatoxin B1 in sheep. Carcinogenesis. 1994;15(5):947–955. doi: 10.1093/carcin/15.5.947. [DOI] [PubMed] [Google Scholar]
- 23.Singha I, Das SK. Free radical scavenging properties of skin and pulp extracts of different grape cultivars in vitro and attenuation of H2O2-induced oxidative stress in liver tissue ex vivo. Indian J Clin Biochem. 2015;30(3):305–312. doi: 10.1007/s12291-014-0442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26(4–5):340–352. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Xiao BH, Shi M, Chen H, Cui S, Wu Y, Gao XH, et al. Glutathione peroxidase level in patients with Vitiligo: a meta-analysis. Biomed Res Int. 2016;2016:3029810. doi: 10.1155/2016/3029810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maithili Karpaga Selvi N, Sridhar MG, Swaminathan RP, Sripradha R. Efficacy of turmeric as adjuvant therapy in type 2 diabetic patients. Indian. J Clin Biochem. 2015;30(2):180–186. doi: 10.1007/s12291-014-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei L, Ren F, Zhang X, Wen T, Shi H, Zheng S, et al. Oxidative stress promotes d-GalN/LPS-induced acute hepatotoxicity by increasing glycogen synthase kinase 3β activity. Inflamm Res. 2014;63(6):485–494. doi: 10.1007/s00011-014-0720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen T, Wu ZM, Liu Y, Tan YF, Ren F, Wu H. Upregulation of heme oxygenase-1 with hemin prevents d-galactosamine and lipopolysaccharide-induced acute hepatic injury in rats. Toxicology. 2007;237(1–3):184–193. doi: 10.1016/j.tox.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Park G, Oh DS, Lee MG, Lee CE, Kim YU. 6-Shogaol, an active compound of ginger, alleviates allergic dermatitis-like skin lesions via cytokine inhibition by activating the Nrf2 pathway. Toxicol Appl Pharmacol. 2016;310:51–59. doi: 10.1016/j.taap.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Wu S, Yue Y, Peng A, Zhang L, Xiang J, Cao X, et al. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food Funct. 2016;7(6):2624–2634. doi: 10.1039/C6FO00419A. [DOI] [PubMed] [Google Scholar]