Abstract
Omentin-1 and prostate specific antigen (PSA) are known to be markers of insulin resistance and hyperandrogenism respectively in polycystic ovary syndrome (PCOS). This study aimed to assess the changes in serum omentin-1 and PSA levels in PCOS patients while on treatment. Serum omentin-1, PSA, BMI and Ferriman gallwey score (FG score) were measured in 80 women with PCOS. The subjects were treated depending on their presenting complaints. The parameters were analysed at baseline and after 3 months of treatment viz. lifestyle modification (Group I), oral contraceptive pills (OCP) (Group II), clomiphene citrate (Group III), medroxy progesterone acetate (Group IV) or metformin (Group V) and was compared using paired-t test. Group II showed significant increase in serum omentin-1 (271.7 ± 112.2 vs 378.1 ± 242 ng/ml; P = 0.025) and decrease in serum PSA (0.014 ± 0.02 vs 0.005 ± 0.008 ng/ml; P = 0.027) after 3 cycles of OCP. Group I and IV also showed a decrease in serum PSA while FG score decreased in group II and group III. There was a negative correlation observed between serum omentin-1 and BMI. To conclude, PCOS women showed increase in serum omentin-1 levels after 3 cycles of OCP while serum PSA decreased after 3 months of lifestyle modification or on treatment with OCP or medroxy progesterone acetate.
Keywords: Hirsutism, Omentin-1, Polycystic ovary syndrome, Prostate specific antigen
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine metabolic disorders affecting 6–10% of women in the reproductive age group [1]. It commonly manifests as menstrual irregularity, obesity, hirsutism and infertility. Insulin resistance is the hallmark in PCOS that leads to hyperandrogenism further contributing to cardiovascular risk and metabolic syndrome. Omentin-1 is a novel adipokine produced by visceral adipose tissue. It is an insulin sensitizer that increases insulin stimulated glucose uptake in human adipocytes [2]. Recent studies have shown that omentin-1 is a good index to confirm insulin resistance and that serum omentin-1 levels are decreased in PCOS, obesity, type-2 diabetes mellitus and atherosclerosis. Hence Omentin would be a better alternative as it is also predictive of metabolic complications associated with obesity [3]. Further, serum Insulin alone cannot explain the inflammatory response in PCOS because adipokines play a central role in the metabolic pathogenesis of metabolic syndrome by affecting vascular contractility and vascular inflammatory response [4]. In PCOS the high insulin levels and Insulin like growth factor-1 (IGF-1) stimulate the LH mediated ovarian androgen synthesis resulting in hyperandrogenism. Various studies have shown that hyperandrogenism in PCOS is associated with elevated serum prostate specific antigen (PSA) levels which correlates with serum testosterone levels [5, 6]. PSA is a glycoprotein produced by prostate gland and is being used as a specific marker of prostatic cancers. PSA is also detectable in some female tissues like breast, ovary, endometrium, amniotic fluid and milk [7]. PSA is a emerging marker of hirsutism. Unlike serum testosterone PSA is not affected by short term ovarian and adrenal stimulation [8]. The fact is still unknown whether the clinical improvement in hirsutism determined by Ferriman Gallwey Score (FG Score) in PCOS can be assessed by a decrease in serum PSA levels.
Women on oral contraceptive pills (OCPs) for menstrual disturbances show a decrease in hirsutism besides return of their regular menstrual cycle. Therefore we intended to explore the role of oral contraceptive pills in decreasing hyperandrogenism and therefore its effect on insulin resistance in the present study. As PCOS women on metformin show increase in insulin sensitivity and decrease in serum testosterone concentrations, a similar role for metformin with reference to omentin-1 levels and serum prostate specific antigen is anticipated in this study. Clomiphene citrate which is still the first line drug for ovulation induction in PCOS with infertility due to anovulation has shown to increase the ovulation rates and pregnancy rates. But its impact on other components of PCOS, namely, hyperandrogenism and insulin resistance is unknown. Medroxy progesterone acetate decreases the gonadotrophin levels by decreasing GnRH levels via hypothalamo-pituitary axis, thereby reducing the estrogen and testosterone synthesis from the ovaries [9]. As various modalities of treatment are in practice to treat PCOS, comparison of effectiveness of these on insulin resistance and hyperandrogenism is worth studying. Data regarding the use of these novel markers viz. omentin and PSA for monitoring therapy is deficient. Hence the aim of the present study was to assess the changes in serum prostate specific antigen and omentin-1 levels in patients with PCOS while on treatment.
Materials and Methods
The study was a prospective cohort study, conducted in the Department of Biochemistry in collaboration with the Department of Obstetrics and Gynecology, JIPMER, Puducherry, India. After approval by JIPMER research committee and Institute Ethics sub-committee (human studies) a written informed consent was obtained from the study subjects before recruitment from the gynecology out patient department based on Rotterdam ESHRE-ASRM (2004) criteria for diagnosis of PCOS [10]. Newly diagnosed PCOS cases as well as those who were previously diagnosed and receiving OCP for menstrual disturbances and hirsutism and those who were on Metformin or Clomiphene citrate for infertility for a period less than 6 months were included. Patients with hypothyroidism, late onset congenital adrenal hyperplasia, Cushing’s syndrome, ovarian tumours or adrenal tumour, patients with PCOS who conceived during the study and multipara with PCOS were excluded. 85 subjects were recruited in the study. 4 of them conceived during treatment and were excluded and one was lost during follow up. At the first visit, a detailed history regarding the presenting complaints, nature, duration, compliance of treatment and Ferriman Gallwey scoring (FG Score) for hirsutism, were recorded. FG scoring was done by assigning scores from 0 (no hair) to 4 (frankly virile) for hair distribution in each of the nine androgen sensitive areas namely upper lip, chin, upper arm, chest, thighs, upper abdomen, lower abdomen, upper back and lower back. Summating the scores, FG score ≥ 8 was considered to be hirsutism [11]. Anthropometric measurements like height and weight were measured and their BMI was calculated using the formula, weight (kg)/height (m2).
A fasting blood sample of 5 ml was collected from the antecubital vein, serum separated and preserved at − 80 °C before analysis. The subjects were treated depending on their presenting complaints such as infertility or oligomenorrhea with either metformin or clomiphene citrate or hormonal pills. Some of them did not receive any medical therapy and were advised lifestyle modification by weight reduction and dietary management. OCP composed of ethinyl estradiol 30 μg and levonorgestrel 150 μg was given 1 tablet OD from day 2 of cycle for 3 cycles. Metformin was given at a dose of 500 mg OD for 3 months. Clomiphene citrate 150 mg was given from Day 2 to Day 6 of menstrual cycle for three cycles. Medroxy progesterone acetate 10 mg oral was given from Day 16 to Day 26 for 3 cycles. During their next visit after 3 months anthropometric measurements were recorded and second sampling was done to assess the changes in the levels of the parameters.
Serum glucose (glucose oxidise peroxidise method), cholesterol (cholesterol oxidase method) and triglyceride (lipase method) were analysed in Beckman Coulter 680 autoanalyser. Serum Omentin-1 was assayed with enzyme linked immunosorbant assay with kits from Biovendor, Czech Republic. Serum FSH, LH, testosterone, T3, T4, TSH, PSA was assayed by direct chemiluminescence using reagents from Siemens Medical Solutions Diagnostics, USA as per the manufacturer’s instructions (ADVIA Centaur CP Siemens).
Statistical Analysis
The distribution of data on age, BMI and treatment of the patients were expressed as frequencies and percentages. Serum omentin-1, serum PSA and BMI were expressed as mean with standard deviation, FG Score as median and inter quartile range (IQR). Comparison of baseline and post treatment data was done using students paired-t test for normal distributions and Wilcoxon signed rank test was used for non normal data. Independent student-t test was used to compare the parameters between the age and BMI groups. One way analysis of variance with Bonferroni post hoc analysis was carried out for comparing the parameters between the different BMI groups. Karl Pearson’s coefficient of correlation was used to assess the linear relationship between the parameters. All statistical analyses were carried out using SPSS-16.0 version and P value < 0.05 was considered to be statistically significant. (SPSS Inc., Chicago, IL)
Results
80 women with PCOS were included in the study. Based on the treatment received during the 3 months period, PCOS patients were divided into following five groups namely Group I who did not receive any medical treatment but were advised life style modification (n = 31), Group II who were put on oral contraceptive pills (OCP) (n = 17), Group III who received clomiphene citrate for ovulation induction (n = 12), Group IV who received medroxy progesterone acetate (MPA) for withdrawal bleeding (n = 15) and Group V who received Metformin (n = 5). The general characteristics of the study subjects are shown in Table 1.
Table 1.
General characteristics of study subjects at baseline
| Variables | Mean | SD |
|---|---|---|
| Age (years) | 22 | 4.2 |
| Height (cm) | 154.8 | 5.9 |
| Weight (kg) | 59.8 | 13.6 |
| BMI (kg/m2) | 25 | 5.7 |
| Glucose (mg/dl) | 74.8 | 18.8 |
| T. cholesterol (mg/dl) | 149.4 | 28.6 |
| Triacylglycerol (mg/dl) | 100.6 | 52.5 |
| HDL-cholesterol (mg/dl) | 48 | 10.4 |
| LDL-cholesterol (mg/dl) | 71.1 | 34.7 |
| VLDL-cholesterol (mg/dl) | 17.6 | 11.8 |
| T3 (pg/ml) | 3 | 0.4 |
| T4 (ng/dl) | 1.6 | 2.1 |
| TSH (µIU/ml) | 2.4 | 1.5 |
| FSH (IU/L) | 9.5 | 20.1 |
| LH (IU/L) | 12.3 | 10.6 |
| Prolactin (ng/ml) | 14.8 | 14.7 |
| Testosterone (ng/dl) | 44.7 | 30.5 |
SD standard deviation
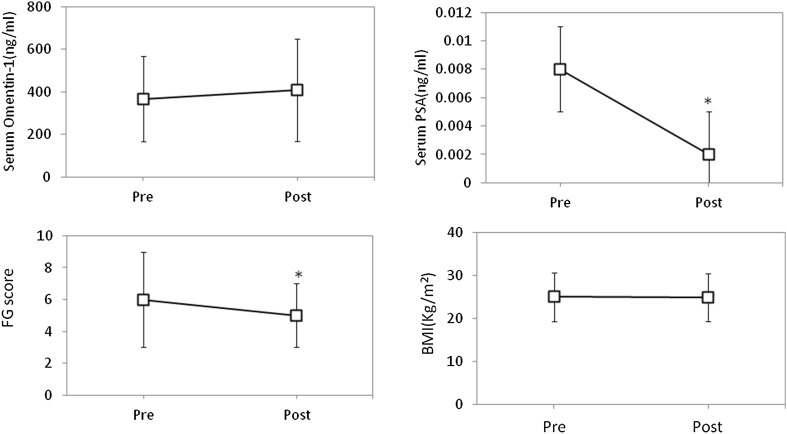
The mean serum omentin-1 level in PCOS women during recruitment was 366.4 ± 199.8 ng/ml. After 3 months of treatment there was a significant increase in serum omentin-1 level with a mean of 409.5 ± 241.1 ng/ml (P = 0.027). Serum PSA was detectable in 36.25% (n = 29) of the study subjects at the time of recruitment with a mean of 0.008 ± 0.015 ng/ml. After 3 months of therapy, PSA was detectable in only 17.5% (n = 14) with the mean 0.002 ± 0.004 ng/ml (P < 0.0001). There was a significant decrease in FG score [6 (3–9) vs 5 (3–7), P = 0.036] after 3 months compared to the baseline value while BMI did not show any change (Fig. 1).
Fig. 1.
Change in study parameters after treatment. Figure shows the change in serum Omentin-1, serum PSA, FG score and BMI in the study subjects after 3 months of treatment for PCOS depending on their presenting complaints.*P < 0.05; Pre—Baseline data; Post—Post treatment data
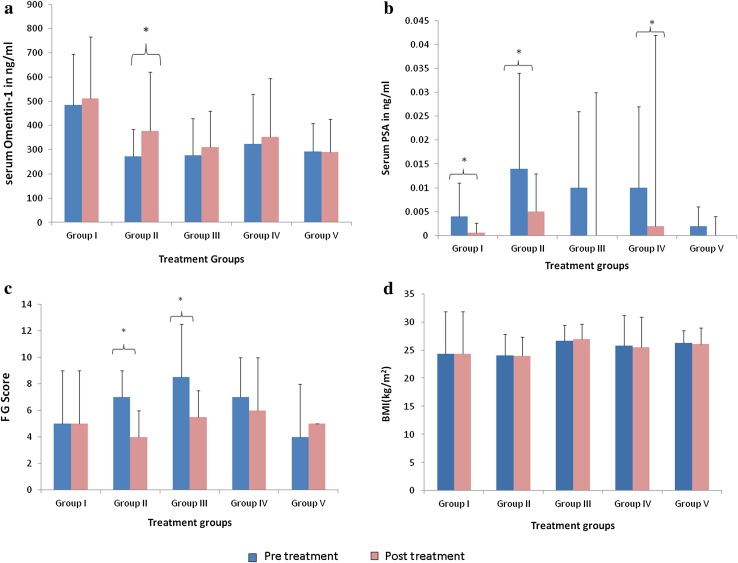
Among the treatment groups it was observed that there was a significant increase in serum omentin-1 levels in Group II (n = 17, 271.7 ± 112.2 vs 378.1 ± 242.5 ng/ml, P = .025) (Fig. 2a). Serum PSA level significantly decreased after 3 months of treatment in Group I (n = 31, 0.004 ± 0.007 vs 0.0006 ± 0.002 ng/ml, P = .003), Group II (0.014 ± 0.02 vs 0.005 ± 0.008, P = .027) and Group IV (n = 15, 0.01 ± 0.018 vs 0.002 ± 0.04, P = .047) (Fig. 2b). Improvement in hirsutism with significant decrease in FG score was seen in Group II [7 (3.5–9) vs 4 (3–6), P = .002] and Group III [8.5 (4.25–12.25) vs 5.5 (4–7.5), P = .040] (Fig. 2c). The BMI of the patients did not differ significantly in any of the treatment groups, as observed in the present study (Fig. 2d).
Fig. 2.
Change in study parameters after treatment in various treatment groups. Figure shows group wise change in parameters in the study subjects after 3 months of treatment for PCOS depending on their presenting complaints. a Significant increase in serum Omentin-1in OCP treated group II, b significant decrease in serum PSA in Lifestyle modification group I, OCP treated group II, MPA treated group IV, c significant decrease in FG score in OCP treated group II and clomiphene citrate treated group III, d no change in BMI in any of the groups. *P < 0.05; Pre—Baseline data; Post—Post treatment data
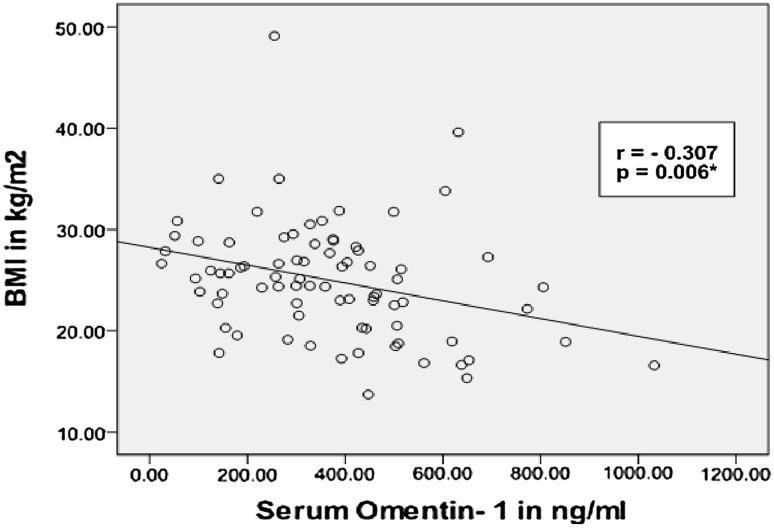
Based on their BMI at baseline, serum omentin-1 was significantly high in underweight subjects (BMI < 18.5, n = 11) compared to overweight subjects (BMI 25.0–29.9, n = 30) (525.3 ± 228.6 vs 285.2 ± 159.9 ng/ml, P = .003) Table 2. There was also a negative correlation between serum omentin-1 and BMI (Pearson’s correlation; r = − 0.307; P = 0.006) (Fig. 3).
Table 2.
Comparison of study parameters between different BMI groups at baseline
| Parameters | Underweight (n = 11) | Normal (n = 28) | Overweight (n = 30) | Obese (n = 11) | P value |
|---|---|---|---|---|---|
| Serum Omentin-1 (ng/ml) | 525.3 ± 228.6 | 401.1 ± 196.4 | 285.2 ± 159.9 | 340.1 ± 181.8 | 0.003* |
| Serum PSA (ng/ml) | 0.011 ± 0.016 | 0.07 ± 0.013 | 0.008 ± 0.017 | 0.007 ± 0.011 | 0.886 |
Values expressed as mean ± SD
*Significant difference between underweight and overweight group
Fig. 3.
Correlation between serum Omentin-1 and BMI. Figure shows significant negative correlation between serum omentin-1 and BMI in the study subjects at baseline
Discussion
PCOS is currently managed symptomatically to regularize the menstrual cycles, to decrease the androgen levels and to restore fertility. Metabolic derangements in the circulating adipokines and hormones which lead to long term risks in PCOS are extensively studied now. Hence, the present study was conducted with an aim to assess the changes in the serum levels of omentin-1 and prostate specific antigen with treatment in PCOS. It is evident from the present study that majority of PCOS patients (38.8%) were only advised life style modification. The second largest treatment group was that treated with OCP (21.2%). It is to be noted that only 15% of the patients were treated with clomiphene citrate and 6.2% were treated with metformin.
Omentin is a secretory glycoprotein composed of 296 amino acids and N-linked oligosaccharides, secreted by stromal vascular cells in omental adipose tissue but not by subcutaneous adipose tissue. It acts as an insulin sensitizer by increasing insulin signal transduction by activating protein kinase AKt/PKB pathway thereby enhancing insulin stimulated glucose uptake by human adipocytes. It is also expressed in the heart, lungs, ovary, placenta and in the paneth cells in small intestine of neonates [2]. Serum omentin-1 which is considered as a good indicator of insulin resistance dramatically increased with 3 cycles of oral contraceptive pills which enables us to interpret regularization of menstrual cycle by OCPs can bring about insulin sensitization by increasing serum omentin-1 levels. This is supported by a study conducted by Mahde et al. [3] who reported a significant difference in serum omentin-1 levels in PCOS patients with regular menstrual cycle compared to those with irregular menstrual cycles without the use of OCP. Dynamic estimation of serum omentin-1 during various phases of menstrual cycles may explain the interplay of adipokines with ovarian hormones and the role of omentin-1 in regulating menstrual function.
PSA, a serine protease belonging to human glandular kallikrein family is a 33 kDa glycoprotein with chymotrypsin like activity. It is encoded by hklk-3 gene located in chromosome 19 and has a steroid hormone binding site which is inducible by androgen, progesterone, vitamin D3 and probably estrogen [12]. PSA which was considered to be specific to prostate gland is now found to be produced in various tissues like breast, placenta, endometrium, sweat glands, salivary glands, adrenals, ovary and periurethral glands (Skene’s gland) in women [13]. Though Guzelmeric et al. [14] have previously shown that serum PSA cannot be used to monitor hirsutism treated with contraceptive pills we found a decrease in serum PSA level and FG score with 3 cycles of oral contraceptive pills which may be due to the fact that the previous study included non PCOS hirsute women. The beneficial role of OCPs in hirsutism is already known because of the fact that the progesterone component of OCP suppresses LH and decreases the ovarian androgen production. The estrogen component increases the SHBG which decreases the free androgen levels [9]. Since androgens were known to induce PSA production in females [14–17], decrease in serum PSA levels in these patients may be explained by the decrease in hyperandrogenism as depicted by a change in FG Score. Additionally, LH stimulated excess androgen production is decreased with insulin sensitization leading to decrease in FG score of hirsutism.
Previously, Vural et al. have found a high level of serum PSA in PCOS women compared to normal controls. The serum PSA also positively correlated with FG score, DHEAS, total testosterone, LH:FSH ratio, free androgen index. They have concluded that PSA is a useful marker of androgen excess in PCOS women [4]. Furthermore, Obiezu et al. [18] have proved urine PSA to be a promising marker of hyperandrogenism in PCOS women by demonstrating high urine PSA in PCOS women compared to controls. They have also shown a weak positive correlation between urine PSA and serum testosterone. Both these studies were case control studies and did not evaluate the change in PSA with treatment in PCOS women while, the present study has found a decrease in serum PSA in PCOS patients after 3 months of treatment. This possibly makes PSA, a reliable marker to monitor the effectiveness of therapy in PCOS women. However the present study did not aim to compare serum PSA with serum testosterone as a marker to monitor therapy of hirsutism in PCOS. This offers scope for future research on this speculation. Probably ours is the first prospective study to show that serum PSA can be used as a prognostic marker of treatment in PCOS women with hirsutism. The results of the present study is similar to a previous study by Metawie et al. [19] who had shown than serum PSA predicts hirsutism associated with PCOS and does not predict idiopathic hirsutism. In their study involving PCOS women with infertility who were put on ovulation induction with clomiphene citrate, they had shown that serum PSA decreased among responders than non responders to clomiphene citrate. The present study also found a significant decrease in FG score in those patients treated with clomiphene citrate for ovulation induction, though there was no significant change in serum PSA levels. This may be due to the fact that local androgen production in the skin can also manifest as hirsutism in addition to circulating androgens. The genetic and molecular interaction of PSA with androgens needs detailed study.
Regularisation of menstrual cycles by exercise and weight reduction or by MPA has resulted in a decrease in serum PSA. This may be due to the uterine regulatory function of PSA as PSA inhibits IGFBP-3 by its protease action. IGFBP-3 is an inhibitor of IGF-1 which plays a role in endometrial proliferation and differentiation. Hence PSA regulates uterine function locally by its protease action on IGFBP-3 [20]. Patients who received metformin did not show any change in the serum omentin-1, serum PSA and FG Score. Tan et al. and Shaker et al. have shown an increase in serum omentin-1 levels with metformin therapy. It is clear from the present study that only a small number of PCOS patients (6.2% in the present study) were started with metformin therapy. Also those studies showing decrease in insulin resistance with metformin were achieved by higher dose of metformin for longer duration. Tan et al. administered metformin 850 mg bd for 6 months and Shaker et al. for 3 months [21, 22]. Whereas in the present study, subjects received only 500 mg/day for 3 months, which may not be sufficient to bring about change in insulin resistance and hyperandrogenism. Additionally, their study postulated that increase in omentin-1 after metformin therapy may be due to decrease in BMI but the present study did not find any change in BMI after 3 months of metformin therapy. This could also be the reason for no change in serum omentin-1 levels. Further the group which received metformin in the present study was small.
BMI did not change significantly with 3 months of medical treatment and lifestyle modification which reflects that omentin-1 is not dependent on BMI alone or vice versa. In the present study there was a significantly high serum omentin-1 level in underweight subjects than overweight subjects. But serum omentin-1 levels did not significantly vary between obese and normal weight PCOS women. This is similar to a study by Orlik et al. [23] who has shown that serum omentin-1 was significantly low in PCOS compared to non PCOS women but the levels were similar in obese and non obese PCOS women. Additionally they had also shown that serum omentin-1 level variability was explained by estradiol, LH/FSH ratio, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and Free Androgen index (FAI) but not to anthropometric indices like BMI, WC, fat mass and percentage. Tan et al. [24] has also concluded that omentin-1 is related to inflammatory states of the visceral adipose tissue than to metabolic parameters like BMI. This leads to the speculation that serum omentin-1 is affected by various factors in addition to BMI. However there was a negative correlation between serum omentin-1 and BMI in the present study. Liu et al. and Choi et al. have shown similar negative correlation between serum omentin-1 and BMI in metabolic syndrome and in PCOS respectively [25, 26]. One limitaion of the study is that as blood samples were collected from patients with amenorrhea for 3–4 months, the phase of menstrual cycle could not be deciphered. Substantially, Buruli et al. [27] has found no significant difference in PSA levels according to menstrual phases in healthy premenopausal women. Nevertheless, this is the first study, to the best of our knowledge to compare and study the various modalities of treatment in PCOS and its effect on various aspects of PCOS like insulin resistance, hyperandrogenesim and obesity.
To conclude, PCOS women showed increase in serum omentin-1 after three cycles of oral contraceptives which signifies increase in insulin sensitivity. OCPs also decreased serum PSA and FG Score thus resulting in clinical and biochemical improvement in hyperandrogenism. Three months of dietary modification and exercise decreased serum PSA levels. Medroxy progesterone acetate decreased serum PSA levels but did not affect the clinical presentation of hirsutism and the insulin resistance. Further studies on the long term effect of these biomarkers in the pathogenesis of PCOS and their modification by treatment would help in the reduction of metabolic complications in PCOS women.
Acknowledgements
I thank JIPMER for granting permission and intramural funds for conducting this study.
Funding
The study was funded by the institute’s intramural fund.
Conflict of interest
Dr. Anbalagan Anithasri, Dr. Palghat Harihara Ananthanarayanan and Dr. P. Veena declare that we have no conflict of interest.
Ethical Approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Yang R-Z, Lee M-J, Hu H, Pray J, Wu H-B, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 3.Mahde A, Shaker M, Al-Mashhadani Z. Study of omentin1 and other adipokines and hormones in PCOS patients. Oman Med J. 2009;24:108–118. doi: 10.5001/omj.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–343. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Ukinc K, Ersoz HO, Erem C, Hacihasanoglu AB. Diagnostic value of prostate-specific antigen (PSA) and free prostate specific antigen (fPSA) in women with ovulatory and anovulatory polycystic ovary syndrome. Endocrine. 2009;35:123–129. doi: 10.1007/s12020-008-9130-6. [DOI] [PubMed] [Google Scholar]
- 6.Vural B, Ozkan S, Bodur H. Is prostate-specific antigen a potential new marker of androgen excess in polycystic ovary syndrome? J Obstet Gynaecol Res. 2007;33:166–173. doi: 10.1111/j.1447-0756.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 7.Mardanian F, Heidari N. Diagnostic value of prostate-specific antigen in women with polycystic ovary syndrome. J Res Med Sci. 2011;16:999–1005. [PMC free article] [PubMed] [Google Scholar]
- 8.Escobar-Morreale HF, Serrano-Gotarredona J, Avila S, Villar-Palasí J, Varela C, Sancho J. The increased circulating prostate-specific antigen concentrations in women with hirsutism do not respond to acute changes in adrenal or ovarian function. J Clin Endocrinol Metab. 1998;83:2580–2584. doi: 10.1210/jcem.83.7.4960. [DOI] [PubMed] [Google Scholar]
- 9.Kuzbari O, Dorais J, Peterson C. Endocrine disorders. Novaks Gynecol. Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 1080–1086. [Google Scholar]
- 10.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod Oxf Engl. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 11.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815–830. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, Diamandis EP. Prostate-specific antigen immunoreactivity in amniotic fluid. Clin Chem. 1995;41:204–210. [PubMed] [Google Scholar]
- 13.Nagar R, Msalati AA. Changes in serum PSA during normal menstrual cycle. Indian J Clin Biochem IJCB. 2013;28:84–89. doi: 10.1007/s12291-012-0263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzelmeric K, Seker N, Unal O, Turan C. High serum prostate-specific antigen concentrations in hirsute women do not decrease with treatment by the combination of spironolactone and the contraceptive pill. Gynecol Endocrinol. 2004;19:190–195. doi: 10.1080/09513590400012069. [DOI] [PubMed] [Google Scholar]
- 15.Escobar-Morreale HF, Avila S, Sancho J. Serum prostate-specific antigen concentrations are not useful for monitoring the treatment of hirsutism with oral contraceptive pills. J Clin Endocrinol Metab. 2000;85:2488–2492. doi: 10.1210/jcem.85.7.6664. [DOI] [PubMed] [Google Scholar]
- 16.Melegos DN, Yu H, Ashok M, Wang C, Stanczyk F, Diamandis EP. Prostate-specific antigen in female serum, a potential new marker of androgen excess. J Clin Endocrinol Metab. 1997;82:777–780. doi: 10.1210/jcem.82.3.3792. [DOI] [PubMed] [Google Scholar]
- 17.Negri C, Tosi F, Dorizzi R, Fortunato A, Spiazzi GG, Muggeo M, et al. Antiandrogen drugs lower serum prostate-specific antigen (PSA) levels in hirsute subjects: evidence that serum PSA is a marker of androgen action in women. J Clin Endocrinol Metab. 2000;85:81–84. doi: 10.1210/jcem.85.1.6230. [DOI] [PubMed] [Google Scholar]
- 18.Obiezu CV, Scorilas A, Magklara A, Thornton MH, Wang CY, Stanczyk FZ, et al. Prostate-specific antigen and human glandular kallikrein 2 are markedly elevated in urine of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:1558–1561. doi: 10.1210/jcem.86.4.7378. [DOI] [PubMed] [Google Scholar]
- 19.Metawie M, El Sarafy T, El-Kattan S, Azab H, El-Biely M. Serum-prostatic specific antigen level as a promising marker in infertile women with polycystic ovarian disease. Middle East Fertil Soc J. 2008;13:28–32. [Google Scholar]
- 20.Rutanen EM, Pekonen F, Mäkinen T. Soluble 34K binding protein inhibits the binding of insulin-like growth factor I to its cell receptors in human secretory phase endometrium: evidence for autocrine/paracrine regulation of growth factor action. J Clin Endocrinol Metab. 1988;66:173–180. doi: 10.1210/jcem-66-1-173. [DOI] [PubMed] [Google Scholar]
- 21.Shaker M, Mashhadani ZIA, Mehdi AA. Effect of treatment with metformin on omentin-1, ghrelin and other biochemical, clinical features in PCOS patients. Oman Med J. 2010;25:289–293. doi: 10.5001/omj.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan BK, Adya R, Farhatullah S, Chen J, Lehnert H, Randeva HS. Metformin treatment may increase omentin-1 levels in women with polycystic ovary syndrome. Diabetes. 2010;59:3023–3031. doi: 10.2337/db10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orlik B, Madej P, Owczarek A, Skałba P, Chudek J, Olszanecka-Glinianowicz M. Plasma omentin and adiponectin levels as markers of adipose tissue dysfunction in normal weight and obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2014;81:529–535. doi: 10.1111/cen.12381. [DOI] [PubMed] [Google Scholar]
- 24.Tan BK, Adya R, Randeva HS. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc Med. 2010;20:143–148. doi: 10.1016/j.tcm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Choi J-H, Rhee E-J, Kim K-H, Woo H-Y, Lee W-Y, Sung K-C. Plasma omentin-1 levels are reduced in non-obese women with normal glucose tolerance and polycystic ovary syndrome. Eur J Endocrinol. 2011;165:789–796. doi: 10.1530/EJE-11-0375. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Wang X, Bu P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res Clin Pract. 2011;93:21–25. doi: 10.1016/j.diabres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Burelli A, Cionini R, Rinaldi E, Benelli E, Fiore E, Canale D, et al. Serum PSA levels are not affected by the menstrual cycle or the menopause, but are increased in subjects with polycystic ovary syndrome. J Endocrinol Invest. 2006;29:308–312. doi: 10.1007/BF03344101. [DOI] [PubMed] [Google Scholar]