Abstract
Finally stereoselective: Enantioselective variations have been developed for many multicomponent reactions; however, it has been missing for the Ugi four-component reaction. This has now changed with the discovery of an efficient catalytic enantioselective variant for the four-component reaction of isocyanides, primary amines, aldehydes or ketones, and carboxylic acids.
Keywords: asymmetric catalysis, enantioselective synthesis, isocyanides, multicomponent reactions, Ugi reaction
Graphical Abstract
Multicomponent reactions (MCRs) are powerful synthetic tools for the synthesis of complex and diverse molecules in a one-pot fashion from more than two starting materials. They find widespread applications in the efficient synthesis of biologically active compounds, marketed drugs, natural products as well as in materials. The most prominent MCR is the four-component reaction of isocyanides, primary amines, aldehydes or ketones and carboxylic acids invented by Ivar Ugi. With aldehydes other than formaldehyde and unsymmetrical ketones a new stereocenter is formed. Previously, there was very limited stereocontrol. Now Tan, Houk and co-workers described an efficient catalytic enantioselective Ugi reaction.[1]
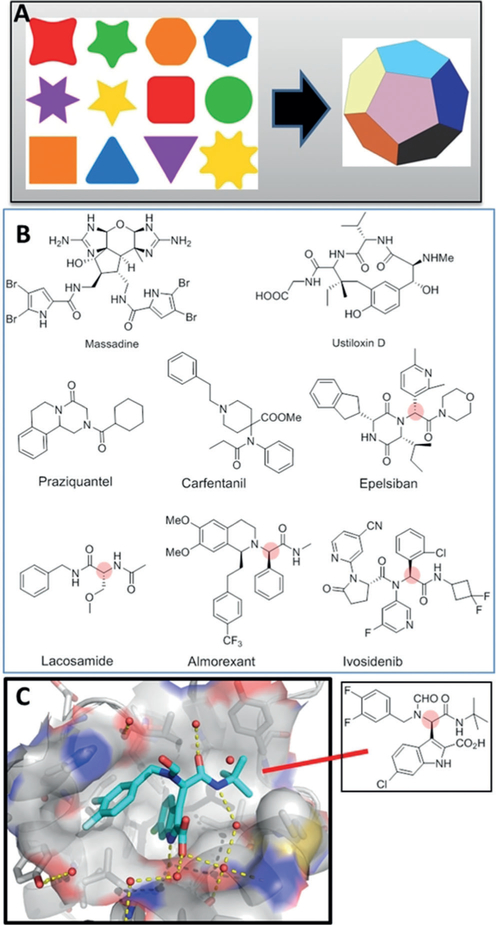
Complex molecules are mostly assembled by sequential one-and two-component transformations and the complexity of the molecules increase with each step. Marketed drugs are synthesized on average in 7–8 steps. An alternative to stepwise synthesis of complex molecules is one-pot multicomponent transformations. Here, at least three starting materials are allowed to react to give a complex product containing most of the atoms of the starting materials. The advantages of MCR over stepwise syntheses include the efficient assembly of complex molecules in just one or few steps (atom and step economy), short reaction times, the overall resource and time saving, low production costs, comparably high yields, great substrate scope, product and scaffold diversity, and the “green conditions” (Figure 1 A).[2] Many MCRs are available in the toolbox of synthetic chemistry, including Strecker, Mannich, Asinger, Gewald, Hantzsch dihydropyridine synthesis, or Biginelli reaction, just to mention a few. Amongst these classical name reactions, the Ugi four-component reaction (U-4CR) is a rather late addition introduced in 1959.[3] Perhaps amongst all MCRs the U-4CR is outstanding in terms of size of chemical space, product and scaffold diversity reachable through secondary reactions, but also in terms of robustness.[4] MCRs and specifically Ugi-type reactions have found widespread applications in drug discovery and natural product synthesis, and more recently in materials science.[5] Many natural products, marketed drugs and clinical drug candidates can be synthesized by Ugi and other MCRs (Figure 1 B).[6] During the U-4CR aldehydes other than formaldehyde and asymmetrical ketones lead to the formation of a new stereocenter. For example, an U-4CR of four simple starting materials yields a potent p53-MDM2 antagonist with one stereocenter (Figure 1 C).[7]
Figure 1.
MCR chemistry and its applications. A) The concept of MCR chemistry involves the convergent assembly of complex products by at least 3 but currently up to 11 starting materials. B) Different natural products, marketed drugs, and clinical candidates that can be efficiently synthesized by the Ugi reaction and other MCRs. C) A chiral R-Ugi product crystallized with the oncogenic MDM2 protein (PDB ID 3TU1).
The R-stereoisomer is more active and can be separated from the racemic reaction mixture by chiral SFC-HPLC in a lengthy and expensive process. To expand the synthetic utility of the U-4CR, for example in drug discovery in the pharmaceutical industry, controlling the stereochemistry is necessary.
The Passerini three-component reaction (P-3CR) of oxo components (aldehydes, ketones), isocyanides and carboxylic acids is related to the U-4CR, where additionally a primary amine is used (Figure 2 A). Mechanistically, however the two reactions differ quite substantially.[8] Whereas the P-3CR prefers apolar-aprotic solvents such as THF or dichloromethane, the U-4CR runs best in polar-protic solvents. The P-3CR mechanism is believed to involve a cyclic uncharged trimeric complex of the three components, where the carboxylic acid increases the carbonyl activity of the oxo component to prepare for the stereogenic step, the nucleophilic attack of the isocyanide. In the U-4CR the key intermediate is the Schiff base which is less reactive than the oxo component in the P-3CR and needs activation through protonation by the carboxylic acid or a Lewis acid and is then attacked by the nucleophilic isocyanide in the stereogenic step. The charged complex of the U-4CR is best stabilized in protic-polar solvents. Consequently, chiral Lewis acid induced Passerini reactions were reported early on and now optimized procedures are available.[8–12] The discovery of catalytic asymmetric variants of the U-4CR was hampered by the polar-protic solvent which competes for the catalyst’s electrophilic center as well as by the strongly nucleophilic and complexing nature of the amine component. Several research groups have tried unsuccessfully for many years to achieve the catalytic enantioselective U-4CR. This is quite remarkable since enantioselective reactions of carbonyl compounds such as additions are quite elaborated tools in organic chemistry. Now Tan, Houk and co-workers have discovered and described an efficient process for the catalytic enantioselective U-4CR in Science.[1] Towards this end they designed conformationally restricted chiral phosphoric acids as organocatalysts (Figure 2 A,B). Two different optimized catalysts were reported, one more suitable for aliphatic aldehydes (CPA6), the other one for aromatic aldehydes (CPA4). The authors propose the formation of a chiral receptor site which forms a hydrogen-bonding-network stabilized complex between the phosphoric acid, the carboxylic acid, and the Schiff base, thus directing the nucleophilic attack of the isocyanide onto the Schiff base carbon atom (Figure 2 C). Overall the phosphoric acid catalyst fulfills two functions, namely, activation of the Schiff base and acceleration of the enantioselective reaction by enhanced carboxylic acid nucleophilicity. The proposed mechanism was supported by DFT calculations and experimentally by comparing the ee of carboxylic acids with different pKa values. The authors proved the synthetic method to be stable in more than 80 examples with chemical yields and ee values ranging from 43 up to 96 % and 83 up to 99 %, respectively. Noteworthy, the solvents used in these procedures are dichloromethane and cyclohexane, atypical Ugi solvents. The results reported are a milestone in isocyanide-based multicomponent reaction chemistry and will greatly enhance future applications in chemical biology, drug discovery, natural product synthesis and materials. Recent applications of the U-4CR range from natural product, drug, stapled peptide synthesis, to stationary phases for mAb separation to sequence-controlled polymers and advanced encryption standard cryptography with molecular steganography to pharmacophore-based virtual screening platforms.[13–15]
Figure 2.
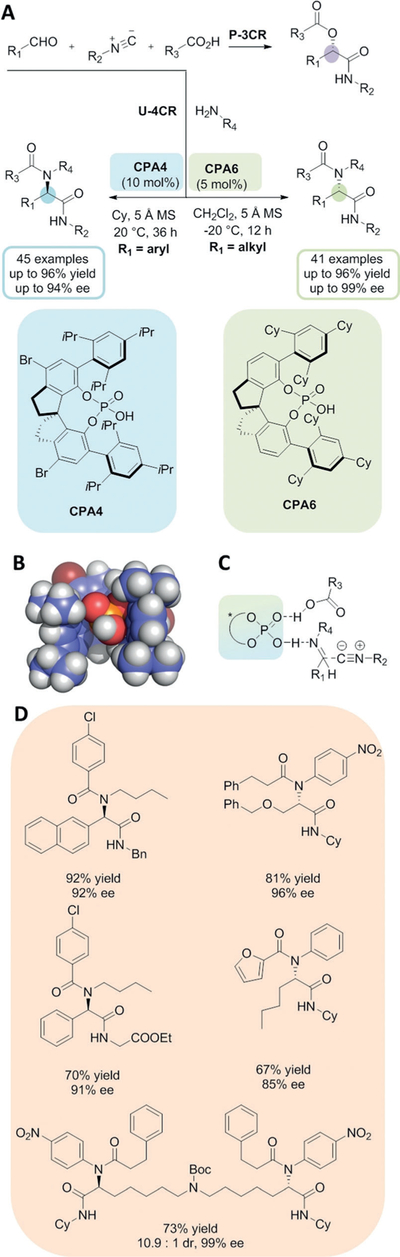
The catalytic enantioselective Ugi reaction. A) Comparison of the Passerini and Ugi reaction. B) Sphere model of CPA4. C) Schematic drawing of the stereocenter-inducing complex. D) Structures, yields, and ee values of representative U-4CR molecules.
All these applications will profit from enhanced stereocontrol. Future work in this area will include the discovery of similar catalytic enantioselective conditions for the multitude of other, less well known but equally important variations of the U-4CR, such as tetrazoles, thiazoles, three-component variations and intramolecular cyclisations.
Acknowledgements
The Dömling laboratory is supported by the National Institute of Health (NIH) (2R01GM097082–05) and the Qatar National Research Foundation (NPRP6–065-3–012). Moreover funding was received through ITN “Accelerated Early stage drug dIScovery” (AEGIS, grant agreement No 675555) and, COFUNDs ALERT and PROMINENT (grant agreements No 665250 and 754425). S.S. is supported by KWF Kankerbestrijding grant No 10504.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Zhang J, Yu P, Li S-Y, Sun H, Xiang S-H, Wang J, Houk KN, Tan B, Science 2018, 361, eaas8707. [DOI] [PubMed]
- [2].Dömling A, Ugi I, Angew. Chem. Int. Ed 2000, 39, 3168–3210; Angew. Chem. 2000, 112, 3300 – 3344. [DOI] [PubMed] [Google Scholar]
- [3].Ugi I, Meyr R, Fetzer U, Steinbrückner C, Angew. Chem 1959, 71, 386. [Google Scholar]
- [4].Cioc RC, Ruijter E, Orru RVA, Green Chem 2014, 16, 2958–2975. [Google Scholar]
- [5].Akritopoulou-Zanze I, Curr. Opin. Chem. Biol 2008, 12, 324–331. [DOI] [PubMed] [Google Scholar]
- [6].Dömling A, Wang W, Wang K, Chem. Rev 2012, 112, 3083–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huang Y, Wolf S, Koes D, Popowicz GM, Camacho CJ, Holak TA, Dömling A, ChemMedChem 2012, 7, 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Q, Wang D-X, Wang M-X, Zhu J, Acc. Chem. Res 2018, 51, 1290–1300. [DOI] [PubMed] [Google Scholar]
- [9].Andreana PR, Liu CC, Schreiber SL, Org. Lett 2004, 6, 4231–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kusebauch U, Beck B, Messer K, Herdtweck E, Dömling A, Org. Lett 2003, 5, 4021–4024. [DOI] [PubMed] [Google Scholar]
- [11].Denmark SE, Fan Y, J. Org. Chem 2005, 70, 9667–9676. [DOI] [PubMed] [Google Scholar]
- [12].Wang S-X, Wang M-X, Wang D-X, Zhu J, Angew. Chem. Int. Ed 2008, 47, 388–391; Angew. Chem. 2008, 120, 394 – 397. [DOI] [PubMed] [Google Scholar]
- [13].Haigh JM, Hussain A, Mimmack ML, Lowe CR, J. Chromatogr. B 2009, 877, 1440–1452. [DOI] [PubMed] [Google Scholar]
- [14].Boukis AC, Reiter K, Frölich M, Hofheinz D, Meier MAR, Nat. Commun 2018, 9, 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Koes D, Khoury K, Huang Y, Wang W, Bista M, Popowicz GM, Wolf S, Holak TA, Dömling A, Camacho CJ, PLOS ONE 2012, 7, e32839. [DOI] [PMC free article] [PubMed] [Google Scholar]