Abstract
OBJECTIVE:
To determine if three-dimensional (3D) printed models can be used to improve acetabular fracture pattern recognition and be a valuable adjunct in orthopedic resident education.
DESIGN:
Fifteen randomized testing stations with each containing plain radiographs (XRs), two-dimensional computed tomography (CT) scans, or 3D model of an acetabular fracture.
SETTING:
Two orthopedic residency programs based at Level 1 trauma centers.
PARTICIPANTS:
Forty-one orthopedic residents, PGY 1–5.
RESULTS:
Senior residents were superior to junior residents at correctly identifying the provided acetabular fracture pattern. Overall, use of CT scans or the 3D model improved fracture classification as compared to standard XRs, but there was no significant difference between use of the CT scans and 3D models. Subjective survey results indicated agreement among residents that 3D models were accurate representations of acetabular fractures and that models would be a desired educational modality.
CONCLUSIONS:
3D models improved the accuracy of acetabular fracture identification compared to XR. In addition, trainees were able to use 3D models to obtain similar accuracy compared to CT scans despite not having previous exposure to the models. Interobserver agreement improved when comparing CT to 3D, but did not provide greater than a fair agreement indicating that fracture patterns were difficult to accurately classify even with the use of 3D models. Residents’ subjective responses indicated a positive experience with the use of 3D models. We conclude that the incorporation of 3D models could be an important adjunct to orthopedic residency education for the evaluation complex fracture patterns, but is not significantly superior to identification with CT scans. ( J Surg Ed 000:1–6. © 2018 Association of Program Directors in Surgery. Published by Elsevier Inc. All rights reserved.)
Keywords: Acetabular fractures, Classification, Pelvis, Three-dimensional, Model
INTRODUCTION
Fractures of the acetabulum are complex and require accurate preoperative identification to determine an optimal treatment plan. Acetabular fractures areroutinely categorized via the Judet and Letournel classification system, with surgical approaches and fixation commonly dictated by fracture type. Thus, the ability to correctly characterize these fractures is an important aspect of an orthopedic resident’s training. Currently, radiographs (XR) and computed tomography (CT) scans are the two-dimensional (2D) imaging modalities utilized to interpret these fractures. However, the complex three-dimensional (3D) geometry and relatively low incidence of these fractures can make it difficult for residents to become adept at correctly identifying acetabular fractures.
With the advent of 3D printing, studies have shown the utility and accuracy of 3D printed models in aiding residents with preoperative decision making in other surgical specialties involving complex and detailed anatomy.1–5 Within orthopedics, studies have shown the use of 3D models as a tool for surgeons during preoperative planning for distal radius and distal humerus fractures, and also as an educational tool for patients to improve understanding of their fractures.6–8 Garrett et al. demonstrated that the use of 3D CT imaging significantly improved resident accuracy in acetabular fracture identification. They also observed an improvement from fair to moderate interobserver agreement when residents used 2D versus 3D CT images to identify acetabular fractures.9 Therefore, the purpose of this study was to evaluate the ability of residents to use 3D printed models to correctly classify acetabular fracture patterns. We hypothesize that 3D models would improve accuracy of acetabular fracture identification compared to XR and CT.
MATERIALS AND METHODS
Five patients with acetabular fractures were identified by the senior authors. Specifically, 1 elementary (transverse) and 4 associated (1 transverse-posterior wall, 1 anterior column posterior hemitransverse, and 2 both column) fracture patterns were selected. The associated fracture patterns were selected due to their relative complexity. We also chose an elementary pattern that involved both columns as this pattern is commonly mistaken for one of the associated fracture patterns. We did not feel that the inclusion of single wall or column patterns would adequately test the ability of 3D models to help residents with acetabular fracture identification.
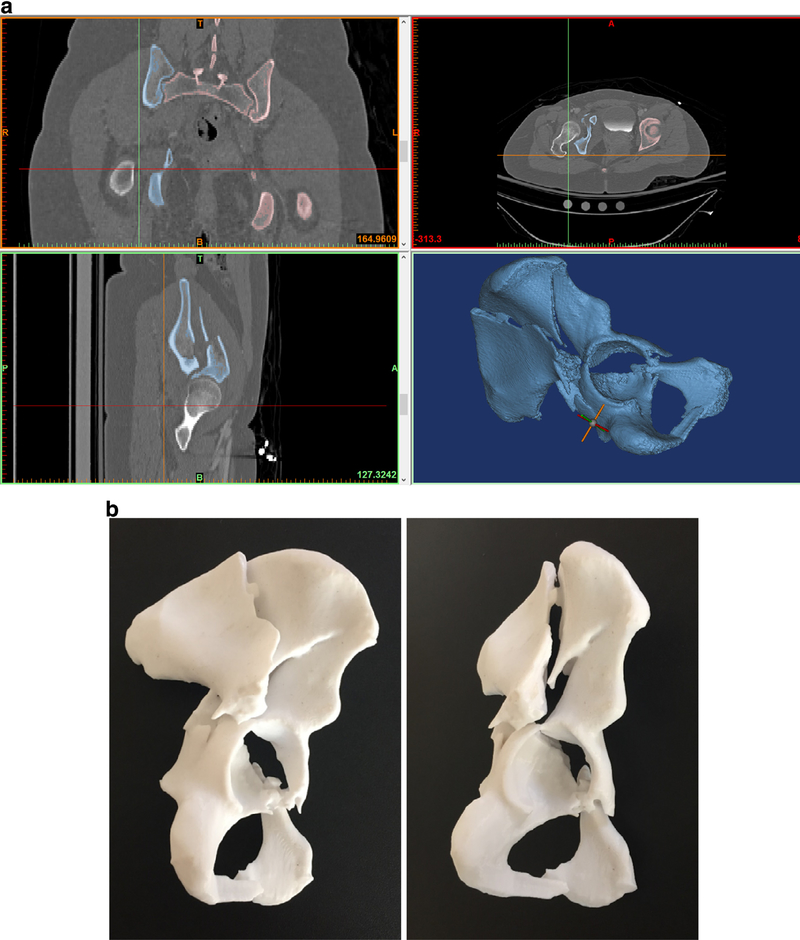
Following patient deidentification, each patient’s pelvis XRs (anterior-posterior and Judet views) and 2D CT scans (axial, sagittal, and coronal reformats) were collected. The CT files were also saved as complete DICOM series and imported into the Materialize Mimics suite (Materialize, Belgium) to create a polycarbonate hemipelvis 3D model containing the respective acetabular fracture (Fig. 1). The models were printed with a Stratasys Fortus 450 MC (Stratasys, Minneapolis, Minnesota).
FIGURE 1.
(a) Screenshot illustrating the use of CT images to render the digital 3D model. (b) Printed 3D model of hemipelvis demonstrating a both column acetabular fracture. The femur was digitally subtracted prior to printing.
Fifteen exam stations were configured. Each station contained either the digital XRs, CT scan images, or a 3D model from each patient. The stations were randomized. Residents were given 90 seconds at each station and allowed to interact with the digital images or model freely. Residents were given an answer sheet that listed the 5 elementary and 5 associated acetabular fracture types and were asked to record their responses for each station. Trainee year was also recorded.
A total of 41 residents from 2 orthopedic training programs based at Level 1 urban trauma centers participated in the study and were divided into 2 groups - junior (PGY1–3) and senior (PGY4–5) trainees. Student t test was used to compare junior and senior groups for collective correct responses and to compare resident capabilities per image modality type. Odds ratios were calculated to determine efficacy of each imaging type to another. Fleiss’ kappa (κ) test was calculated to evaluate the interobserver agreement between imaging modalities and agreement was characterized as described by Landis and Koch: κ value of <0 (poor reliability), 0.00 to 0.20 (slight), 0.21 to 0.40 (fair), 0.41 to 0.60 (moderate), 0.61 to 0.80 (substantial); and 0.81 to 1.00 (near perfect).10
In addition, residents participated in a subjective survey following completion of the exam stations. Responses were recorded on a Likert scale ranging from 1 to 5 (1 being strongly disagree to 5 being strongly agree).
RESULTS
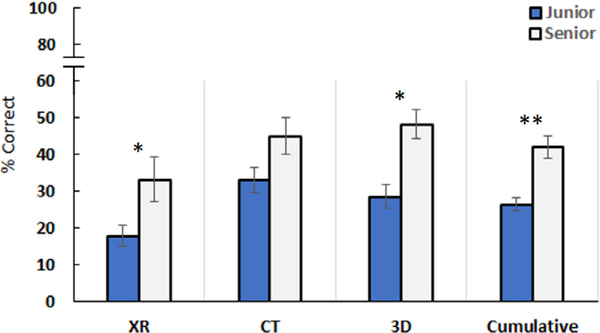
When combining results from all imaging modalities, senior residents scored significantly better than junior residents at correctly identifying the provided acetabular fracture pattern (p < 0.001). Seniors scored significantly higher than junior residents using XRs (p < 0.05) and 3D models (p < 0.05), but not with CT images (Fig. 2). Regardless of the year in training, odds ratios demonstrated significant improvement in fracture identification using CT or 3D models compared to XRs (p < 0.001). There was no significant difference in the odds ratio between CT versus 3D models (Table 1).
FIGURE 2.
Junior and senior resident scores for each imaging modality and all modalities combined (*p < 0.05, **p < 0.001).
TABLE 1.
Odds Ratios Comparing Imaging Modality Effects on Residents’ Ability to Correctly Classify Acetabular Fractures
| Imaging Modality | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| CT vs. XR | 2.0 | (1.5–2.7) | 0.0001 |
| 3D vs. XR | 1.9 | (1.4–2.6) | 0.0001 |
| 3D vs. CT | 0.9 | (0.7–1.3) | 0.7098 |
Evaluation of interobserver agreement showed slight agreement for junior residents when using CT images (κ = 0.172) and an improvement to fair agreement with 3D models (κ = 0.259). Senior residents had fair agreement for both CT (κ = 0.216) and 3D models (κ = 0.351) (Table 2).
Evaluation of interobserver agreement showed slight agreement for junior residents when using CT images (κ = 0.172) and an improvement to fair agreement with 3D models (κ = 0.259). Senior residents had fair agreement for both CT (κ = 0.216) and 3D models (κ = 0.351) (Table 2).
TABLE 2.
Interobserver Agreement When Using or 3D Models (Fleiss Kappa, κ)
| CT | 3D | |
|---|---|---|
| Juniors | 0.172 | 0.259 |
| Seniors | 0.216 | 0.351 |
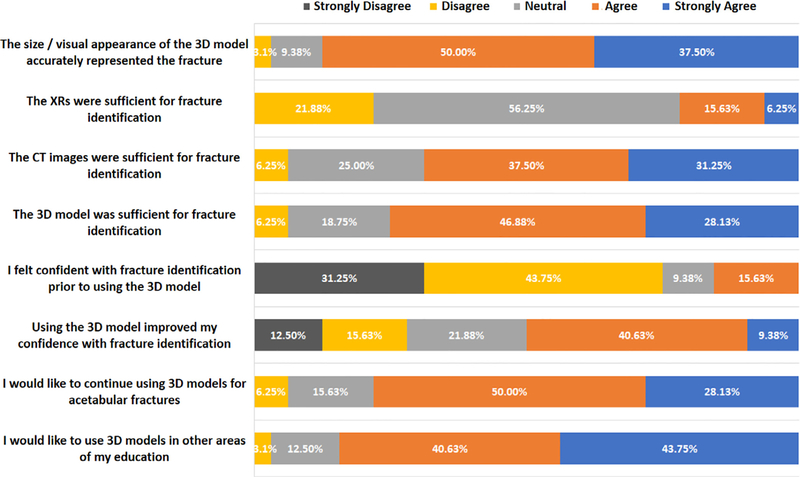
Subjective survey results demonstrated that 87.5% of the residents agreed or strongly agreed with the accuracy and appearance of the 3D model’s representation of acetabular fractures, and 84% desired to further incorporate 3D models into other areas of their education. Residents also agreed with the fact that the CT or 3D model was sufficient for identifying acetabular fractures. Prior to using the 3D model, residents disagreed about feeling confident identifying these fractures. They agreed that the 3D model improved their confidence with fracture identification (Fig. 3).
Subjective survey results demonstrated that 87.5% of the residents agreed or strongly agreed with the accuracy and appearance of the 3D model’s representation of acetabular fractures, and 84% desired to further incorporate 3D models into other areas of their education. Residents also agreed with the fact that the CT or 3D model was sufficient for identifying acetabular fractures. Prior to using the 3D model, residents disagreed about feeling confident identifying these fractures. They agreed that the 3D model improved their confidence with fracture identification (Fig. 3).
FIGURE 3.
Residents’ subjective responses regarding XR, CT, and 3D models.
DISCUSSION
Incorporation of 3D models has been shown to be useful for resident education in surgical specialties such as neurological, thoracic, otorhinolaryngologic, plastic, and hepatic surgery.2,4,5,11,12 Within orthopedics, studies have looked at the potential benefit of using 3D models in cases with significant deformity or complex anatomy such as deformity correction in complex foot and ankle cases or pediatric patients requiring subtrochanteric valgus osteotomy for severe Perthes disease.13,14 Bizzotto et al. demonstrated that 3D models could be printed with sufficient detail and accuracy to aid surgeons with preoperative planning and allow for preselection of distal radius implants which saved time in the operating room. Patients were also surveyed and indicated that 3D models helped them understand the severity of their distal radius fractures.6,7
Overall, our study demonstrates improved resident accuracy in acetabular fracture identification with the use of 3D models compared to XR. When comparing 3D models to CTs, the participants obtained similar accuracy with each modality and both were significantly superior to XRs. However, there was no significant difference between CT and 3D model odds ratios indicating neither modality resulted in superior accuracy.
Studies have clearly demonstrated the importance of experience when attempting to classify acetabular fractures via imaging. Interobserver reliability for the classification of acetabular fracture by orthopedic surgeons who specialize in the treatment acetabular fractures was evaluated and substantial to near perfect agreement was observed (κ range, 0.69–0.83) when using XR and 2D CT images.15,16 Conversely, orthopedic residents and orthopedists who did not regularly manage acetabular fractures showed fair to moderate agreement, respectively.9,15 Inclusion of 3D CT reconstructions improved resident interobserver agreement to moderate agreement (κ range, 0.42–0.44).9 Additionally, Hüfner et al. looked at resident ability to correctly identify acetabular fractures with XR, 2D CT, and 3D CT scans and showed 11%, 30%, and 65% correct responses, respectively.17 Garrett et al. demonstrated similar difficulty by residents with their senior residents correctly identifying only 57.6% of acetabular fractures with 3D CT images.9 The trainees in our study performed similarly and their lower accuracy may be attributable to the complex fracture patterns which were selected for this study. Although interobserver agreement did not exceed fair agreement in this study, there was an improvement when comparing CT to 3D models (Table 2). This data may indicate the overall lack of resident experience with acetabular fractures, but may also corroborate an intuitive quality of 3D models associated with the ability to handle them directly in one’s hands.
Subjectively, 75% of the residents disagreed or strongly disagreed that they felt confident identifying acetabular fractures prior to interacting with the models. However, over 50% agreed or strongly agreed that exposure to the models improved their confidence with fracture identification. This was despite not having any prior experience or exposure to the 3D models. This may be attributable to the tactile and visual feedback inherent to the direct interaction afforded with a 3D printed model. Participants also indicated agreement (87.5%) that the 3D models contained enough detail to represent the acetabular fractures demonstrating that the size and visual appearance of the models were an accurate representation of the fractures. Furthermore, 84% of respondents stated that they either agreed or strongly agreed that they would like to continue using 3D models in other areas of their education. Overall, the subjective data supports the notion that 3D models are perceived as a positive addition to orthopedic resident training.
Accurate representation of complex fracture patterns with 3D printed models can be accomplished with a wide variety of printers that can range from hundreds to hundreds of thousands of dollars for the highest-grade machines. However, studies in both orthopedics and other surgical specialties have been able to use printers with prices below $5000. In this study, a hemipelvis print was approximately $250 including processing and material costs. Even though the current cost of 3D model printing may be prohibitive for some, prices should decrease over time as the technology continues to develop and becomes more prevalent.
Although the results of this study are promising, there are important limitations. First, we had a sample size that was limited to residents from 2 ACGME accredited orthopedic residency programs. Second, we did not test the entire spectrum of acetabular fracture patterns included in Judet and Letournel classification system. Last, the study was designed to only evaluate the utility of 3D models in accurate identification of acetabular fracture types and did not evaluate their use for preoperative planning. Despite these limitations, we believe that 3D printed models are a beneficial tool for enhancing resident education. Future studies may involve the use of this technology to aid in preoperative planning and fixation strategies of these complex fractures.
CONCLUSIONS
In this study, 3D models were equivalent to CT scans for acetabular classification performed by residents; and both modalities were more accurate than XR. Although, based on our results, we cannot recommend the use of 3D models over CT scans, 3D models may become an important adjunct to current imaging modalities and an additional educational tool for residents, especially when first learning about acetabular fractures. The direct tactile and visual feedback afforded by 3D models may be preferred by some residents who learn better through such mediums. Residents’ subjective responses indicate that the models are sufficiently detailed enough to accurately represent acetabular fractures and that they would like to incorporate 3D models into other areas of their education. With the cost of 3D printing continuing to decrease, it may soon be cost effective for residency programs to regularly create 3D models to enhance resident education especially for fractures with complex anatomic geometry.
Acknowledgments
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
DISCLOSURES AND CONFLICT OF INTEREST
Graham S. Stephenson—
no financial disclosures.
Philip K. Lim—no financial disclosures.
Thomas W. Keown—no financial disclosures.
Connor Byrne—no financial disclosures.
Charles C. Lin—no financial disclosures.
Geoffrey S. Marecek—Globus Medical: Paid consultant. Smith & Nephew: Paid consultant. Stryker: Paid consultant. Synthes: Other financial or material support.
John A. Scolaro—Globus Medical: Paid consultant. Smith & Nephew: Paid consultant. Stryker: Paid consultant. Synthes: Other financial or material support. Zimmer Biomet: Paid consultant.
ACGME CORE COMPETENCY: Practice-Based Learning and Improvement
REFERENCES
- 1.Baskaran V, Štrkalj G, Štrkalj M, Di Ieva A. Current applications and future perspectives of the use of 3D printing in anatomical training and neurosurgery. Front Neuroanat. 2016;10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chae MP, Rozen WM, McMenamin PG, Findlay MW, Spychal RT, Hunter-Smith DJ. Emerging applications of bedside 3D printing in plastic surgery. Front Surg. 2015;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das S, Mitchell P. Comparison of three aids for teaching lumbar surgical anatomy. Br J Neurosurg. 2013;27(4):475–478. [DOI] [PubMed] [Google Scholar]
- 4.Kong X, Nie L, Zhang H, et al. Do three-dimensional visualization and three-dimensional printing improve hepatic segment anatomy teaching? A randomized controlled study. J Surg Educ. 2016;73 (2):264–269. [DOI] [PubMed] [Google Scholar]
- 5.Ploch CC, Mansi CS, Jayamohan J, Kuhl E. Using 3D printing to create personalized brain models for neurosurgical training and preoperative planning. World Neurosurg. 2016;90:668–674. [DOI] [PubMed] [Google Scholar]
- 6.Bizzotto N, Sandri A, Regis D, Romani D, Tami I, Magnan B. Three-dimensional printing of bone fractures: a new tangible realistic way for preoperative planning and education. Surg Innov. 2015;22 (5):548–551. [DOI] [PubMed] [Google Scholar]
- 7.Bizzotto N, Tami I, Tami A, et al. 3D Printed models of distal radius fractures. Injury. 2016;47(4):976–978. [DOI] [PubMed] [Google Scholar]
- 8.Brouwer KM, Lindenhovius AL, Dyer GS, Zurakowski D, Mudgal CS, Ring D. Diagnostic accuracy of 2- and 3-dimensional imaging and modeling of distal humerus fractures. J Shoulder Elbow Surg. 2012;21 (6):772–776. [DOI] [PubMed] [Google Scholar]
- 9.Garrett J, Halvorson J, Carroll E, Webb LX. Value of 3-D CT in classifying acetabular fractures during orthopedic residency training. Orthopedics. 2012;35(5):e615–e620. [DOI] [PubMed] [Google Scholar]
- 10.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33 (1):159–174. [PubMed] [Google Scholar]
- 11.Wiesel O, Jaklitsch MT, Fisichella PM. Three-dimensional printing models in surgery. Surgery. 2016;160(3):815–817. [DOI] [PubMed] [Google Scholar]
- 12.Da Cruz MJ, Francis HW. Face and content validation of a novel three-dimensional printed temporal bone for surgical skills development. J Laryngol Otol. 2015;3(129 Suppl):S23–S29. [DOI] [PubMed] [Google Scholar]
- 13.Jastifer JR, Gustafson PA. Three-dimensional printing and surgical simulation for preoperative planning of deformity correction in foot and ankle surgery. J Foot Ankle Surg. 2017;56 (1):191–195. [DOI] [PubMed] [Google Scholar]
- 14.Starosolski ZA, Kan JH, Rosenfeld SD, Krishnamurthy R, Annapragada A. Application of 3-D printing (rapid prototyping) for creating physical models of pediatric orthopedic disorders. Pediatr Radiol. 2014;44(2):216–221. [DOI] [PubMed] [Google Scholar]
- 15.Beaulé PE, Dorey FJ, Matta JM. Letournel classification for acetabular fractures. Assessment of interobserver and intraobserver reliability. J Bone Joint Surg Am. 2003;85-A(9):1704–1709. [PubMed] [Google Scholar]
- 16.Hutt JR, Ortega-Briones A, Daurka JS, Bircher MD, Rickman MS. The ongoing relevance of acetabular fracture classification. Bone Joint J. 2015;97-B (8):1139–1143. [DOI] [PubMed] [Google Scholar]
- 17.Hüfner T, Pohlemann T, Gänsslen A, Assassi P, Prokop M, Tscherne H. The value of CT in classification and decision making in acetabulum fractures. A systematic analysis. Unfallchirurg. 1999;102(2):124–131. [DOI] [PubMed] [Google Scholar]