Abstract
Adjuvant endocrine therapy (AET) is used to prevent recurrence and reduce mortality for women with hormone receptor positive breast cancer. Poor adherence to AET is a significant problem and contributes to increased medical costs and mortality. A variety of problematic symptoms associated with AET are related to non-adherence and early discontinuation of treatment. The goal of this study is to test a novel, telephone-based coping skills training that teaches patients adherence skills and techniques for coping with problematic symptoms (CST-AET). Adherence to AET will be assessed in real-time for 18 months using wireless smart pill bottles. Symptom interference (i.e., pain, vasomotor symptoms, sleep problems, vaginal dryness) and cost-effectiveness of the intervention protocol will be examined as secondary outcomes. Participants (N=400) will be recruited from a tertiary care medical center or community clinics in medically underserved or rural areas. Participants will be randomized to receive CST-AET or a general health education intervention (comparison condition). CST-AET includes ten nurse-delivered calls delivered over 6 months. CST-AET provides systematic training in coping skills for managing symptoms that interfere with adherence. Interactive voice messaging provides reinforcement for skills use and adherence that is tailored based on real-time adherence data from the wireless smart pill bottles. Given the high rates of non-adherence and recent recommendations that women remain on AET for 10 years, we describe a timely trial. If effective, the CST-AET protocol may not only reduce the burden of AET use but also lead to cost-effective changes in clinical care and improve breast cancer outcomes.
Keywords: Adjuvant endocrine therapy, adherence, breast cancer, self-management, RCT
INTRODUCTION
Adjuvant endocrine therapy (AET) is a crucial component of treatment used to prevent recurrence and reduce breast cancer-related mortality for women with hormone receptor positive (HR+) disease.1 In these women, AET reduces breast cancer recurrence by 34-50% and mortality by 24-35%.2 Agents used in AET include tamoxifen and the aromatase inhibitors (AIs) anastrozole, letrozole, and exemestane. AET is delivered in pill form daily for 5-10 years.3,4 Updated guidelines from the American Society of Clinical Oncology recommend that women with HR+ breast cancer remain on AET for up to 10 years5 due to the clinical advantages (e.g., lower risk of recurrence, lower risk of contralateral breast cancer) seen with 10 versus 5 years of treatment. In one recent study, extending AET use from 5 to 10 years resulted in 34% lower risk of recurrence and contralateral breast cancer.6
Rates of non-adherence to AET are high, ranging from 28-59% in clinical settings.7 Similar rates of non-adherence have been found in clinical trials,8-10 epidemiologic studies,7,11-15 and studies of patient-reported adherence or pill counts.13,16,17 Adherence to AET decreases over time,5,18 with research suggesting that only 50% of women are adherent by the fourth year of therapy.18 Most of this research is based on prior guidelines recommending five years of AET; rates of nonadherence may be even greater with guidelines extending AET use to 10 years.19 Early discontinuation or poor adherence (<80% of doses) to AET leads to worse outcomes, including a significantly increased risk of mortality.2,20-25 A recent economic evaluation of tamoxifen adherence found that poor adherence resulted in significant loss of quality-adjusted life-years, increased medical costs, reduced time to recurrence, and increased mortality.25
Women report that AET-related symptoms significantly interfere with daily functioning and are strongly related to AET non-adherence.12,13,16,26-29 These symptoms include persistent pain, vasomotor symptoms, sleep problems, fatigue, and vaginal dryness.30 For women taking AIs, joint pain and stiffness are also major concerns.31-33 Additionally, while women with breast cancer tend to experience more severe menopausal symptoms than women without cancer,34 AET can exacerbate these symptoms.30,35 Our preliminary data showed that 65% of women taking AET reported joint pain, 55% reported hot flashes, and 49% reported night sweats.36 AET-related symptoms significantly interfere with important areas of life including household, family, recreational, and occupational activities, 34, 37 and patients indicate that AET-related symptoms are the most frequent and important reason for stopping treatment.13,26,29 Although systematic training in skills for coping with symptoms potentially could be beneficial in enhancing adherence to AET, to date, no study has evaluated the effects of such training. The efficacy of prior interventions aimed at improving AET adherence has been limited, as these interventions have predominantly focused on providing educational information and reminders, and failed to address the important side effects experienced by women on AET.38,39 The present study aims to evaluate a novel coping skills training intervention protocol (CST-AET) for improving women’s abilities to adhere to AET and reduce the degree to which symptoms interfere with quality of life and daily activities. The content of this protocol is based on our prior work developing and evaluating coping skills interventions for managing symptoms 41-48 and improving adherence 49-56 as well as input from user testing with breast cancer patients. This manuscript details the rationale, design, methods and planned analyses of this National Institutes of Health-funded (1R01CA193673-01A1) randomized clinical trial (RCT).
MATERIALS AND METHODS
A. Study Aims
Our first aim is to investigate the impact of the CST-AET protocol on adherence to AET. Our second aim is to examine the impact of the the CST -AET intervention protocol on symptom interference. Our third aim is to examine the impact of the CST -AET protocol on theory-based measures of perceived barriers to AET medication, beliefs about AET medication, and self-efficacy. Finally, our fourth aim is to estimate short-term costs of implementing the CST-AET intervention and investigate long-term cost-effectiveness of the CST-AET intervention.
B. Patient Selection
a. Eligibility Criteria
Clinical trial participants are recruited from a tertiary medical center and through community cancer clinics affiliated with a cancer clinic network associated with the tertiary medical center. These community cancer clinics are located in medically underserved or rural communities. Eligible participants meet the following inclusion criteria: a) diagnosis of Stage I to III breast cancer, b) hormone receptor positive tumor defined as any positivity of estrogen or progesterone receptor, c) completed surgery, chemotherapy, and radiation, d) within 12 months of beginning AET, and e) at least 18 months of AET recommended. Exclusion criteria are: a) <21 years of age, b) severe cognitive or hearing impairment that is documented in the medical record, or c) unable to provide meaningful consent (e.g., severe cognitive impairment). Though considered, we chose not to include eligibility criteria related to the level or type of symptoms women are experiencing as symptoms can fluctuate and change over the course of AET and may differ by type of AET treatment.57-59
b. Subject Recruitment
This study was approved by the Institutional Review Board. Recruitment procedures comply with HIPAA guidelines. Patients meeting eligibility criteria are provided information about the study in one of two ways: 1) the study brochure that briefly describes the study and letter describing the study is given to women by a member of their treatment team at the time of an oncology follow-up visit, or 2) the study brochure and a letter from their doctor introducing the study is mailed to them. Prospective participants are telephoned by study staff and asked if they are interested in hearing about the study. For women who express interest, study staff arrange an in-person meeting to further describe the study, confirm eligibility, and obtain informed consent.
C. Procedures
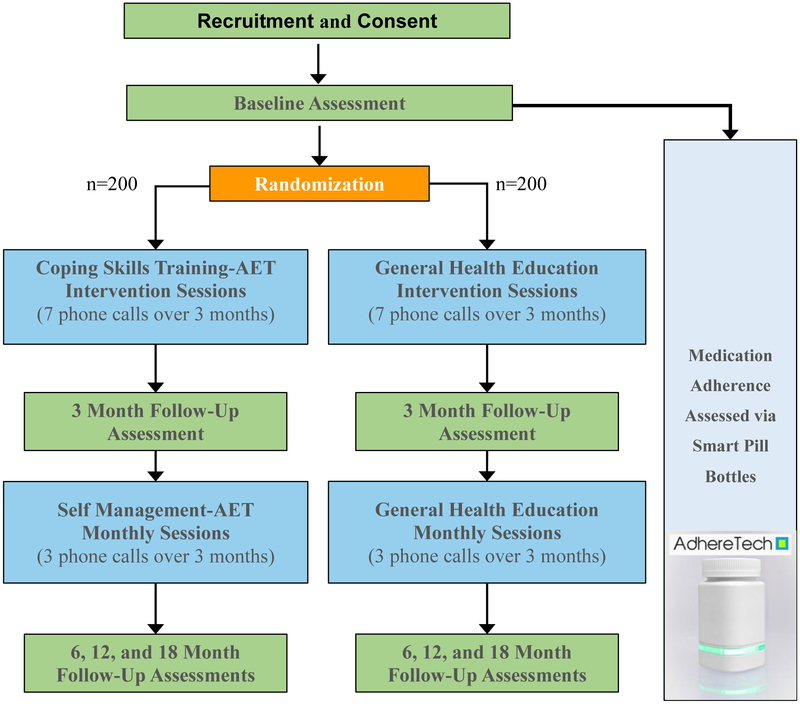
The study design and timeline are presented in figure 1. For this RCT, 400 women who are prescribed AET for breast cancer are randomized with equal allocation to one of two groups: 1) a coping skills training intervention for enhancing skills to improve adherence and reduce symptom interference (CST-AET; active intervention group), or 2) general health education (comparison group that controls for time and attention). Randomization is determined by a randomization program. Participants complete assessments in their treating clinic at baseline, 3 months (at conclusion of intervention sessions), 6 months (at conclusion of maintenance calls), 12 months, and 18 months (one year after conclusion of maintenance calls). A set of questionnaires takes approximately 45-60 minutes to complete and objective physical assessments (i.e., six minute walk test, timed get up and go test, and grip strength test) take approximately 14-20 minutes to complete. Whenever possible, assessments are completed in-person so that participants can complete the physical assessments and research staff can assist those with low literacy in completing items. If participants are unable to attend the in-person visit due to illness or other circumstances, the questionnaire set can be completed online or over the phone. A description of the measures included in the assessments are presented below.
Figure 1.
Study Design and Timeline
Participants also receive AdhereTech’s smart pill bottles. AdhereTech’s bottles are HIPAA-compliant, FDA Class I medical devices that provide electronic tracking of adherence by measuring the bottle opening (date/time) and the contents of the bottle. These bottles were chosen to accommodate the population of women recruited for this study—specifically, women recruited from community clinics who may not have consistent internet access. AdhereTech’s bottles automatically collect adherence data in real-time; as participants use the bottles, adherence data (i.e., bottle opening, cellular connectivity, battery power, bottle functioning) is wirelessly sent from the bottles to AdhereTech’s servers using sensors and a built-in cellular chip. All data and analytics are available 24/7 on the real-time dashboard.
There are several other benefits of the AdhereTech bottles that made it the ideal choice for the present study. First, the pill bottles hold a charge for approximately 6 months meaning that participants do not have to remember to charge the bottles. Second, data related to the status of the bottle’s battery is automatically sent to members of the study team; the study team can then contact participants to remind them to charge the battery in the event that the system identifies a low battery bottle. When thinking about which of the many objective measures of adherence to use, it is important to consider the patient population and barriers to use and/or data collection.
Following randomization and the baseline assessment, women are provided with the intervention materials and are contacted by a study nurse to begin intervention sessions. All participants receive usual medical care.
D. Interventions
The CST-AET and general health education interventions include ten nurse-delivered, individual, phone-based sessions. Each intervention is delivered over 6 months with 3 weekly sessions (month 1), 4 biweekly sessions (months 2-3), and 3 monthly sessions (months 4-6). This faded contact approach is based on recommendations for promoting long-term intervention effects.60,61 Nurses are assisted with delivering the phone-based sessions through the use of an interactive study portal. The customized portal includes manualized session content that has been scripted to assist with intervention delivery. Participants’ responses are used as prompts to guide session content (e.g., symptoms patients are experiencing, whether the study PI or patients’ providers are contacted due to reported symptoms, and answers to questions regarding use of educational information or home practice of intervention techniques). Participants are also provided with intervention material through pre-recorded interactive voice messages. Additional information about the interactive voice messages is provided below.
a. CST-AET Intervention
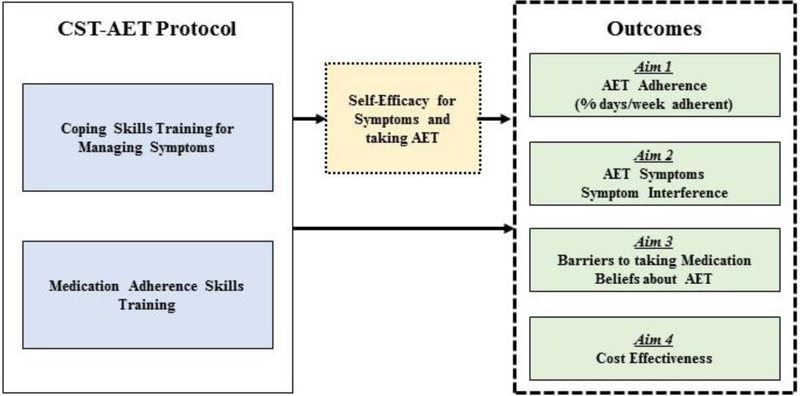
Our work and the resulting CST-AET protocol were guided by social cognitive theory,62 which suggests that the mechanisms responsible for adherence and symptom management are skills that can be learned and applied by patients.62,63 Thus, the CST-AET protocol includes key theory-based approaches: 1) coping skills training for managing symptoms and 2) medication adherence skills training (see Figure 2). The CST-AET protocol combines the use of phone calls, an intervention workbook, audio-recorded relaxation exercises, and interactive voice messaging based on real-time adherence data from AdhereTech bottles and content covered during the sessions. In addition to the aforementioned interactive study portal, nurses delivering the CST-AET intervention also have access to the AdhereTech dashboard which provides data collected via the AdhereTech smart pill bottles, (e.g., scheduled dosage time, bottle open, refill status, bottle check-in, etc.; see Figure 3). This information is used in part to guide discussions regarding potential barriers to adherence during the study intervention sessions.
Figure 2.
CST-AET Intervention Protocol Components
Figure 3.
Screenshot of the portal used by nurses to deliver study session content including data related to participant adherence
Table 1 provides an overview of intervention sessions. Each intervention session is delivered using a four-section structure. 1) Symptom and Adherence Assessment (5 min): A review of symptoms and AET adherence is conducted. Patients are reinforced for using adherence and symptom management skills. If non-adherence is noted, brief problem solving is conducted that directs patients to the relevant adherence skills (e.g., cueing strategies). This approach is consistent with recommendations for increasing the effectiveness of adherence interventions.64 The nurse will immediately notify the patient’s oncologist of major health issues. 2) Symptom-specific strategies (15 min): Each session includes rationales for specific coping and adherence skills, medication education (e.g., why AET is prescribed, evaluation of the benefits and costs of AET), symptom education, and strategies for managing specific symptoms (e.g., strategies for managing hot flashes). 3) Skills Training (15 min): Coping and adherence skills training addresses cognitive, behavioral, social, and emotional factors that influence adherence and heighten symptom interference. Instruction, modeling, and guided practice with formal shaping procedures and differential reinforcement are used to teach patients skills for managing symptoms and improving adherence. 4) Skills Application (10 min): Behavioral rehearsal and home practice plans are used to enhance the patient’s self-efficacy and abilities for applying learned skills to their specific symptoms and adherence concerns. This section concludes by working with the patient to identify goals for home practice and develop action plans for applying learned skills to symptom management and adherence challenges.
Table 1.
Overview of CST-AET Intervention Sessions
| Session | Psycho-Education | Skills Training | Skills Application |
|---|---|---|---|
| 1 | AET Education | Medication Adherence Skills (e.g., routine, cueing) | Rehearsal and application of adherence skills |
| 2 | Pain Education | Relaxation-Based Skills (e.g., progressive muscle relaxation) | Rehearsal and application of relaxation skills for managing pain |
| 3 | Vasomotor Symptom Education | Breathing and Brief Relaxation Skills (e.g., paced breathing) | Rehearsal and application of breathing and brief relaxation skills to manage vasomotor symptoms, other symptoms, and stress |
| 4 | Fatigue Education | Activity Pacing Skills (e.g., activating rest cycle) | Rehearsal and application of activity pacing for managing fatigue |
| 5 | Depression Education | Behavioral Activation Skills (e.g., pleasant activity scheduling, setting SMART goals) | Rehearsal and application of behavioral activation for managing depression and fatigue |
| 6 | Sleep Education | Cognitive Restructuring Skills Part 1 (e.g., identifying unhelpful thoughts, shifting to more neutral thoughts) | Rehearsal and application of cognitive restructuring skills |
| 7 | Concentration and Memory Education | Cognitive Restructuring Skills Part 2 (e.g., letting go of and replacing unhelpful thoughts) | Rehearsal and application of cognitive restructuring skills |
| 8 | Sexual Side Effects and Body Image Education | Communication Skills Training (e.g., talking about sensitive topics) | Rehearsal and application of skills for communicating with healthcare providers about symptoms/concerns |
| 9 | Maintenance Education | Problem Solving Training (e.g., identifying a problem and choosing a strategy to deal with the problem) | Developing a maintenance plan, rehearsal and application of problem solving skills for coping with setbacks |
| 10 | Maintenance Education | Values and Long-Term Goal Setting (e.g., reviewing SMART goals strategy) | Developing a maintenance plan, using proximal goals and reinforcement principles to reach valued long-term goals |
Each participant is provided with the CST-AET workbook, which includes written information, pictures, and diagrams for all content delivered during sessions and supplemental content. The workbook provides guided exercises to help women apply content to their particular concerns. Participants also receive audio recordings in the format of their choice (i.e., download from study website, on compact discs, or on a flash drive) of relaxation exercises (e.g., Progressive Muscle Relaxation).
Sessions 1 – 8 of CST-AET focus on review of home practice with learned skills followed by introducing and applying new skills while sessions 9 and 10 focus on applying skills to cope with potential barriers to adherence and maintaining use of intervention skills. Calls include both psycho-education (e.g., review and re-evaluation of AET benefits/costs, relapse prevention education, etc.) and skills training components (e.g., identifying personal goals and goal setting, problem solving skills, developing a coping plan for setbacks).
Interactive voice messaging is provided during the 6 months of intervention delivery. Messaging is delivered via phone, and can be delivered via mobile phone or landline. Women can select the phone number used for messaging and can change this phone number during the study. Using a voice messaging interface, women hear tailored messages and interact with the system through voice recognition software and their phone’s keypad. Study staff have access to a report indicating the dates and times of the calls, the status of these phone calls (e.g., message played in full, bad number, voicemail), and call length. If the message is unable to be played in full, the system will make two further attempts to reach the participant in a 24-hour period.
CST-AET participants receive two types of interactive messaging. First, they receive adherence-based medication reminders that are tailored using pill bottle data. If the pill bottle data indicates that a participant has opened their pill bottle at least 80% of days over a two week period, she receives a message providing reinforcement for adherence (e.g., you are doing a great job remembering to use the study pill bottle when taking your hormone therapy medicine). A message providing a reminder of the importance of continuing to take the medication every day is used when pill bottle data indicate non-adherence (i.e., bottle opening less than 80% of days). Women receive twelve adherence-based messages over the first 24 weeks of participation. The second type of interactive voice messaging provides a reminder of important topics and skills covered during the study phone sessions (e.g., using relaxation exercises to cope with pain). Participants receive nine of these calls over the first 24 weeks of participation.
b. General Health Education Intervention (comparison condition)
General health education was chosen as a comparison condition, controlling for attention and time. This general approach to an education comparison has been used successfully in many behavioral studies.41-44,65-67 Use of an education comparison enhances the scientific credibility of the study by testing whether the content and skills training provided in the experimental intervention effectively improve adherence and reduce symptom interference over that seen in patients who receive a similar dose of time and attention, but no specific skills training. Health education sessions use a presentation and discussion format similar to that described by Allen et al.68 and Porter et al.69 The protocol combines the use of phone calls, a workbook, and interactive voice messaging that reviews information from sessions.
Each session is delivered using a 4-section structure. 1) Check-In and Review (5 min): Sessions begin with a brief check-in and review regarding any questions the patient may have about previous material. 2) Symptom Assessment (5 min): A brief symptom assessment is conducted. The nurse will immediately notify the patient’s oncologist of major health issues. 3) Health Education (25 min): Each session covers a general health topic (see Table 2). 4) Questions (5 min): Patients are given an opportunity to ask questions.
Table 2.
Overview of General Health Education Sessions CONTROL ARM
| Session | Topics | Content Covered |
|---|---|---|
| 1 | Introduction: Cardiovascular Health: Cholesterol and Blood Pressure | Improving cardiovascular health by managing blood pressure and cholesterol (e.g., making lifestyle changes, monitoring blood pressure) |
| 2 | Food and Nutrition | Benefits of good nutrition and improving healthy eating (e.g., reading a food label, managing portion size) |
| 3 | Physical Activity Guidelines and Fitness | Benefits of physical activity (e.g., importance of strength training, avoiding injury when exercising) |
| 4 | Diabetes | Managing/preventing diabetes and recognizing the signs/symptoms (e.g., importance of monitoring blood sugar) |
| 5 | Screening and Preventative Health Care | Cancer-related screening and preventative measures (e.g., mammogram guidelines, remembering appointments) |
| 6 | Injury Prevention and Response | Fall prevention (e.g., removing tripping hazards) and emergency plans (e.g., carrying your cellphone with you) |
| 7 | Health Care Information | Obtaining and understanding healthcare information and overcoming barriers to receiving medical care (e.g., understanding medical bills, medication costs) |
| 8 | Maintenance Education: Cardiovascular Health/Food and Nutrition | Reinforcing the importance of managing cardiovascular health and eating a healthy, balanced diet |
| 9 | Maintenance Education: Physical Activity Guidelines and Fitness/Diabetes | Reinforcing the importance of increasing physical activity and managing diabetes |
| 10 | Maintenance Education: Screening and Preventative Health Care/Injury Prevention and First Aid | Reinforcing the importance of preventative health care and preventing falls from occurring |
Content-based interactive voice messaging will be provided via phone during the 6 months of intervention delivery. Consistent with the CST-AET protocol, participants receive nine of these calls over the first 24 weeks of participation. The nine messages include a review of the general health information topics covered during the previous phone session with study nurses (e.g., using exercise to help improve blood pressure and cholesterol). In the event that the participant is unavailable, the system will make two further attempts to reach the participant in a 24-hour period.
E. Intervention Training and Fidelity
Several steps have been taken to ensure consistency and fidelity of intervention delivery. First, the study nurses received didactic instruction for each intervention session and participated in role plays of intervention content with mock patients. Second, manualized session content delivered through the customized study portal has been scripted to ensure participants receive standard language to describe skills presented as well as ensure that information provided by participants triggers a consistent set of responses from the study nurses. Third, as part of our user testing of the intervention, each study nurse was assigned 5 training cases during which time they completed each of the 10 sessions with the participant. Fourth, study nurses receive ongoing supervision with the study PI (R.S.) twice per month. Finally, the intervention sessions are audio recorded. The study PI (R.S.) reviews approximately 20% of the sessions using a fidelity checklist; the study nurses receive feedback following the session review.
F. Measures
The measures included in the assessments for the present study have extensive reliability and validity data. Table 3 provides a timeline of assessments.
Table 3.
Timeline of Assessments and Self-report Measures
| Assessments | ||||||
|---|---|---|---|---|---|---|
| Construct & Measure | Daily | Baseline | 3-months | 6-months | 12-months | 18-months |
| Aim 1 | ||||||
| AET Adherence | ||||||
| AdhereTech Smart Pill Bottle | X | |||||
| Medication taking Behavior | ||||||
| Revised Medication Adherence Rating Scale | X | X | X | X | X | |
| Aim 2 | ||||||
| Subjective Reports of Symptoms & Symptom Interference | ||||||
| Menopause Specific Quality of Life Questionnaire | X | X | X | X | X | |
| Brief Pain Inventory- Short Form | X | X | X | X | X | |
| Brief Pain Inventory-Short Form, Pain Severity Subscale | X | X | X | X | X | |
| Arthritis Impact Measurement Scale-II | X | X | X | X | X | |
| Insomnia Severity Index | X | X | X | X | X | |
| Patient Reported Outcomes Information System Fatigue Scale | X | X | X | X | X | |
| Patient Reported Outcomes Information System Depression Scale | X | X | X | X | X | |
| Patient Reported Outcomes Information System Anxiety Scale | X | X | X | X | X | |
| Objective Measures of Physical Functioning and Symptom Interference | ||||||
| 6-minute walk test | X | X | X | X | X | |
| Get up and go test | X | X | X | X | X | |
| Grip Strength | X | X | X | X | X | |
| | ||||||
| Aim 3 | ||||||
| Barriers to Taking AET | ||||||
| Barriers to taking medication | X | X | X | X | X | |
| Treatment Interference | ||||||
| Treatment Burden Questionnaire | X | X | X | X | X | |
| Beliefs about Medication | ||||||
| Beliefs about Medicines Questionnaire | X | X | X | X | X | |
| Self-efficacy for managing symptoms and taking AET | ||||||
| Self-Efficacy for managing symptoms | X | X | X | X | X | |
| Self-Efficacy for Appropriate Medication Use Scale | X | X | X | X | X | |
| Aim 4 | ||||||
| Cost-Effectiveness | ||||||
| 5-level EuroQol-5 Dimensions (EQ-5D-5L) health questionnaire | X | X | X | X | X | |
| Other Measures | ||||||
| Treatment Credibility and Satisfaction | ||||||
| Treatment Credibility Questionnaire | X | |||||
| Satisfaction with Therapy and Therapist Scale | X | |||||
| Potential Covariates | ||||||
| Participant Characteristics | ||||||
| Medical Characteristics (e.g., change in AET type, dosage) | X | X | X | X | X | |
| Demographic Characteristics | X | |||||
| Self-Report Disease Burden Scale | X | X | X | X | X | |
| Treatments for comorbidities | X | |||||
| Rapid Estimate of Adult Literacy in Medicine | X | |||||
| Newest Vital Sign | X | |||||
| Chronic Life Stressors Scale | X | X | X | X | X | |
| Economic Pressures/Concerns | X | X | X | X | X | |
Aim 1: Investigate the impact of the CST-AET protocol on Adherence to AET
AET adherence:
Daily adherence is assessed using AdhereTech’s smart pill bottles.
Medication taking behaviors:
A revised 16-item measure based on the Medication Adherence Rating Scale70 and our prior studies71 is used to assess self-reported adherence. Items were revised to refer specifically to participants’ AET medication, assess medication-taking behaviors related to adherence (e.g., forgetting), and capture intentional and unintentional nonadherence over the past month.
Aim 2: Examine the Impact of the CST-AET on Symptoms and Symptom Interference
Subjective, Self-report measures
Menopausal Symptoms and Symptom Interference:
The 32-item Menopause Specific Quality of Life Questionnaire (MENQOL)72 assesses the degree of symptom interference in the past week related to four domains: physical symptoms, vasomotor symptoms, psychosocial symptoms, and sexual symptoms.
Pain and Joint Stiffness/Aching and Symptom Interference:
The Brief Pain Inventory – Short form (BPI-SF) 73 is used to assess pain interference in the past week across 9 areas (e.g., general activity, mood, sleep, enjoyment of life). Five items adapted from the Arthritis Impact Measurement Scale-II (AIMS-II; e.g., How would you describe the joint aching that you usually had?; How often did joint aching make it difficult to sleep?) 74,75 and four items modified from the BPI-SF pain severity scale (e.g., Please rate stiffness in your joints by selecting the one number that best describes your stiffness at its worst in the past week) 73 are used to measure aching and joint stiffness.
Sleep Problems:
The 7-item Insomnia Severity Index (ISI)76 is a measure of participants’ perceived severity of sleep difficulties and the interference of these difficulties with emotional distress, daily functioning, and quality of life in the last two weeks.
Fatigue:
The 8-item Patient Reported Outcomes Information System Fatigue Scale (PROMIS Fatigue) 77 assesses fatigue over the past seven days.
Psychological distress:
The eight-item Patient Reported Outcomes Information System Depression Scale (PROMIS Depression)77 assesses depressive symptoms. The eight-item Patient Reported Outcomes Information System Anxiety Scale (PROMIS Anxiety)77 is used to assess symptoms of anxiety.
Objective Assessments
Physical Functioning and Symptom Interference:
The 6-minute walk test assesses women’s abilities to exert effort in activity and the degree of pain experienced during activity.78,79 Women are asked to walk along an indoor hallway for 6-minutes with the goal being to walk as far as possible within the allotted time. Women also complete the timed get up and go test during which time they are asked to stand up from a chair, walk 10 feet, turn, walk back to the chair, and sit down.80 Grip strength is assessed using a latex free JAMAR hydraulic hand dynamometer. The participant sits with her forearm in a neutral position, and wrist between 0° and 30° dorsiflexion and between 0° and 15° ulnar deviation.81 She then squeezes the handle of the dynamometer as hard as she can. Data is recorded in pounds. One trial is conducted with each hand.
Aim 3: Examine the impact of the CST-AET protocol on theory-based measures of perceived barriers to AET medication, beliefs about AET medication, and self-efficacy
Barriers to taking AET medication:
Eleven items assess barriers to taking medication over the past month.82,83 Specifically, women rate how often certain situations (e.g., forgetfulness, problematic side effects, being out of routine) make it difficult for them to take their medications every day.
Treatment interference:
Five items from the Treatment Burden Questionnaire 84 assess how often, time spent, frequency, or inconveniences associated with recommended health care present a problem for the participant.
Beliefs about medication:
The 10-item Beliefs about Medicines Questionnaire (BMQ)85,86 assesses perceived necessity and concerns about AET (e.g., my health depends on my medicine, my medicine protects me from becoming ill).
Self-Efficacy for managing symptoms and taking AET:
An 8-item scale will measure self-efficacy for managing symptoms.87 Participants are asked to rate how confident they are that they can manage common symptoms (e.g., aches and plain, sexual side effects and hot flashes/sweating) related to AET. The 13-item Self-Efficacy for Appropriate Medication Use Scale88 assesses self-efficacy for taking medications across various situations (e.g., when no one reminds you to take the medicine, when you have a busy day planned, when you are away from home).
Aim 4: Estimate the short-term costs of implementing the CST-AET intervention and investigate long-term cost-effectiveness of the CST-AET intervention
The 5-level EuroQol-5 Dimensions (EQ-5D-5L) health questionnaire89 assesses current health status across five domains that map to a 0 (dead) to 1 (perfect health) scale representing the relative utility (or desirability) of health-related quality of life. These utility weights are used to derive quality-adjusted life years (QALYs).
Other measures
Treatment Credibility and Satisfaction.
The Treatment Crediblity Questionnaire90-92 is a 5-item measure of the degree to which participants perceive a treatment as credible and expect positive outcomes (e.g., how helpful does the therapist seem to you?; how confident are you that this treatment will help you manage your symptoms and health concerns?). The Satisfaction with Therapy and Therapist Scale93 is a 13-item measure that has been modified to obtain participants’ satisfaction with the intervention and the nurse delivering the intervention sessions. Items assess satisfaction with and global improvement (i.e., how much did the intervention help with your symptoms and health concerns) after intervention.
Participant sociodemographic and medical characteristics, health literacy, numeracy, health problems, and chronic life stressors will be obtained (see Table 3). These variables will be considered as potential covariates in planned analyses. Participants are also asked about their pariticpation in other programs that may interfere with the results of the present study.
Demographic and medical information.
Participants will be asked to provide information regarding their demographic characteristics (e.g., age, race/ethnicity, education, employment status, marital status). Type and duration of AET, discontinuation or change in AET medication, and reasons for medication change or discontinuation will be abstracted from the medical record and recorded.
Comorbidities.
The Adult Comorbidity Evaluation Scale94 is a 27-item comorbidity index for patients with cancer that assesses the severity of comorbidities with data abstracted from patients’ medical records. The Self-Report Disease Burden Scale 95 measures subjective comorbidity burden based on the degree to which 25 common chronic conditions (e.g., stroke, asthma, high cholesterol) interfere with daily activities in the past month. Treatments for comorbid conditions are collected via medical record and self-report.
Literacy and Numeracy.
The Rapid Estimate of Adult Literacy in Medicine (REALM)96 is measure of health literacy. During this word recognition test, participants are asked to de-code or pronounce health-related words. The Newest Vital Sign (NVS97) is a 6-item measure of numeracy and literacy in adults. Participants are given a nutrition label and asked to answer four questions involving understanding and using numbers and two questions involving reading and understanding text.
Chronic life stress:
The nine-item Chronic Life Stressors Scale98 is used to assess stressors across nine domains: general/ambient problems, financial, work, relationship, parental concerns, family, social life, residence, and health issues. Participants also complete a questionnaire asking them to rate the economic pressures and concerns they have personally experienced in the past 12 months or since the last study assessment (e.g., During the past 12 months, how much difficulty have you had paying bills?; I have enough money to afford the kind of food I should have; I am concerned because I cannot afford adequate health insurance).99-102
G. Statistical Analysis
a. Analyses of aims 1, 2, and 3
The primary hypothesis is that breast cancer survivors who receive CST-AET will be more adherent to AET. Analyses will examine treatment group differences in AET adherence using linear mixed-effects models. The outcome variable for Aim 1 will be AET adherence (% days adherent per week) collected via smart bottles over time; longitudinal models examine weekly adherence over the 18 months. Patient effects will be entered as variance components to model within-patient correlations over time. We will fit marginal models that account for within-patient correlations using an appropriate correlation matrix without introducing random-effects. As a model building strategy, we will first test a main-effects only model and then test group by time interaction effects when appropriate. We will follow up significant interactions using procedures described in Aiken and West103 and Preacher et al.104 Best models will be selected using BIC information criterion to strike a balance between goodness of fit and model complexity. Marginal models will be fitted as mixed-effects models in SAS.
Secondary hypotheses are that breast cancer survivors who receive CST-AET will experience greater reductions in AET symptoms and symptom interference (Aim 2) and show greater reductions in barriers to taking medication, increases in perceived necessity of AET, reductions in concerns about AET, and improved self-efficacy (Aim 3). Outcome variables for Aims 2 and 3 will be patient reported measures (i.e., symptom interference, barriers, beliefs about AET, and self-efficacy) collected at 3, 6, 12, and 18 months. As with adherence, we will examine treatment group differences in symptoms and symptom interference, barriers, beliefs about AET and selfefficacy using linear mixed-effects models using a similar analytic strategy to that described above for to test aim 1.
It is also hypothesized that pre to post-intervention changes in self-efficacy for taking AET medication will mediate the impact of treatment on adherence and symptom interference. We will use similar linear mixed-effects or marginal models as described above. The mediational hypothesis will be addressed using a set of three models: 1) the effect of treatment on adherence and symptom interference, 2) the effect of treatment arm on self-efficacy, and 3) the effect of self-efficacy on adherence and symptom interference both measured as differences from their baseline. Following recent work on modeling and timing in mediational models105-109 we will fit more parsimonious autoregressive cross-lag (ACL) models using SAS.
b. Analyses of aim 4
When accounting for the costs of the CST-AET intervention, it is hypothesized that, if efficacious, the intervention will provide good value with an incremental cost-effectiveness ratio below $50,000 per quality-adjusted life-year (QALY). The first component of the economic evaluation will focus on estimating direct costs of providing the CST-AET protocol, including the mean cost per patient and the total cost per site. We will estimate intervention costs using the TEAM-HF Costing Tool 110. The tool was designed to estimate costs for patient-centered interventions using scientifically-sound economic principles to assign costs to personnel, facilities, equipment, supplies, patient incentives and other items.
In addition to intervention-related costs, we will estimate patient-level costs associated with AET medications based on adherence data. Patient time costs associated with the CST-AET intervention will be valued using the average hourly wage in the US.111 We will compare mean estimates of short-term quality-adjusted life-years (QALYs) between study groups by converting responses to the EQ-5D-5L to health utility weights and computing patient-level quality-adjusted survival over the 18-month follow-up period. We will also examine utilities for each of the five domains measured by the EQ-5D-5L, and will examine how patient factors (e.g., chronic life stress, comorbidity burden, symptoms) impact these utilities.
We will develop a probabilistic decision-analytic simulation model, similar to a Markov model that adheres to good modeling practices,112 to examine the long-term cost-effectiveness of the CST-AET intervention versus education control. The simulation model will be designed prior to the conclusion of the 18-month follow-up period. It will incorporate published hazard ratios that represent the inverse relationship between adherence to AET and cancer recurrence,23-25 as well as relationships between breast cancer recurrence and disease-specific mortality and other-cause mortality. In addition to utility weights measured in the study for women without disease recurrence, the model will also incorporate costs and utility weights from the published medical literature to account for costs and quality of life decrements associated with disease recurrence. To avoid underestimating the incremental costs associated with CST-AET in practice, costs for the general health education intervention will not be included in the base-case analysis. Sensitivity analyses will include varying the magnitude of improvement in adherence with the CST-AET intervention versus education, varying the duration over which improvements in adherence persist, and varying CST-AET intervention costs.
DISCUSSION
This paper details the rationale, design, methods and analyses of a randomized clinical trial evaluating a novel skills training intervention for improving patients’ abilities to adhere to AET and reduce symptom interference. This study is innovative and important as it may lead to changes in clinical care, improve outcomes for breast cancer patients, and stimulate new research. This study will provide important information about strategies to improve adherence that will be of interest to patients, providers, health systems and payors. The cost of non-adherence to AET is high in terms of lost quality-adjusted life years, increased medical costs, and increased mortality.25 Thus, if the CST-AET protocol is found to improve AET adherence, it also has the potential to improve survival for women with breast cancer. Such a finding could greatly heighten awareness of the role of self-management in promoting adherence to AET, and it could lead to more openness to including behavioral interventions for adherence and symptom management in the routine medical care of breast cancer patients. Finally, it could facilitate early referral to self-management interventions such as CST-AET before non-adherence compromises the benefits of treatment.
Strengths of the Present Study
This study has several strengths that support its innovation. First, AdhereTech’s smart pill bottles will be used as an objective measure of AET adherence. Prior studies have assessed AET adherence using self-report measures, which may limit the validity of the adherence data.113,114 While Medication Event Monitoring Systems (MEMs) are frequently used as an objective measure of adherence, data obtained from these devices must be downloaded in-person and then examined on a computer.115 There is no way to obtain the data if the MEMs Cap is not returned. The smart pill bottles used in the present study automatically collect adherence data in real time and send this data to AdhereTech’s servers wirelessly using a built-in cellular chip. All data and analytics are available 24/7 on the real-time dashboard. The nurses delivering the intervention can view the data and provide real-time feedback to intervention participants. This also allows for the intervention sessions to be delivered to patients remotely via phone as patients’ adherence data will not have to be downloaded in-person as is the case with MEMs. As the technology continues to advance, the functionality of these smart pill bottles will grow (e.g., enhanced reminders, integration with pharmacy records), as will the number of commercially available products. Thus, the results of the present study will provide important information about participants’ use of and interaction with these bottles and about how real-time adherence data can be used to inform clinical practice.
Second, nurses have been chosen to deliver the intervention. Many patients have medical concerns related to their use of AET and in relation to the side effects experienced from their cancer treatments. In most clinical settings, nurses are trained to conduct symptom assessments and provide patients with specific recommendations to address these symptoms. Further, nurses are often more readily accessible and available to patients than other behavioral medicine specialists; by having nurses serve as the interventionists, the intervention may more readily match the real-world clinic setting. The intervention portal developed for this study is novel and provides nurses with manualized session content to assist with intervention delivery; we believe this will facilitate dissemination of the intervention if it is found to be efficacious.
Third, women will be recruited within the first year of AET and then followed for an additional 18 months. Endocrine therapy adherence decreases with each year after initiation of treatment; however, risk of all-cause and breast cancer-specific mortality decrease the longer patients are adherent to AET.116,117 If CST-AET is found to be efficacious for improving patient adherence, early intervention with CST-AET may result in significant benefits for patient outcomes including increased effectiveness of the medication and decreased mortality.
Finally, this study is being conducted at a major medical center as well as through community oncology clinics in medically underserved areas. Patients treated in the community clinics tend to have lower socioeconomic status (SES), less formal education, and lower literacy levels. Thus, involving this range of clinics increases the generalizability of our study findings. Additionally, lessons learned from this work will not only inform future research in this area but also provide information related to the efficacy of this type of intervention across clinic type (large academic medical center vs. medically underserved community clinic), types of communities (urban vs. rural), and patient sociodemographic characteristics (e.g., low vs. high SES, low vs. high literacy).
Limitations of the Present Study
There are several limitations that warrant acknowledgement. First, the study is limited to breast cancer survivors receiving treatment in North Carolina. Although this may limit our ability to generalize to patients treated in other regions of the country, we are recruiting participants from both a major academic medical center and community oncology clinics. This will allow for us to capture the effect of the intervention on the experiences of women on endocrine therapy who live in diverse settings. Further, many women continue on endocrine therapy for up to 10 years; however, we will follow women for 18 months following enrollment. If the intervention is shown to be efficacious, future studies may benefit from a longer follow-up period. Finally, while the AdhereTech’s smart pill bottles will serve as an objective measure of medication adherence and are more feasible than MEMs caps, we will not have access to patients’ medication refill record; thus, we are unable to calculate other objective measures of assessment like the medication possession ratio and proportion of days covered. Future studies may benefit from collecting data on these variables.
Clinical Implications
Future studies could adapt this intervention to address medication adherence problems affecting other cancer treatments. Studies suggest that treatment-related symptoms are a key contributor to early discontinuation and non-adherence to other cancer therapies that are orally administered (e.g., tyrosine kinase inhibitors, androgen deprivation therapies, capecitabine).118 Improving patients’ abilities to adhere to orally administered cancer therapies is increasingly important as the use of these treatments is rapidly expanding, and is the standard of care for certain cancers.119-123 Non-adherence to these treatments can have a substantial impact on disease progression, recurrence, and survival.123 The CST-AET protocol could readily be adapted to address specific challenges to adherence for these treatments, such as complicated dosing schedules or difficult side effects. For example, imatinib (a tyrosine kinase inhibitor used for certain types of leukemia)123 is often taken twice per day with a meal, dose changes often occur during the course of treatment, and it is associated with many difficult side effects (e.g., upset stomach, swollen joints, weakness, insomnia). The CST-AET protocol could be adapted for patients taking imatinib by emphasizing medication adherence skills to address its more complex dosing regimen, developing psycho-education modules for common symptoms associated with taking it, and focusing the application of coping skills training on these symptoms.
If this trial finds that the CST-AET protocol improves AET adherence, then several new lines of research could be pursued. The CST-AET protocol involves multiple components and future studies could examine which of these components contribute most to treatment effects. Future studies could examine the best strategies for maximizing the efficacy of this treatment. These studies, for example, could test how many sessions are needed, how often sessions should be delivered, and how long the intervention should last to effectively improve adherence and reduce symptom interference. Future studies could also test the efficacy of innovative methods of delivering CST-AET that minimize the time and resources needed to deliver the intervention (e.g. brief, self-paced, web-based strategies). The decision to deliver CST-AET by telephone with interactive voice messaging was made because telephone access is widely available and feasible for most patients. As other forms of communication (e.g., video-conferencing) become more widely available in the study population, the CST-AET protocol could be easily adapted and evaluated for these methods of delivery.
Acknowledgments
Grant acknowledgement, Source of Support: This work was supported by the National Institutes of Health [1R01CA193673-01A1].
Footnotes
Trials registration: ClinicalTrials.gov, NCT02707471, registered 3/3/2016
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collaborative Group on Hormonal Factors in Breast Cancer. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials. Lancet. 2005;365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 2.Abe O, Abe R, Enomoto K, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. May 14 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 3.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: Status report 2004. Journal of Clinical Oncology. January 20 2005;23(3):619–629. [DOI] [PubMed] [Google Scholar]
- 4.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. March 9 2013;381(9869):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein HJ, Temin S, Anderson H, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. Journal of Clinical Oncology. 2014;32(21):2255–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss PE, Ingle JN, Pritchard KI, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med. July 21 2016;375(3):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: systematic review. Breast Cancer Research and Treatment. July 2012;134(2):459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crivellari D, Sun Z, Coates AS, et al. Letrozole compared with tamoxifen for elderly patients with endocrine-responsive early breast cancer: The BIG 1–98 trial. Journal of Clinical Oncology. April 20 2008;26(12):1972–1979. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. February 23 1989;320(8):479–484. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher M, Bastert G, Bojar H, et al. Randomized 2×2 trial evaluating hormonal treatment and the duration of chemotherapy in node-positive breast cancer patients. German Breast Cancer Study Group . Journal of Clinical Oncology. October 1994;12(10):2086–2093. [DOI] [PubMed] [Google Scholar]
- 11.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. Journal of Clinical Oncology. February 15 2003;21(4):602–606. [DOI] [PubMed] [Google Scholar]
- 12.Owusu C, Buist DSM, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. Journal of Clinical Oncology. February 1 2008;26(4):549–555. [DOI] [PubMed] [Google Scholar]
- 13.Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Research and Treatment. September 2006;99(2):215–220. [DOI] [PubMed] [Google Scholar]
- 14.Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. Journal of Clinical Oncology. July 20 2009;27(21):3445–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. Journal of Clinical Oncology. September 20 2010;28(27):4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: Predictors of use, side effects, and discontinuation in older women. Journal of Clinical Oncology. January 15 2001;19(2):322–328. [DOI] [PubMed] [Google Scholar]
- 17.Waterhouse DM, Calzone KA, Mele C, et al. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. Journal of Clinical Oncology. June 1993;11(6):1189–1197. [DOI] [PubMed] [Google Scholar]
- 18.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. February 15 2003;21(4):602–606. [DOI] [PubMed] [Google Scholar]
- 19.Fontein DBY, Nortier JWR, Liefers GJ, et al. High non-compliance in the use of letrozole after 2.5 years of extended adjuvant endocrine therapy. Results from the IDEAL randomized trial. European Journal of Surgical Oncology. 2012;38(2):110–117. [DOI] [PubMed] [Google Scholar]
- 20.Thompson AM, Dewar J, Fahey T, et al. Association of poor adherence to prescribed tamoxifen with risk of death from breast cancer. Breast Cancer Symposium. 2007;Abstract 130. [Google Scholar]
- 21.Barron TI, Cahir C, Sharp L, et al. A nested case-control study of adjuvant hormonal therapy persistence and compliance, and early breast cancer recurrence in women with stage I-III breast cancer. British journal of cancer. September 17 2013;109(6):1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haque R, Ahmed SA, Fisher A, et al. Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer. Cancer medicine. December 2012;1(3):318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Research and Treatment. April 2011;126(2):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh KP, Chen LC, Cheung KL, et al. Interruption and non-adherence to long-term adjuvant hormone therapy is associated with adverse survival outcome of breast cancer women--an Asian population-based study. PloS one. 2014;9(2):e87027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makubate B, Donnan PT, Dewar JA, et al. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. British journal of cancer. April 16 2013;108(7):1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunfeld EA, Hunter MS, Sikka P, et al. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Education and Counseling. October 2005;59(1):97–102. [DOI] [PubMed] [Google Scholar]
- 27.Kahn KL, Schneider EC, Malin JL, et al. Patient centered experiences in breast cancer - Predicting long-term adherence to tamoxifen use. Medical Care. May 2007;45(5):431–439. [DOI] [PubMed] [Google Scholar]
- 28.Garreau JR, Delamelena T, Walts D, et al. Side effects of aromatase inhibitors versus tamoxifen: The patients' perspective. American journal of surgery. October 2006;192(4):496–498. [DOI] [PubMed] [Google Scholar]
- 29.Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer. September 2006;42(14):2271–2276. [DOI] [PubMed] [Google Scholar]
- 30.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Research and Treatment. January 2008;107(2):167–180. [DOI] [PubMed] [Google Scholar]
- 31.Fallowfield L, Cella D, Cuzick J, et al. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. Journal of Clinical Oncology. November 1 2004;22(21):4261–4271. [DOI] [PubMed] [Google Scholar]
- 32.Donnellan PP, Douglas SL, Cameron DA, et al. Aromatase inhibitors and arthralgia. Journal of Clinical Oncology. May 15 2001;19(10):2767. [PubMed] [Google Scholar]
- 33.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. Journal of Clinical Oncology. September 1 2007;25(25):3877–3883. [DOI] [PubMed] [Google Scholar]
- 34.Hunter MS, Grunfeld EA, Mittal S, et al. Menopausal symptoms in women with breast cancer: Prevalence and treatment preferences. Psycho-Oncology. November 2004;13(11):769–778. [DOI] [PubMed] [Google Scholar]
- 35.Gallicchio L, MacDonald R, Wood B, et al. Menopausal-type symptoms among breast cancer patients on aromatase inhibitor therapy. Climacteric : the journal of the International Menopause Society. August 2012;15(4):339–349. [DOI] [PubMed] [Google Scholar]
- 36.Shelby RA, Edmond SN, Wren AA, et al. Self-efficacy for coping with symptoms moderates the relationship between physical symptoms and well-being in breast cancer survivors taking adjuvant endocrine therapy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. May 13 2014. [DOI] [PubMed] [Google Scholar]
- 37.Boonstra A, van Zadelhoff J, Timmer-Bonte A, et al. Arthralgia during aromatase inhibitor treatment in early breast cancer patients: prevalence, impact, and recognition by healthcare providers. Cancer nursing. January-February 2013;36(1):52–59. [DOI] [PubMed] [Google Scholar]
- 38.Ziller V, Kyvernitakis I, Knöll D, et al. Influence of a patient information program on adherence and persistence with an aromatase inhibitor in breast cancer treatment--the COMPAS study. BMC cancer. 2013;13:407–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekinci E, Nathoo S, Korattyil T, et al. Interventions to improve endocrine therapy adherence in breast cancer survivors: what is the evidence? Journal of Cancer Survivorship. June 01 2018;12(3):348–356. [DOI] [PubMed] [Google Scholar]
- 40.Komatsu H, Yagasaki K, Yamaguchi T. Effects of a nurse-led medication self-management programme in cancer patients: protocol for a mixed-method randomised controlled trial. BMC nursing. 2016;15:9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keefe FJ, Caldwell DS, Williams DA, et al. Pain coping skills training in the management of osteoarthritic knee pain: A comparative study. Behavior Therapy. Win 1990;21(1):49–62. [Google Scholar]
- 42.Keefe FJ, Caldwell DS, Baucom D, et al. Spouse-assisted coping skills training in the management of osteoarthritic knee pain. Arthritis care and research : the official journal of the Arthritis Health Professions Association. August 1996;9(4):279–291. [DOI] [PubMed] [Google Scholar]
- 43.Keefe FJ, Caldwell DS, Baucom D, et al. Spouse-assisted coping skills training in the management of knee pain in osteoarthritis: Long-term followup results. Arthritis care and research : the official journal of the Arthritis Health Professions Association. April 1999;12(2):101–111. [DOI] [PubMed] [Google Scholar]
- 44.Keefe FJ, Blumenthal J, Baucom D, et al. Effects of spouse-assisted coping skills training and exercise training in patients with osteoarthritic knee pain: a randomized controlled study. Pain. August 2004;110(3):539–549. [DOI] [PubMed] [Google Scholar]
- 45.Carson JW, Keefe FJ, Affleck G, et al. A comparison of conventional pain coping skills training and pain coping skills training with a maintenance training component: a daily diary analysis of short- and long-term treatment effects. J Pain. September 2006;7(9):615–625. [DOI] [PubMed] [Google Scholar]
- 46.Abernethy AP, Keefe FJ, McCrory DC, et al. Behavioral therapies for the management of cancer pain: A systematic review In: Flor H, Kalso E, Dostrovsky JO, eds. Proceedings of the 11th World Congress on Pain. Seattle, WA: IASP Press, 2006:789–798. [Google Scholar]
- 47.Campbell LC, Keefe FJ, Scipio C, et al. Facilitating research participation and improving quality of life for African American prostate cancer survivors and their intimate partners. A pilot study of telephone-based coping skills training. Cancer. January 15 2007;109(2 Suppl):414–424. [DOI] [PubMed] [Google Scholar]
- 48.Blumenthal JA, Keefe FJ, Babyak MA, et al. Caregiver-assisted coping skills training for patients with COPD: background, design, and methodological issues for the INSPIRE-II study. Clinical Trials. April 2009;6(2):172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosworth HB, Olsen MK, Neary A, et al. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns. March 2008;70(3):338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bosworth HB, Olsen MK, Dudley T, et al. Patient education and provider decision support to control blood pressure in primary care: A cluster randomized trial. American Heart Journal. March 2009;157(3):450–456. [DOI] [PubMed] [Google Scholar]
- 51.Powers BJ, King JL, Ali R, et al. The Cholesterol, Hypertension, and Glucose Education (CHANGE) study for African Americans with diabetes: study design and methodology. Am Heart J. September 2009;158(3):342–348. [DOI] [PubMed] [Google Scholar]
- 52.Heisler M, Hofer TP, Klamerus ML, et al. Study protocol: the Adherence and Intensification of Medications (AIM) study--a cluster randomized controlled effectiveness study. Trials. 2010;11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reed SD, Li Y, Oddone EZ, et al. Economic evaluation of home blood pressure monitoring with or without telephonic behavioral self-management in patients with hypertension. American journal of hypertension. February 2010;23(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosworth HB, Powers BJ, Olsen MK, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Archives of internal medicine. July 11 2011;171(13):1173–1180. [DOI] [PubMed] [Google Scholar]
- 55.Allen KD, Bosworth HB, Brock DS, et al. Patient and provider interventions for managing osteoarthritis in primary care: protocols for two randomized controlled trials. BMC musculoskeletal disorders. 2012;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zullig LL, Melnyk SD, Stechuchak KM, et al. The Cardiovascular Intervention Improvement Telemedicine Study (CITIES): Rationale for a Tailored Behavioral and Educational Pharmacist- Administered Intervention for Achieving Cardiovascular Disease Risk Reduction. Telemedicine journal and e-health : the official journal of the American Telemedicine Association. December 4 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glaus A, Boehme C, Thürlimann B, et al. Fatigue and menopausal symptoms in women with breast cancer undergoing hormonal cancer treatment. Annals of Oncology. 2006;17(5):801–806. [DOI] [PubMed] [Google Scholar]
- 58.ATAC Trialists' Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. The Lancet. 2005/01/01/ 2005;365(9453):60–62. [DOI] [PubMed] [Google Scholar]
- 59.Kyvernitakis I, Ziller V, Hars O, et al. Prevalence of menopausal symptoms and their influence on adherence in women with breast cancer. Climacteric : the journal of the International Menopause Society. June 2014;17(3):252–259. [DOI] [PubMed] [Google Scholar]
- 60.Eakin EG, Reeves MM, Marshall AL, et al. Living Well with Diabetes: a randomized controlled trial of a telephone-delivered intervention for maintenance of weight loss, physical activity and glycaemic control in adults with type 2 diabetes. BMC public health. 2010;10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eakin EG, Winkler EA, Dunstan DW, et al. Living well with diabetes: 24-month outcomes from a randomized trial of telephone-delivered weight loss and physical activity intervention to improve glycemic control. Diabetes care. August 2014;37(8):2177–2185. [DOI] [PubMed] [Google Scholar]
- 62.Bandura A Health promotion from the perspective of social cognitive theory. Psychology & Health. 1998 1998;13(4):623–649. [Google Scholar]
- 63.Bosworth HB, Oddone EZ. A model of psychosocial and cultural antecedents of blood pressure control. Journal of the National Medical Association. April 2002;94(4):236–248. [PMC free article] [PubMed] [Google Scholar]
- 64.Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. September 2011;162(3):412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalton JA, Keefe FJ, Carlson J, et al. Tailoring cognitive-behavioral treatment for cancer pain. Pain management nursing : official journal of the American Society of Pain Management Nurses. March 2004;5(1):3–18. [DOI] [PubMed] [Google Scholar]
- 66.Martire LM, Schulz R, Keefe FJ, et al. Feasibility of a dyadic intervention for management of osteoarthritis: a pilot study with older patients and their spousal caregivers. Aging & mental health. January 2003;7(1):53–60. [DOI] [PubMed] [Google Scholar]
- 67.Turner JA, Jensen MP. Efficacy of cognitive therapy for chronic low back pain. Pain. February 1993;52(2):169–177. [DOI] [PubMed] [Google Scholar]
- 68.Allen KD, Oddone EZ, Coffman CJ, et al. Telephone-based self-management of osteoarthritis: A randomized trial. Annals of internal medicine. November 2 2010;153(9):570–579. [DOI] [PubMed] [Google Scholar]
- 69.Porter LS, Keefe FJ, Garst J, et al. Caregiver-Assisted Coping Skills Training for Lung Cancer: Results of a Randomized Clinical Trial. Journal of pain and symptom management. September 9 2011;41:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krousel-Wood M, Islam T, Webber LS, et al. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. The American journal of managed care. January 2009;15(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- 71.Kimmick G, Edmond SN, Bosworth HB, et al. Medication taking behaviors among breast cancer patients on adjuvant endocrine therapy. Breast. October 2015;24(5):630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas. March 14 2005;50(3):209–221. [DOI] [PubMed] [Google Scholar]
- 73.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore. March 1994;23(2):129–138. [PubMed] [Google Scholar]
- 74.Meenan RF, Gertman PM, Mason JH. Measuring health status in arthritis. The arthritis impact measurement scales. Arthritis Rheum. February 1980;23(2):146–152. [DOI] [PubMed] [Google Scholar]
- 75.Guillemin F, Coste J, Pouchot J, et al. The AIMS2-SF. A short form of the arthritis impact measurement scales 2. Arthritis & Rheumatism. 1997;40(7):1267–1274. [DOI] [PubMed] [Google Scholar]
- 76.Morin CM. Treatment Manuals for Practitioners Insominia: Psychological assessment and management. New York: Guilford Press, 1993. [Google Scholar]
- 77.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010/11/01/ 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du H, Newton PJ, Salamonson Y, et al. A review of the six-minute walk test: its implication as a self-administered assessment tool. European journal of cardiovascular nursing : journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology. March 2009;8(1):2–8. [DOI] [PubMed] [Google Scholar]
- 79.ATS statement: guidelines for the six-minute walk test. American journal of respiratory and critical care medicine. July 1 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 80.Mathias S, Nayak CIS, Isaacs B. Balance in elderly patients: the "get-up and go" test. Archives of physical medicine and rehabilitation. June 1986;67(6):387–389. [PubMed] [Google Scholar]
- 81.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age and ageing. July 2011;40(4):423–429. [DOI] [PubMed] [Google Scholar]
- 82.Ogedegbe G, Schoenthaler A, Richardson T, et al. An RCT of the effect of motivational interviewing on medication adherence in hypertensive African Americans: rationale and design. Contemporary clinical trials. February 2007;28(2):169–181. [DOI] [PubMed] [Google Scholar]
- 83.Ogedegbe G, Harrison M, Robbins L, et al. Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study. Ethnicity & disease. Winter 2004;14(1):3–12. [PubMed] [Google Scholar]
- 84.Tran V-T, Montori VM, Eton DT, et al. Development and description of measurement properties of an instrument to assess treatment burden among patients with multiple chronic conditions. BMC Medicine. July 04 2012;10(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horne R Patients' beliefs about treatment: the hidden determinant of treatment outcome? Journal of psychosomatic research. December 1999;47(6):491–495. [DOI] [PubMed] [Google Scholar]
- 86.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychology & Health. 1999/01/01 1999;14(1):1–24. [Google Scholar]
- 87.Lorig K, Chastain RL, Ung E, et al. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. January 1989;32(1):37–44. [DOI] [PubMed] [Google Scholar]
- 88.Risser J, Jacobson TA, Kripalani S. Development and psychometric evaluation of the Self-efficacy for Appropriate Medication Use Scale (SEAMS) in low-literacy patients with chronic disease. J Nurs Meas. 2007;15(3):203–219. [DOI] [PubMed] [Google Scholar]
- 89.Oemar M, Janssen B. EQ-5D-5L User Guide: Basic Information on How to Use the EQ-ED-5L Instrument. Rotterdam, The Netherlands: EuroQol Group, 2013. [Google Scholar]
- 90.Borkoveck TD, Nau SD. Credibility of Analogue Therapy Rationales. J Behav Ther Exp Psy. 1972;3(4):257–260. [Google Scholar]
- 91.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. Journal of behavior therapy and experimental psychiatry. June 2000;31(2):73–86. [DOI] [PubMed] [Google Scholar]
- 92.Keefe FJ, Shelby RA, Somers TJ, et al. Effects of coping skills training and sertraline in patients with non-cardiac chest pain: a randomized controlled study. Pain. April 2011;152(4):730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oei TPS, Green AL. The Satisfaction With Therapy and Therapist Scale--Revised (STTS-R) for Group Psychotherapy: Psychometric Properties and Confirmatory Factor Analysis. Prof Psychol-Res Pr. 2008;39(4):435–442. [Google Scholar]
- 94.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA : the journal of the American Medical Association. May 26 2004;291(20):2441–2447. [DOI] [PubMed] [Google Scholar]
- 95.Bayliss EA, Ellis JL, Steiner JF. Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument. Health and quality of life outcomes. 2005;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parker RM, Baker DW, Williams MV, et al. The test of functional health literacy in adults: a new instrument for measuring patients' literacy skills. Journal of general internal medicine. October 1995;10(10):537–541. [DOI] [PubMed] [Google Scholar]
- 97.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. November-December 2005;3(6):514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Turner RJ, Wheaton B, Lloyd DA. The epidemiology of social stress. American Sociological Review. 1995;60:104–125. [Google Scholar]
- 99.Conger RD, Wallace LE, Sun Y, et al. Economic pressure in African American families: A replication and extension of the family stress model. Developmental Psychology. 2002;38(2):179–193. [PubMed] [Google Scholar]
- 100.D CR, Xiaojia G, H EG, et al. Economic Stress, Coercive Family Process, and Developmental Problems of Adolescents. Child Development. 1994;65(2):541–561. [PubMed] [Google Scholar]
- 101.Conger RD, Elder GH Jr.,. Families in troubled times: Adapting to change in rural America. Hillsdale, NJ: Aldine, 1994. [Google Scholar]
- 102.Spilman SK, Peng L. Critcial Transitions Project G3 Technical Reports, FY14 2007-2008. Ames, IA: Iowa State University;2009. [Google Scholar]
- 103.Aiken LS, West SG. Multiple regression: Testing and interpretting interactions. Newbury Park, CA: Sage Publications, 1991. [Google Scholar]
- 104.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- 105.Yuan Y, MacKinnon DP. Bayesian mediation analysis. Psychol Methods. December 2009;14(4):301–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.MacKinnon DP, Lockwood CM, Hoffman JM, et al. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. March 2002;7(1):83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mackinnon DP. Integrating mediators and moderators in research design. Res Soc Work Pract. November 2011;21(6):675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. March 2007;12(1):23–44. [DOI] [PubMed] [Google Scholar]
- 110.Reed SD, Li Y, Kamble S, et al. Introduction of the Tools for Economic Analysis of Patient Management Interventions in Heart Failure Costing Tool: a user-friendly spreadsheet program to estimate costs of providing patient-centered interventions. Circ Cardiovasc Qual Outcomes. January 2012;5(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.United States Department of Labor BoLS. Occupational Employment Statistics (OES) Research Estimates. Available at: http://www.bls.gov/oes/current/oes_research_estimates_2012.htm, May 28, 2014.
- 112.Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices--overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--1. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. September-October 2012;15(6):796–803. [DOI] [PubMed] [Google Scholar]
- 113.Oberguggenberger AS, Sztankay M, Beer B, et al. Adherence evaluation of endocrine treatment in breast cancer: methodological aspects. BMC Cancer. October 15 2012;12(1):474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. March 2009;20(3):431–436. [DOI] [PubMed] [Google Scholar]
- 115.Ailinger RL, Black PL, Lima-Garcia N. Use of electronic monitoring in clinical nursing research. Clin Nurs Res. May 2008;17(2):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Makubate B, Donnan PT, Dewar JA, et al. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. British journal of cancer. 03/21/online 2013;108:1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Winn AN, Dusetzina SB. The association between trajectories of endocrine therapy adherence and mortality among women with breast cancer. Pharmacoepidemiology and Drug Safety. 2016;25(8):953–959. [DOI] [PubMed] [Google Scholar]
- 118.Verbrugghe M, Verhaeghe S, Lauwaert K, et al. Determinants and associated factors influencing medication adherence and persistence to oral anticancer drugs: a systematic review. Cancer treatment reviews. October 2013;39(6):610–621. [DOI] [PubMed] [Google Scholar]
- 119.Smieliauskas F, Chien CR, Shen C, et al. Cost-Effectiveness Analyses of Targeted Oral Anti-Cancer Drugs: A Systematic Review. PharmacoEconomics. May 13 2014. [DOI] [PubMed] [Google Scholar]
- 120.Bassan F, Peter F, Houbre B, et al. Adherence to oral antineoplastic agents by cancer patients: definition and literature review. European journal of cancer care. January 2014;23(1):22–35. [DOI] [PubMed] [Google Scholar]
- 121.Mathes T, Pieper D, Antoine SL, et al. Adherence influencing factors in patients taking oral anticancer agents: A systematic review. Cancer epidemiology. June 2014;38(3):214–226. [DOI] [PubMed] [Google Scholar]
- 122.Mathes T, Antoine SL, Pieper D, et al. Adherence enhancing interventions for oral anticancer agents: a systematic review. Cancer treatment reviews. February 2014;40(1):102–108. [DOI] [PubMed] [Google Scholar]
- 123.Al-Barrak J, Cheung WY. Adherence to imatinib therapy in gastrointestinal stromal tumors and chronic myeloid leukemia. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. August 2013;21(8):2351–2357. [DOI] [PubMed] [Google Scholar]