Abstract
Background
Brain microvessel endothelial cells constitute an important component in the blood-brain barrier. Cell-culture-based models of the blood-brain barrier (BBB) have been extensively applied in pharmacology, pathology and physiology. This study investigated effects of anti-N-methyl-D-aspartic acid receptor 2 (anti-NR2), N-methyl-D-aspartic acid (NMDA) receptor antibodies, NMDA receptor antagonists, and NMDA receptor agonists on brain microvessel endothelial cell models, and verified the effect of anti-NR2 antibody on the BBB as a receptor agonist.
Material/Methods
The primary brain microvessel endothelial cells were isolated and cultured, and an in vitro BBB model was established based on microvessel endothelial cells. Anti-NR2 antibody, glutamic acid, ifenprodil, and memantine were added in the BBB model to analyze changes in transepithelial electrical resistance (TEER) and to examine the permeability of the brain microvessel endothelial cell model.
Results
The results showed that TEER values were significantly decreased by the addition of anti-NR2 antibody and glutamate, but were significantly increased by the addition of ifenprodil and memantine. TEER values showed no changes when treated by anti-NR2 antibody and ifenprodil, as well as anti-NR2 antibody and memantine. When dexamethasone was added, the TEER values increased by 23.8%, 39.4%, and 29.6% by treating with anti-NR2 antibody, positive cerebrospinal fluid, and positive serum, respectively.
Conclusions
Our findings show that anti-NR2 antibody in neuropsychiatric lupus serum can damage the BBB and enter the brain.
MeSH Keywords: Blood-Brain Barrier, Microvessels, Neuropsychiatry, Permeability
Background
Systemic lupus erythematosus (SLE) is a multi-system inflammatory disorder characterized by the presence of autoantibodies directed against DNA. Anti-DNA antibodies cross-react with N-methyl-D-aspartic acid receptor 2 (NR2) and damage neuronal cells via an apoptotic pathway [1,2]. However, not all anti-DNA antibodies are able to cross-react with NR2 completely. The frequency of anti-NR2 antibody positivity has been reported to be ~30% in patients with SLE. Although anti-NR2 antibody in cerebrospinal fluid (CSF) has been reported to be associated with diffuse psychiatric/neuropsychological SLE, no significant correlation has been found between serum anti-NR2 antibody positivity and cognitive dysfunction [3,4]. In contrast, serum anti-NR2 antibody has been associated with depression [5,6].
N-methyl-D-aspartic acid (NMDA) receptors are part of the glutamate receptor family, responsible for the majority of excitatory synaptic transmission in the central nervous system (CNS). NMDA receptors have been implicated as mediators of neuronal damage caused by excess glutamate in a number of neurologic disorders, including stroke, epilepsy, trauma, and neurodegenerative disorders [4]. DeGiorgio et al. reported that the injection of anti-NR2 glutamate receptor-binding antibodies (purified antibodies from the sera of SLE patients, and 1 CSF sample from an SLE patient with progressive cognitive decline) into mouse brains resulted in apoptosis of the neuronal cells, without signs of inflammation [7]. In addition, it has been reported that anti-NMDA receptor antibodies might be involved in amygdala damage in human SLE [3,6]. Of note, Kowal et al. recently demonstrated that mice with antigen-induced anti-NR2 expression have no neuronal damage until breakdown of the BBB takes place. Presumably, an intact BBB prevents the transport of anti-NR2 from the systemic circulation into the brain [8]. These data therefore could account for the previous observation that cognitive decline in SLE does not parallel systemic disease activity.
Cell-culture-based models of the BBB have extensively applied in pharmacology, pathology, and physiology since they were first established in 1973 [9,10]. The BBB mainly provides nutrients and ionic homeostasis, which are necessary for appropriate functioning of the central nervous system (CNS). The BBB protects the CNS from the xenobiotics and modulates neuro-active mediator levels [11,12]. Several immortalized human BBB models have been developed with good expression of BBB markers but they generally have a lower trans-endothelial electrical resistance (TEER) than most animal models [13–15]. Models derived from rats provide useful comparison with in vivo studies, the rat still being the most widely used animal model for experimental study, including for pharmaceutical applications and pharmacokinetic investigations [16,17]. Based on the above descriptions, the aim of this study was to isolate and culture the primary brain microvessel endothelial cells from rats, and to establish an in vitro BBB model based on microvessel endothelial cells. The changes in transepithelial electrical resistance (TEER) for the brain microvessel endothelial cell model are also discussed.
Material and Methods
Chemicals and materials
DMEM was obtained from the Hyclone (Logan, UT, USA). Collagenase II was purchased from Gibco BRL (Grand Island, New York, USA). Bovine serum albumin (BSA), Gelatin, and glutamic acid were purchased from Amresco (Solon, OH, USA). Polyvinylidene fluoride (PVDF) were obtained from Prospec (ProSpec, Ness-Ziona, Israel). NMDAR2b was purchased from Chemicon (Temecula, CA, USA). Memantine hydrochloride and ifenprodil tartrate salt were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Other chemicals were of the highest purity available.
Isolation and culture of primary brain microvessel endothelial cells
Male or female Wistar rats (130–140 g) were obtained from the Animal Facility of the Second Affiliated Hospital of Harbin Medical University, Harbin, China. The protocols of feeding were formed in accordance with the Guidelines of the Second Affiliated Hospital of Harbin Medical University Animal Research Committee. This study was also approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University, Harbin, China.
Petri dishes, iris scissors, conjunctiva tweezers, and hemostatic clamps were surface sterilized with 75% ethenol. Then, we performed the following: 1) To isolate the cerebral cortex, the rats were anesthetized using 2 ml chloral hydrate (10%), decapitated, and sterilized with 75% ethanol (mainly to sterilizes the hairs and skin) for 3–5 min. The brains were obtained and placed in cold D-Hanks liquid in disposable plastic petri dishes, and the cerebellum and diencephalon were carefully removed. The brains were replaced in cold D-Hanks solution, and the white matter and pia mater were removed. Last, the cerebral cortex was obtained. 2) For enzymatic digestion, the cerebral cortex was rinsed several times with cold D-Hanks solution, and 2 ml DMEM culture medium was added. The cerebral cortex was cut into pieces (about 1 mm3), and placed into centrifuge tubes. Collagenase II (0.1%, 2 ml) was added, mixed, and digested at 37° in an incubator for 1.5 h. 3) To isolate brain microvessels, the digested tissue was centrifuged at 1000 rpm for 8 min at room temperature, and the supernatant was collected. The BSA (20%, 2 ml) was added and mixed, and the mixture was centrifuged at 3000 rpm for 20 min at 4°C. The large pelagic nerve tissue and blood vessels were removed, and the sediment (brain microvessels) was collected. 4) To perform enzymatic digestion, the isolated microvessels were added to 2 ml collagenase II solution (0.1%) and mixed. The suspension was digested at 37°C in an incubator for 60 min. 5) Cell culture was performed by centrifuging the digested mixture at 1000 rpm for 5 min at room temperature, and the supernatant was discarded. DMEM medium was added and mixed, and the cell suspension was seeded in gelatin-coated plastic culture flasks, which were then incubated at 37°C and 5% CO2. After 6 h, the medium was changed, and then the medium was changed every other day. After the formation of a single layer, the culture medium was removed. The cell was digested with 0.25% trypsin and seeded.
Microvessel endothelial cells identification
The cell growth and morphology were observed by inverted-phase contrast microscopy after inoculation for 4 h, 12 h, and 24 h.
For immunocytochemistry analysis, the sterile coverslip was placed in a disposable petri dish, and the microvessel endothelial cells were seeded on a disposable petri dish. These petri dishes were incubated at 37°C and 5% CO2 for 48–72 h. When the cells were observed on the coverslip, the cover-slips were rinsed with PBS buffer (pH 7.0) and then fixed with 4% paraformaldehyde for 30 min. Then, the H2O2 (3%), normal goat serum, rabbit anti-human Coagulation Factor VIII (1: 100), anti-mouse IgG/Bio, and streptavidin-horseradish peroxidase (HRP) were added step by step. The results were colored using diaminobenzidine (DAB) reagent, and the stained endothelial cells were observed under an optical microscope.
In vitro BBB model
An in vitro BBB model was established based on microvessel endothelial cells. The BBB model consisted of inner and outside pools, and the inner pool is a plug-in cell culture dish (Millicell, Cat. No. #PICM03050, Millipore, Boston, MA, USA), and the outside pool is wells of 24-well culture plates. The Millicell was inserted in the wells and sterilized using ultraviolet radiation. The endothelial cell suspension (cell density 5×104/cm2) was inoculated in the inner pool, followed by adding the DMEM medium. They were incubated at 37°C and 5% CO2 until confluence (endothelial cells formed a dense single layer). This system is called the in vitro BBB model. After establishing the BBB model, a leak test was used to analyze the form of the BBB. DMEM medium was added to the inner pool and maintained the difference value of the liquid surface (>0.5 cm). After 4 h, the value remained unchanged, indicating that the BBB model had been established successfully.
Membrane resistance of BBB model
In the present study, the 2 electrodes were placed on the surface and overleaf of membrane, and 3 ml of DMEM complete medium endothelial cells was added to the Millicell. The initial values of membrane resistance were measured using a multimeter (UT39A, UNIT Co., Hongkong, China). The microvessel endothelial cells were digested, centrifuged, and counted, and the endothelial cell suspension (cell density 5×104/cm2) was inoculated in the Millicell (diameter 12 mm, pore size 0.4 μm, suitable for 24-well plates, Millipore Corporation, USA). The TEER values were measured daily, and were calculated using the following equation: TEER=(daily resistance value-initial value)/Millicell bottom area.
Effects of additives on the permeability
Rat brain microvessel endothelial cells were cultured until confluence, and the positive holes were selected for permeability experiments based on the results of leak tests. The experiment holes containing culture medium were separated into 3 groups. The experiment holes of Group A were added with anti-NR2 antibody (100 μl, 10 μg/ml), positive cerebrospinal fluid (CSF, 100 μl), negative CSF (100 μl), positive serum (100 μl), negative serum (100 μl), and normal serum (100 μl). The experiment holes of Group B were added with anti-NR2 antibody (100 μl, 10 μg/ml), glutamate (5 mM), ifenprodil (10 μg/ml), memantine (10 μg/ml), ifenprodil (10 μg/ml) + anti-NR2 antibody (100 μl, 10 μg/ml), and memantine (10 μg/ml) + anti-NR2 antibody (100 μl, 10 μg/ml). The experiment holes of Group C were added with anti-NR2 antibody (100 μl, 10 μg/ml), positive cerebrospinal fluid (CSF, 100 μl), and positive serum (100 μl), as well as plus dexamethasone. After culture for 12 h, the TEER values were measured.
Statistical analysis
All experimental data are expressed as mean ± standard error of mean (SEM) and were obtained from at least 3 independent experiments. The data were analyzed using SPSS 16.0 statistical analysis software (SPSS, Chicago, IL, USA). The t test was used to compare the differences between 2 groups. Tukey’s post hoc test was used to validate the ANOVA for comparing measurement data among groups. p<0.05 represents a significant difference.
Results
Morphology and identification of brain microvessel endothelial cells
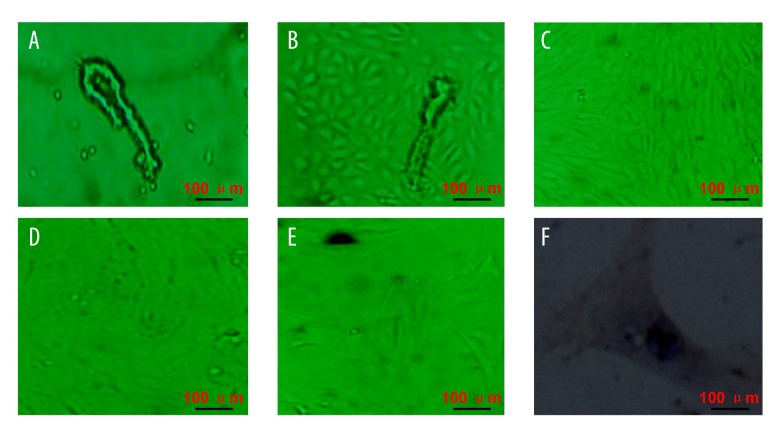
The primary brain microvessel endothelial cells were seeded on disposable plastic flasks containing 1% gelatin, and a single or multi-branch-like microvessel formed by round endothelial cells. Moreover, single cell and/or tissue fragments were also observed (Figure 1A). After culture for 24–48 h, the short spindle and/or polygonal cells were observed around microvessel segments, and showed a regional monolayer growth trend (Figure 1B). When the medium was changed, the number of microvessel segments decreased gradually with the replacement times. Therefore, the number of fibroblasts and pericytes also decreased gradually. The continued proliferation of endothelial cells was observed with increasing culture time, and these cells showed a vortex-like shape. After about 6–8 days, the number of short spindle cells was more than 90%, and showed paving stone-like change and no overlap (Figure 1C). After about 10–12 days, these endothelial cells showed a monolayer growth tread, and no tendency of multilayer growth or overlap were observed when these endothelial cells formed pieces (Figure 1D). These cells showed as bright spheres suspended in culture medium after digestion. After culture for 2 h, most cells showed adherent growth and uniform distribution. After 3–6 h, some cells became larger and flatter. After 24 h, most cells expanded, and the cell body becomes larger and showed a proliferation trend (Figure 1E). These cells formed a monolayer again at about 7 days. The cells became more uniform with the increasing number of cell passages, and the permeability was enhanced under an inverted microscope. Factor VIII-related antigen (FVIII-RAG) was present in the cytoplasm of endothelial cells, and immunohistochemical staining for FVIII-RAG with the peroxidase-antiperoxidase technique was used as a marker for endothelial cells in a variety of nevoid, reactive, and malignant vascular cutaneous proliferations [18]. In the present study, the brown cytoplasm and nucleus were observed in the cultured brain microvessel endothelial cells after DAB staining, and showed a vacuolized structure (Figure 1F).
Figure 1.
Isolation and identification of rat brain microvessel endothelial cells. (A) Isolated microvessel segments and/or single cells. (B) Short spindle and/or polygonal cells were observed around microvessel fragments. (C) Paving stone-like cells. (D) Monolayer growth endothelial cells. (E) Passaged rat brain microvessel endothelial cells. (F) Immunocytochemistry identified brain microvessel endothelial cells. Scale bars are illustrated in figures.
Dynamic changes in TEER in brain microvessel endothelial cells
The resistance values in the Millicell were measured using the UT39A Multi-Meter, and results are shown in Figure 2. The TEER values were calculated using the following equation: TEER=(daily resistance value- initial value)/1.13 cm2. As shown in Figure 2, the TEER values in the rat BMECs increased gradually up to day 4, and the highest value was about 91.26±6.87 KΩ/cm2. After culture for 4 day, the TEER values decreased gradually with increasing time. Based on these results, the permeability experiments were carried out when the TEER values were the highest, that is, the cells were cultured at day 4 (91.26±6.87 KΩ/cm2) or day 5 (89.72±6.19 KΩ/cm2).
Figure 2.
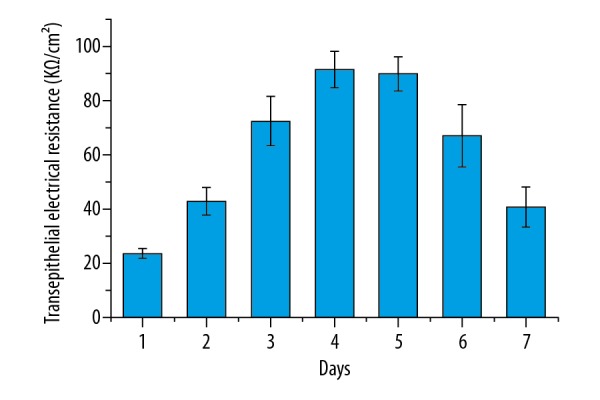
The dynamic changes in transepithelial electrical resistance in the brain microvessel endothelial cells. Results are averages of 3 independent experiments. Data are represented as mean ±SEM.
Effects of different additives on permeability in the BBB model
Based on the results of leak tests in the BBB model, the permeability experiments were carried out. The effects of different additives on permeability in the BBB model are shown in Figure 3. As shown in Figure 3, the TEER values showed different changes at the additives of anti-NR2 antibody, positive cerebrospinal fluid, negative CSF, positive serum, negative serum, and normal serum. The TEER values in the BBB model after the addition of anti-NR2 antibody, positive cerebrospinal fluid, and positive serum decreased by 54.6%, 57.5%, and 59.6%, respectively, compared to the control. The values after the addition of negative CSF and negative serum also showed decreasing treads, and the values decreased by 22% and 24.1%, respectively, compared to the control. However, the TEER values showed no significant change after the addition of normal serum compared to the control. The effects of NMDA receptor antagonists and agonists on permeability in the BBB model are shown in Figure 4. As shown in Figure 4, the TEER values with the addition of glutamate decreased by 40.8% compared to the control. The values with the additions of ifenprodil and memantine increased by 22.5% and 19.7%, respectively, compared to the control. However, the values showed no significant changes when the ifenprodil + anti-NR2 antibody and/or and memantine + anti-NR2 antibody were added. These results show that changes in the TEER values were clearly correlated with these additives.
Figure 3.
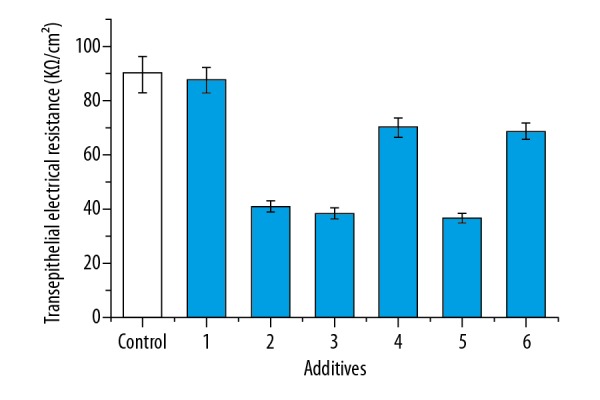
The effects of different additives on transepithelial electrical resistance in the brain microvessel endothelial cells. 1: normal serum (100 μl), 2: anti-NR2 antibody (100 μl, 10 μg/ml), 3: positive cerebrospinal fluid (CSF, 100 μl), 4: negative CSF (100 μl), 5: positive serum (100 μl), 6: negative serum (100 μl). After culture for 12 h, the TEER was measured and measured. Results are averages of 3 independent experiments. Data are represented as mean ± SEM.
Figure 4.

The effects of NMDA receptor antagonists and agonists on transepithelial electrical resistance in the brain microvessel endothelial cells. 1: anti-NR2 antibody (100 μl, 10 μg/ml), 2: glutamate (5 mM), 3: ifenprodil (10 μg/ml), 4: memantine (10 μg/ml), 5: ifenprodil (10 μg/ml) + anti-NR2 antibody (100 μl, 10 μg/ml), 6: memantine (10 μg/ml) + anti-NR2 antibody (100 μl, 10 μg/ml). After culture for 12 h, the TEER was measured. Results are averages of 3 independent experiments. Data are represented as mean ±SEM.
Effects of dexamethasone on transepithelial electrical resistance in the brain microvessel endothelial cells
Changes of the TEER values in the BBB model after the addition of dexamethasone are shown in Figure 5. As shown in Figure 5, the TEER values increased significantly after the addition of dexamethasone compared to those with no addition of dexamethasone. After dexamethasone treatment, the TEER values increased by 23.8%, 39.4%, and 29.6% after the addition of anti-NR2 antibody, positive cerebrospinal fluid, and positive serum. These results indicate that dexamethasone plays important role in repair of the BBB.
Figure 5.
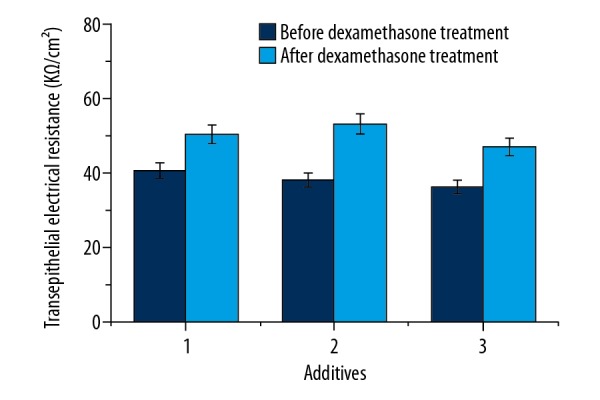
The effects of dexamethasone on transepithelial electrical resistance in the brain microvessel endothelial cells. 1: anti-NR2 antibody (100 μl, 10 μg/ml), 2: positive cerebrospinal fluid (CSF, 100 μl), 3: positive serum (100 μl). The holes with increased permeability in the blood-brain barrier were added by dexamethasone (1 μg), and the TEER was measured after 12 h. Results are averages of 3 independent experiments. Data are represented as mean ±SEM.
Discussion
In the present study, the method of isolated microvessel sections was first tried using tissue homogenate. However, the isolated endothelial cells are easily contaminated and have lower survival rates. Thus, we selected the isolation method using 0.1% collagenase II digestion, and further isolated endothelial cells from the cerebral cortex. Collagenase helps to release contact between endothelial cells and the basement membrane, and furthers destruction of the tight junction interaction endothelial cells. Compared to the tissue homogenate, the enzymatic digestion method may avoid tissue and endothelial cell damage and helps to improve cells viability [19,20]. After collagenase digestion, the nerve tissue, large blood vessels, and microvessel sections were separated using 20% bovine serum albumin and/or 15% dextran, and microvessel sections were harvested. Our results show that the number and state of microvessel sections using 20% bovine serum albumin (BSA) are higher and better than those achieved using 15% dextran. Digestion time is a key factor determining the state of microvessel sections. Digestion for too long will affect the viability of isolated endothelial cells, and a digestion time that is too short will affect cell proliferation for several undigested microvessel pericytes, such as astrocytes. Thus, the digestion times were about 1.5 and 2 h at first and second treatments, respectively, which obtained higher viability of isolated endothelial cells and low viability of microvessel pericytes. High purity of endothelial cells is the primary condition for in vitro studies of cell culture and BBB modeling. In the present study, the pia mater and vessels were carefully removed, and then the alba also removed, which will reduce presence of other cells and maintains the purity of isolated endothelial cells. Earlier reports suggested that brain microvessel endothelial cells start to adhere after passaging for 1 h, and the adherence rate reached about 60% after culture for 4 h, and the glial cells are usually adherent after passaging for 6 h [21]. Thus, the media was changed after passaging for 4 h, and this helped to obtain higher-purity endothelial cells.
There are a variety of ways to identify endothelial cells based on the morphology characteristics and specific antigens [22]. The present results assessed the morphology of endothelial cells using an inverted microscope, and the cells were polygonal or short spindle, in a monolayer with contact inhibition characteristics, and showed paving stone shape (Figure 1). Immunocytochemistry is the most effective way to identify brain microvessel endothelial cells. Our results indicate that the cytoplasm and the nucleus of cultured brain microvessel endothelial cells are brown, and vacuolization nucleus structure was observed after DAB staining (Figure 1F). In the present study, we performed a series of steps, include collagenase digestion, 20% bovine serum albumin gradient centrifugation, passage and culture, morphology and immunocytochemical identification, and further established an in vitro BBB model by using microvessel endothelial cells. The results of the present study will help to study and understand the physiological, biochemical, and pharmacological changes of brain endothelial cells, and will aid in establishing in vitro BBB models.
In the present study, in vitro BBB models were followed with subsequent steps, and the third generation of high-purity rat brain microvessel endothelial cells was digested and planted in the cell culture dish. When the cells were cultured to confluence, the BBB model was successfully established. Results of leakage test experiments suggested that the significant external surface difference in the Millicell was observed after 4 h, which limits water molecules passing freely. These results suggest that the BBB has been formed and can be used as in vitro model to study the permeability of the BBB. A comparative study of the TEER values in the brain microvessel endothelium and other endothelial cells showed that the ability of different endothelial cell barrier is different; thus, the TEER values are an index of endothelial cell permeability [24]. Our results indicated that the TEER value increased slowly with the increasing cell density in the early inoculation endothelial cells. However, the value increased rapidly when the cells began fusion, and the highest value was observed when the cells were completely fused. The maximum level was maintained for about 1–2 days, and then decreased (Figure 2). Thus, the changes in TEER value may reflect the state of cell growth, which will help to study permeability of the BBB.
The present study established an in vitro model of cerebral microvessel endothelial cells and BBB, and compared the effects of anti-NR2 antibody and its antagonists and agonists on the brain microvessel endothelial cells, and further verified effect of serum anti-NR2 antibody in lupus encephalopathy patients on cognitive dysfunction and memory loss.
In the present study, the TEER values significantly decreased in the rat brain microvessel endothelial cell model after the addition of anti-NR2 antibodies and glutamate, which suggests that the permeability increased (Figure 4). Our findings indicate that anti-NR2 antibody, as an NMDA receptor agonist, like glutamate, may result in increased permeability in the BBB model. These changes may result in degeneration, necrosis, delayed neuron death (DND), and/or apoptosis, and further lead to a series of clinical symptoms of neuropsychiatric systemic lupus. Therefore, these changes may also finally cause the cognitive dysfunction and memory loss. However, the mechanism by which anti-NR2 antibody affects the BBB model is complex and needs further study.
Glutamate (Glu) is the major mediator of excitatory synaptic transmission in the mammalian brain. Glu release changes the structure and function of the BBB under cerebral ischemia, such as barrier function impairment and increased permeability. These changes will result in substances crossing the BBB and reaching interstitial spaces, further causing cerebral edema and secondary hemorrhagic infarction. However, increased levels of glutamate, which results in extensive stimulation of NMDA receptors, is implicated in many diseases, including epilepsy, schizophrenia, and various neurodegenerative disorders [25,26]. Neuronal death in CNS diseases mediated by glutamate excitotoxicity may be blocked by an NMDA receptor antagonist. Ifenprodil is a novel NMDA receptor antagonist that selectively inhibits receptors containing the NR2B subunit. As such, it has become widely used to study subtypes of NMDA receptors both in vitro and in vivo, and as a tool for use in molecular studies of the properties and regulation of NMDA receptors [27]. Memantine is also an uncompetitive NMDAR receptor antagonist with strong voltage-dependency and fast kinetics, approved for clinical use in moderate to severe Alzheimer’s disease. It can block the neuro-toxicity of glutamate, but does not affect normal physiological functions [28]. In the present study, the TEER values increased significantly in the rat brain microvessel endothelial cell model after the addition of ifenprodil and memantine, which suggests that the permeability decreases. However, the TEER values with the addition of anti-NR2 antibody and ifenprodil, as well as anti-NR2 antibody and memantine, showed no significant changes compared to these values when there were no additions (Figure 4). Based on the above findings, it appears that the antagonistic effects of ifenprodil and memantine on anti-NR2 antibodies and glutamate are observed in the BBB model. These findings may help to identify new drugs for the treatment of early-stage SEL. NMDA receptor antagonists such as selfotel, aptiganel, eliprodil, licostinel, and gavestinel have been applied in the clinical treatment of stroke and brain injury [29,30], but the efficacy is not satisfactory. This may be because the treatment time was too short. This might also be due to the deficient properties of the molecules that entered human trials and to inappropriate design of clinical studies. Ikonomidou and Turski reported that glutamate should be administered in the acute neuro-destructive phase that occurs immediately after traumatic or ischemic injury. However, glutamate may enter a period of slow growth after a certain time, which will let it inhibit neuronal regeneration [31,32]. The unsatisfying results on the efficacy of NMDA receptor antagonists during clinical treatment may also be due to the concentrations used and serious adverse reactions. Thus, NMDA receptor antagonists may have a wide range of therapeutic applications in SEL when these questions are resolved.
Conclusions
The present study suggests that the anti-NR2 antibody in neuropsychiatric lupus serum can damage the BBB and enter the brain. Therefore, we speculate that anti-NR2 antibody results in memory loss and cognitive dysfunction. This study will help to better understand the responses of TEER and permeability in the brain microvessel endothelial cell model.
Footnotes
Source of support: This research was supported by the National Natural Science Foundation of China (Grant No. 81202340)
Conflict of interest
None.
References
- 1.Liang Y, Leng RX, Pan HF, et al. Associated variables of myositis in systemic lupus erythematosus: A cross-sectional study. Med Sci Monit. 2017;23:2543–49. doi: 10.12659/MSM.902016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertsias GK, Boumpas DT. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat Rev Rheumatol. 2010;6:358–67. doi: 10.1038/nrrheum.2010.62. [DOI] [PubMed] [Google Scholar]
- 3.Omdal R, Brokstad K, Waterloo K, et al. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol. 2005;12:392–98. doi: 10.1111/j.1468-1331.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 4.Lapteva L, Nowak M, Yarboro CH, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2505–14. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- 5.Husebye ES, Sthoeger ZM, Dayan M, et al. Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Ann Rheum Dis. 2005;64:1210–13. doi: 10.1136/ard.2004.029280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gono T, Kawaguchi Y, Kaneko H, et al. Anti-NR2A antibody as a predictor for neuropsychiatric systemic lupus erythematosus. Rheumatology (Oxford) 2011;50:1578–85. doi: 10.1093/rheumatology/keq408. [DOI] [PubMed] [Google Scholar]
- 7.DeGiorgio LA, Konstantinov KN, Lee SC, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–93. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 8.Kowal C, DeGiorgio LA, Nakaoka T, et al. Cognition and immunity; Antibody impairs memory. Immunity. 2004;21:179–88. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Deli MA, Abrahám CS, Kataoka Y, et al. Permeability studies on in vitro blood-brain barrier models: Physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa S, Deli MA, Kawaguchi H, et al. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54:253–63. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Roux F, Couraud PO. Rat brain endothelial cell lines for the study of blood-brain barrier permeability and transport functions. Cell Mol Neurobiol. 2005;25:41–58. doi: 10.1007/s10571-004-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa S, Deli MA, Nakao S, et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27:687–94. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Förster C, Burek M, Romero IA, et al. Differential effects of hydrocortisone and TNF alpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol. 2008;586:1937–49. doi: 10.1113/jphysiol.2007.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sano Y, Shimizu F, Abe M, et al. Establishment of a new conditionally immortalized human brain microvascular endothelial cell line retaining an in vivo blood-brain barrier function. J Cell Physiol. 2010;225:519–28. doi: 10.1002/jcp.22232. [DOI] [PubMed] [Google Scholar]
- 15.Zhou HY, Zhu H, Yao XM, et al. Metformin regulates tight junction of intestinal epithelial cells via MLCK-MLC signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:5239–46. doi: 10.26355/eurrev_201711_13847. [DOI] [PubMed] [Google Scholar]
- 16.Perrière N, Demeuse P, Garcia E, et al. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J Neurochem. 2005;93:279–89. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- 17.Perrière N, Yousif S, Cazaubon S, et al. A functional in vitro model of rat blood-brain barrier for molecular analysis of efflux transporters. Brain Res. 2007;1150:1–13. doi: 10.1016/j.brainres.2007.02.091. [DOI] [PubMed] [Google Scholar]
- 18.von Beust BR, Suter MM, Summers BA. Factor VIII-related antigen in canine endothelial neoplasms: An immunohistochemical study. Vet Pathol. 1988;25:251–55. doi: 10.1177/030098588802500401. [DOI] [PubMed] [Google Scholar]
- 19.Inagami T, Naruse M, Hoover R. Endothelium as an endocrine organ. Annu Rev Physiol. 1995;57:171–89. doi: 10.1146/annurev.ph.57.030195.001131. [DOI] [PubMed] [Google Scholar]
- 20.van Beijnum JR, Rousch M, Castermans K, et al. Isolation of endothelial cells from fresh tissues. Nat Protoc. 2008;3:1085–91. doi: 10.1038/nprot.2008.71. [DOI] [PubMed] [Google Scholar]
- 21.Biegel D, Spencer DD, Pachter JS. Isolation and culture of human brain microvessel endothelial cells for the study of blood-brain barrier properties in vitro. Brain Res. 1995;692:183–89. doi: 10.1016/0006-8993(95)00511-n. [DOI] [PubMed] [Google Scholar]
- 22.Craig LE, Spelman JP, Strandberg JD, et al. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvasc Res. 1998;55:65–76. doi: 10.1006/mvre.1997.2045. [DOI] [PubMed] [Google Scholar]
- 23.Grant GA, Abbott NJ, Janigro D. Understanding the physiology of the blood-brain barrier: In vitro models. News Physiol Sci. 1998;13:287–93. doi: 10.1152/physiologyonline.1998.13.6.287. [DOI] [PubMed] [Google Scholar]
- 24.Weksler BB, Subileau EA, Perrière N, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–74. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 25.de Groot J, Sontheimer H. Glutamate and the biology of gliomas. Glia. 2011;9:1181–89. doi: 10.1002/glia.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue H, Field CJ. New role of glutamate as an immunoregulator via glutamate receptors and transporters. Front Biosci (Schol Ed) 2011;3:1007–20. doi: 10.2741/205. [DOI] [PubMed] [Google Scholar]
- 27.Ogden KK, Traynelis SF. New advances in NMDA receptor pharmacology. Trends Pharmacol Sci. 2011;32:726–33. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molinuevo JL, Lladó A, Rami L. Memantine: Targeting glutamate excitotoxicity in Alzheimer’s disease and other dementias. Am J Alzheimers Dis Other Demen. 2005;20:77–85. doi: 10.1177/153331750502000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams K. Ifenprodil, a novel NMDA receptor antagonist: Site and mechanism of action. Curr Drug Targets. 2001;2:285–98. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- 30.Parsons CG, Stöffler A, Danysz W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system – too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–86. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 32.Gao J, Wang H, Liu Y, et al. Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress. Med Sci Monit. 2014;20:499–512. doi: 10.12659/MSM.890589. [DOI] [PMC free article] [PubMed] [Google Scholar]