Abstract
Background
Diabetes is a risk factor for coronary atherosclerosis and coronary heart disease. Resveratrol (RESV) is a natural compound with anti-inflammatory effects. The objective of this study is to evaluate the cardio protective effects of RESV in a diabetic rat model with coronary heart disease.
Material/Methods
Diabetic rat model with coronary heart disease was constructed by feeding high-fat and high-calorie diet, followed by injection of streptozotocin. The diabetic rats received RESV or DMSO as treatment. Insulin, total cholesterol, and total triglyceride levels in serum were measured using enzyme-linked immunosorbent assay (ELISA) to evaluate the effect of RESV in alleviating diabetic symptoms. Inflammatory factors, including tumor necrotic factor α, interleukin-6, interleukin-8, intracellular adhesion molecule 1, vascular-cell adhesion molecule 1, and monocyte chemoattractant protein-1 were assayed using ELISA. Real-time polymerase chain reaction and western blot analysis were performed to evaluate the impact of RESV treatment on the TLR4/MyD88/NF-κB signaling pathway (toll-like receptor 4/myeloid differentiation factor 88/nuclear factor kappa B signaling pathway). Hematoxylin and eosin staining was used to document pathological changes in cardiovascular muscles.
Results
RESV preserved pancreatic tissue, which therefore reduced levels of glucose and triglycerides glyceride in serum. Inflammatory factors were also suppressed by RESV. TLR4/MyD88/NF-κB signaling pathway was downregulated after RESV treatment.
Conclusions
RESV offers protective effects of cardiovascular tissues in the diabetic rat model with coronary heart disease. Those effects are mediated by downregulating the TLR4/MyD88/NF-κB signaling pathway.
MeSH Keywords: Anti-Inflammatory Agents; Coronary Disease; Diabetes Mellitus, Type 2
Background
Coronary heart disease is one of the leading cause of death worldwide [1]. Overweight individuals now constitute a dangerously high proportion of the population, particularly in developed countries, with diabetes being a well-known health problem of obesity and a notorious contributor to cardiovascular diseases including coronary heart disease [2]. Excessive lipid accumulation in skeletal muscle and liver leads to insulin resistance and predisposes to the development of type 2 diabetes, resulting in elevated oxidative stress and inflammatory responses in multiple organs. Tissues of coronary arteries possess limited anti-oxidative capacity and are vulnerable to damages from oxidative stress and inflammatory attack. It is recognized that coronary heart disease increases mortality of myocardial infarction by a factor of 3 to 7 [3]. Therefore, clinical management of coronary heart disease demands effective strategies in lowering lipid, glucose, and triglyceride levels, as well as alleviating oxidative stress and inflammatory response to counteract disorders associated with diabetes.
Resveratrol (3,5,4′-trihydroxystilbene, RESV) is a naturally occurring phytoalexin enriched in grapevine products and peanuts. It is shown to extend the lifespan of a wide spectrum of species, ranging from simple organisms, such as Caenorhabditis elegans, to mammal animals such as mice [4]. The increased survival may be attributable to the effects of RESV in promoting weight loss and counteracting the deleterious effects of high-calorie and high-fat uptake [5]. Further, RESV is considered an effective anti-inflammatory compound [6]. Recent clinical trials indicate that RESV increases serum adiponectin and downregulates inflammatory genes, thus stabilizing coronary artery disease [7]. Besides, the anti-inflammatory ability of RESV has been employed for the treatment of arthritis [8], cancer [9], and fibrotic diseases [10]. Despite the recognition of the clinical utility of RESV in regulating diabetes-related inflammatory responses, the underlying mechanism of this regulation remains largely unknown.
In the present study, we aimed to corroborate the cardioprotective effects of RESV in a diabetic heart coronary disease rat model, and further investigate the role of TLR4/MyD88/NF-κB signaling pathway (toll-like receptor 4/myeloid differentiation factor 88/nuclear factor kappa B signaling pathway) in this regulation. TLR4/MyD88/NF-κB signaling pathway is an important activator of a wide range of inflammatory factors. RESV helped reduce injury of pancreas as it worked on the heart tissue. Our data could possible shed light on how RESV exerts protection against coronary heart disease, and broaden the clinical application of RESV in patients.
Material and Methods
Animals
All animal experiments were performed in accordance to standards defined in Experimental Animal Environmental and Facility Regulations of China (GB14925-2001) and Beijing Experimental Animal Regulations. This study was approved by the ethics committee of Qilu Hospital of Shandong University (Qingdao). Sprague Dawley (SD) male rats (SPF level) were acquired from Experimental Animal Resource Center of Chinese Academy of Science (Shanghai, China). SD rats of 4 weeks old (n=30) were randomly assigned to received regular food (control group; n=10) or high-fat diet (HFD group; n=20) for 8 weeks. Rats fed with high-fat food developed insulin resistance at the 8th week. Streptozotocin (STZ) (40 mg/kg) was given and followed by measuring glucose level at 3 days and 7 days. If the measured glucose levels at 3 days and 7 days were higher than 11.6 mmol/L, these rats were considered to possess diabetes. Those rats were then subcutaneously injected with isoprenaline hydrochloride at the dose of 1 mg/kg/day for 10 days. Blood was collected at 5 day and 10 day from posterior orbital venous plexus, which was used for analysis of creatine kinase (CK) and creatine kinase-myocardial band (CK-MB) content in blood. CK and CK-MB levels in DMCHD group at 5 day was much higher than that in the control group, which indicates the severe damage of cardiovascular muscle. Rats with diabetes and cardiovascular damage were then grouped to received DMSO (n=10; DMCHD group) or RESV (n=10; RESV group). RESV (Runcheng Biotechnology Co., Ltd., Shanghai, China) was dissolved in DMSO (Sigma Aldrich, Saint Louis, MO, USA) to a stock concentration of 10 mg/mL. RESV was intravenously administered at the dose of 10 mg/kg/day. All rats in 3 groups were subsequently fed with regular food and appropriate treatments for 8 weeks.
ELISA (enzyme-linked immunosorbent assay)
After the last dosage, rats went through fasting for 12 hours before blood was collected from abdominal artery. Collected blood was centrifuged at 3000 rpm for 10 minutes and the serum was separated. Glucose, insulin, total cholesterol (TC), total triglyceride (TG), tumor necrotic factor α (TNF-α), interleukin-6 (IL-6), intracellular adhesion molecule 1 (ICAM-1), vascular-cell adhesion molecule 1 (VCAM-1) and monocyte chemoattractant protein-1 (MCP-1) levels were then determined using corresponding enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, USA).
Hematoxylin and eosin (H&E) staining
Hematoxylin and eosin were purchased from Solabio (Beijing, China). Hearts of the rats were harvested after blood collection, which were embedded in paraffin and sectioned at the thickness of 5 μm. These heart tissue slices mounted on glass then underwent deparaffinization and rehydration using standard procedures, stained with hematoxylin for 5 minutes, incubated with hydrochloric acid in ethanol for 30 seconds, soaked in water at 50ºC for 5 minutes, before being stained with eosin for 2 minutes. The slides were then dehydrated by treatment with 95% ethanol (1 minute), 100% ethanol sequentially (1 minute) and xylene (3 minute). The tissues were then covered with a thin glass and imaged using an inverted light scope.
Real-time polymerase chain reaction (RT-PCR)
Heart tissues were homogenized, and total RNA was extracted with the TRIzol RNA Extraction Kit (Thermo Fisher). Purified RNA was then used for cDNA synthesis using the Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA). Real-time polymerase chain reaction (RT-PCR) was performed using the SYBR Green master mixture and 1 μL of synthesized cDNA. The PCR cycles were conducted using the following parameters: denaturing (94ºC for 30 seconds), annealing (30 seconds) and extension (68ºC for 90 seconds). Pre-denaturing (94ºC for 2 minutes) and final extension (68ºC for 12 minutes) were added before and after of cycles. A total of 30 cycles were completed. The following primers were used for qPCR: TLR4: 5′-TCAGAGCCGTTGGTGTATCTT-3′ (forward); 5′-TGTCCTCCCATTCCAGGTAG-3′ (reverse). MyD88: 5′-TGGTGG TGGTTGTTTCTGAC-3′ (forward); 5′-AGTCCTTCTTCATCGCCTTG-3′ (reverse). ICAM-1: 5′-CAAGGCCTCAGTCAGTGTGA-3′ (forward); 5′-CCTCTGGCTTCGTCAGAATC-3′ (reverse). The products of PCR were analyzed using agarose gel electrophoresis and ethidium bromide (EB) staining. The mRNA levels of TLR4, MyD88, ICAM-1 relative to β-actin were quantified using ImageJ.
Western blot analysis
Heart tissue lysates (10 μg) were boiled in the Laemmli buffer for 5 minutes, and loaded into 10–20% ready gels (Biorad). Protein bands were resolved by SDS-PAGE at 100 V. Electrotransferring was performed at 100 V for 30 minutes onto PVDF membranes. After washing, PVDF was resolved proteins were blocked with 5% non-fat milk. These membranes were then used for detection of the proteins of interest. Rabbit anti-mouse TLR4 antibody, rabbit-anti-MyD88 antibody, and goat anti-rabbit IgG antibody were acquired from Abcam (England). The following dilutions of primary antibodies were used: TLR4 (1: 1000), MyD88 (1: 500), NF-κB-p65 (1: 2000), and p-NF-κB-p65 (1: 1000). Primary antibodies were used to incubate the membranes at 4ºC overnight. After TBST washing, the goat anti-rabbit antibodies were added. Chemiluminescent substrates were then added for visualization of protein bands. Expression levels of TLR4, MyD88, and NF-κB were quantified after normalization to the level of β-actin.
Statistical analysis
SPSS 19.0 was used for statistical analysis of data. All data were represented as mean ±SD. Difference between 2 groups were analysis using the least significant difference (LSD) method, and the P value of less than 0.05 indicated significant difference between groups.
Results
Construction of diabetic rat model with coronary heart disease
We first constructed the diabetic rat model with coronary heart disease by feeding high-glucose and high-fat diet to rats for 8 weeks, followed by injection of STZ. STZ is toxic to the insulin-producing cells of pancreas, which facilitated the development of diabetes. Blood glucose level at 3 days and 7 days after STZ injection (rats were considered insulin resistant if both measurement yielded blood glucose above 11.6 mmol/L). After injecting isoprenaline hydrochloride (1 mg/kg/day) for 5 days and 10 days, serum CK and CK-MB levels were measured. As indicated in Table 1, CK level in DMCHD group (119±4.09 ng/mL) at 10 days was much higher than that in the control group (P<0.01). CK level in the DMCHD group also higher than that of the control group at 5 days (P<0.01). Similarly, DMCHD group demonstrated a significant increase in CK-MB level compared to the control group at 5 days (P<0.01). Whereas the CK-MB level of DMCHD group dramatically decreased at 10 days (P<0.01) (even lower than that of the control group) (P<0.05). This sharp decrease in CK-MB level at 10 days was a strong indication that severe cardiovascular damage had occurred.
Table 1.
Serum CK and CK-MB levels in rat diabetic coronary heart disease model.
| Groups | CK (ng/mL) | CK-MB (pg/mL) | ||
|---|---|---|---|---|
| 5 day | 10 day | 5 day | 10 day | |
| Control | 29.3±1.13 | 35.6±1.18 | 112.4±5.08 | 113.6±5.15 |
| DMCHD | 32.2±3.12 | 119.8±4.09*# | 146.8±6.96* | 37.9±2.02*# |
Vs. control group
P<0.01; vs. 5 day
P<0.01.
RESV alleviated insulin resistance and reduces of blood glucose, insulin, TC, and TG levels
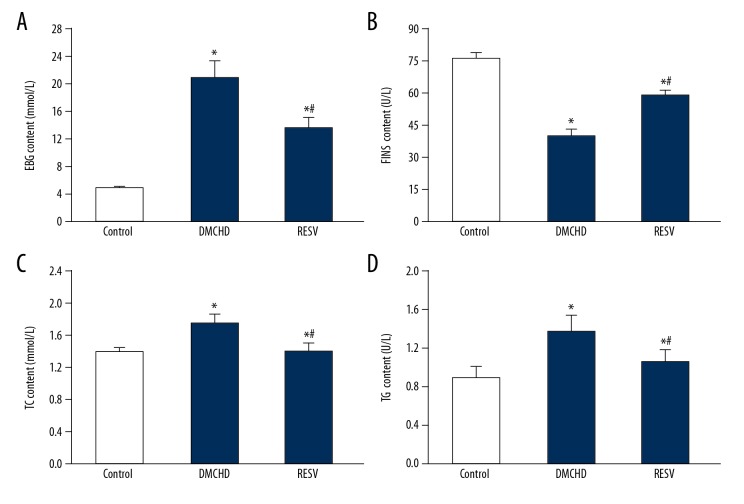
We next assessed the serum glucose, insulin, TC, and TG levels in the rats to further confirm the successful construction of the diabetic models. As shown in Figure 1, the DMCHD group demonstrated a 48.1% decrease in insulin level compared to the control group. As a consequence, serum glucose, TC and TG increased 4.18, 1.28, and 1.52 times, respectively compared to the control group. Notably, although RESV group also had abnormal insulin, glucose, TC, and TG levels compared to the control group, the glucose, TC, and TG levels were markedly lower than the DMCHD groups, suggesting that RESV ameliorated the insulin resistance in diabetic rats.
Figure 1.
Resveratrol (RESV) alleviated insulin resistance in diabetic rats. Serum levels of glucose (FBG) (A), insulin (FINS) (B) total cholesterol (TC) (C) and total triglycerides (TG) (D) in control, DMCHD and RESV groups are shown. Vs. control group * P<0.01; vs. DMCHD group # P<0.01.
RESV downregulated inflammatory factors
Diabetic coronary heart disease is characterized by elevated expression of inflammatory factors. The hyperactive inflammatory response is one of the contributors to the damage in cardiovascular tissues. Therefore, here we also investigated whether RESV could alleviate inflammatory response by downregulating inflammatory factors. As shown in Figure 2, while DMCHD group upregulated the expression of TNF-α, IL-6, ICAM-1, VCAM-1, and MCP-1 for 1.49, 1.48, 1.82, 1.52, and 1.78 times, respectively (P<0.01), RESV pronouncedly lowered those levels (P<0.01). The expression levels of those inflammatory factors were similar to that of the control group (P>0.01). It is clear that RESV alleviated the inflammatory response from diabetic coronary heart disease.
Figure 2.
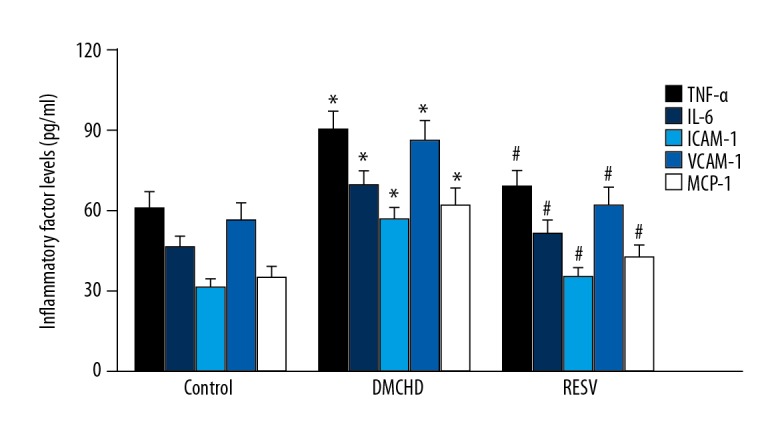
RESV attenuated inflammatory responses by downregulating inflammatory factors. Serum levels of TNF-α, IL-6, VCAM-1, ICAM-1, and MCP-1 are shown. Vs. control group * P<0.01; vs. DMCHD group # P<0.01. RESV – resveratrol; TNF-α – tumor necrotic factor α; IL-6 – interleukin-6; VCAM-1 – vascular-cell adhesion molecule 1; ICAM-1 – intracellular adhesion molecule 1; MCP-1 – monocyte chemoattractant protein-1.
RESV demonstrated cardiovascular protection in diabetic rats
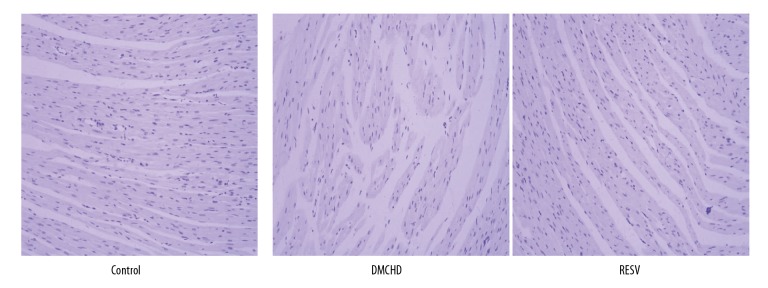
To confirm the cardioprotective effects of RESV, we harvested heat tissues from rats and examined pathological changes using H&E staining. In Figure 3, blue color denotes nucleus and light purple denotes cytoplasm. In contrast to the control group, in which uniform alignment of fibers in heart tissues could be seen, the DMCHD group demonstrated reduced cell density, along with disruption and thickening of the muscle fibers. RESV treatment appeared to restore the normal heart histopathology. This further supported our assumption that RESV can offer protective effects on cardiovascular tissues in diabetic rats.
Figure 3.
Resveratrol (RESV) demonstrated cardiovascular protection. Representative Hematoxylin and eosin staining images of the heart tissues harvested from control, DMCHD and RESV group were shown (100×).
RESV downregulated TLR4, MyD88, and ICAM-1 expression
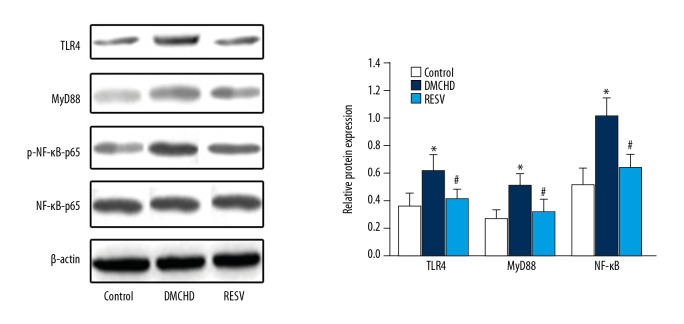
To explore the precise mechanism of the cardiovascular protection offered by RESV, we analyzed TLR4, MyD88, and ICAM-1 expression in heart tissues. As shown in Figure 4, RT-PCR analysis indicated that mRNA levels of TLR4, MyD88, and ICAM-1 in the DMCHD group were 36%, 28%, and 32% higher than those in the control group, respectively (P<0.01). RESV prominently attenuated the increase by downregulating TLR4, MyD88, and ICAM-1 mRNA expression by 17.64%, 14.84%, and 16.67%, respectively, compared to the DMCHD group. No significant differences were seen in the expression of TLR4, MyD88, and ICAM-1 mRNA levels between RESV group and the control group. Consistently, western blot analysis (Figure 5) also suggested that RESV treatment was able to shift the higher TLR4 and MyD88 expression in diabetic rats to normal levels. NF-κB protein expression was also reduced by RESV, which again supported that RESV attenuated inflammatory responses in heart tissues.
Figure 4.
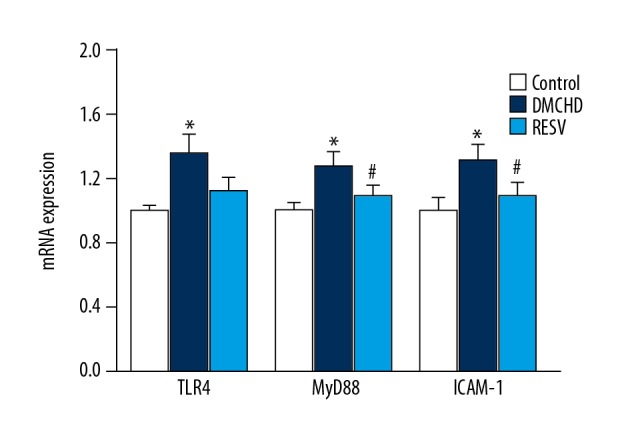
Resveratrol (RESV) downregulates TLR4, MyD88 and ICAM-1 mRNA levels. The relative mRNA expression of TLR4, MyD88 and ICAM-1 was shown. Expression levels in DMCHD and RESV groups were normalized to those in the control group. Vs. control group * P<0.01; vs. DMCHD group # P<0.01. TLR4 – toll-like receptor 4; MyD8 – myeloid differentiation factor 88; ICAM-1 – intracellular adhesion molecule 1.
Figure 5.
Western blot analysis of TLR4, MyD88, and NF-κB protein levels in heart tissues. The level of β-actin was used as a loading control. Vs. control group * P<0.01; vs. DMCHD group # P<0.01. TLR4 – toll-like receptor 4; MyD88 – myeloid differentiation factor 88; NF-κB – nuclear factor kappa B.
Discussion
Diabetes is a metabolic disorder affected by a number of lifestyle factors including exercise, diet, and stress. Current antidiabetic interventions primarily focus on alleviating glycemia and their effects against cardiovascular damages are quite limited [11]. The benefits of moderate consumption of red wine in protection against cardiovascular diseases has been increasingly recognized [12]. In current study, we provide strong evidence that RESV significantly lowers blood glucose, TC, and TG,and increases insulin level in diabetic rats. The aberrant CK and CK-MB levels indicated development of insulin resistance and cardiovascular damages in tissues of coronary arteries. RESV effectively lowered the lipid content (TC and TG levels) in serum, which is important for alleviating lipoprotein retention in the heart coronary.
Based on the interplay between diabetes, inflammation, and coronary heart disease [13,14], we proceeded to assess whether RESV was able to alleviate inflammatory responses. High levels of inflammatory factors result in activation of immune cells, which initiates inflammatory responses in the artery wall. In addition, immune cells, including macrophages, T cells, antigen-presenting dendritic cells, monocytes, and mast cells, constitute an important part of the atheroma, aggravating the risk of cardiovascular malfunction [15]. Here we showed that RESV-treated rats demonstrated reduction in TNF-α, IL-6, VCAM-1, ICAM-1, and MCP-1 levels. Thus, it can be speculated that RESV attenuated inflammatory responses to counteract the diabetes-associated hyperactive immunity in heart. Indeed, H&E staining of heart tissues indicated ameliorating effects of RESV, as evidenced by significantly reduced damages to the integrity of cardiovascular muscle fibers. It is worth noting that this protection was achieved after diabetic rats already developed cardiac and coronary artery tissue injury, indicating that RESV not only exerts preventative effects against cardiovascular diseases, but also alleviates damages resulted from inflammatory attack.
An important task of our study was to investigate how RESV exerts cardio-protection in diabetic rats. TLRs are essential mediators of the innate immunity and recognize a wide range of inflammatory inducers [16]. Following activation, TLRs recruit an adaptor molecule, MyD88, to activate NF-κB pathway for the production of a number of cytokines [17]. Although TLR-4/MyD88 pathway is conventionally thought to regulate microbial and fungal immunity, recent evidences indicate that obesity also triggers TLR-4 signaling [18]. TLR4 plays an important role in insulin resistance and inhibition or deletion of TLR4 confers protection against insulin resistance [19,20]. Therefore TLR4/MyD88/NF-κB stands at the crossroad of insulin resistance and innate immunity [21]. Based on this, we investigated how RESV affects TLR4/MyD88/NF-κB signaling pathway and found that RESV potently downregulated both mRNA and protein levels of TLR4, MyD88 and NF-κB. This accounts for the suppressed expression of inflammatory factors and cardiovascular protection by RESV.
Conclusions
Currently, primary treatment for coronary heart disease is coronary revascularization. The combination of insulin-sensitization or insulin-provision was also used to improve cardiovascular outcomes [22]. However, safe drugs capable of increasing insulin levels, as well as dampening inflammatory responses, are still lacking. In this study, we demonstrated that RESV is an effective cardioprotective compound in diabetic rats. By downregulating a wide range of inflammatory factors, RESV pronouncedly ameliorates coronary injuries by inflammation. Based on the shown effect, RESV might show a beneficial outcome or has a promising effect in diabetic cases with coronary heart disease according to protective effects on cardiovascular tissues, which presents the potential of RESV for clinical management of coronary heart disease.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Vasan RS, Benjamin EJ. The future of cardiovascular epidemiology. Circulation. 2016;133:2626–33. doi: 10.1161/CIRCULATIONAHA.116.023528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Look AHEAD Research Group. Gregg EW, Jakicic JM, Blackburn G, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–21. doi: 10.1016/S2213-8587(16)30162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 4.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arzola-Paniagua MA, García-Salgado López ER, Calvo-Vargas CG, Guevara-Cruz M. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: A randomized controlled trial. Obesity (Silver Spring) 2016;24:1454–63. doi: 10.1002/oby.21523. [DOI] [PubMed] [Google Scholar]
- 6.Yeo SC, Fenwick PS, Barnes PJ, Donnelly LE. Anti-inflammatory effects of resveratrol analogues in cellular models of airway inflammation. Eur Respir J. 2015;46:OA487. [Google Scholar]
- 7.Tome-Carneiro J, Gonzalvez M, Larrosa M, et al. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drug Ther. 2013;27:37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riveiro-Naveira RR, Valcarcel-Ares MN, Almonte-Becerril M, et al. Resveratrol lowers synovial hyperplasia, inflammatory markers and oxidative damage in an acute antigen-induced arthritis model. Rheumatology. 2016;55:1889–900. doi: 10.1093/rheumatology/kew255. [DOI] [PubMed] [Google Scholar]
- 9.Panaro MA, Carofiglio V, Acquafredda A, et al. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-kappa B activation in Caco-2 and SW480 human colon cancer cells. Brit J Nutr. 2012;108:1623–32. doi: 10.1017/S0007114511007227. [DOI] [PubMed] [Google Scholar]
- 10.Mise N, Fernandez IE, Eickelberg O. Resveratrol regulates ECM remodeling in lung fibrosis. Eur Respir J. 2014;44:P3914. [Google Scholar]
- 11.Gæde P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 12.Chiva-Blanch G, Arranz S, Lamuela-Raventos RM, Estruch R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: Evidences from human studies. Alcohol Alcohol. 2013;48:270–77. doi: 10.1093/alcalc/agt007. [DOI] [PubMed] [Google Scholar]
- 13.Dandona P, Aljada A, Chaudhuri A, et al. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–54. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 14.Rana JS, Nieuwdorp M, Jukema JW, Kastelein JJP. Cardiovascular metabolic syndrome – an interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes Metab. 2007;9:218–32. doi: 10.1111/j.1463-1326.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 15.Kriszbacher I, Koppan M, Bodis J. Inflammation, atherosclerosis, and coronary artery disease. New Engl J Med. 2005;353:429–30. [PubMed] [Google Scholar]
- 16.Gantner BN, Simmons RM, Canavera SJ, et al. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adachi K, Tsutsui H, Kashiwamura S, et al. Plasmodium berghei infection in mice induces liver injury by an IL-12-and toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J Immunol. 2001;167:5928–34. doi: 10.4049/jimmunol.167.10.5928. [DOI] [PubMed] [Google Scholar]
- 18.Kim KA, Gu W, Lee IA, et al. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radin M, Sinha S, Bhatt B, et al. Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia. 2008;51:336–46. doi: 10.1007/s00125-007-0861-3. [DOI] [PubMed] [Google Scholar]
- 20.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity. 2008;16:1248–55. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 21.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.BARI 2D Study Group. Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. New Engl J Med. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]