Abstract
Autoimmune hepatitis (AIH) is a disease of unknown aetiology with drug-induced AIH being the most complex and not fully understood type. We present the case of a 57-year-old female patient with acute icteric hepatitis after interferon-beta-1b (IFNβ-1b) administration for multiple sclerosis (MS). Based on liver autoimmune serology, histology and appropriate exclusion of other liver diseases, a diagnosis of AIH-related cirrhosis was established. Following discontinuation of IFNβ-1b, a complete resolution of biochemical activity indices was observed and the patient remained untreated on her own decision. However, 3 years later, after a course of intravenous methylprednisolone for MS, a new acute transaminase flare was recorded which subsided again spontaneously after 3 weeks. Liver biopsy and elastography showed significant fibrosis regression (F2 fibrosis). To our knowledge, this is the first report showing spontaneous cirrhosis regression in an IFNβ-1b-induced AIH-like syndrome following drug withdrawal, suggesting that cirrhosis might be reversible if the offending fibrogenic stimulus is withdrawn.
LEARNING POINTS
Autoimmune hepatitis (AIH) is a very heterogeneous liver disease of unknown aetiology, with drug-induced AIH being the most complex and not fully understood type.
Intravenous methylprednisolone pulse administration may reactivate or unmask previously unrecognised or misdiagnosed AIH and therefore liver autoimmune serology should be sought for every patient with acute or chronic hepatitis in the absence of viral, metabolic, genetic and alcoholic causes of liver disease.
Spontaneous regression of cirrhosis, although controversial, may occur if the offending fibrogenic stimuli are immediately withdrawn as shown in this case of IFNβ-1b-induced AIH.
Keywords: Autoimmune hepatitis, cirrhosis, drug-induced liver injury, interferon-beta, multiple sclerosis
INTRODUCTION
Autoimmune hepatitis (AIH) is an acute or chronic liver disease of unknown aetiology that mainly affects women and is characterized by circulating autoantibodies, interface hepatitis on liver histology, favourable response to immunosuppression and hypergammaglobulinaemia even in the absence of cirrhosis[1,2]. The disease can sometimes demonstrate quite dramatic disease fluctuations with periods of apparent spontaneous remission, acute flares and/or smouldering disease[1,2].
Of the several diagnostic challenges associated with this disease, the issue of drug-induced AIH is the most complex and is not fully understood. There seem to be three scenarios: (a) drug-induced liver injury (DILI) with a strong immune component mimicking AIH; (b) AIH mimicking DILI due to drug exposure in recent weeks and spontaneous improvement after cessation of drug exposure; and (c) AIH triggered by an offending drug (DILI-induced AIH)[2]. On the other hand, although cirrhosis is widely regarded as an irreversible end stage of chronic liver disease, there is recent evidence supporting the possibility of its reversibility or significant regression in cases of effective treatment of the underlying conditions[3]. In this context, some reports have already shown the reversibility of fibrosis and cirrhosis in AIH patients after favourable response to immunosuppression. However, to our knowledge spontaneous cirrhosis regression in DILI-induced AIH-like syndrome following drug withdrawal has not been reported so far.
CASE REPORT
A 57-year-old Caucasian female with a history of multiple sclerosis (MS) under interferon-beta-1b (IFNβ-1b) treatment for the previous 2 years was admitted because of acute icteric hepatitis accompanied by flatulence and considerable fatigue. On admission, she had no fever but she was jaundiced, while the remaining physical examination was unrevealing. She denied ever consumption of herbal agents and/or dietary supplements, intravenous or nasal illicit drugs, or alcohol use.
Total bilirubin (5 mg/dL), direct bilirubin (2.8 mg/dL), AST (474 IU/L), ALT (597 IU/L), γ-GT (144 IU/L) and INR (1.36), were elevated, whereas complement component C4 was characteristically low (13.6 mg/dL; low limit of normal 18 mg/dL). The remaining haematological, virological and biochemical parameters including IgG levels, were within normal limits. Abdominal ultrasonography was also normal. Liver autoimmune serology by indirect immunofluorescence on in-house freshly frozen rodent substrate that includes kidney, liver and stomach according to our standard protocols[1,4], revealed anti-nuclear antibodies (ANA) titre 1:80 (positive titre ≥1:40) and smooth muscle antibodies (SMA) titre 1:320 (positive titre ≥1:40). Additional testing for liver/kidney microsomal antibodies type 1 (anti-LKM1) and type 3 (anti-LKM3), liver cytosol antibodies and antibodies against soluble liver antigens/liver pancreas (anti-SLA/LP) by Western immunoblot using in-house rat liver microsomal and cytosolic extracts showed profound reactivity for anti-LKM3 and anti-SLA/LP antibodies. Liver biopsy revealed interface hepatitis with moderate portal inflammation consisting of lymphocytes and plasma cells, hepatocyte swelling and mild cholangiolar hyperplasia but without inflammatory lymphocytic infiltrate of the bile ducts and incomplete and complete cirrhotic nodules with obvious extensive portal to portal and portal to central bridging fibrosis (Fig. 1 A,B).
Figure 1.
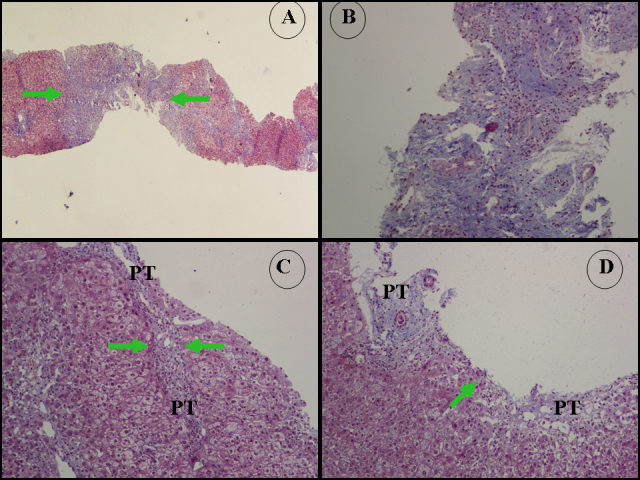
(A) Masson’s trichrome ×20.
(B) Masson’s trichrome ×200. Representative microphotographs from the first biopsy which was 2.2 cm long and contained incomplete and complete cirrhotic nodules and 14 PT, and also showed extensive portal to portal and portal to central bridging fibrosis. There is a thick diaphragm of connective tissue (thickness 0.75 mm, green arrows) implying the presence of a cirrhotic nodule (modified Hepatic Activity Index stage 5–6). Collagen type I accumulation in the PT and fibrous septa was counted as 73.8±11.8. Blue areas represent collagen type I.
(B, C) Representative microphotographs from the second biopsy performed 3 years later which was 2.8 cm long and contained 22 PT. There is portal to portal bridging fibrosis. Green arrows point to thin diaphragms of connective tissue (C: thickness 0.08 mm. D: thickness 0.02 mm). Modified Hepatic Activity Index stage 2 and inflammation grade 8/18.
PT: portal tract.
Based on liver autoimmune serology (presence of ANA, SMA, anti-LKM3 and anti-SLA/LP), the histological findings and appropriate exclusion of other liver diseases, a diagnosis of DILI-induced (IFNβ-1b-induced) AIH-related cirrhosis was established[2]. IFNβ-1b was immediately discontinued and a combination treatment schedule of prednisolone (1 mg/kg/day) along with azathioprine (1 mg/kg/day) was advised, but the patient refused treatment as following discontinuation of IFNβ-1b, a complete resolution of biochemical activity indices was observed.
The patient recovered completely and was discharged in very good health with permanent discontinuation of IFNβ-1b and close follow-up by liver ultrasound at 6-month intervals for hepatocellular carcinoma (HCC) screening, regular screening for complications, and close monitoring of AST, ALT and IgG every 3–6 months including follow-up liver biopsy if ALT and/or IgG levels increased or fluctuated[2].
Three years later, a new episode of acute flare of transaminases (AST 123 IU/L, ALT 264 IU/L) was recorded after a course of intravenous methylprednisolone pulses (1 g/day for 3 consecutive days) for MS exacerbation, which again completely subsided spontaneously after 3 weeks. A second liver biopsy at this time along with transient elastography showed significant regression of fibrosis compared to the first biopsy 3 years previously. Liver histology again showed moderate interface hepatitis, hepatocellular rosette formation, hepatocyte swelling and mild cholangiolar hyperplasia. However, neither incomplete nor complete cirrhotic nodules were seen apart from the presence of focal portal to portal bridging fibrosis (stage 2 according to the Ishak staging system) (Fig. 1 C,D). In line with the histological findings (Ishak fibrosis stage 2/6, inflammation grade 8/18), a treatment schedule was recommended to the patient with corticosteroids in combination with azathioprine but the patient again refused any intervention.
DISCUSSION
The present case raises the following major points: (a) DILI-induced AIH is an intriguing and complex disorder, which can manifest clinically in different phenotypes across the spectrum of the disease; (b) AIH should never be excluded only because of normal IgG levels; (c) spontaneous biochemical remission despite histological evidence of persisting inflammatory activity in AIH, though infrequent, does exist; (d) treatment with intravenous methylprednisolone pulses could potentially reactivate or unmask previously unrecognised or misdiagnosed AIH; and, most importantly, (e) liver cirrhosis might be regressed if the offending fibrogenic stimuli are immediately withdrawn as shown in this case by the spontaneous cirrhosis regression following IFNβ-1b withdrawal confirmed by both liver biopsy and elastography to avoid sampling error. Indeed, although historically liver biopsy has been the only accepted method to evaluate the severity of hepatic fibrosis in patients with liver disease, the procedure is associated not only with sampling error problems because a liver biopsy sample represents only 1/50,000th of total liver volume, but also with other problems because of intra- and inter-observer variability in assessing fibrosis stage. In contrast, transient elastography effectively samples an area of liver stiffness that is approximately of 1 cm in diameter and 2 cm long, leading to a volume 100 times larger than that of a standard liver biopsy specimen and therefore results in more precise assessment of liver fibrosis. Unfortunately, due to a firm diagnosis after the first liver biopsy (IFNβ-1b-induced AIH-related cirrhosis), at that time we did not perform transient elastography which we could have compared with that performed 3 years later and possibly demonstrated a considerable change in liver stiffness.
Liver dysfunction in MS could be due to many factors such as fatty infiltration, viral infection and DILI, and sometimes the autoimmune process. On the other hand, AIH is associated with a variety of autoimmune conditions including MS[1,2]. In particular, MS patients who are being treated with IFNβ or corticosteroid pulses because of a supposed ‘reconstitution’ of the immune system during interpulse periods, seem to carry a higher risk for autoimmune reactions including either DILI-induced AIH or genuine AIH[1,2].
Of the three DILI/AIH scenarios, DILI-induced AIH seems the most likely in our case, although the presence of cirrhosis at the first liver biopsy suggests that genuine AIH may have already been present and IFNβ-1b and/or pulse corticosteroid administration simply provoked and worsened the disease phenotype.
Spontaneous biochemical remission of the disease would not be unexpected as this is consistent with the fluctuating nature of AIH[1,2]. Such spontaneous apparently biochemical remission is a critical issue that may sometimes result in delay and/or underestimation of AIH diagnosis. This disease behaviour may sometimes explain the presence of previously established cirrhosis in almost one third of patients at the time of initial diagnosis[1,2].
Concerning the absence of hypergammaglobulinaemia in our patient, it should be emphasised that although high levels of serum IgG are found in approximately 85% of AIH patients[1,2,4], this prevalence tends to be lower in those patients with acute onset of disease, a higher proportion of whom (25%–39%) have been reported to have normal IgG levels[2].
Finally, for almost two centuries liver cirrhosis, the name of which is derived from the Greek word κίρρος [kirrhos] meaning tawny, has been considered an irreversible end stage of chronic liver disease. However, this issue is currently a matter of debate as cirrhosis itself comprises a broad spectrum of stages such as pre-cirrhosis or incipient cirrhosis, early cirrhosis, fully developed cirrhosis, advanced cirrhosis and decompensated cirrhosis[3]. If cirrhosis reversibility does actually exist, it most likely does not affect all cirrhosis stages equally. Indeed, the incipient stage and the early cirrhotic stage are the better candidates for reversibility. In light of this, our case perhaps represents early stage cirrhosis, although there was collagen type I accumulation in the connective tissue matrix, which is less frequent compared to collagen type III in early cirrhotic stages. Nevertheless, it should be emphasized that our patient could probably have had an even better outcome with complete resolution of fibrosis and a normal or near normal second liver biopsy if apart from immediate discontinuation of IFNβ, she had not refused the suggested immunosuppression which, according to the recent EASL guidelines, should be offered to all patients with active disease in order to achieve complete biochemical and histological resolution of AIH[2].
In conclusion, to our knowledge, this is the first report suggesting spontaneous cirrhosis regression in an IFNβ-induced AIH-like syndrome following drug withdrawal. Although this of course is not, and should not be, standard management for AIH cases, our report indicates that liver cirrhosis might be reversible if the offending fibrogenic stimulus is immediately withdrawn.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
REFERENCES
- 1.Zachou K, Muratori P, Koukoulis GK, Granito A, Gatselis N, Fabbri A, et al. Review article: autoimmune hepatitis - current management and challenges. Aliment Pharmacol Ther. 2013;38:887–913. doi: 10.1111/apt.12470. [DOI] [PubMed] [Google Scholar]
- 2.EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Hytiroglou P, Snover DC, Alves V, Balabaud C, Bhathal PS, Bioulac-Sage P, et al. Beyond “cirrhosis”: a proposal from the International Liver Pathology Study Group. Am J Clin Pathol. 2012;137:5–9. doi: 10.1309/AJCP2T2OHTAPBTMP. [DOI] [PubMed] [Google Scholar]
- 4.Zachou K, Gatselis N, Papadamou G, Rigopoulou EI, Dalekos GN. Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naive patients. J Hepatol. 2011;55:636–646. doi: 10.1016/j.jhep.2010.12.032. [DOI] [PubMed] [Google Scholar]


