Abstract
Objective:
To compare Medicare spending on provider-administered chemotherapy in hospital outpatient departments and physician offices after controlling for cancer type.
Study Design:
Secondary data analysis.
Methods:
We used 2010–2013 claims data for a random sample of Medicare Fee-for-Service beneficiaries who had cancer and received chemotherapy services either in physician offices or in hospital outpatient departments. We constructed two spending measures: 1) spending on chemotherapy drugs; and 2) spending on chemotherapy administration. Each spending measure was the allowed payment, which includes both Medicare reimbursement and patient out-of-pocket spending. We compared the spending measures in the two care settings using regression analysis to control for certain patient risk factors including cancer type. We also compared the number of chemotherapy and administration claims per beneficiary and spending per claim by cancer type to understand differences in utilization patterns in the two care settings.
Results:
Risk-adjusted chemotherapy drug spending per beneficiary was $2,451 lower in hospital outpatient departments compared with physician offices. Risk-adjusted chemotherapy administration spending was $322 higher in hospital outpatient departments than in physician offices. Patients in physician offices received chemotherapy drugs more frequently than those in hospital outpatient departments.
Conclusion:
Chemotherapy drug spending per Medicare beneficiary was lower in hospital outpatient departments than in physician offices, driven by less frequent use of chemotherapy in hospital outpatient departments. As the site of provider-administered chemotherapy shifts from physician offices to hospital outpatient departments, continuing assessment of cancer care spending by site of care is necessary.
Keywords: Medicare Part B drugs, Provider-administered chemotherapy, Site of chemotherapy administration
Precis:
The spending on chemotherapy drugs was lower among Medicare beneficiaries that received chemotherapy in hospital outpatient departments than comparable beneficiaries receiving chemotherapy in physician offices.
Introduction
Chemotherapy is a common cancer treatment modality and a significant contributor to the cost of cancer treatment.1 Many chemotherapy drugs are available in injectable forms, which are administered by providers in clinical settings.2 Provider-administered drugs are usually reimbursed under the medical benefit of an insurance policy instead of the pharmacy benefit.2 In Medicare, they are reimbursed by Part B coverage for outpatient medical services. Providers purchase drugs and then submit claims to Medicare for reimbursement of the drugs and associated administration costs.2 Most Part B-covered drugs are administered in physician offices or hospital outpatient departments (HOPDs).
Over the past decade, the site of provider-administered cancer drugs has shifted from physician offices to HOPDs.3 This trend has led to a concern that cancer care costs may increase because of differences in care costs between HOPDs and physician offices. Spending on cancer care in commercial settings is considerably higher in HOPDs than in physician offices, mainly due to higher payment rates for chemotherapy drugs and other services in HOPDs.4–7
However, these findings may not apply to Medicare. Medicare typically reimburses hospitals and physicians the same fee for Part B-covered drugs: 106% of the manufacturer’s Average Sales Price or ASP (the budget sequestration of 2013 reduced payments received by providers to 104.3% of ASP).8 No consistent pattern exists in Medicare’s reimbursement for drug administration. Some administration codes are paid more in HOPDs, while others are paid more in physician offices. In general, payments are higher in HOPDs. For example, in 2011, 14 of 20 administration codes payable in both settings were paid more in HOPDs.3 However, administration fees are much smaller than chemotherapy drug costs. Thus, differences in chemotherapy-related costs in Medicare Part B mainly come from differences in chemotherapy drug utilization, such as the quantity of chemotherapy or use of more expensive chemotherapeutic agents.
A report by The Moran Company compared spending on chemotherapy between HOPDs and physician offices in Medicare using 2009–2011 claims data.3 The report documented that the average number of chemotherapy claims per patient was slightly higher in HOPDs than in physician offices, and average spending per patient on chemotherapy agents was substantially higher in HOPDs. Based on these findings, the Moran report concluded that more and costlier chemotherapy treatments are used in HOPDs than in physician offices, given the same Medicare fees for chemotherapy drugs in both settings. However, the Moran analysis did not adjust for differences in patient risk factors between the two settings. An important risk factor is cancer type. The distribution of cancer types differs by setting9 and cancer drug utilization patterns differ by cancer type.10 If patients with specific cancer types requiring expensive chemotherapy are more likely to be treated in HOPDs, the Moran report’s conclusion is not valid.
To our knowledge, no study has examined chemotherapy-related spending in Medicare Part B after controlling for patient characteristics such as cancer type. Our study fills this gap. We compare chemotherapy drug and administration spending in HOPDs and physician offices after controlling for cancer type. In addition, we explore differences in chemotherapy utilization patterns between the two settings.
Methods
Data
The primary data sources were the 2010–2013 Medicare Hospital Outpatient file, which contains records for services in HOPDs, and the 2010–2013 Carrier file, which has claims for services by non-institutional providers. Both files contain information on diagnosis, Healthcare Common Procedure Coding System (HCPCS) code, service date, and payments. Medicare Master Beneficiary Summary Files provided beneficiaries’ demographic characteristics and disease indicators including cancer type, and the American Community Survey (ACS) supplied ZIP-level income, education, and unemployment rates.
Study Population
The study population is a random sample of Medicare Fee-for-Service beneficiaries with cancer between 2010 and 2013. To select the sample, the Centers for Medicare and Medicaid Services (CMS) first identified all patients with cancer from 100% of Medicare claims based on the standard algorithm used to create cancer indicators in the Medicare Chronic Condition Warehouse (CCW): having ≥ 1 inpatient or skilled nursing facility claim with a cancer diagnosis or ≥ 2 Carrier or Hospital Outpatient claims with a cancer diagnosis in a given year. Next, CMS provided us with the data for a random sample of those patients.
We restricted the sample to patients who had at least one chemotherapy claim identified by HCPCS Level II (J-codes) in the Hospital Outpatient or Carrier data. Chemotherapy includes all anti-neoplastic drugs (immune-, hormonal, and target therapy). We selected claims with both cancer diagnosis and chemotherapy J-codes to exclude cases using cancer drugs for other conditions. All cancer diagnosis codes and chemotherapy J-codes used are reported in Appendix A. Claims for chemotherapy reported in both Carrier and Hospital Outpatient files using the same J-code on the same day were considered duplicates, and duplicate claims in the Carrier file were excluded to avoid double counting. We considered Carrier claims with the service place code of hospital outpatient departments as HOPD claims.
We further restricted the sample to cancer patients who had both Medicare Part A and Part B coverage for the full year, and excluded those that died within three months of diagnosis. We excluded enrollees in Medicare Advantage (MA) plans because their claims data are not available to researchers.
The study sample was categorized into two groups depending on the site of chemotherapy administration: “HOPD Only” if they received chemotherapy in hospital outpatient departments only; and “Office Only” if they received chemotherapy in physician offices only. Patients receiving chemotherapy in both settings, who accounted for 4.4% of the sample, were excluded to make a clean comparison of costs between HOPDs and physician offices.
Outcome Measures
We constructed two outcome measures: 1) chemotherapy drug spending per beneficiary; and 2) chemotherapy administration spending per beneficiary. These spending variables are “allowed” payments, including both Medicare reimbursements and patient out-of-pocket spending. We constructed chemotherapy drug spending by summing the allowed payments across each patient’s chemotherapy claims with a cancer diagnosis code. Chemotherapy administration spending was created as the sum of the allowed payments across each patient’s claims with chemotherapy administration codes and a cancer diagnosis.
Analyses
We began with a descriptive analysis of six cancer types (prostate, breast, lymphoma, colon, lung, and leukemia) for which Part B chemotherapy is frequently used. First, we compared the distribution of cancer types between HOPDs and physician offices. Second, we compared chemotherapy drug and administration spending per beneficiary between the two settings for the entire sample and for each cancer type.
We used a linear regression model with clustered standard errors within a ZIP. Our unit of analysis was a patient-year. The dependent variables were chemotherapy drug spending and chemotherapy drug administration spending. The key explanatory variable was a binary indicator equal to 1 if the patient received provider-administered chemotherapy only in HOPDs and 0 if she/he received chemotherapy only in physician offices. Key control variables were cancer type indicators, an indicator of cancer metastasis, and the number of cancer-related hospitalizations and outpatient visits in the prior year. To identify metastasis, we used the criterion of ≥ 2 diagnosis codes of metastatic disease (ICD-9-CM 196–199), separated by 30 days or more. 12–14
The regression also controlled for patient age, gender, race, state buy-in status, indicators of chronic conditions (ischemic heart disease, diabetes, hypertension, hyperlipidemia, depression, heart failure, chronic pulmonary disease, and cataract), and the number of chronic conditions. Area-level variables were average income, percent college educated, and unemployment rates at the ZIP level. Finally, we used year dummies to control for year-specific effects that are common to all patients.
Using the regression results, we obtained risk-adjusted spending in each setting. To calculate risk-adjusted chemotherapy drug and administration spending in physician offices, we computed predicted spending by setting the HOPD indicator to zero and all other covariates to their mean values. Similarly, we obtained risk-adjusted spending in HOPDs by computing predicted spending with the HOPD indicator equal to one and the means of all other covariates.
To explore whether chemotherapy utilization patterns differed between HOPDs and physician offices, we compared the number of chemotherapy and administration claims per beneficiary by cancer type. We also assessed spending per claim for chemotherapy drug and administration by cancer type.
Sensitivity Checks
We performed the following sensitivity checks. First, we performed the regression analysis by year to check if differences in a particular year were driving the overall regression results. We used the same variables as in the primary analysis (except year-specific dummies), and calculated risk-adjusted spending in HOPDs versus physician offices for each year.
Second, we performed the regression analysis separately for each of the six cancers (prostate, breast, lymphoma, colon, lung, and leukemia) to check if the results were consistent across major cancer types.
Third, we limited the analysis to separately reimbursable chemotherapy drugs (drugs that are not bundled into a payment group under the Medicare Hospital Outpatient prospective payment system (OPPS)). Medicare determines separately reimbursable drugs based on a threshold daily cost (> $80 in 2013). Chemotherapy drugs whose daily cost is below the threshold are considered as a dependent or ancillary service to the drug administration. Their cost is “bundled” into an Ambulatory Payment Classification15 and is arbitrarily allocated by hospitals. Including them in the analysis may lower the estimates of chemotherapy drug spending per beneficiary in HOPDs. We thus excluded non-separately reimbursable drugs from both the Carrier and Hospital Outpatient files and checked the sensitivity of the results.
Last, we identified patients with ICD-9 codes of surgeries for certain cancers for which there is evidence of better outcomes.16 Individuals who underwent these surgeries are likely to use chemotherapy drugs as adjuvant therapy. We conducted the regression analysis on this sub-population who are relatively homogenous (in terms of cancer severity) and checked the sensitivity of the results.
Results
Figure 1 shows the distribution of cancer types among Medicare chemotherapy users in HOPDs and physician offices. Six cancers accounted for over 90% of all chemotherapy users in both HOPDs and physician offices, but the distribution of cancer types differed by setting. Prostate cancer accounted for 25% of chemotherapy users in HOPDs but more than 55% of chemotherapy users in physician offices. The right panel of Figure 1 indicates that physician offices were the dominant place of services for all cancer types. Eighty-four percent of prostate cancer patients received chemotherapy in physician offices, and about 60% of patients with other cancers used physician offices.
Figure 1.

Distribution of cancer types between hospital outpatient departments (HOPDs) and physician offices among Medicare beneficiaries who used chemotherapy
Figure 2 shows that unadjusted average chemotherapy drug spending per beneficiary in the entire sample was about 34% higher in HOPDs than in physician offices ($15,058 vs. $11,219). However, chemotherapy drug spending for patients with the same cancer type was higher in physician offices than in HOPDs for most cancer types except prostate cancer (the right panel of Figure 2).
Figure 2.
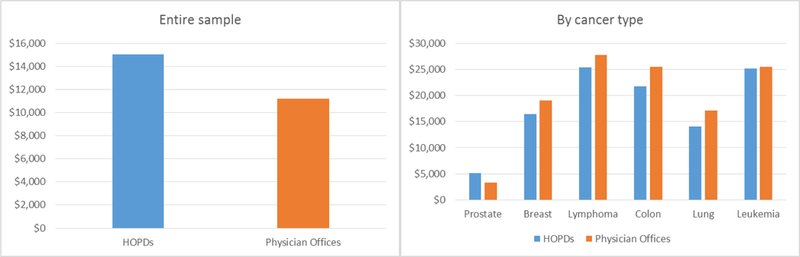
Unadjusted chemotherapy drug spending per beneficiary in hospital outpatient departments (HOPDs) versus physician offices
Figure 3 depicts descriptive data on chemotherapy administration spending per beneficiary. Unadjusted average chemotherapy administration spending per beneficiary was higher in HOPDs compared with physician offices, both in the full sample and among patients with the same cancer type.
Figure 3.

Unadjusted chemotherapy administration spending per beneficiary in hospital outpatient departments (HOPDs) versus physician offices
Figure 4 presents risk-adjusted chemotherapy spending in each setting based on the regression results. Risk-adjusted chemotherapy drug spending showed very different patterns than unadjusted spending. Risk-adjusted chemotherapy drug spending per beneficiary was $2,451 lower in HOPDs than in physician offices ($10,658 versus $13,109). Risk-adjusted chemotherapy administration spending per beneficiary was $322 higher in HOPDs compared with physician offices ($1,543 versus $1,221).
Figure 4.
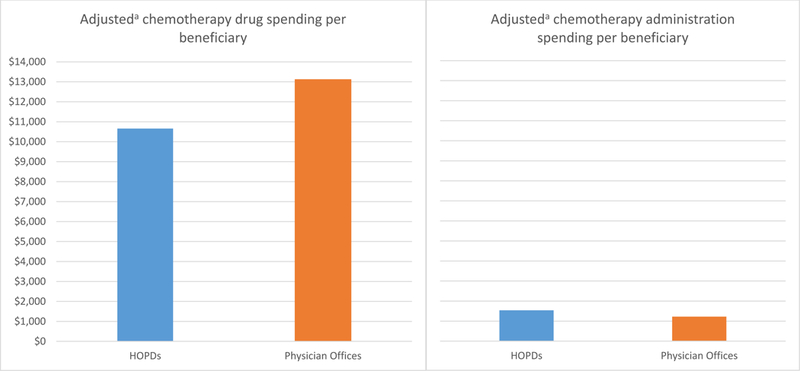
Adjusted chemotherapy drug and administration spending per beneficiary in hospital outpatient departments (HOPDs) versus physician offices a Spending was adjusted for patient and market characteristics, and year-specific effects. Patient characteristics were age, gender, race, state buy-in status, cancer type (breast cancer, leukemia, lung cancer, colon cancer, skin cancer, pancreatic cancer, sarcoma, prostate cancer, kidney cancer, ovarian cancer, and lymphoma), indicators of common chronic conditions (ischemic heart disease, diabetes, hypertension, hyperlipidemia, depression, heart failure, chronic pulmonary disease, and cataract), the number of chronic conditions, the number of cancer-related hospitalizations in the prior year, and the number of cancer-related physician office visits in the prior year. Market factors were average income, percent college educated, and the unemployment rate.
Table 1 reports the number of claims per beneficiary and spending per claim on chemotherapy drugs and administration by cancer type. These data help explain why chemotherapy drug spending was lower in HOPDs compared with physician offices after controlling for cancer type. The frequency of chemotherapy among chemotherapy users with the same cancer type was higher in physician offices than HOPDs for most cancer types except prostate cancer. For example, colon cancer patients had 19 chemotherapy drug claims per beneficiary in physician offices versus 13 in HOPDs, on average. On the other hand, spending per claim among colon cancer patients was $367 higher in HOPDs compared with physician offices. For other cancers, spending was between $257 and $737 higher in HOPDs than in physician offices. Thus, the difference in spending per claim between HOPDs and physician offices is much smaller compared with the difference in spending from adding one more claim, which exceeds $1,000 across all cancer types in both settings. These data imply that additional drug claims are an important driver of total chemotherapy drug spending per patient, and more frequent use of chemotherapy led to higher spending in physician offices than HOPDs, after controlling for cancer type.
Table 1.
Number of claim lines and spending per line for chemotherapy drugs and administration
| Variable | Number of lines/Beneficiary | Spending per claim line ($) | ||
|---|---|---|---|---|
| HOPDsa | Physician Offices | HOPDsa | Physician Offices | |
| Chemotherapy Drugs: | ||||
| Prostate | 3.8 | 3.1 | 1,328.65 | 1,071.42 |
| Breast | 9.6 | 13.8 | 1,709.36 | 1,372.63 |
| Lymphoma | 8.3 | 10.8 | 3003.96 | 2,521.80 |
| Colon | 12.8 | 19.2 | 1,688.05 | 1,321.41 |
| Lung | 8.9 | 14.0 | 1,629.19 | 149.12 |
| Leukemia | 8.2 | 10.8 | 3,106.72 | 2,370.20 |
| Chemotherapy Administrations: | ||||
| Prostate | 4.0 | 3.4 | 138.30 | 64.30 |
| Breast | 10.8 | 16.2 | 203.19 | 111.13 |
| Lymphoma | 11.2 | 15.3 | 194.49 | 106.22 |
| Colon | 21.2 | 34.1 | 181.55 | 100.02 |
| Lung | 10.2 | 16.3 | 217.04 | 111.67 |
| Leukemia | 11.0 | 14.7 | 224.17 | 112.99 |
HOPDs – Hospital outpatient departments
Similarly, the number of chemotherapy administration claims per beneficiary was higher in physician offices than in HOPDs for most cancer types, except prostate cancer. On the other hand, average spending per administration claim was almost twice as high in HOPDs compared with physician offices for most cancer types. For example, spending per chemotherapy administration claim for colon cancer was $182 in HOPDs compared with $100 in physician offices. This difference in spending per claim is large, considering that most administration claims were less than $200. Thus, higher chemotherapy administration costs per beneficiary in HOPDs compared with physician offices are largely driven by more costly administration claims in HOPDs.
The results of the sensitivity analysis supported the findings described above. Across all years, risk-adjusted chemotherapy drug spending per beneficiary was lower in HOPDs than in physician offices, and risk-adjusted chemotherapy administration spending per beneficiary was consistently higher in HOPDs compared with physician offices (Appendix Table A1). Results from the analysis of each cancer (Appendix Figure A1) were also consistent with the main analysis. The analysis using only separately reimbursable chemotherapy produced very similar results to the primary analysis (Appendix Figure A2). Risk-adjusted chemotherapy drug spending per beneficiary was $2245 lower in HOPDs than in physician offices, driven by the smaller number of separately reimbursable chemotherapy claims in HOPDs. The analysis using patients undergoing a cancer-related surgery also produced consistent results with the main analysis.
Discussion
Analyzing 2010–2013 Medicare claims data, we found that risk-adjusted chemotherapy drug spending was lower for patients receiving chemotherapy in HOPDs than for patients using physician offices. We explored two contributors to these differences – differences in the number of chemotherapy drug claims and spending per chemotherapy claim. We found that chemotherapy users in physician offices received more chemotherapy than those in HOPDs for most cancer types, and average spending per chemotherapy drug claim was slightly higher in HOPDs than in physician offices for all cancer types. However, the differences in average spending per claim were not large enough to make substantial differences in total chemotherapy drug spending per beneficiary. These findings indicate that lower utilization per beneficiary was an important driver of lower risk-adjusted chemotherapy drug spending in HOPDs than in physician offices.
Our findings differ from the Moran report, which concluded that more and costlier chemotherapy treatments are used in HOPDs than in physician offices.3 It is important to note that the Moran report did not adjust for patient risk factors including cancer type. As our results and prior literature indicate, the distribution of cancer types differs by setting9 and cancer drug utilization patterns differ by cancer type.10 Further, our data indicated that the frequency of chemotherapy among chemotherapy users with the same cancer was higher in physician offices than HOPDs for most cancer types except prostate cancer. Thus, adjusting for cancer type is of utmost importance in explaining the cost and utilization differences between the two settings.
Our findings also differ from prior research in commercial settings, which consistently found that chemotherapy costs were higher in HOPDs than physician offices.4–7 However, as mentioned earlier, spending differences in commercial settings are driven by price differences between HOPDs and physician offices rather than differences in the quantity of services.4,5 Medicare uses the same reimbursement rates for chemotherapy drugs in both settings. It is thus not surprising that chemotherapy spending per Medicare beneficiary is lower in HOPDs than physician offices.
Our analysis also showed that higher chemotherapy spending in physician offices was due to higher utilization. This result is consistent with research in commercial settings. Hayes et al. (2015) found that the mean number of chemotherapy sessions in employer-sponsored plans was higher in community oncology clinics than in HOPDs.17 To our knowledge, our analysis is the first to explore differences in chemotherapy utilization by care setting and cancer type in Medicare.
Limitations
We note several limitations of our study. First, we did not consider costs for other services that patients may have used when receiving chemotherapy. Prior research suggests that patients visiting HOPDs are likely to receive additional services (e.g., lab tests) that might not be offered in physician offices.9,18 We did not analyze spending on those services. Second, our findings are not generalizable to the commercial sector, where payment rates for chemotherapy drugs differ substantially by care site.4,5 Third, we could not completely adjust for cancer severity, such as cancer stage, because detailed clinical information is not available in Medicare data. We partially addressed this issue by using a metastasis indicator, but our approach of identifying metastasis from diagnosis codes may have limited validity.19–21 Third, the choice of chemotherapy site could depend on patients’ preferences. Patients may prefer to use HOPDs because of the availability of other services or a short travel distance. Such patient characteristics might be related to chemotherapy use and spending to some extent. However, our study did not control for those factors. Finally, there was a shift in the site of cancer care from office-based to hospital outpatient-based due to hospitals’ acquisition of physician practices during the study period.11 While examining chemotherapy use and spending in those practices acquired by hospitals would be informative, it is beyond the scope of our analysis, and we leave it to future research.
Conclusion
Chemotherapy drug spending per Medicare beneficiary was lower in hospital outpatient departments than in physician offices, driven by less frequent use of chemotherapy. As the site of provider-administered chemotherapy shifts from physician offices to hospital outpatient departments, continuing assessment of cancer care spending by care site is necessary.
Take-Away Points:
Using 2010–2013 Medicare claims data, this study demonstrates that:
The spending on chemotherapy drugs was $2,451 lower for Medicare beneficiaries receiving chemotherapy in hospital outpatient departments than patients in physician offices.
The spending on chemotherapy administration was $322 higher for Medicare beneficiaries receiving chemotherapy in hospital outpatient departments than patients in physician offices.
As chemotherapy infusions are increasingly provided in the hospital outpatient setting, policy makers and payers should be aware that this shift in the site of chemotherapy may influence cancer care patterns and spending.
Appendix A
-
Cancer diagnosis codes used in the study:
Breast Cancer: 174.0, 174.1, 174.2, 174.3, 174.4, 174.5, 174.6, 174.8, 174.9, 175.0, 175.9, 233.0, V10.3
Colon Cancer: 153.0, 153.1, 153.2, 153.3, 153.4, 153.5, 153.6, 153.7, 153.8, 153.9, 154.0, 154.1, 230.3, 230.4, V10.05, V10.06
Prostate Cancer: 185, 233.4, V10.46
Lung Cancer: 162.2, 162.3, 162.4, 162.5, 162.8, 162.9, 231.2, V10.11
Leukemia: 204, 205, 206, 207, 208, V10.60, V10.61, V10.62, V10.63, V10.69
Lymphoma: 200, 202, V10.71, V10.79
Others: 157, V10.09, 183, V10.43, 172, V10.82, 189.0, V10.52, 171
-
Chemotherapy J-codes used in the study:
J9000-J9999, J8521, J8560, J8520, and J8530
-
Chemotherapy administration codes (HCPCS Level I codes) used in the study:
96xxxx
Figure A1. Adjusted chemotherapy drug and administration spending per beneficiary in hospital outpatient departments (HOPDs) versus physician offices by cancer type.

aSpending was adjusted for patient and market characteristics, and year-specific effects. Patient characteristics were age, gender, race, state buy-in status, cancer type (breast cancer, leukemia, lung cancer, colon cancer, skin cancer, pancreatic cancer, sarcoma, prostate cancer, kidney cancer, ovarian cancer, and lymphoma), indicators of common chronic conditions (ischemic heart disease, diabetes, hypertension, hyperlipidemia, depression, heart failure, chronic pulmonary disease, and cataract), the number of chronic conditions, the number of cancer-related hospitalizations in the prior year, and the number of cancer-related physician office visits in the prior year. Market factors were average income, percent college educated, and the unemployment rate.
Figure A2. Adjusted spending per beneficiary on separately reimbursable chemotherapy drugs in hospital outpatient departments (HOPDs) versus physician offices.
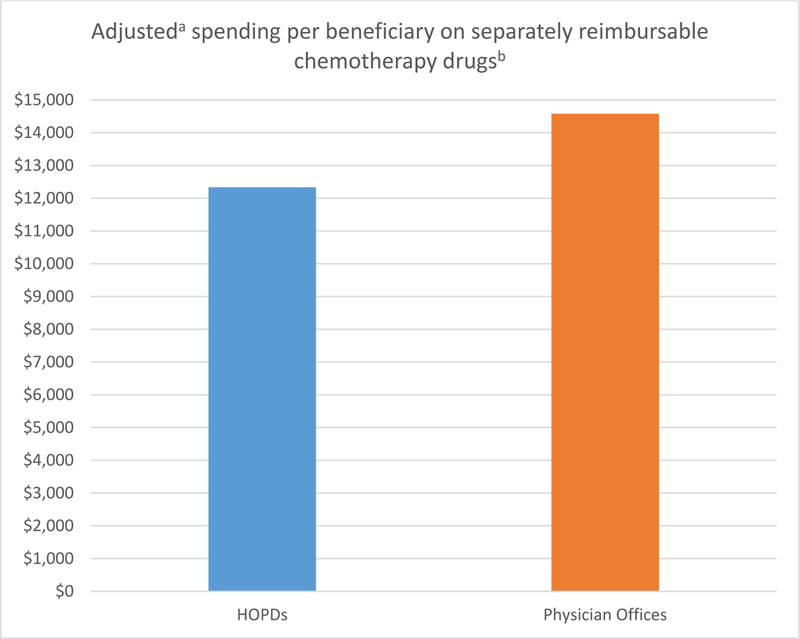
a Spending was adjusted for patient and market characteristics, and year-specific effects. Patient characteristics were age, gender, race, state buy-in status, cancer type dummies (breast cancer, leukemia, lung cancer, colon cancer, skin cancer, pancreatic cancer, sarcoma, prostate cancer, kidney cancer, ovarian cancer, and lymphoma), indicators of common chronic conditions (ischemic heart disease, diabetes, hypertension, hyperlipidemia, depression, heart failure, chronic pulmonary disease, and cataract), the number of chronic conditions, the number of cancer-related hospitalizations in the prior year, and the number of cancer-related physician office visits in the prior year. Market factors were average income, percent college educated, and the unemployment rate.b Separately reimbursable chemotherapy drugs are chemotherapy drugs that are not bundled into a payment group under the Medicare Hospital Outpatient prospective payment system
Table A1.
Adjusted chemotherapy drug and administration spending per beneficiary in hospital outpatient departments (HOPDs) versus physician offices by year
| Variable | Adjusted spending a /beneficiary | |||
|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | |
| Chemotherapy Drug Spending: | ||||
| HOPDs | $9,850.23 | $10,451.7 | $11,188.26 | $11,000.49 |
| Physician Offices | $12,661.31 | $13,318.21 | $13,437.24 | $13006.85 |
| Chemotherapy Administration Spending: | ||||
| HOPDs | $1,600.71 | $1,473.96 | $1,485.19 | $1,602.40 |
| Physician Offices | $1,267.20 | $1,266.83 | $1,171.09 | $1,167.75 |
Spending was adjusted for patient and market characteristics. Patient characteristics were age, gender, race, state buy-in status, cancer type (breast cancer, leukemia, lung cancer, colon cancer, skin cancer, pancreatic cancer, sarcoma, prostate cancer, kidney cancer, ovarian cancer, and lymphoma), indicators of common chronic conditions (ischemic heart disease, diabetes, hypertension, hyperlipidemia, depression, heart failure, chronic pulmonary disease, and cataract), the number of chronic conditions, the number of cancer-related hospitalizations in the prior year, and the number of cancer-related physician office visits in the prior year. Market factors were average income, percent college educated, and the unemployment rate.
Contributor Information
Yamini Kalidindi, Pennsylvania State University.
Jeah Jung, Pennsylvania State University.
Roger Feldman, University of Minnesota.
References:
- 1.American Cancer Society. The costs of cancer: Addressing patient costs. https://www.acscan.org/sites/default/files/Costs%20of%20Cancer%20-%20Final%20Web.pdf. Published 2017. Accessed July 1, 2017.
- 2.American Society of Clinical Oncology. ASCO in action brief: Physician administered drugs - the evolution of buy & bill. https://www.asco.org/advocacy-policy/asco-in-action/asco-action-brief-physician-administered-drugs-%E2%80%94-evolution-buy-bill. Published 2013. Accessed July 1, 2017.
- 3.The Moran Company. Cost differences in cancer care across settings. https://www.communityoncology.org/UserFiles/Moran_Cost_Site_Differences_Study_P2.pdf. Published 2013. Accessed July 1, 2017.
- 4.Bach PB, Jain RH. Physician’s office and hospital outpatient setting in oncology: It’s about prices, not use. Journal of Oncology Practice. 2017;13:4–5. [DOI] [PubMed] [Google Scholar]
- 5.Fisher MD, Punekar R, Yim YM, et al. Differences in health care use and costs among patients with cancer receiving intravenous chemotherapy in physician offices versus in hospital outpatient settings. Journal of Oncology Practice. 2017;13:e37–e46. [DOI] [PubMed] [Google Scholar]
- 6.Millman. Cost drivers of cancer care: A retrospective analysis of Medicare and commercially insured population claim data 2004–2014. http://www.milliman.com/insight/2016/Cost-drivers-of-cancer-care-A-retrospective-analysis-of-Medicare-and-commercially-insured-population-claim-data-2004-2014/.Published 2016. Accessed July 1, 2017.
- 7.Jain RH, Bach PB. Hospital outpatient versus physician office cost for physician administered cancer drugs. Evidence Driven Drug Pricing Project. http://drugpricinglab.org/wp-content/uploads/2017/01/HOPDSvsPO_010417.pdf. Published 2017. Accessed July 1, 2017.
- 8.Medicare Payment Advisory Commission. Medicare Part B drug and oncology payment policy issues. http://www.medpac.gov/docs/default-source/reports/chapter-5-medicare-part-b-drug-and-oncology-payment-policy-issues-june-2016-report-.pdf?sfvrsn=0. Published 2015. Accessed October 1, 2017.
- 9.Avalere Health LLC. Cost of cancer care by setting of therapy. http://216.230.117.100/hmd/~/media/Files/Activity%20Files/Disease/NCPF/June%202014%20Workshop/Hammelman.pdf. Published 2014. Accessed July 1, 2017.
- 10.Jung J, Feldman R, McBean M. The price elasticity of specialty drug use: Evidence from Medicare Part D enrollees with cancer. Forum for Health Economics and Policy. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avalere Health LLC. Hospital acquisitions of physician practices and the 340B program. http://340breform.org/userfiles/Avalere%20Acquisition.pdf. Published 2015. Accessed October 1, 2017.
- 12.Rao S, Kubisiak J, Gilden D. Cost of illness associated with metastatic breast cancer. Breast Cancer Research and Treatment, 2004; 83(1), 25–32. doi: 10.1023/B:BREA.0000010689.55559.06 [DOI] [PubMed] [Google Scholar]
- 13.Jacobson M, O’Malley A J, Earle C C, Pakes J, Gaccione P, Newhouse J P. Does reimbursement influence chemotherapy treatment for cancer patients? Health Affairs, 2006; 25(2), 437–443. doi: 10.1377/hlthaff.25.2.437 [DOI] [PubMed] [Google Scholar]
- 14.Goldman D P, Jena A B, Lakdawalla D N, Malin J L, Malkin J D, Sun E. The value of specialty oncology drugs. Health services research, 2010; 45(1), 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services. Hospital outpatient prospective payment system. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/HospitalOutpaysysfctshtTextOnly.pdf. Published 2016. Accessed July 1, 2017.
- 16.California HealthCare Foundation. Cancer Surgery Volume Study: ICD-9 and CPT Codes. http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/PDF%20C/PDF%20CancerSurgeryCaHospitalsICD9Codes.pdf. Published 2017. Accessed October 1, 2017.
- 17.Hayes J, et al. Cost differential by site of service for cancer patients receiving chemotherapy. The American journal of managed care. 2015; 21(3):e189–e196 [PubMed] [Google Scholar]
- 18.Avalere Health, LLC. Total cost of cancer care by site of service: physician office vs outpatient hospital. Community Oncology Alliance website. http://www.communityoncology.org/pdfs/avalere-cost-ofcancer-care-study.pdf. Published March 2012. Accessed July 1, 2017.
- 19.Thomas S K, Brooks S E, Daniel Mullins C, Baquet C R, Merchant S. Use of ICD‐9 coding as a proxy for stage of disease in lung cancer. Pharmacoepidemiology and Drug Safety, 2002; 11(8), 709–713. doi: 10.1002/pds.759 [DOI] [PubMed] [Google Scholar]
- 20.Nordstrom B L, Whyte J L, Stolar M, Mercaldi C, Kallich J D. Identification of metastatic cancer in claims data. Pharmacoepidemiol Drug Saf, 2012; 21: 21–28. doi: 10.1002/pds.3247 [DOI] [PubMed] [Google Scholar]
- 21.Chawla N, Yabroff K R, Mariotto A, McNeel T S, Schrag D, Warren J L. Limited validity of diagnosis codes in medicare claims for identifying cancer metastases and inferring stage. Annals of Epidemiology, 2014; 24(9), 666–672.e2. doi: 10.1016/j.annepidem.2014.06.099 [DOI] [PMC free article] [PubMed] [Google Scholar]


