Abstract
Background/Aims
Statins have been postulated to lower the risk of colorectal neoplasia. No studies have examined any possible chemopreventive effect of statins in patients with inflammatory bowel disease (IBD) undergoing colorectal cancer (CRC) surveillance. This study examined the association of statin exposure with dysplasia and CRC in patients with IBD undergoing dysplasia surveillance colonoscopies.
Methods
A cohort of patients with IBD undergoing colonoscopic surveillance for dysplasia and CRC at a single academic medical center were studied. The inclusion criteria were IBD involving the colon for 8 years (or any colitis duration if associated with primary sclerosing cholangitis [PSC]) and at least two colonoscopic surveillance exams. The exclusion criteria were CRC or high-grade dysplasia (HGD) prior to or at enrollment, prior colectomy, or limited (<30%) colonic disease. The primary outcome was the frequency of dysplasia and/or CRC in statin-exposed versus nonexposed patients.
Results
A total of 642 patients met the inclusion criteria (57 statin-exposed and 585 nonexposed). The statin-exposed group had a longer IBD duration, longer follow-up period, and more colonoscopies but lower inflammatory scores, less frequent PSC and less use of thiopurines and biologics. There were no differences in low-grade dysplasia, HGD, or CRC development during the follow-up period between the statin-exposed and nonexposed groups (21.1%, 5.3%, 1.8% vs 19.2%, 2.9%, 2.9%, respectively). Propensity score analysis did not alter the overall findings.
Conclusions
In IBD patients undergoing surveillance colonoscopies, statin use was not associated with reduced dysplasia or CRC rates. The role of statins as chemopreventive agents in IBD remains controversial.
Keywords: Hydroxymethylglutaryl-CoA reductase inhibitors, Neoplasia, Chemoprevention, Epidemiology, Prevention and control
INTRODUCTION
Long-standing inflammatory bowel disease (IBD) that affects the colon, including ulcerative colitis (UC), Crohn’s disease (CD), and IBD unclassified colitis (IBD-U), is a well-established risk factor for colorectal dysplasia and cancer.1–4 While the incidence of colorectal neoplasia in the IBD population appears to be decreasing, there is still an estimated 2-fold higher risk of colorectal cancer (CRC) compared to non-IBD populations in both referral-based and population-based studies.5 Patients with concomitant primary sclerosing cholangitis (PSC) carry an even higher risk of dysplasia and CRC, estimated to be 3-fold higher than the IBD population without PSC.6–8 Enrollment in a surveillance protocol with colonoscopies at routine intervals is therefore recommended according to clinical practice guidelines for patients with long-standing IBD or PSC with colonic involvement irrespective of IBD disease duration. Despite its limitations, colonoscopy dysplasia surveillance is currently the most effective way to prevent cancer in these high-risk patients.
Chronic inflammation of the colon creates a “field effect,” whereby any part of the colon that is currently, or was previously, inflamed, is at risk for neoplastic transformation.9,10 As such, unless dysplasia is visible and can be resected endoscopically with continued close surveillance, colectomy has traditionally been recommended for occult dysplasia or unresectable dysplasia due to the high likelihood of either a synchronous lesion or progression to cancer in the short-term.11–14 Because of the field effect in IBD, medications with putative chemopreventive properties that have favorable safety profiles are an attractive addition to regular colonoscopic surveillance. To date, medications such as 5-aminosalicylates (5-ASAs), ursodeoxycholic acid, thiopurines, anti-tumor necrosis factor agents, and folate have been suggested as chemopreventive agents, but data are often conflicting as to their effectiveness.15,16
Statins are prescribed for an increasing proportion of the U.S. population, primarily for their lipid-lowering effect through inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase). Because HMG-CoA reductase is over-expressed in CRC and other cancers, statins seem to be rational agents to explore as chemopreventive agents.17–20 Indeed, in addition to their anti-hyperlipidemic effect, statins also exhibit anti-inflammatory, anti-proliferative and pro-apoptotic effects, among other posited antineoplastic mechanisms. While there is a body of literature supporting the antineoplastic effect of statins in vitro, in vivo data are more limited and variable.21–29 With respect to CRC risk among individuals without IBD, some studies have suggested small CRC risk reduction,30–32 while others, including a large population-based study, failed to demonstrate any significant benefit in this respect.33–37 The literature describing the chemopreventive effect of statins on CRC in the IBD population is quite limited and includes only two recent studies to our knowledge.38,39 While both studies notably reported a reduced risk of CRC among IBD patients, either self-reported IBD39 or according to International Classification of Disease (ICD)-9 code,38 neither of these studies were designed specifically as CRC surveillance studies in the setting of established colonic IBD.
To further explore statins as chemopreventive agents in IBD, the present study investigated statin exposure in patients with confirmed IBD at increased risk of dysplasia and CRC who were undergoing active colonoscopic surveillance.
MATERIALS AND METHODS
1. Case identification
Mount Sinai Hospital in New York is a tertiary referral center where physicians regularly perform surveillance colonoscopies for patients with longstanding colonic IBD, or with PSC and any duration of colonic IBD. We queried the Mount Sinai Data Warehouse, an electronic health record-linked database that includes both inpatient and outpatient encounters, for all cases of IBD who had at least two colonic pathology reports available electronically between January 2005 and December 2016. Diagnosis was initially via ICD-9 (555.*-558.*) and/or ICD-10 codes (K50.*-K52.*) and free-text search for IBD, CD, and UC. Pathology from both colonoscopic biopsies and surgical colectomy specimens were captured. “Date of enrollment” was considered to be the date of the first IBD-related gastroenterology (GI) encounter closest to January 1, 2005 and included either an office visit or colonoscopy procedural encounter.
2. Inclusion and exclusion criteria
After initial identification through the electronic query, individual charts were manually reviewed. Inclusion criteria were: (1) diagnosis of IBD (UC, CD, IBD-U) with colonic involvement by standard clinical, endoscopic, and histologic criteria; (2) confirmed disease ≥8 years (non-PSC) or any duration of confirmed colonic involvement (PSC); (3) enrollment in dysplasia surveillance protocol at Mount Sinai or affiliated practices with electronic access to clinical, endoscopic, and histologic data; (4) at least two colonic pathology specimens from surveillance exams read by the Mount Sinai Division of GI-Pathology; (5) no history of colectomy prior to enrollment; or (6) no history of advanced colorectal neoplasia (ACRN), which includes high-grade dysplasia (HGD) and/or CRC, at or prior to enrollment. Exclusion criteria were: CD without colonic involvement; UC with limited proctitis and no extension, and IBD-U or CD with <33% colonic involvement; or ACRN, defined as HGD or CRC on the first colonoscopy at enrollment.
3. Data abstraction
For individuals who met inclusion criteria, the following baseline information was abstracted from the medical record at the time of enrollment: demographic information and baseline disease-related characteristics (age, sex, date of IBD diagnosis, IBD type [UC, CD, IBD-U], extent of disease [Montreal classification], personal history of dysplasia [indefinite for dysplasia, IND], low-grade dysplasia [LGD]; history of HGD were excluded), diagnosis of PSC by histologic or radiologic criteria, IBD-related medication exposure (biologics, thiopurines, mesalamines, 5-ASAs), and nonsteroidal anti-inflammatory drug (NSAID) exposure. Statin exposure was recorded as exposed or non-exposed, with the former defined as any use greater than 3 months and determined based on clinical documentation or review of electronic medical prescriptions.
Duration of follow-up and mean number of colonoscopies by the end of follow-up were recorded. Each follow-up encounter was defined as a surveillance colonoscopy with pathology report available. Both colonoscopic and histologic findings were recorded including: date of exam, proximal-most extent examined, gross findings for each anatomic colonic segment and also overall impression of endoscopic activity (remission, mild, moderate, and severe) on colonoscopy, presence of dysplasia (IND, LGD, and HGD) or cancer and severity of inflammation (normal mucosa, inactive/remission, mild, moderate, and severe) on pathology. All histologic diagnoses were as reported in the original pathology report; no specimens were re-reviewed or altered for the purpose of this study. As noted, all pathology was read by one expert IBD GI pathology group at Mount Sinai according to accepted criteria established by the IBD Morphology Study Group.40 It is routine practice at our institution that all cases concerning for dysplasia or neoplasia are reviewed at the time of diagnosis and agreed upon by at least two pathologists within the group. Members of the Mount Sinai Division of GI Pathology have previously demonstrated negligible inter-observer variability (kappa 0.9) for interpreting colorectal neo-plasia in IBD.41
All patients were followed until either the last recorded colonic pathology report or colectomy.
4. Grading of histologic inflammation and determination inflammation score
The highest score for each segment was recorded. The degree of inflammation for each biopsy site was scored as follows: 0, normal or inactive/remission; 1, mildly active; 2, moderately active; or 3, severely active. The inflammation score (IS) for each colonoscopy was calculated as the sum of highest histologic activity in every segment divided by the number of segments examined (“mean IS”).9
5. Primary outcome
The primary outcome was development of any new dysplasia (IND, LGD) and/or ACRN (HGD, CRC) since enrollment.
6. Statistical analyses
Categorical variables were reported as count and percentages and compared using the chi-square test. Continuous variables were reported as mean±standard deviation and compared using the Student t-test. Given the differences in mean time of follow-up between the statin-exposed and non-exposed groups, time-to-event analyses were performed up to 5 years of follow-up. Cumulative event rates were estimated using Kaplan-Meier methods. Standardized incidence rates at 5 years for the study endpoints were reported as 100 patient-years of follow-up. Hazard ratios and 95% confidence intervals for the statin-exposed and non-exposed groups were generated with proportional hazard models (Cox regression, primary analytic model). In order to account for baseline confounding between the two groups, multivariable Cox regression models were constructed and included candidate covariates determined a priori, including statin exposure, age, sex, PSC, IBD duration, mean IS, and number of colonoscopies, as well as any variables with p<0.10 in the univariate model. Covariates were included in the multivariable model using a conservative 1:8 covariate-to-event ratio in order to minimize model overfitting. Multicollinearity was evaluated by visual inspection of the correlation matrix and estimation of the variance inflation factor, with >10 used as a threshold to define significant multicollinearity. The proportionality assumption for the Cox models was verified using the Schoenfeld residuals method. Analyses were performed with STATA version 12.0 (StataCorp., College Station, TX, USA). A two-sided p-value of <0.05 was considered statistically significant for all analyses.
7. Propensity score-based matching
Given the retrospective, non-randomized nature of the surveillance cohort with respect to statin exposure, we also performed an exploratory propensity score based analysis (sensitivity analysis) using a 1:2 statin exposed:statin non-exposed ratio matched on variables decided a priori–age (±10 years), sex, inflammation extent, mean IS, IBD duration (±5 years), and number of colonoscopies. Kaplan-Meier curves were generated to compare the time to dysplasia and/or ACRN development between the two matched groups. The propensity score-based analyses were performed in SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
8. Ethical considerations
The Icahn School of Medicine at Mount Sinai Institutional Review Board (IRB number: HS#: 15-00688, GCO#1: 15-1509(0001)) approved this retrospective longitudinal cohort study, which was performed in accordance with Health Insurance Portability and Accountability Act guidelines.
RESULTS
1. Full cohort
A total of 642 IBD patients (57 statin exposed, 585 statin non-exposed at the time of inclusion) undergoing CRC surveillance met full inclusion criteria (Table 1). The two groups were comparable with respect to the proportion of male patients and extent of colitis. The statin exposed group tended to be older and had longer disease duration compared to the statin non-exposed group (p<0.0001). Statin-exposed patients more often had UC and a lower frequency of PSC than the non-exposed groups. The statin exposed group had slightly longer follow-up duration and underwent more colonoscopies (4.1 vs 3.2, p=0.003), but had slightly lower mean IS score (0.6 vs 0.8, p=0.03) than the non-exposed group. The two groups had comparable use of NSAIDs and 5-ASAs but the statin-exposed group had significantly lower use of thiopurines and biological medicines.
Table 1.
Baseline Characteristics in Statin-Exposed versus Nonexposed Patients
| Characteristics | Statin exposed (n=57) | Statin non-exposed (n=585) | p-value |
|---|---|---|---|
| Age at the time of enrollment, yr | 59.4±9.6 | 39.5±14.4 | <0.0001 |
| Male sex | 31 (54.4) | 296 (50.6) | 0.59 |
| IBD diagnosis | <0.0001 | ||
| Ulcerative colitis | 43 (75.4) | 272 (46.5) | |
| Crohn’s disease | 11 (19.3) | 292 (49.9) | |
| Indeterminate colitis | 3 (5.3) | 21 (3.6) | |
| Primary sclerosis cholangitis | 4 (7.0) | 118 (20.2) | 0.02 |
| Disease extent | 0.15 | ||
| Limited | 4 (7.0) | 54 (9.3) | |
| Intermediate | 15 (26.3) | 157 (27.2) | |
| Extensive/pancolitis | 27 (47.4) | 311 (53.8) | |
| IBD duration, yr | 21.1±12.5 | 14.1±9.6 | <0.0001 |
| Follow-up duration, yr | 5.0±2.8 | 3.8±2.8 | 0.003 |
| No. of colonoscopies at follow-up | 4.1±2.4 | 3.2±2.2 | 0.003 |
| Inflammation score at follow-up | 0.6±0.6 | 0.8±0.7 | 0.03 |
| Biologics use | 10 (17.5) | 274 (46.8) | <0.0001 |
| Thiopurine use | 19 (33.3) | 343 (58.6) | <0.0001 |
| 5-ASA use | 49 (86.0) | 489 (83.6) | 0.64 |
| NSAID use | 5 (8.8) | 48 (8.2) | 0.11 |
Data are presented as mean±SD or number (%).
IBD, inflammatory bowel disease; 5-ASA, 5-aminosalicylates; NSAID, nonsteroidal anti-inflammatory drug.
Differences between categorical variables were tested with the two-sided chi-square test. Differences between continuous variables were tested with the two-sided student t-test.
2. Primary outcome
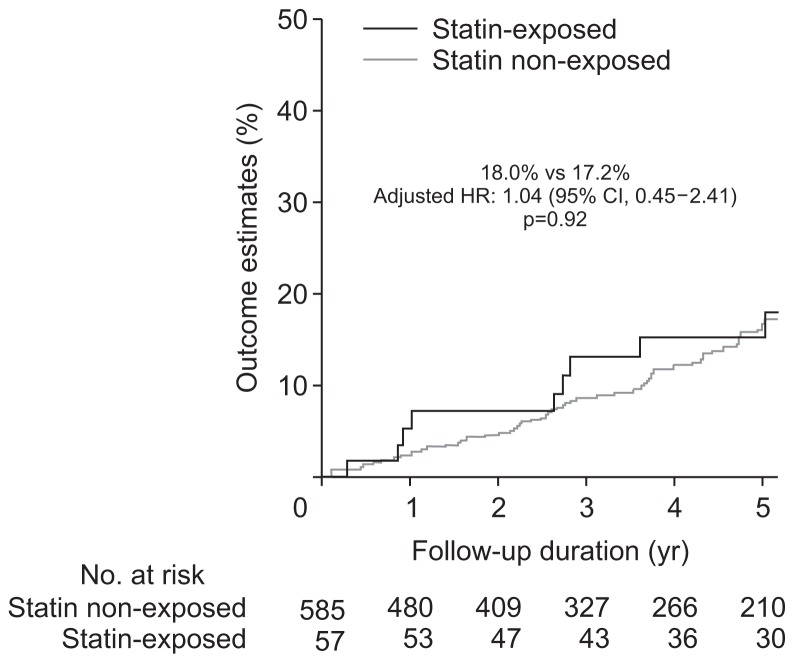
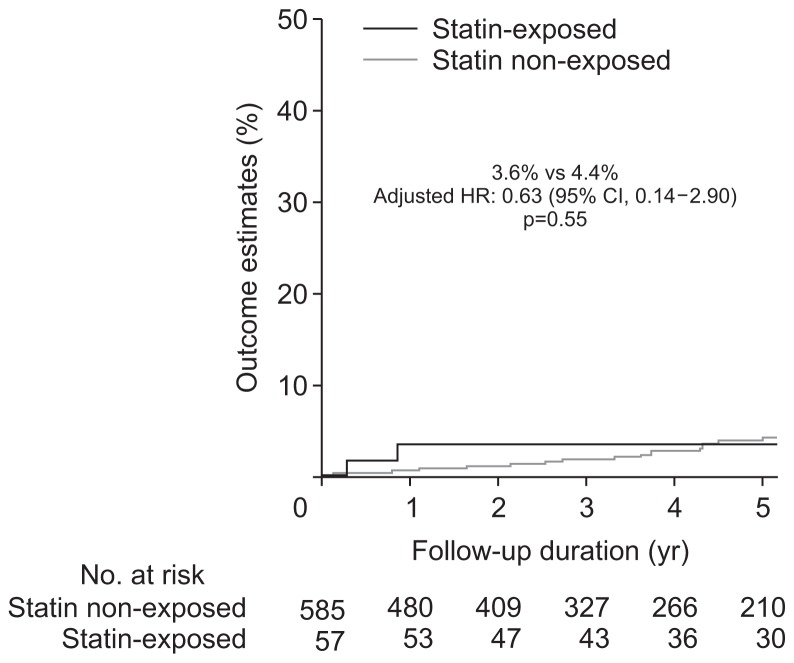
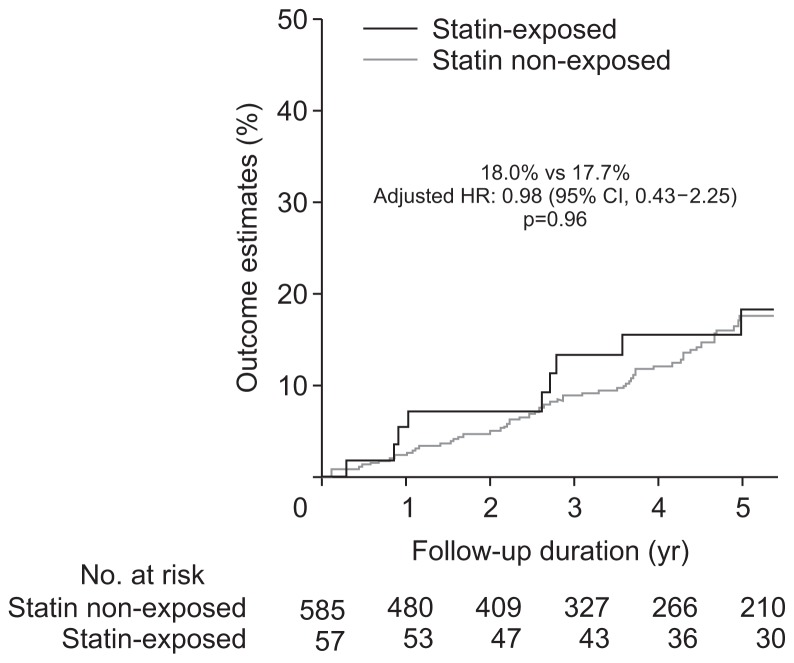
Unadjusted univariate analysis demonstrated no difference in the rates of dysplasia or CRC development between the statin exposed and non-exposed groups (Table 2). After adjusting for age, sex, PSC, IBD duration, mean IS, number of colonoscopies, thiopurine and biologic exposure, there still was no difference in the rate of any dysplasia and/or CRC according to statin exposure (Table 2). The incidence rate per 100 patient years of follow-up for ACRN was 0.9% and 0.8%, while IND/LGD was 4.1% and 3.5% in the statin exposed and non-exposed groups, respectively. Nor was there a difference in 5-year rates of IND/LGD (Fig. 1) or ACRN (Fig. 2) between the two groups. Notably, the 5-year estimated rate of ACRN was 3.6% to 4.4% for the whole cohort irrespective of statin exposure, while that of IND/ LGD was 17.2% to 18% (Fig. 3).
Table 2.
Clinical Outcomes in Statin-Exposed versus Nonexposed Patients
| Statin exposed (n=57) | Statin non-exposed (n=585) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | p-value* | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 5-yr KM estimate | 100 patient-yr rate | 5-yr KM estimate | 100 patient-yr rate | ||||
| CRC or HGD | 2 (3.6) | 2 (0.9) | 15 (4.4) | 15 (0.8) | 1.08 (0.25–4.74) | 0.63 (0.14–2.90)† | 0.55 |
| CRC | 1 (1.8) | 1 (0.5) | 5 (1.5) | 5 (0.3) | 1.62 (0.19–13.9) | 0.56 (0.06–4.92)† | 0.60 |
| HGD | 1 (1.8) | 1 (0.5) | 10 (2.9) | 10 (0.5) | 0.81 (0.10–6.36) | 0.72 (0.08–6.29)† | 0.76 |
| IND or LGD | 9 (18.0) | 9 (4.1) | 64 (17.2) | 64 (3.5) | 1.14 (0.57–2.28) | 1.04 (0.45–2.41) | 0.92 |
| IND | 4 (7.4) | 4 (1.8) | 35 (9.6) | 35 (1.9) | 0.94 (0.33–2.64) | 0.93 (0.31–2.79)‡ | 0.90 |
| LGD | 8 (16.5) | 8 (3.6) | 50 (14.0) | 50 (2.7) | 1.28 (0.61–2.70) | 0.95 (0.38–2.34) | 0.91 |
| CRC, HGD, IND or LGD | 9 (18.0) | 9 (4.1) | 66 (17.7) | 66 (3.6) | 1.10 (0.55–2.21) | 0.98 (0.43–2.25) | 0.96 |
Data are presented as number (%).
KM, Kaplan-Meier; HR, hazard ratio; CI, confidence interval; CRC, colorectal cancer; HGD, high-grade dysplasia; IND, indefinite dysplasia; LGD, low-grade dysplasia.
The median follow-up time was 5.0 years (interquartile range [IQR], 2.8–7.3 years) in the statin-exposed group and 3.5 years (IQR, 1.4–5.9 years) in the statin nonexposed group. HR were generated by Cox regression modeling. Multivariable Cox regression models included the following covariates: statin use, age, sex, primary sclerosing cholangitis, duration of inflammatory bowel disease, mean inflammatory score, number of colonoscopies, thiopurine exposure and biologic exposure.
Adjusted p-value;
Adjusted only for age and sex to minimize overfitting;
Adjusted for age, sex and duration of inflammatory bowel disease to minimize overfitting.
Fig. 1.
Development of indefinite dysplasia or low-grade dysplasia on follow-up according to statin exposure status.
HR, hazard ratio; CI, confidence interval.
Fig. 2.
Development of high-grade dysplasia or colorectal cancer on follow-up according to statin exposure status.
HR, hazard ratio; CI, confidence interval.
Fig. 3.
Development of indefinite dysplasia, low-grade dysplasia, high-grade dysplasia, or colorectal cancer on follow-up according to statin exposure status.
HR, hazard ratio; CI, confidence interval.
3. Propensity-matched cohort
Demographic characteristics for the 1:2 statin exposed:non-exposed propensity score matched cohort are detailed in Supplementary Table 1. In this cohort matched on age (±10 years), sex, inflammation extent, mean IS, IBD duration (±5 years), and number of colonoscopies, there was no difference in the frequency of dysplasia or CRC (Supplementary Table 2). ACRN (HGD and/or CRC) developed in 7% of the statin exposed group compared to 9.6% of the statin non-exposed group (p=0.57) over the follow up period, while 31.6% and 36%, respectively, developed IND and/or LGD (p=0.57). There was no difference in the rate of ACRN development between the matched groups (p=0.90).
DISCUSSION
Statins are highly pleiotropic agents and have been shown to have several additional in vitro and in vivo effects above the potent anti-hyperlipidemic and cardioprotective benefit achieved via their reliable inhibition of HMG CoA reductase. In this large retrospective study of patients with confirmed IBD actively undergoing colonoscopic CRC surveillance, we found no significant chemopreventive benefit of statin use even after controlling for potentially clinically relevant confounders. Despite the statin-exposed group having longer disease duration, somewhat longer follow up, and more colonoscopies per patient, the incidence of neoplasia was no different than the non-exposed individuals. However, it should be noted that although our population was high-risk based on disease duration or concomitant PSC, the incidence of dysplasia, particularly HGD, and CRC during follow-up in our surveillance population was low.
With respect to use of statins as chemopreventive agents in sporadic CRC, the data are equivocal. Because no studies have assessed CRC prevention as a primary outcome of statin use, studies are seldom powered for this outcome. A recent meta-analysis of 11 randomized clinical trials (RCTs) with nearly 100,000 patients failed to show a significant CRC chemopreventive benefit from statin use (relative risk [RR], 0.94; 95% confidence interval [CI], 0.86 to 1.04; I2, 23%; p=0.23),17 findings that are consistent with other meta-analyses.33,42 The duration of most studies included was approximately 5 years, with only two of the 11 studies lasting longer than 8 years, raising the question of whether the study durations were too short to see benefit in an average risk population. When RCTs were combined with observational studies (case-control and cohort) comprising over 30 studies and 2.5 million patients, there was a 9% risk reduction in CRC between statin exposed and non-exposed individuals (adjusted RR, 0.91; 95% CI, 0.87 to 0.96; p<0.001).17 Population-based studies have also shown non-statistically significant benefit for sporadic CRC risk reduction.37,43 Statins have further been explored as adenoma-preventive agents, but their effect on adenoma incidence or recurrence remains controversial at best.17,44–47
Studies analyzing the chemopreventive effect of statins specifically in the IBD population are more limited. To our knowledge, the current study is the only one performed in an IBD population with colonic involvement confirmed endoscopically and histologically and undergoing active CRC surveillance. One study did demonstrate a surprisingly large risk reduction in those with self-reported IBD (odds ratio, 0.07; 95% CI, 0.01 to 0.78).39 While this begs the question of recall bias, the authors also reported a marked risk reduction of 51% in statin users without self-reported IBD. In addition to the recall and misclas-sification bias associated with self-reporting, that study also did not account for disease duration, disease location (including whether or not there was colonic involvement), concomitant PSC, medications, inflammation extent and severity, among other important factors. A more recent study by Ananthakrishnan et al.38 analyzed data from two tertiary referral centers in the Boston area and included 11,000 IBD patients by ICD-9 diagnoses and found that statin exposure was associated with a nearly 60% risk reduction in CRC. In that study, the IBD diagnosis was based on ICD-9 codes without stated confirmation of colonic involvement, disease extent, or severity of inflammation. This was also not a representative surveillance population given that the median duration of IBD was less than 8 years in both groups, and only 35% to 40% had had a colonoscopy within 3 years of their CRC diagnosis. Lack of colonic involvement, or disease extent limited to proctitis, and colonic involvement <8 years in the absence of concurrent PSC are not thought to place IBD patients at significantly increased CRC risk above the baseline population.48 Thus, it is possible that some patients in this cohort who did not have colonic involvement had reduction in their sporadic CRC risk as opposed to IBD associated CRC risk. Nevertheless, the magnitude of this group’s findings is notable and warrants validation in larger surveillance cohorts. In our surveillance cohort, the small number of patients on statins and the low overall rates of dysplasia and CRC, despite a high-risk cohort with nearly 20% with concomitant PSC overall, limited our power to detect even a small difference in either dysplasia or ACRN between the groups in both the unmatched cohort and the propensity score matched cohort. While the statin exposed group did have lower mean ISs compared to the non-exposed patients, both groups had relatively quiescent disease histologically, which is associated with lower risk of dysplasia and CRC development.9,10,49,50 Furthermore, our low dysplasia and CRC incidence in this otherwise high-risk population also speaks to the efficacy of a colonoscopic surveillance protocol as a cancer protective strategy. That the mean number of colonoscopies over the follow-up period was 3.1 to 4.1 (mean follow-up, 3.8 to 5 years) suggests adherence with the recommended yearly or biennial surveillance interval.
Our study has several strengths. The patients in this surveillance cohort had thorough characterization of their IBD history, including confirmation of disease duration, endoscopic extent and histologic activity, with the latter determined by expert pathology review and confirmation. It is the first to reliably control for colonic inflammation using histologic data, rather than surrogates such as serum C-reactive protein.48 Moreover, our study directly addressed statin exposure and occurrence of any dysplasia, and therefore any neoplastic transformation among IBD patients undergoing active surveillance for longstanding disease or PSC-related colitis of any duration.
These points notwithstanding, our study does have some limitations. First, despite being a rather large surveillance cohort, our study may have had inadequate power to detect differences. Another limitation may be tertiary referral basis, which may limit generalizability. Duration, dose, and type of statin use were also not captured in the present study. While comorbidities were not specifically recorded, age is an acceptable surrogate for the primary purpose of this study and, along with IBD duration, were each controlled for in the multivariable model; both were also designated as a priori factors for the propensity-score analysis. Although the statin non-exposed cohort was younger than the statin-exposed group, this group notably also had over 20% with concomitant PSC and was thus at particularly high risk for colonic neoplasia independent of other factors. While it is plausible that PSC patients have a higher likelihood of abnormal liver chemistries and may be less likely to be prescribed statins, there was no difference in dysplasia rate in the multivariate model. The retrospective nature of our study inherently limits our ability to fully control for both unmeasured and measured confounders, yet we did account for factors deemed most clinically relevant with respect to colonic neoplasia risk for this population including age, IBD duration, PSC, colonic inflammation, number of colonoscopies, and thiopurine and biological medication use, among others. Finally, had our study duration been longer than 5 years post-enrollment, it is possible that we might have detected a difference in the late development of colonic neoplasia between statin exposed and non-exposed patients.
In conclusion, our study did not find a significant chemopreventive benefit of statin use in a well-characterized surveillance cohort of patients with confirmed IBD at increased risk for neoplasia. While biologic plausibility exists for the antineoplastic benefit of statins, presently there is insufficient evidence to support their use in IBD patients for chemoprevention alone. The long duration of follow-up needed given the slow development of neoplasia in IBD compounded by the relatively low incidence of neoplasia overall limits the feasibility of clinical trials and adequate powering for chemoprevention as the primary outcome. Moving forward, well-designed and adequately powered prospective studies looking at the highest risk populations, such as IBD patients who have already developed dysplasia or CRC, may prove worthwhile.
Supplementary Material
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Karlén P, Löfberg R, Broström O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047–1052. doi: 10.1111/j.1572-0241.1999.01012.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::AID-CNCR1073>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer: a population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 4.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutgens MW, van Oijen MG, van der Heijden GJ, et al. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789–799. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 6.Brentnall TA, Haggitt RC, Rabinovitch PS, et al. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1996;110:331–338. doi: 10.1053/gast.1996.v110.pm8566577. [DOI] [PubMed] [Google Scholar]
- 7.Broomé U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31–41. doi: 10.1055/s-2006-933561. [DOI] [PubMed] [Google Scholar]
- 8.Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 9.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489–501.e26. doi: 10.1016/j.gie.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Wanders LK, Dekker E, Pullens B, Bassett P, Travis SP, East JE. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:756–764. doi: 10.1016/j.cgh.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Odze RD, Farraye FA, Hecht JL, Hornick JL. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol. 2004;2:534–541. doi: 10.1016/S1542-3565(04)00237-X. [DOI] [PubMed] [Google Scholar]
- 14.Blonski W, Kundu R, Furth EF, Lewis J, Aberra F, Lichtenstein GR. High-grade dysplastic adenoma-like mass lesions are not an indication for colectomy in patients with ulcerative colitis. Scand J Gastroenterol. 2008;43:817–820. doi: 10.1080/00365520801909686. [DOI] [PubMed] [Google Scholar]
- 15.Bezzio C, Festa S, Saibeni S, Papi C. Chemoprevention of colorectal cancer in ulcerative colitis: digging deep in current evidence. Expert Rev Gastroenterol Hepatol. 2017;11:339–347. doi: 10.1080/17474124.2017.1292129. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich AC, Patel S, Meillier A, Rothstein RD, Friedenberg FK. Chemoprevention of colorectal cancer in inflammatory bowel disease. Expert Rev Anticancer Ther. 2017;17:247–255. doi: 10.1080/14737140.2017.1283987. [DOI] [PubMed] [Google Scholar]
- 17.Bardou M, Barkun A, Martel M. Effect of statin therapy on colorectal cancer. Gut. 2010;59:1572–1585. doi: 10.1136/gut.2009.190900. [DOI] [PubMed] [Google Scholar]
- 18.Stanilov N, Miteva L, Mintchev N, Stanilova S. High expression of Foxp3, IL-23p19 and survivin mRNA in colorectal carcinoma. Int J Colorectal Dis. 2009;24:151–157. doi: 10.1007/s00384-008-0588-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC. Survivin and molecular pathogenesis of colorectal cancer. Lancet. 2003;362:205–209. doi: 10.1016/S0140-6736(03)13910-4. [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Wang CY, Wang XH, Fu ZX. Oncolytic adenovirus mediated Survivin knockdown by RNA interference suppresses human colorectal carcinoma growth in vitro and in vivo. J Exp Clin Cancer Res. 2009;28:81. doi: 10.1186/1756-9966-28-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CY, Shui HA, Chang TC. In vivo evidence of duality effects for lovastatin in a nude mouse cancer model. Int J Cancer. 2010;126:578–582. doi: 10.1002/ijc.24760. [DOI] [PubMed] [Google Scholar]
- 22.Jang HJ, Hong EM, Park SW, et al. Statin induces apoptosis of human colon cancer cells and downregulation of insulin-like growth factor 1 receptor via proapoptotic ERK activation. Oncol Lett. 2016;12:250–256. doi: 10.3892/ol.2016.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauthaman K, Richards M, Wong J, Bongso A. Comparative evaluation of the effects of statins on human stem and cancer cells in vitro. Reprod Biomed Online. 2007;15:566–581. doi: 10.1016/S1472-6483(10)60390-2. [DOI] [PubMed] [Google Scholar]
- 24.Narisawa T, Fukaura Y, Terada K, et al. Prevention of 1,2-dimethylhydrazine-induced colon tumorigenesis by HMG-CoA reductase inhibitors, pravastatin and simvastatin, in ICR mice. Carcinogenesis. 1994;15:2045–2048. doi: 10.1093/carcin/15.9.2045. [DOI] [PubMed] [Google Scholar]
- 25.Asakage M, Tsuno NH, Kitayama J, et al. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor (pravastatin) inhibits endothelial cell proliferation dependent on G1 cell cycle arrest. Anticancer Drugs. 2004;15:625–632. doi: 10.1097/01.cad.0000131680.83518.91. [DOI] [PubMed] [Google Scholar]
- 26.Skaletz-Rorowski A, Walsh K. Statin therapy and angiogenesis. Curr Opin Lipidol. 2003;14:599–603. doi: 10.1097/00041433-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Nübel T, Dippold W, Kleinert H, Kaina B, Fritz G. Lovastatin inhibits Rho-regulated expression of E-selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J. 2004;18:140–142. doi: 10.1096/fj.03-0261fje. [DOI] [PubMed] [Google Scholar]
- 28.Cho SJ, Kim JS, Kim JM, Lee JY, Jung HC, Song IS. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. Int J Cancer. 2008;123:951–957. doi: 10.1002/ijc.23593. [DOI] [PubMed] [Google Scholar]
- 29.Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–641. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Poynter JN, Gruber SB, Higgins PD, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 31.Farwell WR, Scranton RE, Lawler EV, et al. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100:134–139. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmeister M, Chang-Claude J, Brenner H. Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: a population-based case-control study. Int J Cancer. 2007;121:1325–1330. doi: 10.1002/ijc.22796. [DOI] [PubMed] [Google Scholar]
- 33.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 34.Boudreau DM, Koehler E, Rulyak SJ, Haneuse S, Harrison R, Mandelson MT. Cardiovascular medication use and risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3076–3080. doi: 10.1158/1055-9965.EPI-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinogradova Y, Hippisley-Cox J, Coupland C, Logan RF. Risk of colorectal cancer in patients prescribed statins, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 inhibitors: nested case-control study. Gastroenterology. 2007;133:393–402. doi: 10.1053/j.gastro.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 36.Yang YX, Hennessy S, Propert K, Hwang WT, Sarkar M, Lewis JD. Chronic statin therapy and the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2008;17:869–876. doi: 10.1002/pds.1599. [DOI] [PubMed] [Google Scholar]
- 37.Singh H, Mahmud SM, Turner D, Xue L, Demers AA, Bernstein CN. Long-term use of statins and risk of colorectal cancer: a population-based study. Am J Gastroenterol. 2009;104:3015–3023. doi: 10.1038/ajg.2009.574. [DOI] [PubMed] [Google Scholar]
- 38.Ananthakrishnan AN, Cagan A, Cai T, et al. Statin use is associated with reduced risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:973–979. doi: 10.1016/j.cgh.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samadder NJ, Mukherjee B, Huang SC, et al. Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer. 2011;117:1640–1648. doi: 10.1002/cncr.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–968. doi: 10.1016/S0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 41.Fiel M, Qin L, Suriawinita A, et al. Histologic grading of disease activity in chronic IBD: inter- and intra-observer variation among pathologists with different levels of experience. Mod Pathol. 2003;16:118A. [Google Scholar]
- 42.Bonovas S, Filioussi K, Flordellis CS, Sitaras NM. Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J Clin Oncol. 2007;25:3462–3468. doi: 10.1200/JCO.2007.10.8936. [DOI] [PubMed] [Google Scholar]
- 43.Friis S, Poulsen AH, Johnsen SP, et al. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 44.Bertagnolli MM, Hsu M, Hawk ET, Eagle CJ, Zauber AG Adenoma Prevention with Celecoxib (APC) Study Investigators. Statin use and colorectal adenoma risk: results from the adenoma prevention with celecoxib trial. Cancer Prev Res (Phila) 2010;3:588–596. doi: 10.1158/1940-6207.CAPR-09-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung YS, Park CH, Eun CS, Park DI, Han DS. Statin use and the risk of colorectal adenoma: a meta-analysis. J Gastroenterol Hepatol. 2016;31:1823–1830. doi: 10.1111/jgh.13393. [DOI] [PubMed] [Google Scholar]
- 46.Siddiqui AA, Nazario H, Mahgoub A, Pandove S, Cipher D, Spechler SJ. The long-term use of statins is associated with a decreased incidence of adenomatous colon polyps. Digestion. 2009;79:17–22. doi: 10.1159/000203636. [DOI] [PubMed] [Google Scholar]
- 47.Wei JT, Mott LA, Baron JA, Sandler RS Polyp Prevention Study Group. Reported use of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors was not associated with reduced recurrence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2005;14:1026–1027. doi: 10.1158/1055-9965.EPI-03-0080. [DOI] [PubMed] [Google Scholar]
- 48.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 49.Nieminen U, Jussila A, Nordling S, Mustonen H, Färkkilä MA. Inflammation and disease duration have a cumulative effect on the risk of dysplasia and carcinoma in IBD: a case-control observational study based on registry data. Int J Cancer. 2014;134:189–196. doi: 10.1002/ijc.28346. [DOI] [PubMed] [Google Scholar]
- 50.Rubin DT, Huo D, Kinnucan JA, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol. 2013;11:1601–1608.e4. doi: 10.1016/j.cgh.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.