Abstract
Background:
Death with graft function remains an important cause of graft loss among kidney transplant recipients (KTRs). Little is known about the trend of specific causes of death in KTRs in the recent years.
Methods:
We analyzed United States Renal Data System data (1996 −2014) to determine 1-year and 10-year all-cause and cause-specific mortality in adult KTRs who died with a functioning allograft. We also studied one-year and ten-year trends in the various causes of mortality.
Results:
Of 210,327 KTRs who received their first kidney transplant from 1996 to 2014, 3.2% died within 1 year after transplant. Cardiovascular deaths constituted the majority (24.7%), followed by infectious (15.2%) and malignant (2.9%) causes; 40.1% of deaths had no reported cause. Using 1996 as referent year, all-cause as well as cardiovascular mortality declined whereas mortality due to malignancy did not. For analyses of 10-year mortality, we studied 94,384 patients who received a first kidney transplant from 1996 to 2005. Of those, 22.1% died over 10 years and the causative patterns of their causes of death were similar to one-year mortality.
Conclusions:
Despite the downtrend in mortality over the last two decades, a significant percentage of KTRs die in ten-years with a functioning graft, and cardiovascular mortality remains the leading cause of death. These data also highlight the need for diligent collection of mortality data in KTRs.
Keywords: Kidney transplant, cause specific death, cardiovascular disease, malignancy
Background
In 2015, there were 124,114 newly reported cases of end-stage kidney disease (ESKD) in the United States1. Kidney transplantation offers the best survival for selected patients who develop ESKD. Although the number of deceased donor kidney transplants increased from 12,279 in 2015 to 13,501 in 2016 2, there is substantial organ shortage. As of December 21, 2015, approximately 84,000 patients on dialysis were on the waiting list for a transplant, and 8% of the patients listed in 2013 died before they could receive a transplant 1. Hence, it is vital to maximize graft and patient survival in patients who have undergone kidney transplantation.
Death with graft function (DWGF), defined as death of a recipient without prior graft failure, re-transplantation, or return to dialysis, leads to simultaneous loss of the recipient’s life and the functioning kidney. Identifying cause of death patterns in these patients will help develop strategies to improve patient and graft survival by focusing resources on specific causes. Available evidence on this subject is limited to single center studies, or outdated data from patients who received older induction and maintenance transplant medications 3–5. It is unclear if factors leading to mortality have changed in the last two decades, especially with the introduction of newer technologies and immunosuppressive agents. Mortality and contributions from various causes could have been affected by a myriad of factors over the years. According to the annual data report of Scientific Registry of Transplant Recipients (SRTR), 18.4% of kidney transplant recipients (KTRs) in 2016 were ≥65 years of age compared to around 10% in 2004. Older patients and those with diabetes are at higher risk of cardiovascular disease and infections. A significant proportion of KTRs have pre-transplant dialysis durations exceeding 5 years. In addition to change in patient characteristics, there were significant changes in induction and maintenance protocols to minimize acute rejection episodes over the past two decades. The use of T-cell depleting antibody for induction has increased from ~50% in 2005 to 70% in 2016 2. Compared to interleukin-2 receptor antibodies, T-cell depleting antibodies are associated with more opportunistic infections 6 and malignancies.
Therefore, we hypothesized that the 1-year and 10-year mortality rates would have declined whereas the causes of death could have changed over time. To address these hypotheses, we examined the 1-year and 10-year mortality rates in KTRs with functioning graft in US over almost two decades of observation. In addition, we studied the causes of death post-transplantation and examined the trends in 1-year and 10-year cause specific mortality among KTRs.
Material and Methods
Source population and study design
We conducted the present study using the United States Renal Data System (USRDS), which is the national registry of patients with ESKD and includes almost all patients requiring renal replacement therapy - either dialysis or a kidney transplant. We restricted the study cohort to adult (age ≥18) first-time KTRs who received their allograft between January 1, 1996 and December 31, 2014. Patients who received kidney and pancreas transplantation, any solid organ transplantation or bone marrow transplantation prior to the kidney transplantation were excluded.
Outcome of interest
The death events reported in the USRDS were obtained from several sources, including the Centers for Medicare and Medicaid Services (CMS) Medicare Enrollment Database, CMS forms 2746 and 2728, Organ Procurement and Transplantation Network (OPTN) transplant follow-up forms, CROWNWeb database, Social Security Death Master File, and inpatient claims. For the USRDS ESKD patient cohort, cause of death is determined from the Death Notification form (CMS-2746). We classified the cause of death into categories according to the primary cause of death documented in the USRDS ‘patients’ file: cardiovascular, infectious, malignancy and others (Supplemental table 1). A large proportion of deaths had a primary cause of death reported as unknown or this information completely missing in the ‘patients’ file. We classified them as separate categories.
Exposure and covariates
We used calendar year of transplantation as exposure. Demographics including age, sex, race and health related characteristics of recipients such as hypertension, diabetes, and several other comorbidities were abstracted from the ‘patients’ and Medicare Evidence Report files in the USRDS. Demographic characteristics of donors were obtained from the transplant files in USRDS along with additional information from OPTN.
Statistical analysis
We described the characteristics of recipients at the time of transplantation. Means and standard deviations or medians and interquartile ranges were used for continuous variables and absolute numbers and percentages were used for categorical variables. Recipients were followed for death event with a functioning graft. We studied mortality in both the short term (1-year) and long-term (10-year) duration. In the 1-year mortality analysis, patients were censored when they lost graft function or at the end of 1-year after the first transplantation, whichever was earlier. We divided the number of death-events by the number of recipients at baseline to calculate the proportion of deaths in the 1-year period. In the 10-year mortality analysis, we limited patient population to KTRs between January 1, 1996 and December 31, 2005 to allow 10-year follow-up time for patients in each transplantation year. They were censored when they lost graft function or at the end of 10 years after the initial transplantation, whichever came first.
We described the proportion of deaths among recipients by donor type (deceased, living and unknown), cause of ESKD (diabetes, hypertension, glomerulonephritis and others) and recipient age (<65 vs. ≥65 years). Proportions of each category of the cause of death were described among death-events. We estimated cumulative incidence of all-cause mortality during the first year after transplantation, then cumulative incidence of each cause-specific death considering death from other causes as competing events. We estimated the hazard ratio (HR) of all-cause death according to transplantation year with year 1996 as the referent (HR of 1) from the Cox proportional hazard model. Then, we applied the cause-specific hazard regression model to estimate the cause-specific hazard ratio for each cause of death, considering death due to other causes as competing events. Hazard ratios were adjusted for age (18–24, 30–49, 50–69, ≥70), sex and race (White, Black, Asian, other). To test for linear trend of all-cause and cause-specific mortality over time, we included the calendar year as a continuous variable in the adjusted model. We repeated the analyses described above for 10-year mortality data as well.
Due to concern that the use of anti-lymphocyte polyclonal antibodies (i.e. depleting agents) may increase the risk of malignancy and thus mortality, we stratified the 1-year and 10-year mortality analyses by the use of depleting agents. Patients who received induction by anti-lymphocyte globulin (ALG), anti-thymocyte globulin (ATG) and Moromunab (OKT3) were included in the “depleting agents” group, while patients who did not receive these agents were included in the “non-depleting agents” group. As death due to malignant disease in the first year after kidney transplantation is a rare event, we collapsed transplantation years into three 5-year periods, using 1996–2001 as a reference group to evaluate one year mortality, stratified by depleting agents.
The study was approved by an institutional review board at Baylor College of Medicine (protocol #H-36408), and an active Data Use Agreement with the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) was in place. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc. Cary, NC).
Results
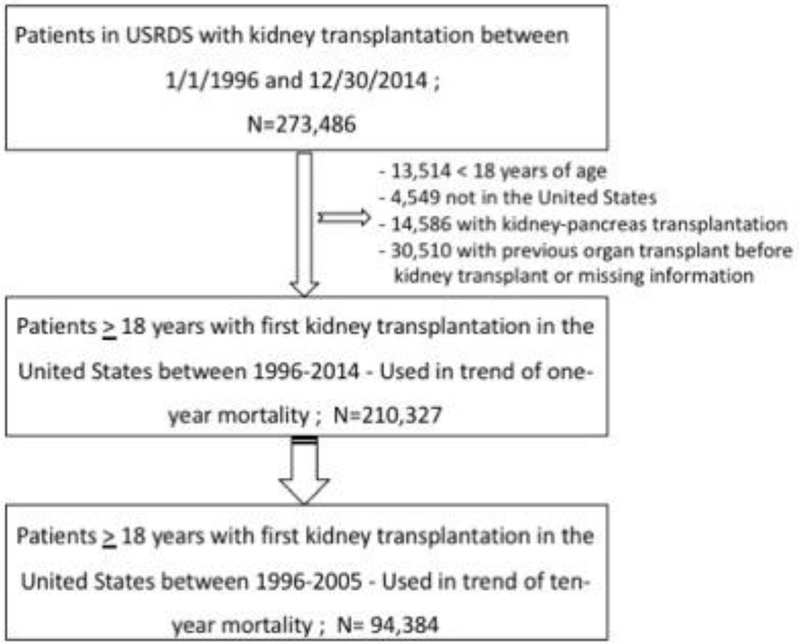
Of 429,717 patients who were recorded to have received a kidney transplant in the USRDS, 156,231 patients were excluded whose first kidney transplantation occurred outside the study period (1/1/1996 to 12/30/2014) (Figure 1.A total of 210,327 KTRs who had transplantation from 1996–2014 were included in the study of 1-year mortality and 94,384 (transplanted between 1996–2005) were studied to assess 10-year mortality.
Figure 1:
Flow chart showing how patients were selected for this study
Baseline Characteristics
Table 1 describes the baseline characteristics of the study population. Mean age of KTRs was 50.5 years (SD 13.4) with the majority being Caucasian (65.6%) and male (60.7%). Diabetes was the leading reported cause of ESKD (27% of patients), followed by glomerulonephritis (25%) and hypertension (21.2%). The narrower sample of KTRs from 1996–2005 who were studied for 10-year mortality are also described in detail in Table 1.
Table 1.
Baseline characteristics of kidney transplant recipients
| Characteristics | Sample for 1-year mortality Kidney transplant recipients, 1996–2014 N = 210,327 | Sample for 10-year mortality Kidney transplant recipients, 1996–2005 N = 94,384 |
|---|---|---|
| Age, year | ||
| mean (SD) | 50.5 (13.4) | 48.6 (13.1) |
| median (interquartile range) | 52.0 (41.0, 61.0) | 50.0 (39.0, 58.0) |
| range | (18, 102) | (18, 86) |
| Female | 82720 (39.3) | 37805 (40.1) |
| Race | ||
| White | 137951 (65.6) | 62340 (66.1) |
| Black | 56417 (26.8) | 25129 (26.6) |
| Asian | 12214 (5.8) | 4969 (5.3) |
| Other | 3432 (1.6) | 1813 (1.9) |
| missing | 313 (0.2) | 133 (0.1) |
| Hispanic | 29593 (14.1) | 11896 (12.6) |
| Cause of ESKD | ||
| Diabetes | 56697 (27.0) | 24644 (26.1) |
| Hypertension | 44627(21.2) | 19198 (20.3) |
| Glomerulonephritis | 52605 (25.0) | 25029 (26.5) |
| Other | 53812 (25.6) | 24698 (26.2) |
| Missing | 2586 (1.2) | 815 (0.9) |
| Dialysis before transplantation | 181276 (86.2) | 84937 (90.0) |
| Vintage of dialysis before transplantation, year | ||
| mean (SD) | 2.8 (2.8) | 2.7 (2.5) |
| median (interquartile range) | 2.2 (0.8, 4.2) | 2.1 (0.9, 3.8) |
| range | (0, 34.8) | (0, 31.2) |
| Donor type | ||
| Deceased | 144498 (68.7) | 67263 (71.3) |
| Living | 65705 (31.2) | 27017 (28.6) |
| Unknown | 124 (0.1) | 104 (0.1) |
Causes and Trend of 1-year mortality
Of the 210,327 patients, 6774 (3.2%) died within 1 year after receiving a kidney transplant; 756 (11.2%) patients’ cause of death was reported as unknown while 1959 (28.9%) patients cause of death was missing (Table 2). Among KTRs with a known cause of death, one quarter died from cardiovascular causes (24.7%), followed by infectious (15.2%) and malignant causes (2.9%). Other known causes constitute 17.1% of the known causes of death.
Table 2.
Death in 1 year after the first kidney transplant while the graft is functioning, among recipients in 1996–2014
| Cause of ESKD[1] |
Donor type[2] |
Recipient age |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All recipients(N = 210,327) | Diabetes (N = 56,697) | Hypertension (N = 44,627) | Glomerulonephritis (N = 52,605) | Other (N = 53,812) | Missing (N = 2586) | Deceased (N = 144,498) | Living (N = 65,705) | <65 years (N=177,523) | ≥65 years (N=32,804) | |
| All-cause death | 6774 (3.2) | 2810 (5.0) | 1492 (3.3) | 977 (1.9) | 1372 (2.6) | 123 (4.7) | 5767 (4.0) | 1006 (1.5) | 4798 (2.7) | 1976 (6.0) |
| Proportion of cause among death | ||||||||||
| Cardiovascular | 1672 (24.7) | 841 (29.9) | 348 (23.3) | 203 (20.8) | 272 (19.8) | n/a | 1417 (24.6) | 255 (25.4) | 1191 (24.8) | 481 (24.3) |
| Infection | 1031 (15.2) | 376 (13.4) | 231 (15.5) | 172 (17.6) | 241 (17.6) | n/a | 904 (15.7) | 127 (12.6) | 692 (14.4) | 339 (17.2) |
| Malignancy | 194 (2.9) | 59 (2.1) | 37 (2.5) | 30 (3.1) | 65 (4.7) | n/a | 147 (2.5) | 47 (4.6) | 127 (2.7) | 67 (3.4) |
| Other | 1162 (17.1) | 416 (14.8) | 257 (17.2) | 206 (21.1) | 275 (20.1) | n/a | 999 (17.3) | 162 (16.1) | 811 (16.9) | 351 (17.7) |
| Unknown | 756 (11.2) | 367 (13.1) | 177 (11.9) | 97 (9.9) | 111 (8.1) | n/a | 646 (11.2) | 110 (10.9) | 541 (11.3) | 215 (10.9) |
| Missing | 1959 (28.9) | 751 (26.7) | 442 (29.6) | 269 (27.5) | 408 (29.7) | 89 (72.4) | 165 (28.7) | 305 (30.3) | 1436 (29.9) | 523 (26.5) |
Cells with count <10 were not presented.
124 subjects with missing donor type were not listed. Only one of them died with functioning graft during one-year follow-up.
To assess the impact of etiology of ESKD on the mortality rate and cause of death, we subdivided the KTR population according to the leading causes of ESKD (Table 2). Of note, 5% of the patients who had ESKD secondary to diabetes died in the first-year post-transplant, with the majority (29.9%) of deaths secondary to cardiovascular causes.
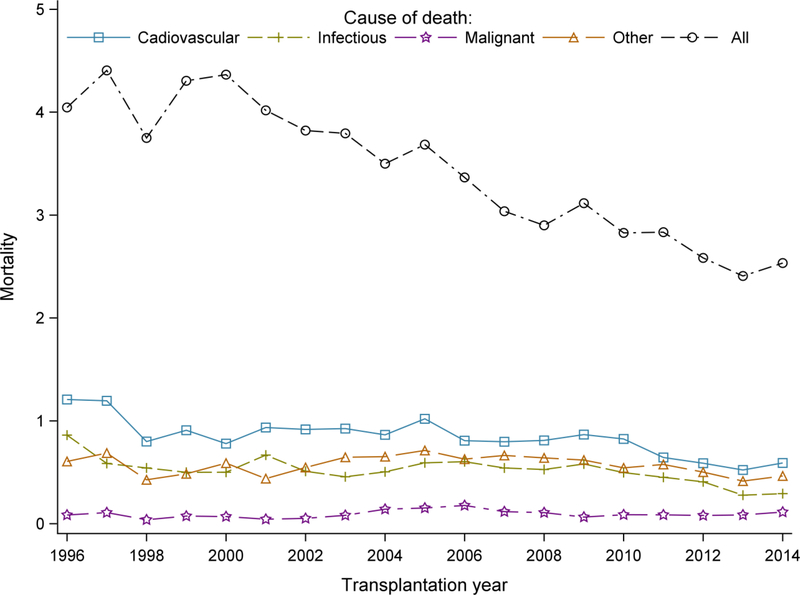
Figure 2 shows the trends in 1-year all-cause and cause specific mortality for the patients who received kidney transplantation from 1996–2014. All-cause mortality decreased over the years, from a mortality of 4.1% in 1996 to 2.5% in 2014 (Supplemental Table 2). The results also show a decline in mortality due to cardiovascular and infectious causes. However, mortality due to malignancy did not decrease.
Figure 2:
Trends of 1-year all cause and cause-specific mortality among KTRs
Causes and Trend of 10-year Mortality
Ten-year mortality data is presented in Table 3. Almost two thirds (61.5%) of patients had an unknown or missing cause of death. Death from cardiovascular causes constituted the majority (14.6%) of known causes, followed by infectious (8.2%) and malignant causes (5.4%). Other known causes were reported for 10.3% of deaths.
Table 3.
Death in 10 years after the first kidney transplant while the graft is functioning, among recipients in 1996–2005
| Cause of ESKD[1] |
Donor type[2] |
Recipient age |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cause of death | All(N =94,384) | Diabetes(N =24,644) | Hypertension(N = 19,198) | Glomerulonephritis(N = 25,029) | Other(N =24,698) | Missing(N =2586) | Deceased(N =67,263) | Living (N =27,017) | <65 years (N=83,584) | ≥65 years (N=10,800) |
| All-cause mortality | 20849 (22.1) | 8946 (36.3) | 4183 (21.8) | 3329 (13.3) | 4196 (17.0) | 195(23.9) | 16582 (24.7) | 4259(15.8) | 15550 (18.6) | 5299 (49.1) |
| Proportion of cause among death | ||||||||||
| Cardiovascular | 3038(14.6) | 1545(17.3) | 574 (13.7) | 423 (12.7) | 491 (11.7) | n/a | 2444 (14.7) | 594(14.0) | 2311 (14.9) | 727 (13.7) |
| Infectious | 1702(8.2) | 711 (8.0) | 336 (8.0) | 291 (8.7) | 360 (8.6) | n/a | 1421 (8.6) | 280 (6.6) | 1200 (7.7) | 502 (9.5) |
| Malignant | 1125(5.4) | 289 (3.2) | 227 (5.4) | 257 (7.7) | 347 (8.3) | n/a | 844 (5.1) | 281 (6.6) | 792 (5.1) | 333 (6.3) |
| Other | 2157 (10.3) | 812 (9.1) | 443 (10.6) | 404 (12.1) | 492 (11.7) | n/a | 1742 (10.5) | 412 (9.6) | 1581 (10.2) | 576 (10.9) |
| Unknown | 2945 (14.1) | 1418 (15.9) | 594 (14.2) | 426 (12.8) | 502 (12.0) | n/a | 2410 (14.5) | 535(12.6) | 2160 (13.9) | 785 (14.8) |
| Missing | 9882 (47.4) | 4171 (46.6) | 2009 (48.0) | 1528 (45.9) | 2004 (47.8) | 170(87.2) | 7721 (46.6) | 2157(50.7) | 7506 (48.3) | 2376 (44.8) |
Cells with count <10 were not presented.
104 subjects with donor type missing were not listed. Eight of them died with functioning graft during ten-year follow-up.
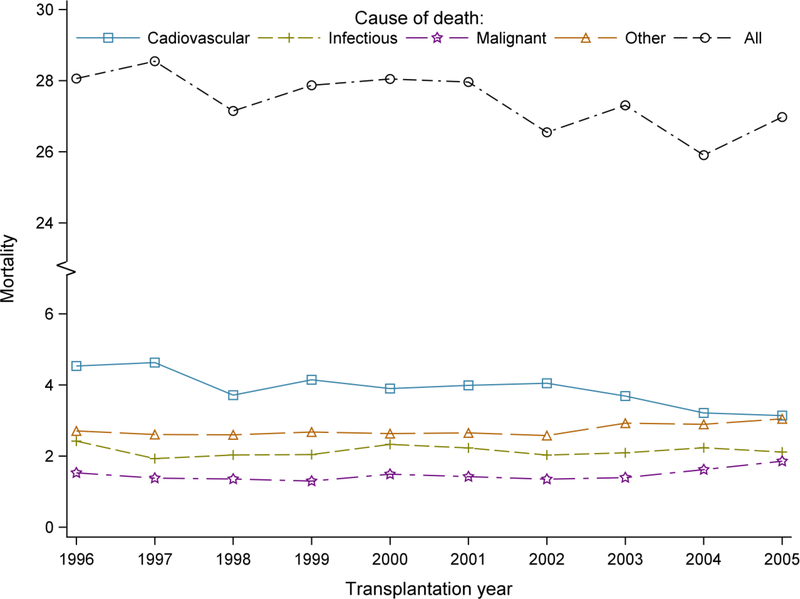
Figure 3 examines the trend of 10-year all-cause and cause-specific mortality for the transplantation years 1996–2005. Ten-year all-cause mortality was 28.1% for transplantation year 1996 while it was 27.0% for transplantation year 2005 (Supplemental Table 3). Ten-year cardiovascular mortality showed a downtrend since 2002. By contrast, 10-year mortality due to malignant causes was higher (1.86%) for transplantation year 2005 than it was for year 1996 (1.53%).
Figure 3:
Trends of 10-year all cause and cause-specific mortality among KTRs
Cause-specific Hazard Ratios for Different Causes of Mortality According to Transplantation year
Cox-proportional hazard models showed a decline over time in adjusted 1-year and 10-year all-cause mortality as shown in Tables 4 and 5. To formally consider competing events, we used cause-specific hazard models. Table 4 shows the model for 1-year cause-specific death. Using 1996 as the referent year, the hazard ratios (HR) for CV and infectious mortality have steadily and significantly (p-value for trend <0.001) declined and the respective HRs were 0.38 (CI 0.28–0.51) and 0.25 (CI 0.17–0.38) in 2014, respectively. However, the HR for malignant causes remained comparable (albeit with wide confidence limits) during the same period with a HR of 0.86 (CI 0.33–2.23) for 2014. The cause specific hazard model for 10-year cause-specific death showed a similar pattern (Table 5). A significant linear trend was observed for 1-year mortality due to all causes, cardiovascular, infections and other causes (p<0.0001); and for 10-year mortality due to all causes, cardiovascular and infections causes (p<0.0001).
Table 4.
Transplantation year and 1-year cause of death with a functioning graft in kidney transplant recipients
| Hazard ratio (95% CI) from adjusted model* |
|||||||
|---|---|---|---|---|---|---|---|
| Transpla ntation year | All-cause[1] | Cardiovascular[2] | Infectious[2] | Malignant[2] | Other[2] | Unknown[2] | Missing[2] |
| 1996 | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| 1997 | 1.06 (0.90, 1.24) | 0.97 (0.72, 1.29) | 0.66 (0.45, 0.98) | 1.22 (0.42, 3.52) | 1.11 (0.74,1.66) | 1.63 (0.92, 2.86) | 1.31 (0.97, 1.76) |
| 1998 | 0.88 (0.75, 1.04) | 0.63 (0.46, 0.87) | 0.60 (0.41, 0.89) | 0.42 (0.11, 1.69) | 0.68 (0.43, 1.06) | 0.92 (0.49, 1.73) | 1.56 (1.17, 2.07) |
| 1999 | 1.00 (0.85, 1.16) | 0.71 (0.52, 0.96) | 0.54 (0.37, 0.81) | 0.80 (0.26, 2.47) | 0.76 (0.49, 1.16) | 0.79 (0.42, 1.51) | 1.93 (1.47, 2.53) |
| 2000 | 0.99 (0.85, 1.15) | 0.59 (0.43, 0.81) | 0.53 (0.36, 0.78) | 0.71 (0.23, 2.20) | 0.90 (0.60, 1.34) | 0.80 (0.43, 1.50) | 1.95 (1.49, 2.55) |
| 2001 | 0.89 (0.76, 1.04) | 0.69 (0.52, 0.93) | 0.69 (0.48, 0.98) | 0.44 (0.13, 1.58) | 0.65 (0.42, 1.00) | 0.83 (0.45, 1.53) | 1.47 (1.12, 1.94) |
| 2002 | 0.82 (0.71, 0.96) | 0.66 (0.49, 0.89) | 0.51 (0.35, 0.74) | 0.50 (0.15, 1.65) | 0.80 (0.53, 1.19) | 0.95 (0.53, 1.70) | 1.28 (0.97, 1.69) |
| 2003 | 0.80 (0.69, 0.93) | 0.66 (0.49, 0.88) | 0.45 (0.31, 0.66) | 0.85 (0.30, 2.39) | 0.92 (0.63, 1.35) | 0.84 (0.46, 1.52) | 1.18 (0.89, 1.56) |
| 2004 | 0.73 (0.63, 0.85) | 0.62 (0.46, 0.82) | 0.49 (0.34, 0.71) | 1.30 (0.50, 3.35) | 0.92 (0.63, 1.35) | 1.04 (0.59, 1.83) | 0.83 (0.62, 1.12) |
| 2005 | 0.75 (0.65, 0.87) | 0.70 (0.53, 0.93) | 0.56 (0.39, 0.79) | 1.33 (0.52, 3.38) | 0.98 (0.68, 1.42) | 1.60 (0.95, 2.70) | 0.57 (0.42, 0.79) |
| 2006 | 0.67 (0.58, 0.78) | 0.54 (0.41, 0.73) | 0.56 (0.40, 0.79) | 1.49 (0.60, 3.70) | 0.85 (0.58, 1.23) | 1.69 (1.01, 2.82) | 0.48 (0.35, 0.67) |
| 2007 | 0.60 (0.51, 0.70) | 0.53 (0.40, 0.71) | 0.50 (0.35, 0.71) | 0.97 (0.37, 2.53) | 0.89 (0.62, 1.29) | 1.69 (1.01, 2.82) | 0.29 (0.20, 0.42) |
| 2008 | 0.57 (0.49, 0.66) | 0.54 (0.40, 0.72) | 0.48 (0.34, 0.68) | 0.88 (0.33, 2.31) | 0.86 (0.59, 1.24) | 1.20 (0.70, 2.04) | 0.33 (0.23, 0.46) |
| 2009 | 0.60 (0.52, 0.70) | 0.57 (0.43, 0.75) | 0.52 (0.37, 0.74) | 0.53 (0.18, 1.52) | 0.81 (0.56, 1.18) | 1.41 (0.84, 2.36) | 0.39 (0.28, 0.55) |
| 2010 | 0.54 (0.46, 0.63) | 0.53 (0.40, 0.71) | 0.44 (0.31, 0.63) | 0.69 (0.26, 1.87) | 0.70 (0.48, 1.02) | 1.14 (0.67, 1.94) | 0.37 (0.27, 0.52) |
| 2011 | 0.54 (0.46, 0.62) | 0.41 (0.31, 0.56) | 0.40 (0.28, 0.58) | 0.67 (0.25, 1.83) | 0.74 (0.51, 1.07) | 1.47 (0.88, 2.46) | 0.43 (0.31, 0.59) |
| 2012 | 0.49 (0.42, 0.57) | 0.38 (0.28, 0.52) | 0.36 (0.25, 0.52) | 0.62 (0.23, 1.71) | 0.65 (0.44, 0.95) | 1.06 (0.62, 1.82) | 0.48 (0.35, 0.66) |
| 2013 | 0.45 (0.38, 0.530 | 0.33 (0.24, 0.45) | 0.25 (0.16, 0.37) | 0.70 (0.26, 1.86) | 0.52 (0.35, 0.78) | 1.21 (0.72, 2.05) | 0.51 (0.37, 0.69) |
| 2014 | 0.47 (0.41, 0.550) | 0.38 (0.28, 0.51) | 0.25 (0.17, 0.38) | 0.86 (0.33, 2.23) | 0.59(0.40,0.87) | 0.92 (0.53, 1.58) | 0.56 (0.41, 0.75) |
Adjusting age, sex, race
Cox proportional hazard model
Cause specific hazard model
Table 5.
Transplantation year and 10–year cause of death with a functioning graft in kidney transplant recipients
| Hazard ratio (95% CI) from adjusted model* |
|||||||
|---|---|---|---|---|---|---|---|
| Transplantation year | All- cause[1] | Cardiovascular[2] | Infectious[2] | Malignant[2] | Other[2] | Unknown[2] | Missing[2] |
| 1996 | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | |
| 1997 | 1.00 (0.94, 1.07) | 0.99 (0.84, 1.17) | 0.76 (0.60, 0.96) | 0.88 (0.65, 1.19) | 0.95 (0.77, 1.18) | 1.23 (1.00, 1.51) | 1.03 (0.93, 1.13) |
| 1998 | 0.92 (0.86, 0.98) | 0.77 (0.65, 0.91) | 0.78 (0.62, 0.98) | 0.83 (0.61, 1.11) | 0.90 (0.73, 1.12) | 1.19 (0.98, 1.46) | 0.96 (0.87, 1.05) |
| 1999 | 0.93 (0.87, 0.99) | 0.85 (0.72, 1.01) | 0.77 (0.61, 0.96) | 0.80 (0.60, 1.07) | 0.92 (0.74, 1.13) | 1.30 (1.07, 1.59) | 0.93 (0.84, 1.02) |
| 2000 | 0.92 (0.86, 0.98) | 0.79 (0.67, 0.93) | 0.85 (0.68, 1.05) | 0.89 (0.67, 1.18) | 0.90 (0.73, 1.11) | 1.28 (1.05, 1.55) | 0.91 (0.83, 1.00) |
| 2001 | 0.89 (0.84, 0.95) | 0.80 (0.68, 0.94) | 0.81 (0.65, 1.00) | 0.85 (0.64, 1.12) | 0.87 (0.71, 1.07) | 1.41 (1.17, 1.70) | 0.84 (0.77, 0.92) |
| 2002 | 0.81 (0.76, 0.86) | 0.79 (0.67, 0.92) | 0.71 (0.57, 0.88) | 0.75 (0.56, 0.99) | 0.83 (0.68, 1.02) | 1.24 (1.03, 1.50) | 0.75 (0.68, 0.82) |
| 2003 | 0.82 (0.77, 0.87) | 0.70 (0.60, 0.83) | 0.71 (0.57, 0.87) | 0.78 (0.59, 1.02) | 0.92 (0.76, 1.12) | 1.32 (1.10, 1.59) | 0.76 (0.69, 0.83) |
| 2004 | 0.76 (0.71, 0.81) | 0.61 (0.52, 0.72) | 0.75 (0.61, 0.93) | 0.90 (0.69, 1.17) | 0.91 (0.75, 1.10) | 1.20 (1.00, 1.45) | 0.68 (0.62, 0.74) |
| 2005 | 0.76 (0.72, 0.81) | 0.58 (0.49, 0.68) | 0.69 (0.56, 0.85) | 0.99 (0.76, 1.27) | 0.92 (0.76, 1.12) | 1.22 (1.01, 1.46) | 0.69 (0.63, 0.75) |
Adjusting age, sex, race
Cox proportional hazard model
Cause specific hazard model
When stratified according to the use of depleting agents, no significant interaction between time and depleting drug use was observed. As expected, we observed more use of depleting drugs in induction among kidney transplant patients over time, 15.5% in 1996, 38.0% in 2005 and 50.7% in 2014 (Data not shown). When including interaction term of transplant year and use of depleting drugs in the model, we did not find significant interaction between them for 1-year (p=0.550) and 10-year (p=0.142) malignant cause of death. One-year and ten-year mortality decreased due to cardiovascular and infectious causes, but not due to malignant causes, in both, depleting as well as non-depleting agent groups (Supplemental Table 4 and 5).
Discussion
From this comprehensive analysis of national data spanning almost two decades, we conclude that the mortality of first-time KTR with a functioning allograft declined considerably over the study period. Cardiovascular disease remained the leading cause of death followed by death due to infections and malignancies. These results were consistent, both in analyses focusing on short-term and on long-term follow up, as well as among both younger patients and those older than 65 years. Our findings extend previous observations conducted in other studies that used considerably older data and had relatively smaller sample sizes 3,7,8. Ojo et al. examined the UNOS/SRTR and USRDS databases to study death with graft function (DWGF) among patients who received their first kidney transplantation from 1988 to 1997 3. This study included 86,502 patients and showed an overall 31% reduction in DWGF among patients who received a kidney transplant from 1993–1997, compared to patients who were transplanted from 1988–1992. El Husseini et al. studied patient and graft survival in first-time kidney transplant recipients in UNOS database from 2000–2014 7. However, their objective was to compare survival in this group to repeat kidney transplants and kidney-after-nonrenal solid organ transplant, and they studied death with a functioning graft as well as death with graft loss. Our study is the most comprehensive to date that has analyzed death with graft function among more than 200,000 primary KTRs in the USRDS database over the last two decades.
The preponderance of death due to cardiovascular disease is not surprising as KTRs have multiple cardiac risk factors, including diabetes and hypertension pre- and post-transplantation, resulting in cumulative burden of heart and vascular disease over time. Albeit good allograft function improves uremic milieu, most traditional risk factors of atherogenesis still persist after successful transplantation. However, compared to a KTR who underwent kidney transplant in 1996, the one year and 10-year cardiovascular death rates have improved in recent years, not much different from the general population. The decline among KTRs may be due to overall improvement in cardiac care, or be related to evolving immunosuppressive regimens over last two decades 9,10. Cyclosporine has been largely replaced by tacrolimus, and steroid minimization strategies are common. Compared to tacrolimus, cyclosporine causes more hypertension and lipid abnormalities 11,12, but tacrolimus causes islet cell damage and post-transplant diabetes 13,14. Use of steroids in the post-transplant period is associated with a dose-dependent increase in mortality 15. Steroid minimization strategies reduce risk of post-transplant diabetes 16, although there is not enough evidence to suggest that this plays a significant role in the reduction of cardiovascular mortality. Also, reduction in acute rejection episodes minimizes the need for high dose pulse steroids.
Similar to cardiac disease, short-term and long-term infection-related death rates have also declined. This observation is encouraging as attempts to reduce acute rejection rates may lead to a parallel increase in mortality related to opportunistic infections and malignancies. With the introduction of newer potent transplant medications, acute rejection rates within the first-year post-transplant have declined significantly to the lowest level of around 8% in 2014 recipients 17. The use of T-cell depleting antibody for induction has increased from ~50% in 2005 to around 70% in 2015. Also, cyclosporine and azathioprine have been replaced by tacrolimus and mycophenolate, respectively 2. At the same time, the proportion of patients aged 65 years and older has significantly increased among transplant recipients. Elderly patients secare more prone to infection-related complications 18. The observed reduction in infection-related deaths could be due to reduced exposure to T-cell depleting antibodies and pulse-dose steroids as acute rejection rate continues to improve. It could also be secondary to increasing vigilance and aggressive treatment of infections as well as reduction in invasive viral infections. 19. We acknowledge that we cannot deduce from this data that the incidence of infections has reduced in KTRs, mainly due to censoring for graft failure and partially due to missing causes of death. In fact, the follow-up of FAVORIT trial actually demonstrated that infections are the leading cause of death in the late post-transplant period (4–10 years after transplant), especially in diabetic patients 20.
Unlike cardiovascular and infection related mortality, 1-year and 10-year mortality due to malignancy has not decreased over time. These findings are consistent with studies from other countries that have examined mortality trends in KTRs 21,22. Lack of decline in mortality due to malignancy could be multifactorial, including but not limited to aging patient population, prolonged exposure to immunosuppressive medications due to longer graft survival and reduced number of deaths from cardiovascular and infectious causes. In another analysis of the USRDS data (1990–2004), patients who were <50 years had a higher standardized mortality ratio due to higher risk of succumbing to their malignancy. On the other hand, competing risks of other causes of death in older patient populations reduce their risk of dying from cancer 23. However, our study did not show a reduced mortality in older patient population and, in fact, people above 65 years of age had a higher mortality due to cancer. In our sensitivity analyses, mortality due to malignant causes did not decline over the years in patients who received depleting agents during induction, as opposed to mortality secondary to cardiovascular and infectious causes. Further studies are required to better investigate the relationship between use of depleting agents and mortality due to malignant causes.
Being a population-based study with few selection criteria, its results are broadly generalizable to KTRs in the United States. USRDS data are drawn from a multitude of sources, including CMS, OPTN, and the ESRD networks. As it analyzes the mortality over the last twenty years, the results reliably reflect the impact of our current practices in transplant, including preoperative evaluations and contemporary immunosuppression regimens. Our study carries the inherent limitations that are associated with studies involving large national datasets and registries 24. Missingness of key data is one of them, and according to data obtained from USRDS registry, the cause of death is either missing or unknown in about 40% for patients who die within the first year with a functioning graft. Despite a low overall death rate of 3.2% in this population, delineating a precise cause of death may help develop strategies to reduce immediate post-transplant mortality. During ten-year follow up, when KTRs are less frequently followed in a transplant center, cause of death is missing or unknown in an even higher proportion of patients.
Although alarming, a high fraction of missing or unknown cause of death was also shown by previous studies involving national registries 3,7,23. Missing data does not seem to have affected the contribution of various causes of death, as our results are generally consistent with other studies of mortality where cardiovascular disease, infection and malignancy are the three leading causes of death in patients with KTRs 3,7. A large retrospective study in an integrated health system (IHS) in the United States (with no missing data) had similar conclusion regarding the causes of mortality 8. Studies done outside US, including Spain 21, Australia and New Zealand (ANZDATA) 22, and India 25 have shown very similar patterns.
In summary, cardiovascular mortality remains the leading cause of death in KTRs at 1 and 10 years post-transplant and surpasses infection and malignancy at all times. Although mortality due to cardiovascular and infectious causes has been trending down, there is still a substantial proportion of transplant recipients, especially patients over 65 years of age with a deceased donor, who remain at increased risk of death due to these two factors. Remarkably, the mortality due to malignancy has essentially remained unchanged and may become the most significant cause of death with a functioning graft as the transplant population continues to age and the cumulative exposure to immunosuppressive medications continues to rise. Moreover, we are losing a large proportion of KTRs without adequately delineating the factors that lead to mortality in this high-risk patient population. The current study not only gives a unique insight into the various causes of death in patients with kidney transplant, but it also highlights the significant gaps that currently exist in our knowledge of transplant mortality. This void needs to be filled through improved reporting and collection of mortality data in KTRs.
Supplementary Material
Acknowledgments
Funding Sources: None
Footnotes
Disclosures: The authors disclose no conflicts of interest relevant to this manuscript.
Conflicts of interest
The results presented in this paper have not been published previously in whole or part, except in abstract format- presented as a poster at the 2017 Kidney Week held in New Orleans, LA on Nov 3rd, 2017.
Disclaimer
This work was conducted under a data use agreement between Dr. Winkelmayer and the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK). An NIDDK officer reviewed this manuscript for research compliance and approved of its submission for publication. Data reported herein were supplied by the USRDS. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
References:
- 1.United States Renal Data System. 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2017. [Google Scholar]
- 2.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 Annual Data Report: Kidney. Am J Transplant. 2018;18 Suppl 1:18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57(1):307–313. [DOI] [PubMed] [Google Scholar]
- 4.Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001;16(8):1545–1549. [DOI] [PubMed] [Google Scholar]
- 5.Howard RJ, Patton PR, Reed AI, et al. The changing causes of graft loss and death after kidney transplantation. Transplantation. 2002;73(12):1923–1928. [DOI] [PubMed] [Google Scholar]
- 6.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D, Thymoglobulin Induction Study G. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–1977. [DOI] [PubMed] [Google Scholar]
- 7.El-Husseini A, Aghil A, Ramirez J, et al. Outcome of kidney transplant in primary, repeat, and kidney-after-nonrenal solid-organ transplantation: 15-year analysis of recent UNOS database. Clin Transplant 2017;31(11). [DOI] [PubMed] [Google Scholar]
- 8.Kahwaji J, Bunnapradist S, Hsu JW, Idroos ML, Dudek R. Cause of death with graft function among renal transplant recipients in an integrated healthcare system. Transplantation. 2011;91(2):225–230. [DOI] [PubMed] [Google Scholar]
- 9.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. 2014;35(42):2929. [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356(23):2388–2398. [DOI] [PubMed] [Google Scholar]
- 11.Takeda Y, Miyamori I, Wu P, Yoneda T, Furukawa K, Takeda R. Effects of an endothelin receptor antagonist in rats with cyclosporine-induced hypertension. Hypertension. 1995;26(6 Pt 1):932–936. [DOI] [PubMed] [Google Scholar]
- 12.Textor SC, Canzanello VJ, Taler SJ, et al. Cyclosporine-induced hypertension after transplantation. Mayo Clin Proc. 1994;69(12):1182–1193. [DOI] [PubMed] [Google Scholar]
- 13.Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7(6):1506–1514. [DOI] [PubMed] [Google Scholar]
- 14.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4(4):583–595. [DOI] [PubMed] [Google Scholar]
- 15.Opelz G, Dohler B. Association between steroid dosage and death with a functioning graft after kidney transplantation. Am J Transplant. 2013;13(8):2096–2105. [DOI] [PubMed] [Google Scholar]
- 16.Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y. Patient survival and cardiovascular risk after kidney transplantation: the challenge of diabetes. Am J Transplant. 2008;8(3):593–599. [DOI] [PubMed] [Google Scholar]
- 17.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: Kidney. Am J Transplant. 2017;17 Suppl 1:21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trouillhet I, Benito N, Cervera C, et al. Influence of age in renal transplant infections: cases and controls study. Transplantation. 2005;80(7):989–992. [DOI] [PubMed] [Google Scholar]
- 19.Linares L, Cofan F, Cervera C, et al. Infection-related mortality in a large cohort of renal transplant recipients. Transplant Proc. 2007;39(7):2225–2227. [DOI] [PubMed] [Google Scholar]
- 20.Weinrauch LA, D’Elia JA, Weir MR, et al. Infection and Malignancy Outweigh Cardiovascular Mortality in Kidney Transplant Recipients: Post Hoc Analysis of the FAVORIT. Am J Med. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Mazuecos A, Munoz Terol JM, Garcia Alvarez T, et al. Increase in malignancies as cause of death in renal transplant patients. Transplant Proc. 2009;41(6):2159–2162. [DOI] [PubMed] [Google Scholar]
- 22.Pilmore H, Dent H, Chang S, McDonald SP, Chadban SJ. Reduction in cardiovascular death after kidney transplantation. Transplantation. 2010;89(7):851–857. [DOI] [PubMed] [Google Scholar]
- 23.Kiberd BA, Rose C, Gill JS. Cancer mortality in kidney transplantation. Am J Transplant. 2009;9(8):1868–1875. [DOI] [PubMed] [Google Scholar]
- 24.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prakash J, Ghosh B, Singh S, Soni A, Rathore SS. Causes of death in renal transplant recipients with functioning allograft. Indian J Nephrol. 2012;22(4):264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.