Abstract
The signaling mechanisms mediating myocardial glucose transport are not fully understood. Sucrose non-fermenting AMPK-related kinase (SNARK) is an AMPK-related protein kinase that is expressed in the heart and has been implicated in contraction-stimulated glucose transport in mouse skeletal muscle. We first determined if SNARK is phosphorylated on Thr208, a site critical for SNARK activity. Mice were treated with exercise, ischemia, submaximal insulin, or maximal insulin. Treadmill exercise slightly, but significantly increased SNARK Thr208 phosphorylation. Ischemia also increased SNARK Thr208 phosphorylation, but there was no effect of submaximal or maximal insulin. HL1 cardiomyocytes were used to overexpress wild type SNARK and to knockdown endogenous SNARK. Overexpression of wild type SNARK had no effect on ischemia-stimulated glucose transport; however, SNARK knockdown significantly decreased ischemia-stimulated glucose transport. SNARK overexpression or knockdown did not alter insulin-stimulated glucose transport or glycogen concentrations. To study SNARK function in vivo, SNARK heterozygous knockout mice (SNARK+/−) and wild type littermates performed treadmill exercise. Exercise-stimulated glucose transport was decreased by ~50% in hearts from SNARK+/− mice. In summary, exercise and ischemia increase SNARK Thr208 phosphorylation in the heart and SNARK regulates exercise- and ischemia-stimulated glucose transport. SNARK is a novel mediator of insulin-independent glucose transport in the heart.
Keywords: Akt Substrate of 160 kDa (AS160), glycogen, insulin, HL1 cardiomyocytes
Introduction
Individuals with diabetes have a significant increased risk of developing cardiovascular disease [Bwititi and Nwose, 2014] which is the major cause of mortality associated with diabetes [Morrish et al., 2001]. The precise origin for increased cardiovascular risk in patients with diabetes is unknown; however, altered cardiac glucose metabolism is thought to play an important role. Individuals with diabetes have a decreased ability to increase glucose transport in response to ischemia, which may impair post-ischemic myocardial recovery [Dutka et al., 2006; Saunders et al., 2008]. Therefore, improving the ability of cardiomyocytes to take up and utilize glucose as fuel is an important strategy for the prevention and treatment of heart disease that is associated with insulin resistance and diabetes [Bertrand et al., 2008].
In recent years the AMP-activated protein kinase (AMPK) and its upstream kinase LKB1 have been suggested to be components of a cellular signaling pathway that regulates glucose transport in the heart through an insulin-independent mechanism [Arad et al., 2007; Bravo-Nuevo A MA, 2014; Huang et al., 2013; Ikeda et al., 2009; Jessen et al.; Sakamoto et al., 2006; Xing et al., 2003]. Transgenic mice expressing an inactive AMPK catalytic subunit in the heart have an inability to augment glucose uptake during low-flow ischemia [Bravo-Nuevo A MA, 2014; Xing et al., 2003] and these hearts develop left ventricular dysfunction manifested by an early and more rapid increase in left ventricular end-diastolic pressure [Xing et al., 2003]. While there is a clear cardiac phenotype in AMPK inactive transgenic mice, knockout of LKB1 in the heart results in an even more severe phenotype [Ikeda et al., 2009; Jessen et al.; Sakamoto et al., 2006]. Knockout of heart LKB1 results in reduced heart rates [Ikeda et al., 2009], impaired cardiac function in response to ischemia [Ikeda et al., 2009], increased ventricle diameter [Ikeda et al., 2009], and cardiac hypertrophy and dysfunction [Ikeda et al., 2009; Jessen et al.; Sakamoto et al., 2006]. In addition to functioning as an upstream kinase for AMPK, LKB1 has also been determined to be an upstream kinase for at least 12 AMPK-related kinases [Sun X, 2013]. Thus, given the more pronounced phenotype of the LKB1 KO mice, it is likely that one or more AMPK-related kinases could also be important in cardiac metabolism. However, there has been very limited investigation of the metabolic function of most of the AMPK-related kinases.
The sucrose non-fermenting AMPK-related kinase (SNARK/ RK activity in skeletal muscle [Koh et al.] and knockdown of SNARK by siRNA has no effect on bNUAK2) is the fourth identified member of the AMPK family of protein kinases [Sun X, 2013]. SNARK was initially described as a mediator of the cellular response to metabolic stress [Lefebvre et al., 2001]. SNARK has 41% homology with AMPKα2 in the catalytic domain, and similar to AMPKα2, SNARK is phosphorylated by LKB1 at the characteristic T-loop phosphorylation site (Thr208) [Lizcano et al., 2004]. SNARK is activated by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) and cellular stressors in multiple cell lines [Hurov et al., 2007; Kuga et al., 2008; Lefebvre et al., 2001; Lefebvre and Rosen, 2005]. A recent study showed that SNARK regulates hepatitis C virus replication and pathogenesis through enhancement of TGF-β signaling[Goto K, 2013]. In skeletal muscle SNARK is critical for normal contraction-stimulated glucose uptake [Koh et al.]. Consistent with the concept that insulin- and contraction-stimulated glucose transport work through distinct mechanisms, insulin does not increase SNAasal and insulin-stimulated glucose transport in human primary myotubes [Rune et al., 2009]. Whole body SNARK −/− mice have a high incidence of embryonic lethality, whereas SNARK +/− mice develop mature-onset obesity and metabolic disorders [Tsuchihara et al., 2008]. Taken together, these results suggest that SNARK might play an important role in glucose metabolism.
There have been no published studies to date investigating the regulation or function of SNARK in the heart. Here, we report that SNARK is highly expressed in the heart and HL1 cardiomyocytes. Furthermore, our results demonstrate that SNARK is phosphorylated in response to ischemia and exercise and functions in the regulation of cardiac glucose transport.
Results
Exercise and ischemia increase SNARK Thr208 phosphorylation in mouse hearts.
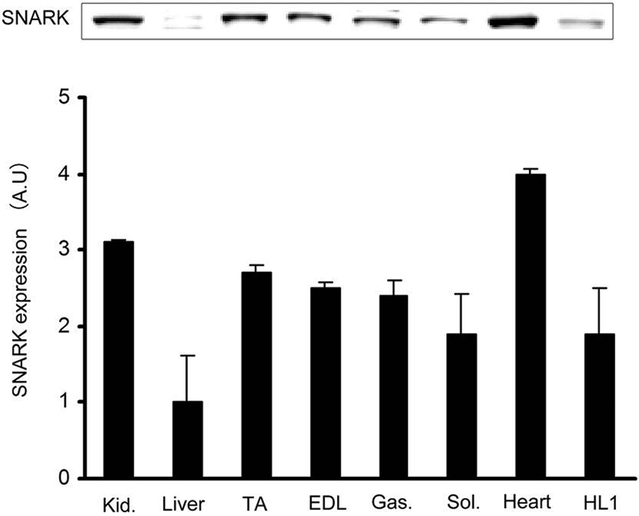
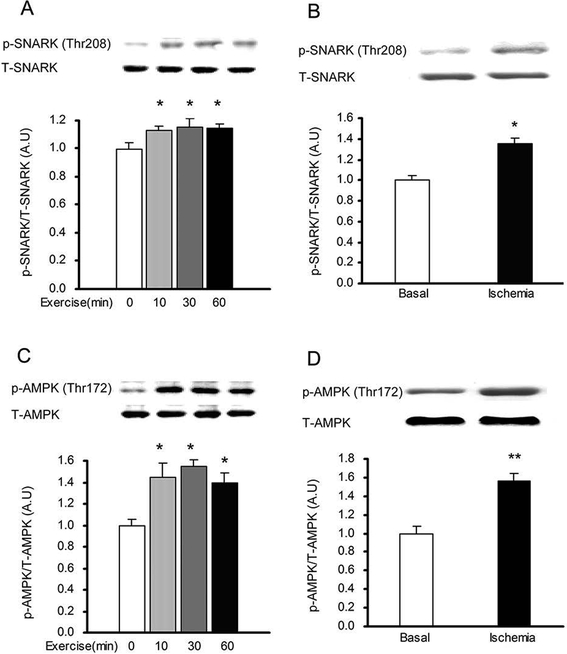
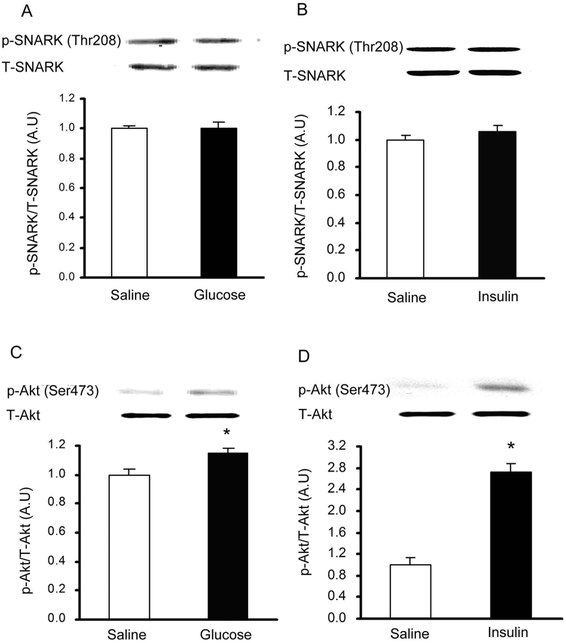
To determine if SNARK was expressed in the heart, multiple muscle types, the kidney and liver, lysates were immunoblotted with an anti-SNARK antibody. We found that SNARK was expressed in all muscles studied including heart sample, tibialis anterior muscle, extensor digitorium longus, gastrocnemius, soleus, and HL1 cardiomyocytes (Fig.1). Next, SNARK phosphorylation on Thr208 was assessed, as the T-loop phosphorylation site of the AMPK family of protein kinases has been shown to be critical for enzyme activity [Davies et al., 1995; Hawley et al., 1996; Stein et al., 2000]. To determine if treadmill exercise increases SNARK Thr208 phosphorylation, mice were exercised on a rodent treadmill at a moderate intensity (0.6 mph, 12.5% grade) for 10, 30, and 60 min. SNARK Thr208 phosphorylation was slightly, but significantly elevated at all three time points (Fig.2A). Global ischemia resulted in a marked increase in SNARK Thr208 phosphorylation (Fig. 2B). Consistent with previous reports, AMPK Thr172 phosphorylation was also increased by both exercise (Fig. 2C) and ischemia (Fig. 2D). In contrast, neither i.p. injection of 1 g glucose/kg bw, which resulted in a submaximal insulin concentration, nor maximal insulin (1 U/kg bw, i.p.) altered SNARK Thr208 phosphorylation (Fig. 3A,B), while Akt Ser473 phosphorylation was significantly increased by insulin (Fig. 3C,D). The stimulation of SNARK phosphorylation by stress stimuli, but not insulin, is consistent with the hypothesis that there are distinct molecular signaling mechanisms regulating the effects of exercise/ischemia and insulin on metabolism.
Fig.1.
SNARK expression in different mouse tissues and HL1 cardiomyocytes. Relative SNARK protein expression in various mouse tissues (Kid, kidney; TA, tibialis anterior muscle; EDL, extensor digitorium longus; Gas, gastrocnemius; Sol, soleus;) and HL1 cardiomyocytes. Data are means ± SEM, n=4.
Fig.2.
SNARK Thr208 phosphorylation was increased by treadmill exercise and ischemia in mouse heart. (A) Mice performed treadmill exercise (10 min, 30 min, and 60 min, at 0.6mph, 12.5% grade). SNARK Thr208 phosphorylation was measured by Western blot. (B) Mice were stimulated with global ischemia. SNARK phosphorylation at Thr208 site was determined by Western blot. (C) Mice performed treadmill exercise (10 min, 30 min, and 60 min, at 0.6mph, 12.5% grade). APMK Thr 172 phosphorylation was measured by Western blot. (D) Mice were stimulated with global ischemia. APMK Thr 172 phosphorylation was determined by Western blot. Data are means ± SEM, n = 6/group. *P < 0.05 and **P< 0.01 vs. corresponding control or basal.
Fig.3.
SNARK Thr208 phosphorylation was not altered by submaximal or maximal insulin in mouse heart. (A) Mice were treated with submaximal insulin (induced by i.p. injection of 1 g glucose/kg bw) or saline. SNARK phosphorylation was determined by Western blot. (B) Mice were injected with maximal insulin (1 U/kg bw, i.p.). SNARK phosphorylation was measured by Western blot. (C) Mice were treated with submaximal insulin (induced by i.p. injection of 1 g glucose/kg bw) or saline. Akt phosphorylation was determined by Western blot. (D) Mice were injected with maximal insulin (1 U/kg bw, i.p.), Akt phosphorylation was measured by Western blot. Data are means ± SEM, n = 6/group. *P < 0.05 vs. corresponding saline.
Overexpression of SNARK increases basal glucose transport in HL1 cardiomyocytes
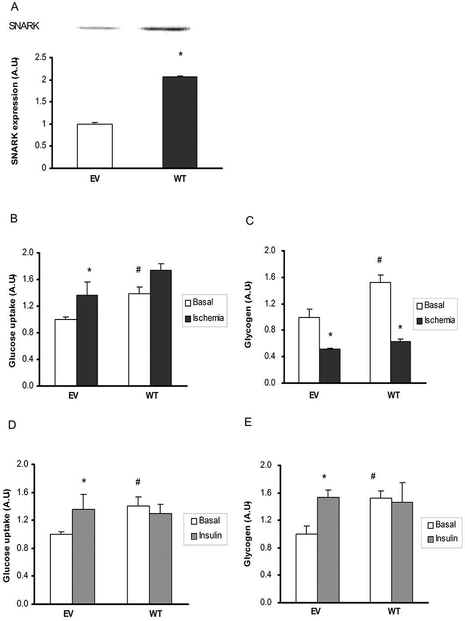
To determine the effects of SNARK overexpression on glucose transport, empty vector (EV) and wild type (WT) SNARK were transfected into HL1 cardiomyocytes and studied in the basal state and in response to ischemia and insulin. Compared with EV, SNARK expression was increased by 2-fold in WT transfected cells (Fig. 4A). Overexpression of WT SNARK resulted in a significant increase in glucose transport under basal conditions, whereas there was no further effect on ischemia-stimulated glucose transport (Fig. 4B). Consistent with increased basal glucose transport, overexpression of WT SNARK markedly increased basal glycogen content (Fig. 4C). Ischemia significantly decreased glycogen concentrations in both EV and WT transfected cells.
Fig.4.
Effects of SNARK overexpression on glucose transport and glycogen concentrations in response to ischemia and insulin. (A) 48 hours after transfection, cells were harvested and lysates were resolved by SDS/PAGE and immunoblotted with anti-SNARK (B and C). The overexpressed cells were stimulated simulated with simulated ischemia by acidic N2 gassed buffer for 45 min. Then glucose uptake (B) and glycogen concentrations (C) were assessed. (D and E) The overexpressed cells were treated with 100nM insulin for 20 min. Then the cells were harvested to assess glucose uptake (D) and glycogen concentrations (E). Data are means ± SEM, n = 4. *P < 0.05 vs. corresponding control or basal; #P<0.05 vs. EV of the basal state.
To determine if SNARK regulates insulin-stimulated glucose transport in cardiomyocytes, HL1 cells overexpressing SNARK were incubated with insulin (100 nM) for 20 min. Similar to the ischemia study, basal glucose transport was significantly increased with WT SNARK overexpression. Likely due to the elevated basal rates of glucose transport in the WT SNARK expressing cells, insulin did not further increase glucose transport. However, insulin-stimulated glucose transport was similar between EV and WT SNARK expressing cells (Fig. 4D). Consistent with these findings, insulin significantly increased glycogen concentrations in EV cells (Fig. 4E), whereas overexpression of WT SNARK increased basal glycogen, but had no additional effect on insulin-stimulated glycogen concentration (Fig. 4E).
In cardiomyocytes, GLUT1 and GLUT4 regulate basal and stimulated glucose transport [Segalen et al., 2008; Shuralyova et al., 2004], and signaling proteins that have been implicated in glucose transport in heart and/or skeletal muscle include AMPK, Akt, and the Rab-GAP protein AS160 [Kramer et al., 2006; Montessuit et al., 2008]. Since we found that overexpression of WT SNARK increased basal rates of glucose transport, we determined if overexpressing WT SNARK affected these signaling molecules, as well as the expression of GLUT1 and GLUT4. For the signaling studies, we measured AMPK phosphorylation on Thr172, the major LKB1 site, ACC phosphorylation on Ser79, the major AMPK site, and AS160 phosphorylation on Ser711, a site we have shown to be regulated by both AMPK and insulin [Treebak et al., 2010]. The basal levels of phosphorylation of AMPK Thr172, ACC Ser79, and AS160 Ser711 were not different between control and WT SNARK overexpressing cells. Ischemia significantly increased phosphorylation AMPK Thr172, ACC Ser79, and AS160 Ser711, and there was no difference between the EV control and WT overexpressing cells (Fig. S1D). Insulin similarly increased phosphorylation of Akt Ser473 and Thr308, and AS160 Ser711 (Fig. S1E). There was no effect of overexpressing wild type SNARK on the expression of AS160, Hexokinase II, GLUT1 and GLUT4 (Fig. S1D, E). Therefore, changes in these signaling proteins and glucose transporter proteins are not the mechanism for increased basal rates of glucose transport in HL1 cells overexpressing SNARK.
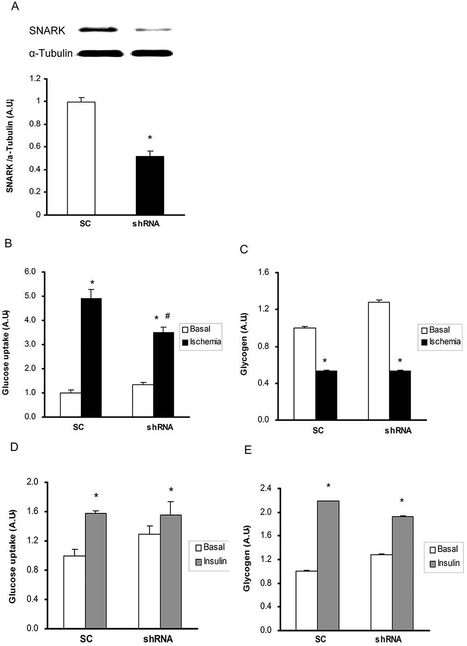
Knockdown of SNARK impairs ischemia, but not insulin-stimulated glucose transport in HL1 cardiomyocytes
We next determined if knockdown of SNARK would alter glucose transport in cardiomyocytes. HL1 cardiomyocytes were infected with retrovirus containing shRNA for SNARK (shRNA) and cells infected with scrambled shRNA were used as controls (SC). SNARK protein expression was decreased by 50% in the shRNA group compared with the SC control cells (Fig. 5A). Ischemia increased glucose transport in control cells and this increase was significantly impaired with knockdown of SNARK (Fig. 5B). There were no differences in glycogen concentrations between control and shRNA cells (Fig. 5C). In contrast to ischemia, insulin-stimulated glucose transport was normal in shRNA cells (Fig. 5D), consistent with previous data showing that SNARK did not affect insulin-stimulated glucose transport in skeletal muscle and C2C12 muscle cells [Koh et al.], as well as human primary myotubes [Rune et al., 2009]. There was also no effect of SNARK knockdown on insulin-stimulated glycogen concentrations (Fig. 5E). These data demonstrate that knockdown of SNARK specifically regulates ischemia-, but not insulin-stimulated glucose transport.
Fig.5.
Effects of SNARK inhibition on glucose transport and glycogen concentrations in response to ischemia and insulin. Retroviruses containing scrambled shRNA (SC) and shRNA for SNARK (shRNA) were infected in HL1 cells. (A) SNARK expression was determined by immunoblot analysis (B and C). The overexpressed cells were stimulated with simulated ischemia by acidic N2 gassed buffer for 45 min. Glucose transport (B) and glycogen concentrations (C) were determined by 2-DG glucose transport measurement and Western blot analysis, respectively (D and E). Cells were treated with insulin (100nM) for 20 min. Glucose transport (D) and glycogen concentrations (E) were measured. Data are means ± SEM, n = 5–7. *P < 0.05 vs. corresponding control or basal; #P<0.05 vs. SC of the ischemia state.
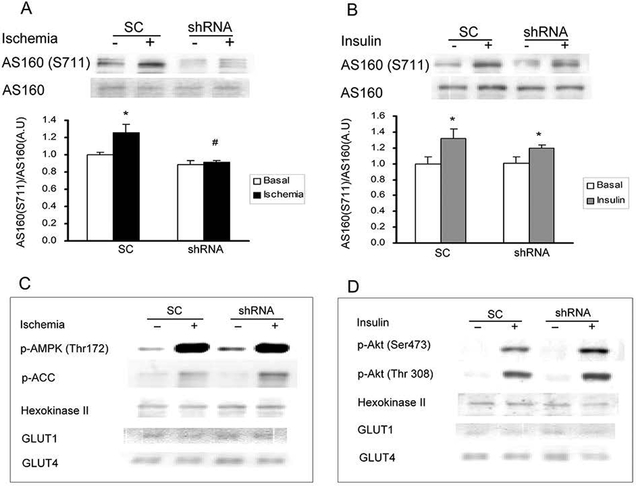
Consistent with glucose transport, ischemia-stimulated AS160 Ser711 phosphorylation was decreased (Fig. 6A), but insulin-stimulated AS160 Ser711 phosphorylation was not significantly changed in SNARK knockdown cells (Fig. 6B). Ischemia significantly increased AMPK Thr172 and ACC Ser79 phosphorylation in the HL1 cardiomyocytes, and there was no difference between the control and shRNA cells (Fig. 6C). Knockdown of SNARK with shRNA did not alter basal and insulin-stimulated Akt Ser473 and Thr308 phosphorylation (Fig. 6D). SNARK knockdown had no effect on Hexokinase II, GLUT1 and GLUT4 protein expression, and short term ischemia and insulin stimulation also had no effect on the expression of these proteins. (Fig. 6C, D).
Fig.6.
Effects of SNARK inhibition on related protein expression in response to ischemia and insulin. Retroviruses containing scrambled shRNA (SC) and shRNA for SNARK (shRNA) were infected in HL1 cells. 48 hours later, the infected cells were stimulated with simulated ischemia by acidic N2 gassed buffer for 45 min or treated with 100nM insulin for 20 min. (A)Ischemia-stimulated AS160 and AS160 S711 phosphorylation were determined by western blot. (B) Insulin-stimulated AS160 and AS160 S711 phosphorylation were measured by Western blot. (C) Ischemia-stimulated AMPK phosphorylation, phosphor-ACC, hexokinase II, GLUT1 and GLUT4 were determined by Western blot. (D) Insulin-stimulated phosphor-Akt (S473 and Thr308), hexokinase II, GLUT1 and GLUT4 were measured by Western blot. Data are means ± SEM, n = 5–7.
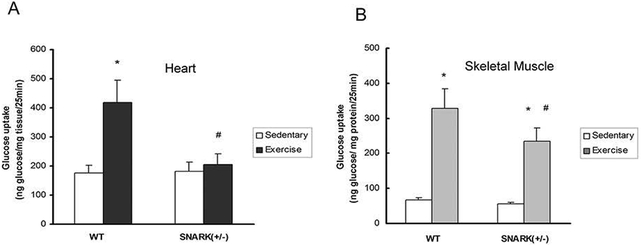
Exercise-stimulated glucose transport is impaired in SNARK heterozygotic knockout (+/−) mice
Because SNARK homozygotic knockout mice are embryonic lethal or develop severe abnormalities, we used SNARK heterozygotic knockout (+/−) mice [Tsuchihara et al., 2008] to study the effects of SNARK on exercise-stimulated glucose transport in the heart. Rates of glucose transport in hearts from the sedentary mice were similar between the two genotypes (Fig. 7A). In contrast, 30 min of exercise significantly increased glucose transport in the hearts of wild type mice, while this increase in glucose transport was completely blunted in SNARK (+/−) mice (Fig. 7A). This blunted glucose transport can not be explained by differences in heart weight (Fig. S1A) or the protein expression of GLUT1 and GLUT4 (Fig. S1B, C), the major glucose transporters expressed in the heart in vivo. In addition, consistent with our previous observations with muscle contraction [Koh et al.], exercise-stimulated glucose transport in gastrocnemius muscle was significantly impaired in SNARK (+/−) mice (Fig. 7B). Thus, similar to the studies of ischemia in HL1 cardiomyocytes, SNARK functions in the regulation of cardiac glucose transport in vivo.
Fig.7.
Exercise-stimulated glucose transport was impaired in SNARK (+/−) mouse heart. SNARK (+/−) mice and control littermates performed 30 min treadmill exercise (0.6 mph, 12.5% grade). Hearts (A) and Gastrocnemius (B) were dissected and glucose transport was measured. Data are means ± SEM, n = 5–7. *P < 0.05 vs. corresponding sedentary control; #P<0.05 vs. WT of the exercise state.
Discussion
Despite the importance of cardiac glucose metabolism in the prevention and recovery from heart disease, there are still many questions regarding the mechanisms that regulate glucose uptake in the heart. SNARK has been shown to play an important role in the regulation of glucose uptake in skeletal muscle [Koh et al.], and some studies have suggested that SNARK may regulate whole body metabolic homeostasis [Bravo-Nuevo A MA, 2014; Tsuchihara et al., 2008]. Accordingly, the current study was designed to determine whether SNARK is part of the mechanism that regulates glucose transport in the heart. Our results show that SNARK is expressed in mouse hearts and HL1 cardiomyocytes, and that treadmill exercise and ischemia, stimuli that increase glucose transport in the heart, also increase SNARK phosphorylation at the Thr208 site that controls SNARK enzymatic activity. Moreover, using cells with knockdown of SNARK and SNARK (+/−) mice, we find that SNARK regulates ischemia- and exercise-stimulated glucose transport. In contrast to the experiments with exercise and ischemia, SNARK did not alter insulin-stimulated glucose transport. This is consistent with previous data demonstrating that there are distinct proximal signals leading to glucose transport by insulin and exercise in skeletal muscle and heart [Goodyear et al., 1995; Goodyear and Kahn, 1998; Hayashi et al., 1998; Lee et al., 1995; Lund et al., 1998; Yeh et al., 1995]. These data implicate SNARK as an important signaling protein for insulin-independent glucose transport in the mouse heart.
Previous studies have indicated that skeletal muscle SNARK may function in the regulation of whole body metabolism [Bravo-Nuevo A MA, 2014; Tsuchihara et al., 2008]. Treatment with azoxymethane results in increased rate of colon carcinogenesis due to increased body weights, increased fat mass, hyperlipidemia and hyperglycemia associated with the loss of one allele of SNARK gene [Tsuchihara et al., 2008]. More recently, the role of SNARK in metabolism was investigated by a two month treatment of KK.Cg-AylJ mice (a model of type 2 diabetes) with the sorbitol derivative meglumine. Meglumine-treated mice had increased skeletal muscle SNARK expression, lower body weights and improved glucose tolerance, consistent with a beneficial metabolic effect of increased SNARK expression in skeletal muscle. However, it is not possible to determine if these metabolic effects were due to a decrease in food consumption since these data were not reported. In the current study we used 20-week old SNARK (+/−) mice, a time point where there were no differences in body weights, blood glucose concentrations and voluntary exercise capacity [Koh et al.]. Thus, whether alterations in SNARK expression in skeletal muscle and heart can affect systemic glucose metabolism is still under debate.
In the current study we found that treadmill exercise-stimulated glucose transport was completely ablated in the hearts of SNARK (+/−) mice, an effect that was not due to inability to complete the exercise protocol, changes in heart or body weights, or glucose transporter expression in the mouse hearts. While exercise-stimulated glucose transport was fully blunted in the hearts of SNARK (+/−) mice, interestingly, treadmill exercise-stimulated glucose transport was only partially blunted in the skeletal muscle of these same animals. Consistent with these findings, our previous study indicated that tetanic contraction-stimulated glucose transport of isolated skeletal muscles of SNARK (+/−) mice was only partially inhibited. The full inhibition of glucose transport in the heart suggests that SNARK may play a more important role in the regulation of glucose transport in the heart compared to skeletal muscle, which is consistent with its greater level of expression in cardiac muscle (Fig. 1).
The mechanism by which SNARK regulates glucose transport in the heart is not known, but the current data suggest that this does not involve alterations in the expression of GLUT1 and GLUT4, the major glucose transporter proteins expressed in the heart. This conclusion is based on our data showing that knockdown of SNARK in both cardiomycytes and SNARK (+/−) hearts, as well as overexpression of WT SNARK in cardiomyocytes, had no effect on total GLUT1 and GLUT4 expression. Using cardiomyoctes, we found that ischemia-stimulated glucose uptake was significantly impaired with SNARK knockdown, an effect that was associated with a decrease in AS160 Ser711 phosphorylation. AS160 is a Rab-GAP protein that is a critical regulator of glucose transport in skeletal muscle and fat [Huang et al., 2013; Li et al., 2013; Treebak et al., 2010]. Currently, the function of AS160 in the heart and the specific function of the Ser711 phosphorylation site in skeletal muscle are still not known [Middelbeek et al., 2013]. Nevertheless, altered function of AS160 is a potential mechanism for impaired glucose uptake with knockdown of SNARK, and the specific role of AS160 in SNARK-regulated glucose transport in the heart will be an important line of future investigation.
In summary, we provide the first direct evidence that SNARK plays a critical role in exercise- and ischemia-stimulated glucose metabolism in mouse heart. Determining the function of SNARK, a previously unexplored metabolic regulator in cardiac muscle, may lead to improved ways to prevent and treat cardiovascular disease.
Materials and Methods
Animals
Male ICR mice 8–12 weeks old (25–35 g) were purchased from Charles River Laboratories (Wilmington, MA). SNARK (+/−) mice have been previously described [Ichinoseki-Sekine et al., 2009]. All mice were housed with a 12h: 12h light: dark cycle and fed standard laboratory chow and water ad libitum. The mice were fasted 5 hours (8:00 to 13:00) prior to study. All animal studies were approved by the Institutional Animal Care and Use Committee of the Joslin Diabetes Center and were in accordance with NIH guidelines.
Male ICR mice were exercised on a treadmill for 0, 10, 30, or 60 min at 0.6 mph up a 12.5% grade. Immediately after exercise, mice were sacrificed and hearts were rapidly removed and quickly frozen in liquid nitrogen. Ischemia stimulation was achieved as described previously [Jessen et al.]. Briefly, animals were anaesthetized with an intraperitoneal injection of pentobarbital (90 mg/kg). Hearts were either freeze-clamped in situ at baseline (basal) or 3 min after cervical dislocation. To study responses to submaximal and maximal insulin, mice were anaesthetized with pentobarbital as described above. Mice were injected i.p. with either: a 20% glucose solution (1.0 g /kg body weight) which results in a submaximal physiological insulin release [Kramer et al., 2006]; a maximal dose of insulin (1 U/kg body weight); or saline as control. Mice were studied 10 min following injection.
Measurement of Glucose Transport in Mouse Hearts in vivo
Glucose transport was measured in mouse hearts as described previously [Kramer et al., 2006; Taylor et al., 2008]. Briefly, mice were fasted from 8:00 to 13:00 and performed treadmill exercise (0.6 mph, 12.5% grade) for 30 min or remained sedentary. Mice were immediately anesthetized with an i.p. injection of pentobarbital sodium (90 mg/kg) and then injected with a 5 ml/kg saline bolus containing 333 μCi/kg [3H]-2-deoxyglucose via the retro-orbital sinus. Basal blood samples were collected from the tail vein prior to retro-orbital injection. Additional blood samples were collected from the tail vein at 5, 10, 15, and 25 min post-injection to determine blood glucose concentrations and [3H]-2-deoxyglucose specific activity. After the final blood draw, the animals were euthanized and the heart and gastrocnemius were removed and frozen in liquid nitrogen. Glucose transport ([3H]-2-deoxyglucose) was determined via a precipitation protocol as previously described [Ferre et al., 1985].
Cell Culture
HL1 cardiomyocytes were generously donated by Dr. William Claycomb (Louisiana State University, New Orleans, LA). These cells are a cardiac muscle cell line derived from an AT-1 mouse atrial cardiomyocyte tumor line, which has phenotypic characteristics of the adult cardiomyocyte [Claycomb et al., 1998]. HL1 cells were maintained in Claycomb medium supplemented with 10% FBS, 0.1 mM Norepinephrine, 2 mM L-Glutamine and 100 U/100μg/ml Penicillin/Streptomycin in a humidified atmosphere containing 5% CO2 at 37℃. To overexpress SNARK in the HL1 cells, human SNARK cDNA was synthesized using the human SNARK sequence and then subcloned into the pcDNA3.1 vector (Invitrogen, USA). Twenty four hours after plating HL1 cells (1×105 cells/well) on 6-well plates, the cells were transfected with empty vector (EV) or wild type SNARK (WT) using lipofectamine (Invitrogen, USA) according to the manufacturer’s instructions. Following a16-hour incubation, transfection medium was replaced with supplemented Claycomb medium. For knockdown of SNARK, shRNA constructs against mouse musculus SNARK in pGFP-V-RS vector were used. Retroviruses containing shRNA for SNARK (shRNA) or scrambled control (SC) were infected in HL1 cardiomyocytes. Following a 16-hour incubation, the retroviruses were replaced with supplemented Claycomb medium.
Forty eight hours after overexpression or knockdown of SNARK, HL1 cells were stimulated with ischemia or insulin. Cells were starved for 3 hours before experiments. Ischemia was simulated using an acidic N2 gassed buffer (pH 6.2) containing (in mM): 118 NaCl, 24 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, 1.2 MgCl2, 20 sodium lactate, 16 KCl and 10 2-deoxyglucose [Das et al., 2006] and the cells were kept under nitrogen gas for 45 min. Insulin treatment was performed by incubation of cells with or without 100 nM insulin in Claycomb medium for 20 min. Glucose transport, glycogen concentrations, and protein expression were measured in these cells.
To measure glucose transport, cells were washed with buffer containing 140 mM NaCl, 20mM HEPES-Na, pH 7.4, 5 mM KCl, 2.5 mM MgSO4, and 1.0 mM CaCl2, followed by the addition of [3H]-2-deoxyglucose for 5 min and then place on ice. Cells were washed with cold saline and harvested in 0.05 N NaOH to determine the net accumulation of [3H]-2-deoxyglucose. Glycogen was measured as previously described [Jessen et al.]. HL1 cells were hydrolyzed in 2 N HCl at 95 ℃ for 2 hours and then neutralized with 2 N NaOH. Glucose content was measured by a hexokinase method using a glucose HK reagent (Eagle Diagnostics, Desoto, Texas, USA).
Western Blot Analysis
Whole hearts were homogenized as previously described [Xing et al., 2003]. Briefly, the frozen hearts were homogenized with a Polytron (Brinkman Instruments) in lysis buffer, lysates were centrifuged at 13,000 × g for 10 min, supernatants were collected, and the protein concentrations were determined by the Bradford assay. The lysates were separated by SDS-PAGE, immunoblotted with primary and secondary antibodies, and immunoblots were developed using ECL reagents (PerkinElmer, USA). The protein bands were scanned by ImageScanner (Amersham Biosciences) and quantitated by densitometry (Fluorchem, 2.0; Alpha Innotech, San Leandro, CA). Antibodies purchased from commercial sources included: p-ACC-Ser79 and AMPK α2 from Upstate; p-AMPK-Thr172, p-Akt-Thr308, p-Akt-Ser473, Phospho (Ser/Thr) Akt substrate (PAS) from Cell Signaling Technology; AS160 from Millipore; Hexokinase II and anti-α-Tubulin from Santa Cruz Biotechnology; and GLUT1 and GLUT4 from Chemicon International. Anti-SNARK and phospho-SNARK-Thr208, and p-AS160-Ser711antibodies were generated by Cell Signaling Technology. Horseradish peroxidase-conjugated anti-rabbit and anti-goat antibodies (Amersham Biosciences) were used to bind and detect all primary antibodies.
Statistical Analysis
Data were expressed as the means ± SEM. Means were compared by Student’s t-test, one-way analysis of variance (ANOVA), or two-way ANOVA. P values < 0.05 were considered statistically significant.
Supplementary Material
Fig.S1. (A) Heart/Body weight was measured in wild type and SNARK (+/−) mice. GLUT1 (B) and GLUT4 (C) protein expression were determined by Western blot. Data are means ± SEM, n = 5-7. (D) 48 hours later, the overexpressed cells were stimulated with simulated ischemia by acidic N2 gassed buffer for 45 min. Ischemia-stimulated AMPK phosphorylation, phosphor-ACC, PAS, AS160 S711 phosphorylation, AS160, hexokinase II, GLUT1 and GLUT4 were determined by Western blot. (E) The overexpressed cells were treated with 100nM insulin for 20 min. Insulin-stimulated phospho-Akt (S473 and Thr308), PAS, AS160 S711 phosphorylation, AS160, hexokinase II, GLUT1 and GLUT4 were measured by Western blot. Data are means ± SEM, n = 4.
Acknowledgements
We greatly appreciate the gift of the HL-1 cardiomyocyte cell line from Dr. William C. Claycomb (Louisiana State University, New Orleans, LA), and Dr. J. Xie (Cell Signaling Technology, Danvers, MA) for generation of the SNARK AS160 antibodies. This work was supported by National Institutes of Health Grants R01 DK068626 and DK101043 (to L.J.G.) and the Joslin Diabetes Center DRC (P30 DK36836). X.L.S. was supported by grants from the China Scholarship Council; S.J.L. was supported by an American Physiological Society Fellowship in Physiological Genomics; D.A. was supported by fellowships from the Canadian Institute for Health Research MFE-83802 and Canadian Diabetes Association Incentive Award PF-3–07–2255-DA; H.J.K. was supported by NIDDK/Harvard Clinical Nutritional Research Center P30-DK040561.
Funding
This work was supported by National Institutes of Health Grants R01 DK068626 and DK101043 (to L.J.G.) and the Joslin Diabetes Center DRC (P30 DK36836). X.L.S. was supported by grants from the China Scholarship Council; S.J.L. was supported by an American Physiological Society Fellowship in Physiological Genomics; D.A. was supported by fellowships from the Canadian Institute for Health Research MFE-83802 and Canadian Diabetes Association Incentive Award PF-3–07–2255-DA; H.J.K. was supported by NIDDK/Harvard Clinical Nutritional Research Center P30-DK040561.
Footnotes
Conflicts of Interest
All authors declare that they have no any conflict of interests.
References
- Arad M, Seidman CE, Seidman JG. 2007. AMP-activated protein kinase in the heart: role during health and disease. Circ Res 100:474–488. [DOI] [PubMed] [Google Scholar]
- Bertrand L, Horman S, Beauloye C, Vanoverschelde JL. 2008. Insulin signalling in the heart. Cardiovasc Res 79:238–248. [DOI] [PubMed] [Google Scholar]
- Bravo-Nuevo A MA HM, Kappler F, Mulgrew J, Laury-Kleintop L, Reichman M, Tobia A, Prendergast GC. 2014. Meglumine exerts protective effects against features of metabolic syndrome and type II diabetes. PLoS One February 27:9(2):e90031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwititi PT, Nwose EU. 2014. Screening of cardiovascular disease risk in diabetes: questions concerning prediabetes and low-mid income countries. N Am J Med Sci 6:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb WC, Lanson NA Jr., Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ Jr. 1998. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A 95:2979–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Smolenski A, Lohmann SM, Kukreja RC. 2006. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem 281:38644–38652. [DOI] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PT, Hardie DG. 1995. 5’-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett 377:421–425. [DOI] [PubMed] [Google Scholar]
- Dutka DP, Pitt M, Pagano D, Mongillo M, Gathercole D, Bonser RS, Camici PG. 2006. Myocardial glucose transport and utilization in patients with type 2 diabetes mellitus, left ventricular dysfunction, and coronary artery disease. J Am Coll Cardiol 48:2225–2231. [DOI] [PubMed] [Google Scholar]
- Ferre P, Leturque A, Burnol AF, Penicaud L, Girard J. 1985. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J 228:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear LJ, Giorgino F, Balon TW, Condorelli G, Smith RJ. 1995. Effects of contractile activity on tyrosine phosphoproteins and PI 3-kinase activity in rat skeletal muscle. Am J Physiol 268:E987–995. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Kahn BB. 1998. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49:235–261. [DOI] [PubMed] [Google Scholar]
- Goto K LW, Zhang L, Jilg N, Shao RX, Schaefer EA, Zhao H, Fusco DN, Peng LF, Kato N, Chung RT. 2013. The AMPK-related kinase SNARK regulates hepatitis C virus replication and pathogenesis through enhancement of TGF-β signaling. J Hepatol. November;59(5):942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. 1996. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271:27879–27887. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Morimoto M, Iwata H, Onodera T. 1998. Interferon-gamma plays a role in pancreatic islet-cell destruction of reovirus type 2-induced diabetes-like syndrome in DBA/1 suckling mice. Int J Exp Pathol 79:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Buckler-Pena D, Nauta T, Singh M, Asmar A, Shi J, Kim JY, Kandror KV. 2013. Insulin responsiveness of glucose transporter 4 in 3T3-L1 cells depends on the presence of sortilin. Mol Biol Cell 24:3115–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurov JB, Huang M, White LS, Lennerz J, Choi CS, Cho YR, Kim HJ, Prior JL, Piwnica-Worms D, Cantley LC, Kim JK, Shulman GI, Piwnica-Worms H. 2007. Loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity in vivo. Proc Natl Acad Sci U S A 104:5680–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinoseki-Sekine N, Naito H, Tsuchihara K, Kobayashi I, Ogura Y, Kakigi R, Kurosaka M, Fujioka R, Esumi H. 2009. Provision of a voluntary exercise environment enhances running activity and prevents obesity in Snark-deficient mice. Am J Physiol Endocrinol Metab 296:E1013–1021. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR, Walsh K. 2009. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J Biol Chem 284:35839–35849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen N, Koh HJ, Folmes CD, Wagg C, Fujii N, Lofgren B, Wolf CM, Berul CI, Hirshman MF, Lopaschuk GD, Goodyear LJ. Ablation of LKB1 in the heart leads to energy deprivation and impaired cardiac function. Biochim Biophys Acta 1802:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HJ, Toyoda T, Fujii N, Jung MM, Rathod A, Middelbeek RJ, Lessard SJ, Treebak JT, Tsuchihara K, Esumi H, Richter EA, Wojtaszewski JF, Hirshman MF, Goodyear LJ, Year, 2010. Sucrose nonfermenting AMPK-related kinase (SNARK) mediates contraction-stimulated glucose transport in mouse skeletal muscle. Proc Natl Acad Sci U S A 107:15541–15546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. 2006. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281:31478–31485. [DOI] [PubMed] [Google Scholar]
- Kuga W, Tsuchihara K, Ogura T, Kanehara S, Saito M, Suzuki A, Esumi H. 2008. Nuclear localization of SNARK; its impact on gene expression. Biochem Biophys Res Commun 377:1062–1066. [DOI] [PubMed] [Google Scholar]
- Lee AD, Hansen PA, Holloszy JO. 1995. Wortmannin inhibits insulin-stimulated but not contraction-stimulated glucose transport activity in skeletal muscle. FEBS Lett 361:51–54. [DOI] [PubMed] [Google Scholar]
- Lefebvre DL, Bai Y, Shahmolky N, Sharma M, Poon R, Drucker DJ, Rosen CF. 2001. Identification and characterization of a novel sucrose-non-fermenting protein kinase/AMP-activated protein kinase-related protein kinase, SNARK. Biochem J 355:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre DL, Rosen CF. 2005. Regulation of SNARK activity in response to cellular stresses. Biochim Biophys Acta 1724:71–85. [DOI] [PubMed] [Google Scholar]
- Li L, Luo Z, Yu H, Feng X, Wang P, Chen J, Pu Y, Zhao Y, He H, Zhong J, Liu D, Zhu Z. 2013. Telmisartan improves insulin resistance of skeletal muscle through peroxisome proliferator-activated receptor-delta activation. Diabetes 62:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. 2004. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 23:833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Pryor PR, Ostergaard S, Schmitz O, Pedersen O, Holman GD. 1998. Evidence against protein kinase B as a mediator of contraction-induced glucose transport and GLUT4 translocation in rat skeletal muscle. FEBS Lett 425:472–474. [DOI] [PubMed] [Google Scholar]
- Middelbeek RJ, Chambers MA, Tantiwong P, Treebak JT, An D, Hirshman MF, Musi N, Goodyear LJ. 2013. Insulin stimulation regulates AS160 and TBC1D1 phosphorylation sites in human skeletal muscle. Nutr Diabetes 3:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montessuit C, Papageorgiou I, Lerch R. 2008. Nuclear receptor agonists improve insulin responsiveness in cultured cardiomyocytes through enhanced signaling and preserved cytoskeletal architecture. Endocrinology 149:1064–1074. [DOI] [PubMed] [Google Scholar]
- Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. 2001. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 44 Suppl 2:S14–21. [DOI] [PubMed] [Google Scholar]
- Rune A, Osler ME, Fritz T, Zierath JR. 2009. Regulation of skeletal muscle sucrose, non-fermenting 1/AMP-activated protein kinase-related kinase (SNARK) by metabolic stress and diabetes. Diabetologia 52:2182–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, Bertrand L. 2006. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab 290:E780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J, Mathewkutty S, Drazner MH, McGuire DK. 2008. Cardiomyopathy in type 2 diabetes: update on pathophysiological mechanisms. Herz 33:184–190. [DOI] [PubMed] [Google Scholar]
- Segalen C, Longnus SL, Baetz D, Counillon L, Van Obberghen E. 2008. 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside reduces glucose uptake via the inhibition of Na+/H+ exchanger 1 in isolated rat ventricular cardiomyocytes. Endocrinology 149:1490–1498. [DOI] [PubMed] [Google Scholar]
- Shuralyova I, Tajmir P, Bilan PJ, Sweeney G, Coe IR. 2004. Inhibition of glucose uptake in murine cardiomyocyte cell line HL-1 by cardioprotective drugs dilazep and dipyridamole. Am J Physiol Heart Circ Physiol 286:H627–632. [DOI] [PubMed] [Google Scholar]
- Stein SC, Woods A, Jones NA, Davison MD, Carling D. 2000. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 345 Pt 3:437–443. [PMC free article] [PubMed] [Google Scholar]
- Sun X GL, Chien HY, Li WC, Zhao J. 2013. The regulation and function of the NUAK family. J Mol Endocrinol. September 10:51(2):R15–22. . [DOI] [PubMed] [Google Scholar]
- Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. 2008. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283:9787–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treebak JT, Taylor EB, Witczak CA, An D, Toyoda T, Koh HJ, Xie J, Feener EP, Wojtaszewski JF, Hirshman MF, Goodyear LJ. 2010. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am J Physiol Cell Physiol 298:C377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihara K, Ogura T, Fujioka R, Fujii S, Kuga W, Saito M, Ochiya T, Ochiai A, Esumi H. 2008. Susceptibility of Snark-deficient mice to azoxymethane-induced colorectal tumorigenesis and the formation of aberrant crypt foci. Cancer Sci 99:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. 2003. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem 278:28372–28377. [DOI] [PubMed] [Google Scholar]
- Yeh JI, Gulve EA, Rameh L, Birnbaum MJ. 1995. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem 270:2107–2111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig.S1. (A) Heart/Body weight was measured in wild type and SNARK (+/−) mice. GLUT1 (B) and GLUT4 (C) protein expression were determined by Western blot. Data are means ± SEM, n = 5-7. (D) 48 hours later, the overexpressed cells were stimulated with simulated ischemia by acidic N2 gassed buffer for 45 min. Ischemia-stimulated AMPK phosphorylation, phosphor-ACC, PAS, AS160 S711 phosphorylation, AS160, hexokinase II, GLUT1 and GLUT4 were determined by Western blot. (E) The overexpressed cells were treated with 100nM insulin for 20 min. Insulin-stimulated phospho-Akt (S473 and Thr308), PAS, AS160 S711 phosphorylation, AS160, hexokinase II, GLUT1 and GLUT4 were measured by Western blot. Data are means ± SEM, n = 4.