Abstract
Background
Frequent whole blood donations increase the prevalence of iron depletion in blood donors, which may subsequently interfere with normal erythropoiesis. The purpose of this study was to evaluate the associations between donation frequency and red blood cell (RBC) storage stability in a racial-ethnically diverse population of blood donors.
Study design
Leukocyte-reduced RBC concentrate-derived samples from 13,403 donors were stored for 39 to 42 days (1–6°C) and then evaluated for storage, osmotic, and oxidative hemolysis. Iron status was evaluated by plasma ferritin measurement and self-reported intake of iron supplements. Donation history in the prior 2 years was obtained for each subject.
Results
Frequent blood donors enrolled in this study were likely to be white, male, and of older age (56.1±5.0 years). Prior donation intensity was negatively associated with oxidative hemolysis (p<0.0001) in multivariate analyses correcting for age, sex and race-ethnicity. Increased plasma ferritin concentration was associated with increased RBC susceptibility to each of the three measures of hemolysis (p<0.0001 for all), whereas self-reported iron intake was associated with reduced susceptibility to osmotic and oxidative hemolysis (p<0.0001 for both).
Conclusions
Frequent blood donations may alter the quality of blood components by modulating RBC predisposition to hemolysis. RBCs collected from frequent donors with low ferritin have altered susceptibility to hemolysis. Thus, frequent donation and associated iron loss may alter the quality of stored RBC components collected from iron deficient donors. Further investigation is necessary to assess post-transfusion safety and efficacy in patients receiving these RBC products.
INTRODUCTION
Biologic heterogeneity in whole blood donors may contribute to differences in the quality of blood components including packed red blood cell (RBC) units for transfusion.1–3 We have recently demonstrated that genetic and biologic variables, such as race-ethnicity, sex or age, can significantly modulate RBC predisposition to hemolysis during routine blood banking cold storage, and in response to osmotic or oxidative stress.4 In addition to genetic polymorphisms, behavioral and other non-genetic factors may contribute to donor-specific differences in RBC characteristics and subsequently to variation in storage stability. For example, repeated phlebotomy in frequent blood donors may severely deplete iron stores and subsequently interfere with normal erythropoiesis.5,6 Recent investigations of frequent blood donors have focused primarily on preventative care against iron deficiency anemia7–12, with limited understanding regarding the impact of frequent donations on RBC characteristics and resilience to cold storage.
Various lab tests used to diagnose iron deficiency anemia have identified iron depletion in frequent blood donors with substantial changes in RBC phenotype, such as the formation of RBCs with low mean corpuscular hemoglobin (MCH) and mean corpuscular volume (MCV).13 However, certain donors exhibit exceptional recovery in response to frequent donations with no apparent impact on RBC indices. These “super donors”14 may donate whole blood every 56 days, the allowed minimum interval for blood donations in the U.S.A, for several years without deferral for low hemoglobin or hematocrit.15 Although the impact of intensive donations on RBC storage stability and transfusion efficacy has not been established in humans, a comparable study in mice has associated frequent phlebotomy with iron-deficient erythropoiesis and reduced 24-h post transfusion recovery of stored murine RBCs.16
In the present study, we evaluated the associations between frequent whole-blood donations and RBC predisposition to hemolysis in 13,403 blood donors from the National Heart, Lung and Blood Institute (NHLBI) Recipient Epidemiology Donor Evaluation Study (REDS)-III Red Blood Cell-Omics (RBC-Omics) study. An overarching goal of the RBC-Omics Study was to define genetic, biochemical, behavioral and metabolic bases for donor-specific differences in RBC storage stability and iron metabolism.4 We report evaluations of donor demographics associated with frequent blood donations, and quantify the impact of prior donations with or without iron intake on three measures of hemolysis, including spontaneous end-of-storage hemolysis and responses of stored RBCs to osmotic and oxidative stress.
MATERIALS AND METHODS
Protection of human subjects:
RBC-Omics was conducted under regulations applicable to all human subject research supported by federal agencies. The Data Coordinating Center (DCC, RTI International, Rockville, MD) of REDS-III supervised the overall compliance of human subjects regulatory protocols including Institutional Review Board approval from all participating blood centers, testing labs and the DCC.
RBC-Omics donor recruitment:
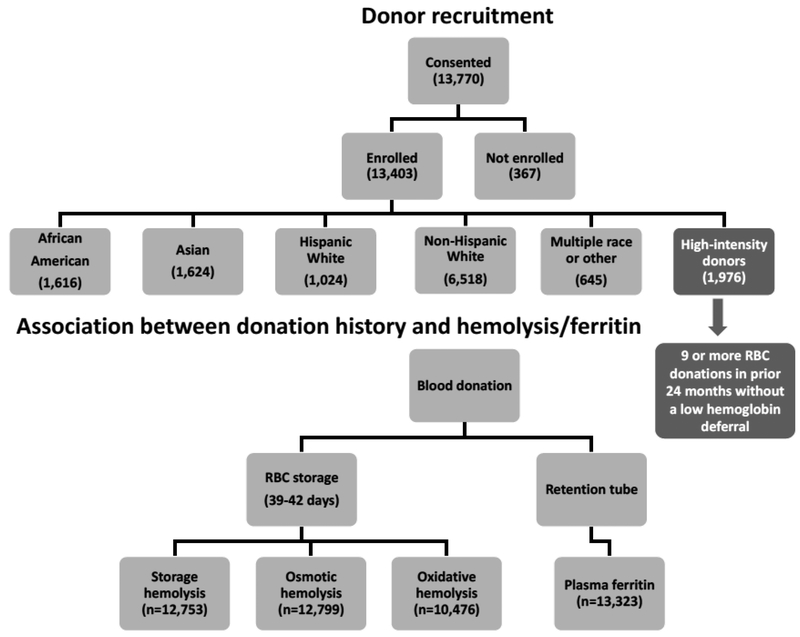
Donor recruitment and testing occurred between December 2013 and December 2015 at four large blood centers: the American Red Cross (ARC, Farmington, CT), the Institute for Transfusion Medicine (ITxM, Pittsburgh, PA), Blood Center of Wisconsin (BCW, Milwaukee, WI), and Blood Centers of the Pacific (BCP, San Francisco, CA). Overall, 97% (13,403) of the whole blood donations provided by 13,770 participant donors ≥18 years of age who provided informed consent were fully evaluable for plasma ferritin levels and measures of hemolysis. Donors were categorized into self-reported racial/ethnic groups: non-Hispanic White, Hispanic White, non-Hispanic African American, non-Hispanic Asian. Donors with multiple races, Hawaiian American, Native American, and other donors were grouped as “Other”. In addition, we recruited a group of 1976 high-intensity donors, who met the specific criteria of 9 or more successful blood donations in the prior 24-months without a low hemoglobin deferral. The overall study design is illustrated in Figure 1.
Figure 1:
Flowchart of the RBC-Omics study cohort and donor testing for hemolysis and ferritin.
Demographic data, such as ethnicity and sex were collected directly from enrollment interviews and recorded in the RBC-Omics Study Management System (SMS) database. Additional demographic data, including weight, height, and the date of birth, as well as the donation history were derived from the blood centers’ routine donor/donation databases and linked through donor ID, donation date, and donation identification number (DIN). Donor age at time of the enrollment donation was derived by calculating the difference between enrollment date and donor date of birth. A Biological Specimen Inventory (BSI) was used to track biospecimens.
Assessment of iron supplements intake:
Consumption of iron supplements in RBC-Omics was assessed by a questionnaire, for which blood donors were asked to report whether they had taken any multiple vitamins with iron or other iron supplements in the past 30 days, the frequency of iron-containing supplements intake (e.g. daily, weekly), and whether they had increased their iron intake after blood donation.
Blood components:
Whole blood units collected at participating blood centers were processed according to each center’s standard operating procedures. An aliquot of whole blood was collected into 10mL EDTA retention tubes for the determination of complete blood count (CBC) using automatic cell counters, and for plasma ferritin levels (ng/mL) determined by batch testing of frozen plasma samples using a quantitative latex agglutination assay on the Beckman Coulter AU680 Chemistry System (Beckman Coulter, Sacramento, CA). Each whole blood unit was filtered to generate a leukocyte-reduced packed RBC (LR-pRBC) unit in additive solution-1 or 3 (AS-1 or AS-3). A representative portion (10–15mL) of RBCs from each LR-pRBC unit was then sterile transferred into a customized transfer bag (Haemonetics, Braintree, MA) created specifically for this study. These transfer bags were made from the same materials as the parent RBC storage bag. Validation studies demonstrated strong correlations in storage outcomes between the parent and the transfer bags.17 The LR-pRBC parent units were released for distribution for transfusion, whereas the transfer bags were sent to RBC-Omics testing labs (University of Pittsburgh, Pittsburgh, PA, and Blood Systems Research Institute, San Francisco, CA).
RBC hemolysis assays:
All transfer bags were stored under routine blood bank conditions (1–6°C) for 39–42 days, after which the content of each bag was transferred into a 15mL conical tube and two aliquots (1mL) were processed immediately for the hemolytic assays. One aliquot was used for the quantification of spontaneous storage hemolysis and the other for the stress-induced hemolysis assays as described before.4 Percent end-of-storage hemolysis was determined according to the following equation:
Sample hematocrit (HCT) was determined by collecting blood samples into capillary tubes, which were centrifuged in a micro-HCT centrifuge (LW Scientific, Lawrenceville, GA). Hbsupernatant refers to the levels of free hemoglobin obtained after centrifugation (1500x g, 10min, 18°C) measured in the supernatant. Hbtotal refers to the total amount of sample hemoglobin before centrifugation. In the entire study, hemoglobin concentrations (micromolar) were determined by the Drabkin’s method.18
For the evaluation of stress-induced hemolysis, stored RBCs were washed (1500x g, 10min, 18°C) three times with phosphate-buffered saline (PBS) to remove plasma and additive solution, and immediately subjected to osmotic or oxidative stress assays.
RBC osmotic hemolysis:
Washed RBCs were incubated under static conditions (4h at 22°C) in pink test buffer19 at a final concentration of 1.6%±0.2% after which samples were centrifuged (1500x g, 10min, 18°C), and percent osmotic hemolysis was determined: , for which Hbosmotic corresponds to supernatant cell-free hemoglobin of pink test-treated RBCs and Hbtotal refers to the total amount of hemoglobin of each sample.
RBC oxidative hemolysis:
RBC susceptibility to oxidative hemolysis was evaluated by incubating RBCs in the presence of 2,2’-azobis-2-methyl-propanimidamide, dihydrochloride (AAPH, 150mmoL). Thermal (37°C) decomposition of AAPH generates peroxyl radicals leading to lipid peroxidation–mediated hemolysis.20 AAPH-induced oxidative hemolysis was determined by: , where HbAAPH corresponds to supernatant cell-free hemoglobin of AAPH-treated RBCs, Hbcontrol corresponds to supernatant cell-free hemoglobin of untreated RBCs, and Hbtotal refers to the total amount of hemoglobin of each sample.
Statistical analyses:
Descriptive Statistics:
To account for center-specific difference in RBC production procedures between hubs, hemolysis measures were adjusted for site specific effects as in the paper in Kanias et al.4 Mean storage, osmotic, and oxidative hemolysis, along with standard errors of the mean estimations were estimated stratifying by donation history and sex (Figure 3), by donation history and age (Figure 4), by donation history, sex, and iron intake (Figure 5), and by donation history and race-ethnicity group (Supplemental Figure 1) using R statistical software version 3.3.1.21 Univariate analyses were conducted to compare the mean hemolysis and ferritin differences between first-time/reactivated donors to each of the donation frequency bins for male and female (Figure 3), and for young donors (< 21 years old; Figure 4). Univariate analyses were also conducted to compare the mean hemolysis and ferritin differences affected by iron intake stratified by donation history and sex (Figure 5). Asterisks represent statistically significant differences in mean hemolysis and ferritin (p < 0.001). However, since univariate statistical analyses does not account for the effect of other covariates, we report only p-values from the multivariate analysis throughout the manuscript. Visuals and graphs were produced by the ggplot2 package in R.22
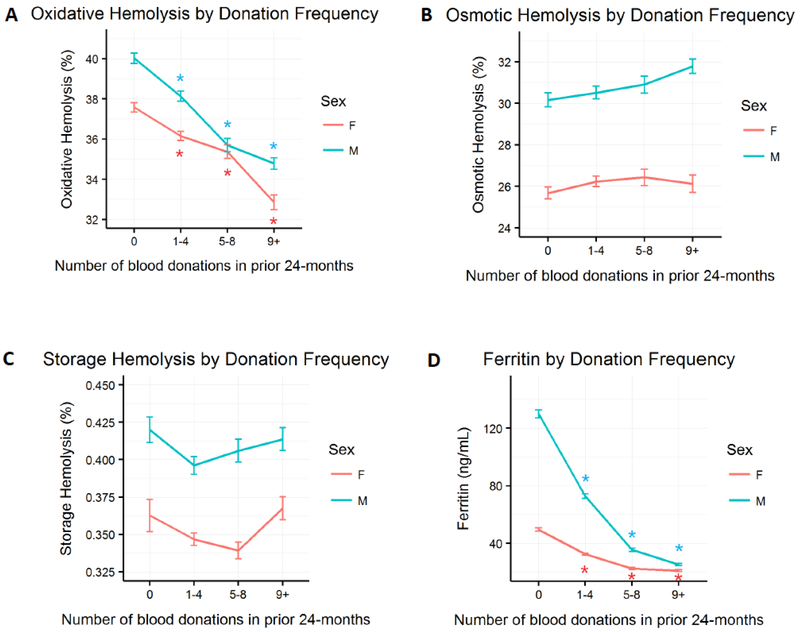
Figure 3: Sex distribution of stress-induced hemolysis, storage hemolysis or donor ferritin levels at selected categories of donation frequency in prior 24 months.
RBC concentrates from male or female donors ages 18–90 years old were stored (1–6°C) for 39–42 days in transfer bags and tested for storage or stress-induced hemolysis as described in Materials and Methods. A. Percent AAPH-induced oxidative hemolysis (150mmol/L, 1.5h, 37°C) (n=10,476). B. Percent osmotic hemolysis (n=12,799). C. Percent spontaneous storage hemolysis (n=12,753). D. Donor plasma ferritin (ng/mL) (n=13,323). Error bars represent standard errors of the mean. Asterisks represent statistical significant difference in mean hemolysis or ferritin between first- time/reactivated donors and each of the donation frequency bin for male and female (p < 0.001). Hemolysis measures were normalized for blood center differences in blood component manufacturing procedures.
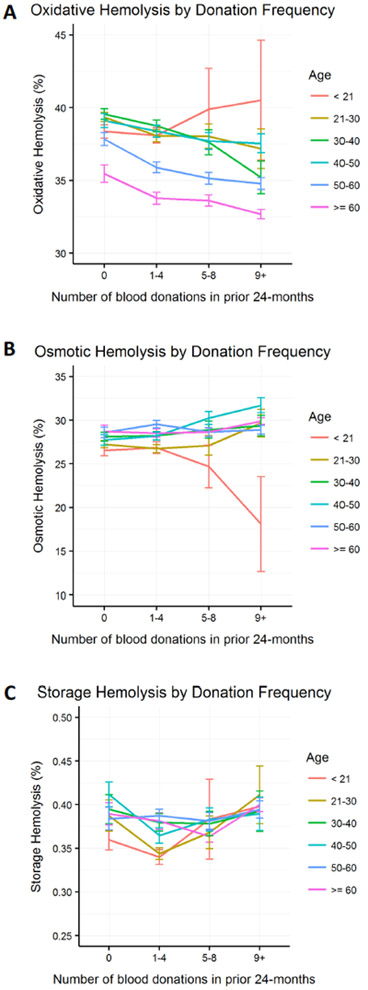
Figure 4: Evaluation of age-specific differences in the rates of stress or storage-induced hemolysis in response to frequent blood donations.
Figure panels represent the amount of A. Percent AAPH-induced oxidative hemolysis (150mmol/L, 1.5h, 37°C) (n=10,476). B. Percent osmotic hemolysis (n=12,799), and C. Percent storage spontaneous hemolysis (n=12,753) at selected age groups (decades) in the 4 categories of donation frequency. Error bars represent standard errors of the mean. A. n=17 and B-C. n=25 young donors (<21 years) at donation categories 5–8 and 9+ combined. Hemolysis measures were normalized for blood center differences in blood component manufacturing procedures.
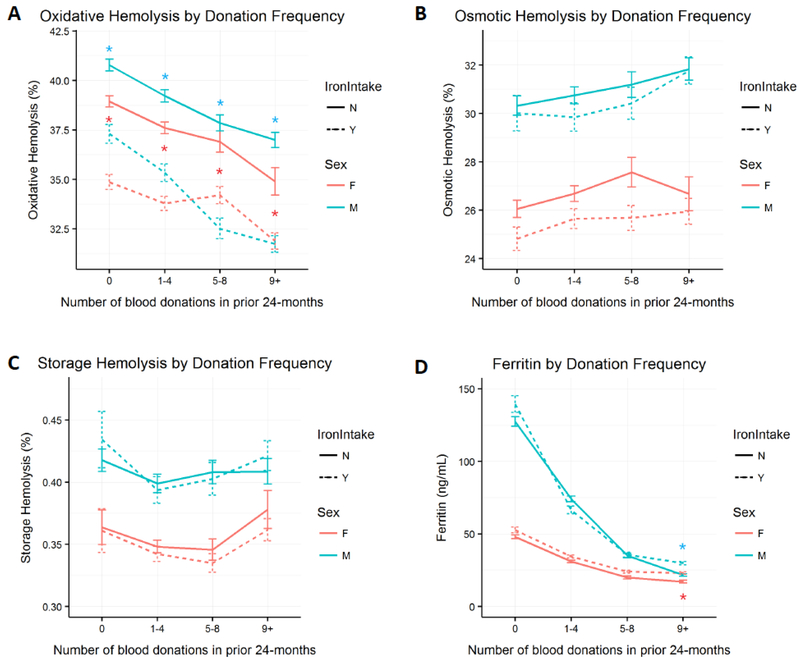
Figure 5: Association of self-reported iron intake with RBC predisposition to stress-induced hemolysis, storage hemolysis or donor ferritin levels.
Data obtained from male (M) and female (F) RBC-Omics donors who responded Yes (Y) or No (N) to the consumption of iron supplements. A. Percent AAPH-induced oxidative hemolysis (150mmol/L, 1.5h, 37°C), B. Percent osmotic hemolysis, C. Percent storage spontaneous hemolysis, and D. Donor ferritin levels (ng/mL). Error bars represent standard errors of the mean at the 4 categories of donation frequency. Asterisks represent statistical significant difference in mean hemolysis or ferritin by iron intake status stratified by donation history and sex (p < 0.001). Hemolysis measures were normalized for blood center differences in blood component manufacturing procedures.
Impact of frequent blood donations in young donors on hemolysis in stored RBCs:
A total of 27 donors younger than 21 years old who donated 5 or more times in the prior 24-months enrolled in the study. This provided the opportunity to evaluate the effect of frequent blood donation in young donors. Differences in hemolysis between young and older frequent donors were compared using the t-test because of the small sample size and consequent limited degrees of freedom in statistical tests.
Association between donation history and measures of hemolysis:
Evaluation of the association between donation history and each hemolysis measurement was performed by multivariate linear model regression analysis using several covariates. Donation frequency was divided into 4 groups: 0 (first-time donors and reference), 1–4, 5–8, and 9 or more donations 24-months prior to enrollment into this study; age was divided into 5 year bins; self-reported race-ethnicity and sex were indicator variables with white females as reference. Ferritin levels were analyzed as continuous measure. The reference groups were white, female, first-time donors, under 21 years of age, and no self-reported intake of iron supplements. Odds ratios and significances for each factor were calculated in the presence of the other covariates (Table 3).
Table 3:
Multivariate linear modeling of hemolysis measures. Differences are considered significant at p<0.001. Reference values were 0 donations, no iron consumption, age < 21, Female, and White race-ethnicity. For continuous measures like ferritin there is no reference value.
| Oxidative Hemolysis | Osmotic Hemolysis | Storage Hemolysis | ||||
|---|---|---|---|---|---|---|
| beta | p | beta | p | beta | p | |
| Donation Frequency 1-4 | −0.7988 | 0.0016 | 0.0998 | 0.7366 | −0.008 | 0.2723 |
| Donation Frequency 5-8 | −0.8267 | 0.0159 | −0.7796 | 0.0477 | 6.00E-04 | 0.9492 |
| Donation Frequency 9+ | −1.569 | < 0.0001 | −1.0589 | 0.0119 | 0.0222 | 0.0315 |
| Ferritin | 0.0071 | < 0.0001 | 0.0098 | < 0.0001 | 3.00E-04 | < 0.0001 |
| Iron Intake Yes | −3.7556 | < 0.0001 | −1.1919 | < 0.0001 | −0.0032 | 0.5911 |
| Age 21-25 | 0.9787 | 0.0535 | −0.6215 | 0.3017 | 0.0237 | 0.1069 |
| Age 25-30 | 0.4887 | 0.3216 | −0.6827 | 0.2414 | 0.0051 | 0.7203 |
| Age 30-35 | 1.0576 | 0.0361 | −0.2312 | 0.6981 | 0.0164 | 0.2608 |
| Age 35-40 | 1.0243 | 0.0475 | 0.3122 | 0.6105 | 0.046 | 0.0021 |
| Age 40-45 | 1.5454 | 0.0026 | 0.285 | 0.6422 | 0.0253 | 0.0917 |
| Age 45-50 | 0.7283 | 0.1516 | 0.9106 | 0.1282 | 0.0386 | 0.0084 |
| Age 50-55 | −0.4248 | 0.3823 | 0.2013 | 0.7267 | 0.0417 | 0.003 |
| Age 55-60 | −1.4726 | 0.0023 | 0.3644 | 0.5223 | 0.0456 | 0.0011 |
| Age 60-65 | −2.3678 | < 0.0001 | 0.1337 | 0.8218 | 0.0261 | 0.072 |
| Age >= 65 | −3.3611 | < 0.0001 | −0.2263 | 0.6964 | 0.0564 | < 0.0001 |
| Race African American | −0.0816 | 0.783 | −13.028 | < 0.0001 | 0.021 | 0.0176 |
| Race Asian | −0.3059 | 0.3513 | −3.2147 | < 0.0001 | 0.0579 | < 0.0001 |
| Race Hispanic | 0.74 | 0.0453 | −1.228 | 0.0051 | 0.0535 | < 0.0001 |
| Sex M | 1.0175 | < 0.0001 | 4.1337 | < 0.0001 | 0.0435 | < 0.0001 |
Multiple comparison adjustment:
To account for the fact that we performed multiple statistical tests in the manuscript (Table 3), and to keep the overall Type I error rate below 0.05, we determined tests with p-values <0.001 to be statistically significant.
RESULTS
Race-ethnicity, sex and age associations with donation frequency:
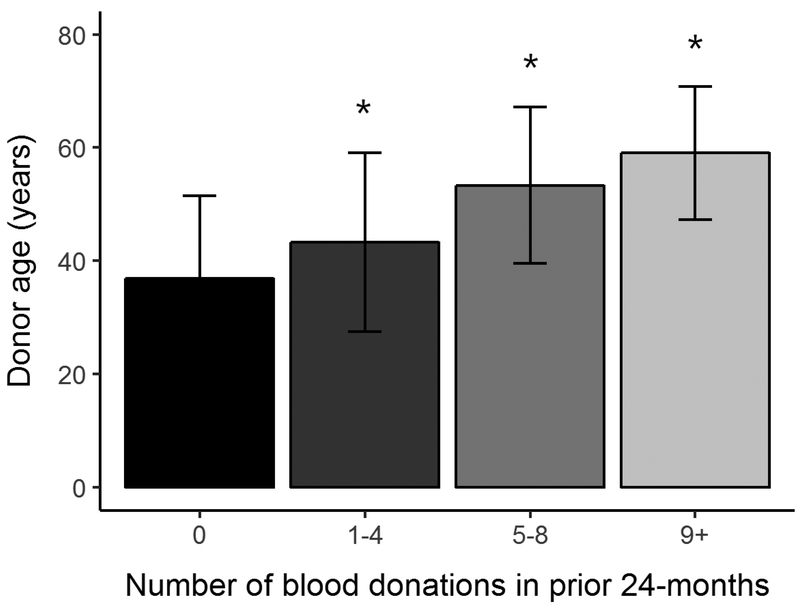
Of the 13,770 consented donors, 13,403 (97%) provided sufficient samples and data for inclusion in the present analysis (further information regarding the study design and population was published by Endres-Dighe et al.23). Participant sex and racial-ethnic distributions stratified by donation history are summarized in Table 1. Donors from both sexes were equally represented in RBC-Omics (50.3% females and 49.7% males). White donors constituted the large majority of subjects who donated 9 or more whole blood units in the 24 months prior to study enrollment. Frequent blood donations (≥5 donations in prior 24 months) were more prevalent in male donors across all racial-ethnic groups. For example, 14.6% of African American male donors compared with 8.5% of African American female donors gave ≥5 donations in the prior 24 months. Older male and female donors of all racial-ethnic groups were more likely to be frequent donors than younger donors (Table 2). The average age of all donors with prior history of 9 or more donations was 56.1±5.0 years versus 35.4±3.3 years in first-time donors and 39.4±4.6 years in donors with 1–4 prior donations (Figure 2).
Table 1:
RBC-Omics blood donor demographics (n=13,403) and donor numbers in each of the 4 categories of donation frequency.
| N | African American |
Asian | White | Hispanic | Other | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Don Freq | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male |
| 0 | 399 | 280 | 406 | 456 | 923 | 751 | 298 | 179 | 172 | 134 |
| 1-4 | 397 | 357 | 273 | 312 | 1567 | 1262 | 275 | 172 | 121 | 110 |
| 5-8 | 66 | 80 | 53 | 96 | 890 | 940 | 34 | 45 | 29 | 38 |
| 9+ | 8 | 29 | 8 | 20 | 792 | 1369 | 8 | 13 | 18 | 23 |
| Sum | 870 | 746 | 740 | 884 | 4172 | 4322 | 615 | 409 | 340 | 305 |
Table 2:
Distribution of donor age (years±SD) stratified by donation frequency, sex, and race-ethnicity in the RBC-Omics cohort (n=13,403).
| Age (years±SD) |
African American | Asian | White | Hispanic | Other | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Don Freq | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male |
| 0 | 36.9±14.7 | 36.0±14.8 | 32.7±11.9 | 35.6±12.1 | 40.1±15.6 | 41.0±15.8 | 31.2±12.0 | 32.5±12.1 | 32.6±12.5 | 35.7±12.8 |
| 1-4 | 42.5±16.1 | 39.9±14.8 | 36.3±13.6 | 37.4±13.0 | 46.5±15.7 | 47.6±15.4 | 34.5±13.2 | 35±14.4 | 37.1±14.7 | 37.6±14.8 |
| 5-8 | 51.4±13.7 | 52.3±13.6 | 50.2±13.1 | 44.3±11.2 | 55.1±13.0 | 54.2±14.0 | 48.2±10. 7 | 42.2±13.5 | 48.6±16.5 | 45.5±15.2 |
| 9+ | 62.0±9.3 | 58.7±11.9 | 49.8±9.0 | 58.2±13.2 | 59.0±11.2 | 59.4±11.9 | 58.3±11.6 | 45.6±15.3 | 53.8±14.9 | 56.1±13.6 |
| Average | 40.8±15.9 | 40.5±15.7 | 35.5±13.5 | 37.7±13.0 | 49.3±15.9 | 51.6±15.6 | 34.0±13.3 | 35.0±13.8 | 36.7±15.0 | 39.1±15.0 |
Figure 2: RBC-Omics donor age (years±SD) at selected categories of donation frequency in the 24 months prior to enrollment.
Asterisks represent statistical significant difference in age (p < 0.001).
Impact of donation frequency, race-ethnicity, and sex on hemolysis and ferritin in stored RBCs:
Frequent blood donations in male or female donors were associated with enhanced resistance to AAPH-induced oxidative hemolysis in both sexes (Figure 3A). This effect was evident by comparison with the average oxidative hemolysis observed among male and female donors with no prior donations (38.8±9.5%) to those with 9 or more donations (34.1±9.9%; p<0.0001). Conversely, no significant associations were observed between frequent blood donations and the amount of osmotic or storage hemolysis (Figure 3B-C). Donor ferritin levels were inversely correlated with donation frequency in both sexes, with the decline in ferritin greater in male donors (Figure 3D). Frequent blood donations had similar impact on RBC storage or stress hemolysis across all race-ethnicity groups. Thus, no race-specific differences in the response to multiple donations were detected (supplemental Figure 1). The levels of storage, oxidative and osmotic hemolysis in each donation frequency category are summarized in Table S1.
Impact of frequent blood donations by young donors on hemolysis in stored RBCs:
High intensity donations (5 or more donations in the prior 24 months) in younger donors (<21 years) were associated with increased susceptibility to AAPH-induced oxidative hemolysis (Figure 4A; n=17 donors) and enhanced resistance to osmotic hemolysis (Figure 4B; n=25 donors). These responses to frequent donations were notably different from donors >21 years, whose RBC exhibited an inverse response to 5 or more donations (i.e. decreased levels of oxidative hemolysis and increased levels of osmotic hemolysis). This same analysis did not reveal age-specific differences in spontaneous storage hemolysis (Figure 4C).
Impact of oral iron supplement use in donors on RBC hemolysis:
Consumption of iron supplements in male and female donors was associated with reduced levels of AAPH-induced oxidative (Figure 5A) hemolysis at all donation frequency categories suggesting that the effect of iron supplements on oxidative hemolysis was independent of donation history. Similar trend was observed with osmotic fragility, for which iron consumption was associated with lower levels of osmotic hemolysis in male donors with donation history of 1–4 and 5–8 units, and in female donors with 0–8 prior donations. No effect of iron supplementation was seen on storage hemolysis. Small increments in ferritin levels were observed in male donors at the extremes of donation history categories (i.e. 0 and 9+), and in female donors at all donation history categories (Figure 5D) as reported before.6
Multivariate analyses of donor demographics associated with hemolysis:
A multivariate linear model was developed to determine the impact of frequent blood donations on each hemolysis end-point.4 This model was adjusted for confounding variables including donor sex, age, race-ethnicity, ferritin, and iron supplements (Table 3). The betas in Table 3 represent the change in the hemolysis measure per unit change of the variable in question compared to the reference value. For example, each increment in ferritin concentration was associated with increases in the rates of oxidative, osmotic or storage hemolysis by 0.007%, 0.01%, and 0.0003%, respectively. Among the 3 hemolysis measures, only oxidative hemolysis was associated with frequent donations, as evident by the negative association with increasing number of prior donations in the last 2 years, evident at less frequent donation frequencies but becoming significant at 9 or more donations. Significant negative associations were also observed between oxidative hemolysis and older age (>60 years) and unexpectedly, with multivitamin with iron or other iron supplement intake (all p values<0.0001). Ferritin and male sex were positively associated with all 3 hemolysis endpoints (all p values<0.0001).
The same analyses demonstrated that average osmotic hemolysis in male RBCs was about 4.1% higher than in females, and osmotic hemolysis was negatively correlated with iron intake and with African American or Asian race-ethnicity. Storage hemolysis was significantly (all p values <0.0001) and positively associated with male sex, older age (>65 years), and with Asian or Hispanic race-ethnicity.
DISCUSSION
Frequent blood donors are essential to maintain a stable supply of RBCs and other blood components for transfusion therapies.24 As each blood donation removes about 200–250 mg of iron, frequent donations over short time intervals expose many blood donors to various health risks related to iron deficiency, such as fatigue, anemia, pica, and restless leg syndrome.5,25 Although ferritin levels progressively decline in response to frequent blood donations, key new findings from this study demonstrated that frequent donations also alter stored RBC susceptibility to oxidative hemolysis, and may have specific impact on young donor RBC susceptibility to osmotic and oxidative hemolysis. Furthermore, we demonstrated direct associations between donor ferritin concentrations and hemolysis, and that predisposition to osmotic or AAPH-induced oxidative hemolysis may be modulated by iron intake.
Frequent donors enrolled in this study were likely to be white, male, and of older age. Female frequent donors were also likely to be of older age and white. These characteristics of frequent donors are similar to recent reports from the Biomedical Excellence for Safer Transfusion (BEST) study of donation patterns in the U.S.26,27, and an assessment of frequent blood donors in Australia.24 Of note, the current study included a cohort of 1976 non-Hispanic white donors categorized as high-intensity donors (Figure 1), who comprised the majority of frequent donors with a history of 9 or more donations in the prior 24-months. The sex dichotomy in donation frequency is likely related to differences in susceptibility to donation-induced iron deficiency and subsequent low hemoglobin deferral28, which is most common in women who have depleted iron stores from menstruation and pregnancy.
The amount of spontaneous storage hemolysis and osmotic hemolysis varied among the four categories of donation frequency, but these differences were not observed in the multivariate model. These observations suggest that frequent blood donations have no apparent impact on RBC membrane integrity or osmotic fragility. By contrast, we found a negative correlation between donation frequency and RBC susceptibility to AAPH-induced oxidative hemolysis. The mechanism behind this phenomenon is not clear and requires further evaluation of the consequences frequent blood donations and iron deficiency impose on RBC antioxidant capacity and rheological properties. Donor age is another factor that can modulate RBC predisposition to AAPH hemolysis.4 While RBCs from young frequent donors (<21 years, Figure 4A) exhibited increased susceptibility to AAPH hemolysis, older donor RBCs (>60 years in particular, Table 3) were relatively resistant to this stressor. Therefore, further investigations should consider age as a significant modifier of hemolysis in stored RBCs.
We have recently reported sex- and race-specific differences in predisposition to hemolysis in the RBC-Omics cohort. For example, RBCs from African American donors exhibited enhanced resistance to osmotic hemolysis, whereas male sex was associated with increased susceptibility to storage and stress hemolysis.4 The current analyses suggested that the sex and race-ethnicity differences in hemolysis were independent of prior donation history, as they were observed across all donation categories. In contrast, our evaluations of age-specific alterations in response to frequent donation (5 or more in the prior 24 months) found that RBCs from younger (18–21 years) donors with frequent prior donations exhibit increased susceptibility to oxidative hemolysis and resistance to osmotic stress. The biochemical mechanisms that underlie such changes in hemolytic profile are unclear but may be related to iron deficiency and stress erythropoiesis in teenage donors, who are at greater risk of adverse reactions in response to frequent blood donations.29 Of note, these analyses did not reach our stringent definition of statistical significance (p>0.001) as they were based on a limited number of young high frequency donors (n=17 in oxidative hemolysis and n=25 in osmotic hemolysis); therefore, further evaluations to assess the impact of frequent blood donations on young adult RBC characteristics and storage stability are needed.
Based on the multivariate analysis, oral iron supplements were associated with reduced oxidative and osmotic hemolysis, an unexpected finding given the positive association between hemolysis and donor ferritin levels reported in Table 3. Iron supplements were also associated with minor increases in plasma ferritin observed primarily in frequent donors. Contrary to intervention studies that demonstrated the effectiveness of oral iron supplements (19 or 38 mg) on donor iron stores and ferritin levels7,30,31, the minimal effect observed in this multivariable analysis may be related to use of a linear regression model which analyzed ferritin across the entire ferritin range, rather than the logistic models used in the previous analyses, which emphasized the likelihood to have low ferritin levels (<12 ng/mL or <26 ng/mL). It is also possible that these donors had less effective self-directed consumption of iron supplements or, perhaps, incorrectly answered the questions about iron supplement use on the survey. Another possible explanation is that the changes observed in predisposition to hemolysis may have resulted from the action of vitamins or antioxidants in multiple vitamin pills rather than the iron content. Unfortunately, the survey did not allow differentiation between taking multivitamins with iron, or separate iron supplements.
Based on the multivariate analyses, a history of 9 or more donations in the prior 24-months and older age (>60 years) was significantly (p<0.0001) and negatively associated with oxidative hemolysis. Conversely, prior donations had no significant impact on storage or osmotic hemolysis suggesting that frequent donations primarily impact the RBC response to oxidative stress. The same analyses revealed positive associations (p<0.0001) between storage or stress hemolysis and donor plasma ferritin. These associations suggest that ferritin levels could predict RBC susceptibility to hemolysis in cold storage; however, further evaluations are required to determine the interactions between ferritin, iron, and hemolysis.
The findings presented in this study are limited to in vitro measurements of hemolysis in stored RBCs and ferritin measurement in donor plasma. Additional peripheral blood testing to assess donor iron status was not performed, because tests such as soluble transferrin receptor, transferrin saturation and total iron binding capacity add little to ferritin alone for assessment of iron status.32,33 Further, blood donors are a healthy population with little underlying inflammatory disease that may cause the ferritin test to be an insensitive indicator of iron stores.
In the absence of rigorous clinical studies that would evaluate transfusion outcomes, our current understanding of possible patient outcomes is limited to animal studies34–36 or to retrospective studies that linked donor characteristics with the risk of transfusion-related mortality.37–40 With regards to frequent blood donations, a recent study has demonstrated that RBCs produced by mice with mild iron deficiency have greatly decreased survival following storage and transfusion.16 Several prospective and ongoing studies by this group and others are in progress to investigate the impact of donor characteristics (sex, donation history, iron repletion therapy) on autologous RBC recovery and survival and patient outcomes following allogeneic RBC transfusions. For example, we have identified patients who received RBC units from RBC-Omics donors, and for whom the REDS-III program has recipient outcome data, including pre-/post-transfusion hemoglobin increments, in the linked donor-recipient database.41–43 In addition, subsequent studies are planned that will recall selected RBC-Omics donors with extremes of hemolysis parameters or identified genetic polymorphisms that were identified in our genome-wide association study to determine in vivo RBC survival based on biotin labeling44–46 and transfusion of autologous stored RBC samples.
Supplementary Material
Supplemental Figure 1: Race-ethnicity distributions of storage or stress-induced hemolysis at selected categories of donation frequency in prior 24 months. RBC concentrates from male or female donors ages 18–90 years old were stored (1–6°C) for 39–42 days in transfer bags and tested for storage or stress-induced hemolysis as described in Materials and Methods. A. Percent AAPH-induced oxidative hemolysis (150mmol/L, 1.5h, 37°C) (n=10,476). B. Percent osmotic hemolysis (n=12,799). C. Percent spontaneous storage hemolysis (n=12,753).
ACKNOWLEDGEMENTS
The authors thank the RBC-Omics research staff at all participating blood centers, the testing laboratories for performing tests and for their contribution to this project, and all blood donors who agreed to participate in this study. This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN2682011–00001I, HHSN2682011–00002I, HHSN2682011–00003I, HHSN2682011–00004I, HHSN2682011–00005I, HHSN2682011–00006I, HHSN2682011–00007I, HHSN2682011–00008I, and HHSN2682011–00009I, which supported the Recipient Epidemiology and Donor Evaluation Study III (REDS-III) RBC-Omics study, and by National Institutes of Health grant R01HL098032–04 from the NHLBI (M.T.G.), which partially supported research staff and assay development for this study.
Sources of support: This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN2682011–00001I, HHSN2682011–00002I, HHSN2682011–00003I, HHSN2682011–00004I, HHSN2682011–00005I, HHSN2682011–00006I, HHSN2682011–00007I, HHSN2682011–00008I, and HHSN2682011–00009I, which supported the Recipient Epidemiology and Donor Evaluation Study III (REDS-III) RBC-Omics study, and by National Institutes of Health grant R01HL098032–04 from the NHLBI (M.T.G.), which partially supported research staff and assay development for this study.
Footnotes
Conflict of interest disclosure: Dr. Darrel Triulzi serves as a paid consultant to Fresenius Kabi. Dr. Alan Mast receives research grant funding from Novo Nordisk. The remaining authors declare no competing financial interests with regards to this manuscript.
REFERENCES
- 1.Jordan A, Chen D, Yi QL, Kanias T, Gladwin MT, Acker JP. Assessing the influence of component processing and donor characteristics on quality of red cell concentrates using quality control data. Vox Sang 2016;111: 8–15. [DOI] [PubMed] [Google Scholar]
- 2.Tzounakas VL, Georgatzakou HT, Kriebardis AG, Voulgaridou AI, Stamoulis KE, Foudoulaki-Paparizos LE, Antonelou MH, Papassideri IS. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion 2016;56: 1274–86. [DOI] [PubMed] [Google Scholar]
- 3.Kanias T, Gladwin MT. Nitric oxide, hemolysis, and the red blood cell storage lesion: interactions between transfusion, donor, and recipient. Transfusion 2012;52: 1388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanias T, Lanteri MC, Page GP, Guo Y, Endres SM, Stone M, Keating S, Mast AE, Cable RG, Triulzi DJ, Kiss JE, Murphy EL, Kleinman S, Busch MP, Gladwin MT. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv 2017;1: 1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiss JE, Birch RJ, Steele WR, Wright DJ, Cable RG. Quantification of body iron and iron absorption in the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion 2017;57: 1656–64. [DOI] [PubMed] [Google Scholar]
- 6.Cable RG, Glynn SA, Kiss JE, Mast AE, Steele WR, Murphy EL, Wright DJ, Sacher RA, Gottschall JL, Tobler LH, Simon TL. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion 2012;52: 702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bialkowski W, Kiss JE, Wright DJ, Cable R, Birch R, D’Andrea P, Bryant BJ, Spencer BR, Mast AE. Estimates of total body iron indicate 19 mg and 38 mg oral iron are equivalent for the mitigation of iron deficiency in individuals experiencing repeated phlebotomy. Am J Hematol 2017;92: 851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cable RG, Brambilla D, Glynn SA, Kleinman S, Mast AE, Spencer BR, Stone M, Kiss JE, National Heart L, Blood Institute Recipient E, Donor Evaluation S, III. Effect of iron supplementation on iron stores and total body iron after whole blood donation. Transfusion 2016;56: 2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bialkowski W, Bryant BJ, Schlumpf KS, Wright DJ, Birch R, Kiss JE, D’Andrea P, Cable RG, Spencer BR, Vij V, Mast AE. The strategies to reduce iron deficiency in blood donors randomized trial: design, enrolment and early retention. Vox Sang 2015;108: 178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cable RG, Birch RJ, Spencer BR, Wright DJ, Bialkowski W, Kiss JE, Rios J, Bryant BJ, Mast AE. The operational implications of donor behaviors following enrollment in STRIDE (Strategies to Reduce Iron Deficiency in blood donors). Transfusion 2017;57: 2440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant BJ, Yau YY, Arceo SM, Daniel-Johnson J, Hopkins JA, Leitman SF. Iron replacement therapy in the routine management of blood donors. Transfusion 2012;52: 1566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radtke H, Tegtmeier J, Rocker L, Salama A, Kiesewetter H. Daily doses of 20 mg of elemental iron compensate for iron loss in regular blood donors: a randomized, double-blind, placebo-controlled study. Transfusion 2004;44: 1427–32. [DOI] [PubMed] [Google Scholar]
- 13.Kiss JE. Laboratory and genetic assessment of iron deficiency in blood donors. Clin Lab Med 2015;35: 73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefrere JJ, Hermine O. The real superdonors. Transfusion 2014;54: 2431–3. [DOI] [PubMed] [Google Scholar]
- 15.Mast AE, Foster TM, Pinder HL, Beczkiewicz CA, Bellissimo DB, Murphy AT, Kovacevic S, Wroblewski VJ, Witcher DR. Behavioral, biochemical, and genetic analysis of iron metabolism in high-intensity blood donors. Transfusion 2008;48: 2197–204. [DOI] [PubMed] [Google Scholar]
- 16.Bandyopadhyay S, Brittenham GM, Francis RO, Zimring JC, Hod EA, Spitalnik SL. Iron-deficient erythropoiesis in blood donors and red blood cell recovery after transfusion: initial studies with a mouse model. Blood Transfus 2017;15: 158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanteri MC, Kanias T, Keating S, Stone M, Guo Y, Page GP, Brambilla DJ, Endres-Dighe SM, Mast AE, Bialkowski W, D’Andrea P, Cable RG, Spencer BR, Triulzi DJ, Murphy EL, Kleinman S, Gladwin MT, Busch MP. NHLBI Recipient Epidemiology Donor Evaluation Study (REDS)-III Program. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: results of recall phase of the REDS-III RBC-Omics study. Transfusion. 2018. November 8. doi: 10.1111/trf.14987. [Epub ahead of print] PubMed PMID: 30408207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zwart A, van Assendelft OW, Bull BS, England JM, Lewis SM, Zijlstra WG. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobinocyanide standard (4th edition). J Clin Pathol 1996;49: 271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Judkiewicz L, Bugala I, Bartosz G. ‘Pink test’ and osmotic fragility test for the diagnosis of hereditary spherocytosis: another view. Eur J Haematol 1989;42: 217. [DOI] [PubMed] [Google Scholar]
- 20.Takebayashi J, Kaji H, Ichiyama K, Makino K, Gohda E, Yamamoto I, Tai A. Inhibition of free radical-induced erythrocyte hemolysis by 2-O-substituted ascorbic acid derivatives. Free Radic Biol Med 2007;43: 1156–64. [DOI] [PubMed] [Google Scholar]
- 21.R: A language and environment for statistical computing. R Foundation for Statistical Computing R Core Team. Vienna, Austria: https://www.r-project.org/. 2016. [Google Scholar]
- 22.Wickham H Ggplot2. 1 ed. New York, NY: Springer-Verlag New York; 2009. [Google Scholar]
- 23.Endres-Dighe SM, Guo Y, Kanias T, Lanteri M, Stone M, Spencer B, Cable RG, Kiss JE, Kleinman S, Gladwin MT, Brambilla DJ, D’Andrea P, Triulzi DJ, Mast AE, Page GP, Busch MP. NHLBI Recipient Epidemiology Donor Evaluation Study (REDS)-III Program. Blood, sweat, and tears: Red Blood Cell-Omics study objectives, design, and recruitment activities. Transfusion. 2018. September 28. doi: 10.1111/trf.14971. [Epub ahead of print] PubMed PMID: 30267427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gemelli CN, Hayman J, Waller D. Frequent whole blood donors: understanding this population and predictors of lapse. Transfusion 2017;57: 108–14. [DOI] [PubMed] [Google Scholar]
- 25.Chansky MC, King MR, Bialkowski W, Bryant BJ, Kiss JE, D’Andrea P, Cable RG, Spencer BR, Mast AE. Qualitative assessment of pica experienced by frequent blood donors. Transfusion 2017;57: 946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yazer MH, Delaney M, Germain M, Karafin MS, Sayers M, Vassallo R, Ziman A, Shaz B, Biomedical Excellence for Safer Transfusion C. Trends in US minority red blood cell unit donations. Transfusion 2017;57: 1226–34. [DOI] [PubMed] [Google Scholar]
- 27.Yazer MH, Vassallo R, Delaney M, Germain M, Karafin MS, Sayers M, van de Watering L, Shaz BH, Biomedical Excellence for Safer Transfusion C. Trends in age and red blood cell donation habits among several racial/ethnic minority groups in the United States. Transfusion 2017;57: 1644–55. [DOI] [PubMed] [Google Scholar]
- 28.Rigas AS, Sorensen CJ, Pedersen OB, Petersen MS, Thorner LW, Kotze S, Sorensen E, Magnussen K, Rostgaard K, Erikstrup C, Ullum H. Predictors of iron levels in 14,737 Danish blood donors: results from the Danish Blood Donor Study. Transfusion 2014;54: 789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloch EM, Mast AE, Josephson CD, Klein HG, Eder AF. Teenage Blood Donors: Are We Asking Too Little and Taking Too Much? Pediatrics 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiss JE, Brambilla D, Glynn SA, Mast AE, Spencer BR, Stone M, Kleinman SH, Cable RG, National Heart L, Blood Institute Recipient E, Donor Evaluation S, III. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA 2015;313: 575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mast AE, Bialkowski W, Bryant BJ, Wright DJ, Birch R, Kiss JE, D’Andrea P, Cable RG, Spencer BR. A randomized, blinded, placebo-controlled trial of education and iron supplementation for mitigation of iron deficiency in regular blood donors. Transfusion 2016;56: 1588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clinical Chemistry 1998;44: 45–51. [PubMed] [Google Scholar]
- 33.Burns ER, Goldberg SN, Lawrence C, Wenz B. Clinical utility of serum tests for iron deficiency in hospitalized patients. Am J Clin Pathol 1990;93: 240–5. [DOI] [PubMed] [Google Scholar]
- 34.Kanias T, Sinchar D, Osei-Hwedieh D, Baust JJ, Jordan A, Zimring JC, Waterman HR, de Wolski KS, Acker JP, Gladwin MT. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion 2016;56: 2571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osei-Hwedieh DO, Kanias T, Croix CS, Jessup M, Xiong Z, Sinchar D, Franks J, Xu Q, E MN, Sertorio JT, Potoka K, Binder RJ, Basu S, Belanger AM, Kim-Shapiro DB, Triulzi D, Lee JS, Gladwin MT. Sickle Cell Trait Increases Red Blood Cell Storage Hemolysis and Post-Transfusion Clearance in Mice. EBioMedicine 2016;11: 239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Wolski K, Fu X, Dumont LJ, Roback JD, Waterman H, Odem-Davis K, Howie HL, Zimring JC. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica 2016;101: 578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chasse M, McIntyre L, English SW, Tinmouth A, Knoll G, Wolfe D, Wilson K, Shehata N, Forster A, van Walraven C, Fergusson DA. Effect of blood donor characteristics on transfusion outcomes: A systematic review and meta-analysis. Transfus Med Rev 2016;30: 69–80. [DOI] [PubMed] [Google Scholar]
- 38.Chasse M, McIntyre L, Tinmouth A, Acker J, English SW, Knoll G, Forster A, Shehata N, Wilson K, van Walraven C, Ducharme R, Fergusson DA. Clinical effects of blood donor characteristics in transfusion recipients: protocol of a framework to study the blood donor-recipient continuum. BMJ Open 2015;5: e007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chasse M, Tinmouth A, English SW, Acker JP, Wilson K, Knoll G, Shehata N, van Walraven C, Forster AJ, Ramsay T, McIntyre LA, Fergusson DA. Association of blood donor age and sex with recipient survival after red blood cell transfusion. JAMA Intern Med 2016;176: 1307–14. [DOI] [PubMed] [Google Scholar]
- 40.Edgren G, Ullum H, Rostgaard K, Erikstrup C, Sartipy U, Holzmann MJ, Nyren O, Hjalgrim H. Association of Donor Age and Sex With Survival of Patients Receiving Transfusions. JAMA Intern Med 2017;177: 854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy MF. The epidemiology of transfusion: where blood goes and why we should care about it. Transfusion 2017;57: 2821–3. [DOI] [PubMed] [Google Scholar]
- 42.Karafin MS, Bruhn R, Westlake M, Sullivan MT, Bialkowski W, Edgren G, Roubinian NH, Hauser RG, Kor DJ, Fleischmann D, Gottschall JL, Murphy EL, Triulzi DJ, National Heart L, Blood Institute Recipient E, Donor Evaluation S, III. Demographic and epidemiologic characterization of transfusion recipients from four US regions: evidence from the REDS-III recipient database. Transfusion 2017;57: 2903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinman S, Busch MP, Murphy EL, Shan H, Ness P, Glynn SA, National Heart L, Blood Institute Recipient E, Donor Evaluation S. The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion 2014;54: 942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mock DM, Matthews NI, Zhu S, Strauss RG, Schmidt RL, Nalbant D, Cress GA, Widness JA. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion 2011;51: 1047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Back DZ, Vlaar R, Beuger B, Daal B, Lagerberg J, Vlaar APJ, de Korte D, van Kraaij M, van Bruggen R. A method for red blood cell biotinylation in a closed system. Transfusion 2018;58: 896–904. [DOI] [PubMed] [Google Scholar]
- 46.Kanias T, Dennis CJ, Meyer EM, Moore L, Sinchar D, Triulzi D, Kiss JE, Gladwin MT, Donnenberg AD. Development of GMP Protocol for the Evaluation of Biotinylated Red Blood Cell Recovery after Transfusion. Transfusion 2017;57: 170A–A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Race-ethnicity distributions of storage or stress-induced hemolysis at selected categories of donation frequency in prior 24 months. RBC concentrates from male or female donors ages 18–90 years old were stored (1–6°C) for 39–42 days in transfer bags and tested for storage or stress-induced hemolysis as described in Materials and Methods. A. Percent AAPH-induced oxidative hemolysis (150mmol/L, 1.5h, 37°C) (n=10,476). B. Percent osmotic hemolysis (n=12,799). C. Percent spontaneous storage hemolysis (n=12,753).