Abstract
A wide range of maternal exposures– undernutrition, obesity, diabetes, stress and infection – are associated with increased risk of metabolic disease in offspring. Developmental influences can cause persistent structural changes in hypothalamic circuits regulating food intake in the service of energy balance. The physiological relevance of these alterations has been called into question because maternal impacts on daily caloric intake do not persist to adulthood. Recent behavioral and epidemiological studies in humans provide evidence that the relative contribution of appetitive traits related to satiety, reward and emotional aspects of food intake regulation changes across the lifespan. This article outlines a neurodevelopmental framework to explore the possibility that cross-talk between developing circuits regulating different modalities of food intake shapes future behavioral responses to environmental challenges.
Introduction
A thorough understanding of the mechanisms that regulate food intake is important because of the increasing incidence of obesity and metabolic disorders. Moreover, the consumption of ‘highly palatable foods’, is becoming more widespread; these foods are usually calorie dense and highly processed, and dysregulate the normal regulation of energy balance and can lead to weight gain. Although most high-profile studies have focused on acute factors influencing energy balance in adult humans and other animals, there is also evidence for developmental influences on energy balance regulation later in life. Indeed, a large body of epidemiological evidence indicates that intrauterine exposure to adverse maternal metabolic conditions such as undernutrition, obesity and psychological stress increases susceptibility of offspring to obesity1,2.
The prevailing theory to explain these observations, the Barker hypothesis, is that maternal influences on the developing brain and peripheral organs produce metabolic adaptations in their progeny that render them vulnerable to nutritional-intake excess later in life3,4. In animal models, manipulations of maternal nutritional status or hormonal status during gestation or the neonatal period produce marked effects on caloric intake at weaning. Exposure to overnutrition leads to increased caloric intake, while exposure to undernutrition leads to decreased caloric intake5,6. This led many to hypothesize that maternal programming of obesity risk in offspring is possible, and is conveyed, in large part, through effects on the developing neuronal circuits regulating feeding behavior3,7,8.
The discovery that the adipokine leptin promotes the formation of key components of circuits that control feeding behavior in the service of energy balance (i.e. homeostatic regulation)9 ushered in a wave of studies of maternal influences on hypothalamic development. This research demonstrated that the neurons in the arcuate nucleus of the hypothalamus (ARH) directly sense circulating signals of metabolic status (i.e. leptin, ghrelin and insulin) and identified critical periods of development when maternal influences on their levels exert a lasting impact on the structure and function of key components of circuits that regulate homeostatic feeding behavior7,8. Given the robustness and persistence of these neuroanatomical effects, it was initially assumed that they underlie maternal programming of obesity risk.
More recently, however, there is a growing appreciation that this model of maternal influences on caloric intake in offspring is over simplistic: although alterations in maternal nutrition are closely correlated with early growth rates, they rarely persist to adulthood10,11. In fact, a recent meta-analysis of 35 rodent studies did not find evidence that maternal influences on susceptibility of offspring to diet-induced obesity are due to changes in caloric intake12. This indicates that the model in which maternal programming of obesity risk occurs by developmental alterations in hypothalamic feeding circuits needs to be reevaluated.
Epidemiological studies in humans and experiments in rodent models demonstrate that maternal influences can increase preferences for high fat foods, independent of caloric intake per se13–15. The mechanism by which information about maternal nutritional status is conveyed to mesolimbic dopamine reward circuits that regulate food choices and motivated feeding behavior is not known. A challenge to identifying conduits for maternal signals to reward circuits, which regulate the motivation to consume food, is that they do not function until the transition to independent feeding. Whereas links between early maternal influences and preferences for “junk food” in childhood are consistent, there are inconsistencies in the degree to which these effects persist and are associated with obesity risk in adults13,15–17.
This article synthesizes data from clinical and epidemiological studies of feeding behavior in humans with neuroanatomical and behavioral studies in rodents to present a neurodevelopmental framework to evaluate the contribution of maternal influences on neuronal development to feeding behavior and obesity risk in adults – a framework that can reconcile the apparently conflicting data. This framework was developed around three core considerations. First, systems that provide orosensory, visceral, reward, homeostatic and emotional control of feeding behavior develop in the same step-wise progression spanning gestation through adolescence in humans and rodents. Second, population-based behavioral studies in humans demonstrate that the relative contributions of different types of feeding control to weight gain changes across the developmental continuum. Third, studies in rodent models show that developmental influences on one control system can produce compensatory adjustments in later-developing systems to maintain stable levels of caloric intake over the long term. In this review, I will first introduce the main systems influencing feeding behaviors and describe current knowledge of the ontogeny of feeding circuit development in rodents and humans. I will then describe maternal influences on feeding circuit development and discuss how crosstalk during development of the different circuits regulating food intake could determine future behavior.
Circuits Regulating Feeding Behaviors
Feeding circuit development in rodents and humans follows loosely similar trajectories, making rodent models appropriate for gaining insight into regulation of human food intake. The ontogeny and developmental influences on many neurobiological and/or behavioral correlates of feeding behavior have been characterized in rodents, and are described in the following sections. Several excellent reviews provide detailed descriptions of circuits that regulate different aspects of feeding behavior18–20. The neurobiological processes that determine food intake can be classified into direct and indirect controls over the size and duration of an individual meal21; these two control systems are covered in detail below.
Direct Control Systems
Direct controls arise from contact between food and sensory receptors during the consummatory phase of ingestion. Orosensory stimuli arising from palatable food (i.e. sweet and fat) provide positive feedback to the brain that increases the rate and amount of food consumption during a single meal. Conversely, post-ingestive stimulation of the gastrointestinal tract stimulates vagal signals (visceral inputs) that provide negative feedback that promotes satiation and meal termination. Direct stimuli transmit information about the quantity and nutrient composition of a meal via direct projections to the dorsal vagal complex in the hindbrain, where positive and negative feedback signals are processed and relayed to central pattern generators in the caudal pons and medulla that regulate somatomotor systems needed to consume food (i.e. swallowing and chewing) (black arrows in Figure 1)(reviewed in22).
Figure 1. Summary diagram illustrating cross-talk between circuits regulating different aspects of feeding behavior.
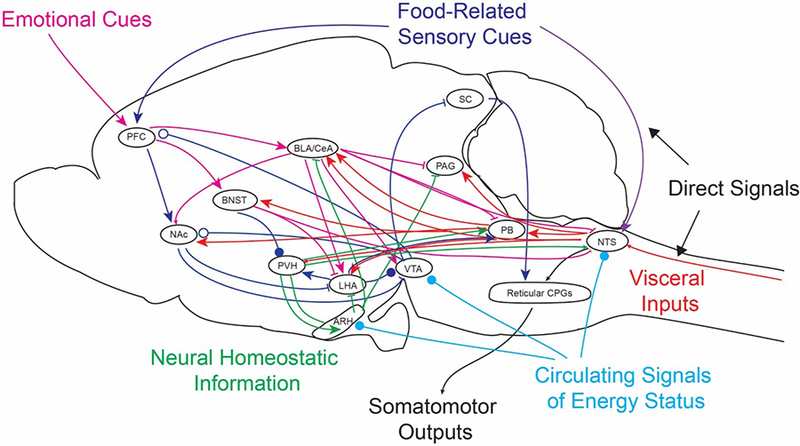
Orosensory (purple) and vagal (red) signals are transmitted directly to the nucleus of the solitary tract (NTS) to promote and terminate feeding, respectively. Visceral signals are also transmitted directly or via the parabrachial nucleus (PB) to higher brain structures, where they converge with other feeding control systems (red arrows). Food-related sensory cues are transmitted from the prefrontal cortex (PFC) to reward circuits (green arrows). Information about metabolic status is transmitted via neural (green arrows) and circulating signals (light blue arrows). Emotional cues are related via the bed nucleus of the stria terminalis (BNST) and central nucleus of the amygdala (CeA) to regulate feeding behavior in response to anxiety and threats in the external environment (pink arrows). Cross-talk and competition between these inputs while they are still forming could impact the balance between circuits regulating different modalities of food intake. Arrowheads represent excitatory inputs, while lines represent inhibitory inputs. Open circles represent dopamine signaling, while closed circles can be inhibitory or excitatory.
Abbreviations: ARH, arcuate nucleus of the hypothalamus; BLA, basolateral nucleus of the amygdala; CPG, central pattern generator; LHA, lateral hypothalamic area; NAc, nucleus accumbens; PAG, periaqueductal gray; PVH, paraventricular nucleus of the hypothalamus; SC, superior colliculus; VTA, ventral tegmental area.
Indirect Control Systems
Indirect control systems modulate the potency of direct positive and negative feedback control systems through connections between the forebrain and hindbrain. They permit the fine tuning of behavior in response to the energy density and palatability of food, anticipated need for energy, and likelihood of securing food.
Visceral Control Systems
In addition to direct (vagal) inputs, post-absorptive visceral inputs are relayed by neural signals and gut peptide factors released into the circulation upon ingestion of a meal that act on distributed sites in the CNS to promote satiation (i.e. meal termination) (green arrows in Figure 1). The nucleus of the solitary tract (NTS) is a key node in circuits regulating satiety. In addition to receiving direct control signals from the vagus, it receives indirect signals from blood-borne gut-derived factors. This information is relayed to nuclei in the rostral brainstem that promote meal termination – the lateral parabrachial nucleus (PB) and periaqueductal gray (PAG). The PB relays visceral signals to reward circuits via the lateral hypothalamic area (LHA), ventral tegmental area (VTA) and nucleus accumbens (NAc)23–25 and to cortico-limbic circuits via the central nucleus of the amygdala (CeA)26–29. The NTS also relays visceral cues to circuits regulating homeostatic feeding behavior via the paraventricular nucleus of the hypothalamus (PVH)30.
Reward Control Systems
Rewards circuits regulate consummatory behaviors related to the “liking” of food (i.e. palatability) and appetitive behaviors relating to “wanting” of food (i.e. food seeking) to guide behavioral strategies to meet energy requirements in an efficient manner (light blue arrows in Figure 1). Information about the sensory properties of food that drive motivated intake is relayed from prefrontal and orbitofrontal cortices via the NAc to the LHA and VTA, critical nodes in the mesolimbic dopamine system. Reward-related signals are relayed to other feeding control systems via projections from the LHA to the PB and PVH31,32.
Homeostatic Control Systems
Homeostatic systems assess nutritional requirements to maintain metabolic homeostasis, by sensing circulating levels of hormones reflecting energy stores (i.e. leptin, insulin and ghrelin)(pink arrows in Figure 1) in conjunction with endocrine and neural correlates of energetically costly processes, such as thermogenesis, growth and reproduction (dark blue arrows in Figure 1). Neurons in the ARH that express neuropeptide Y (NPY), agouti-related protein (AgRP) and γ-aminobutyric acid (GABA) (hereafter called AgRP neurons) are activated by nutrient, hormonal and neural signals of negative energy balance, and conversely, are inhibited by signals of the energetically replete state33,34. They transmit a potent inhibitory signal to many downstream targets to ensure that food-seeking behavior is prioritized over other potential activities. Targets of AgRP neurons include critical nodes in circuits regulating satiety (PVH, PB, PAG), reward (LHA) and emotional (bed nucleus of the stria terminalis (BNST), CeA) aspects of feeding behavior35–37. Over the long term, actions of AgRP neurons are opposed by the actions of proopiomelanocortin (POMC)-expressing neurons in the ARH. In addition to neural projections from the ARH, homeostatic information is conveyed by circulating signals of energy status (i.e. ghrelin, leptin) that act directly on neurons in key nodes in reward (LHA, VTA) and visceral circuits (NTS, PB) to modulate feeding behavior38–43. Homeostatic systems do not regulate food intake per se44 but modulate the strength of reward and satiety circuits to favor motivated behavior in a manner that is appropriate for the situation. For example, food is less rewarding when it follows a meal or is likely poisonous, while it is more rewarding under conditions of negative energy balance, such as fasting or starvation. Functional images studies in humans demonstrate that ghrelin activates regions associated with reward and emotional feeding control systems (i.e. amygdala and orbitofrontal cortex) and that this response is correlated with hunger ratings45.
Emotional Control Systems
Systems that relay information about behavioral emotional states arising from stimuli in the external environment, such as those that predict threats, can override other regulatory systems (red arrows in Figure 1). Sensory cues processed by the prefrontal cortex and anterior cingulate cortex are transmitted to limbic circuits via projections to the basolateral amygdala; this information is then integrated by neurons in the CeA to regulate feeding behavior46. Threat-related stimuli are also conveyed to reward circuits via projections from the CeA to the LHA, VTA and NAC47,48 and from the BNST to the LHA49. These circuits are relatively understudied from a developmental perspective, so will not be discussed further in this review. In the next section, I will discuss how the circuits underlying the direct and indirect systems controlling food intake develop in both rodents and humans.
Ontogeny of Feeding Circuits
To consider the possibility that maternal influences program later feeding behavior in offspring, it is necessary to appreciate the time frame over which different control systems develop. In humans and animal models, systems regulating distinct modalities of food intake develop asynchronously. The most basic types of feeding regulation - direct positive and negative feedback - are established shortly after birth, whereas the emergence of indirect control systems occurs gradually and extends into adolescence.
Feeding circuit development in rodents
The timing of key steps in the maturation of orosensory, visceral, reward and homeostatic feeding control systems in rodents has been characterized at both the behavioral and neuroanatomical levels. Studies of orosensory, visceral and reward circuits have largely utilized rats, whereas studies of homeostatic circuits have largely used mice. There are small (i.e. 1–3 days) differences in developmental timelines in rats vs. mice, but the timepoints corresponding to major developmental stages – gestation, lactation (0–3 weeks), post-weaning (>3 weeks) and pubertal transition (4–6 weeks) – is highly conserved. Thus, references in this review to a particular day in development are an approximation only. Figures 2–5 present an overview of when key neuroanatomical projections are formed within distinct control systems (dotted line), when they actively transmit signals (dashed line) and when their functional properties mature (solid line).
Figure 2. Ontogeny of orosensory inputs in rodents.
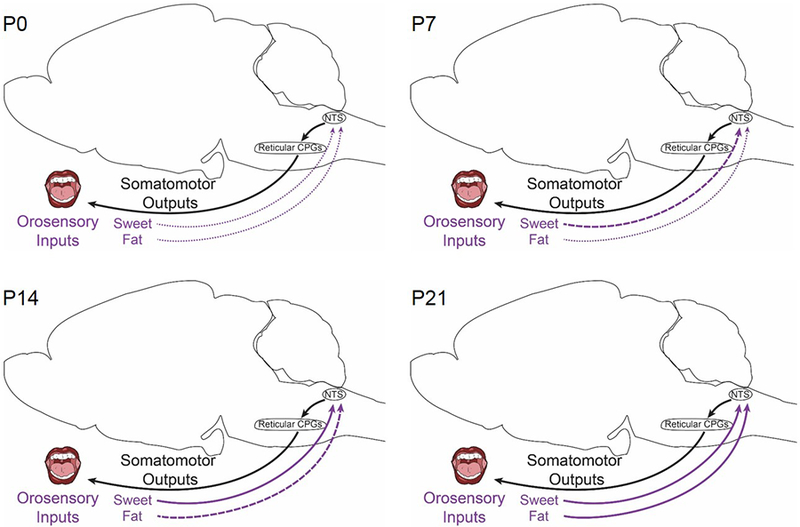
Information about food during the consummatory phase is relayed from the mouth directly to the nucleus of the solitary tract (NTS) (gray arrows), where it is transmitted to reticular central pattern generators (CPGs) that drive motor outputs (red arrows). This direct control system operates within the hindbrain and does not require connections to forebrain circuits. Orosensory circuits are present at birth (postnatal day 0 (P0)) and are refined over the course of lactation (to P21). Projections relaying sweet tastes mature before those relaying fat. Thin dotted lines indicate that the presence of a physical connection, but weak or immature signal transmission. Bold dashed lines represent signal transmission that is robust, but immature. Solid lines represent mature connections.
Figure 5. Ontogeny of homeostatic control systems in rodents.
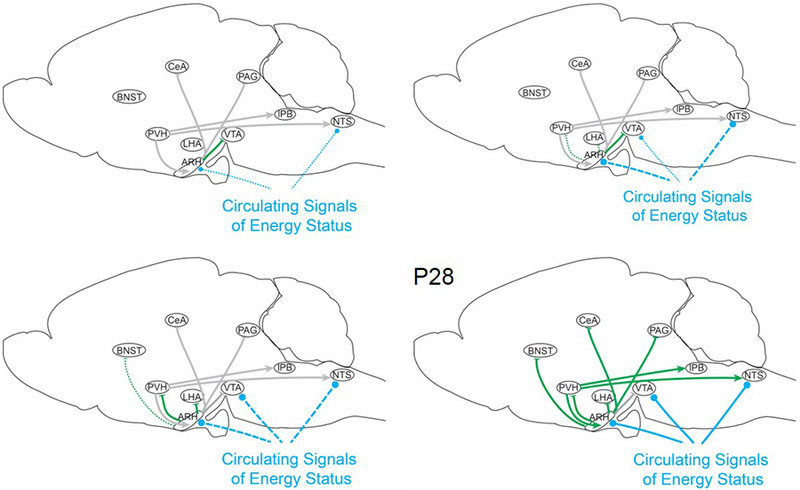
Interoceptive information about long-term energy stores is transmitted via neural projections from agouti-related peptide (AgRP) neurons (green arrows) and direct signaling via circulating factors (light blue arrows). Projections from AgRP neurons to downstream targets are not detected at birth. AgRP neuronal responsiveness to circulating leptin and ghrelin is observed within a week after birth; these signals regulate neurite outgrowth during lactation. A transient projection to the ventral tegmental area (VTA) is formed by postnatal day 7 (P7), and projections to the lateral hypothalamic area (LHA) and paraventricular nucleus of the hypothalamus (PVH) are fully formed by the end of lactation, AgRP circuits do not regulate homeostatic feeding behavior until P28. Thin dotted lines indicate the presence of a physical connection, but weak or immature signal transmission. Bold dashed lines represent signal transmission that is robust, but immature. Solid lines represent mature connections. Gray lines represent projections for which developmental datapoints are missing. Arrowheads represent excitatory inputs, while lines represent inhibitory inputs. Light blue circles represent sites that directly sense circulating signals of energy status (i.e. leptin and ghrelin).
Abbreviations: ARH, arcuate nucleus of the hypothalamus; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; LHA,; NTS, nucleus of the solitary tract; P, postnatal day; PAG, periaqueductal gray; PB, parabrachial nucleus.
Orosensory Control Systems
Influences of maternal diet on infant responses to maternal breast milk50,51 are likely mediated via effects on the development of orosensory control systems. In rodents, irect orosensory positive feedback (Figure 2) resulting from maternal milk stimuli can be detected shortly after birth52,53, although intake in the first postnatal week is driven by thirst and not by nutritional content54. Orosensory systems develop the capability to drive dose-response increases in feeding in response to sweet between 1–2 weeks, with responses to fat maturing between 2–3 weeks55. The rewarding properties of olfactory stimuli associated with milk develop in rats by P6 and diminish after P1256, whereas preferences conditioned by taste stimuli (i.e. sweet and fat) can persist after weaning57,58. These early positive sensory feedback systems depend on opioid signaling59 and precede the development of the mesolimbic dopamine reward system (see below). Sensory stimuli are also transmitted to indirect control systems that regulate reward and emotional aspects of feeding behavior (see below).
Visceral Control Systems
Maternal influences on early patterns of caloric intake are major determinants of meal size and growth in the postnatal period60,61. These effects could, in theory, be mediated through influences on satiety-related control systems. At birth, the cytoarchitecture of the NTS in rodents (Figure 3) is largely mature62 and the density and distribution of vagal sensory inputs to the NTS is established63,64. In neonates, direct negative feedback from gastric distension and gut-derived peptides (i.e. cholecystokinin) (visceral inputs) provide the only inhibitory control over feeding54,65,66. This direct negative feedback system does not require communication with forebrain circuits67,68 and declines in strength after P1469, concomitant with the development of indirect visceral control systems that engage forebrain circuits.
Figure 3. Ontogeny of visceral control systems in rodents.
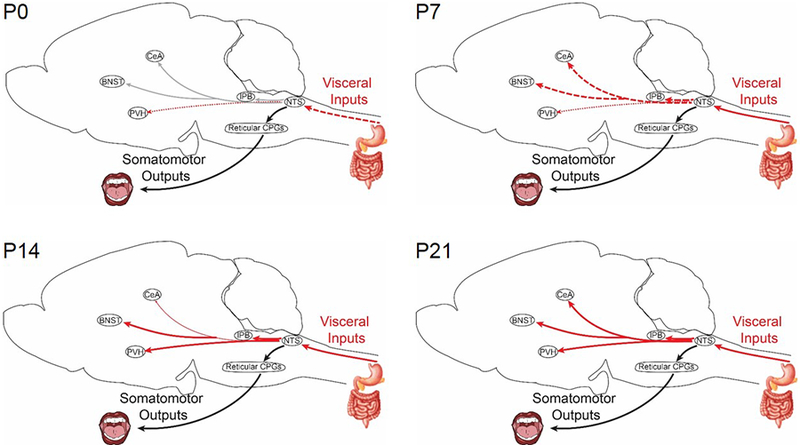
Visceral inputs are transmitted through direct and indirect control systems. Direct transmission via the vagus to the nucleus of the solitary tract (NTS) is sufficient to regulate meal termination and is present from birth (postnatal day 0, P0). Indirect control systems that transmit visceral signals from the NTS to higher brain centers develop across lactation (P7-P21). Projections from the NTS to the paraventricular nucleus of the hypothalamus (PVH) can be detected at birth. Visceral signals that are transmitted to key nodes in emotional control systems (bed nucleus of the stria terminalis (BNST) and central nucleus of the amygdala (CeA)) via the parabrachial nucleus (PB) are not fully functional until the end of lactation (P21). Thin dotted lines indicate the presence of a physical connection, but weak or immature signal transmission. Bold dashed lines represent signal transmission that is robust, but immature. Solid lines represent mature connections. Light gray lines represent projections for which developmental datapoints are missing.
Abbreviations: CPG, central pattern generator.
Transmission of visceral signals to indirect control systems in the brainstem (PB) and limbic regions (CeA, BNST) rapidly expand across the first two postnatal weeks69. Signaling to the PB, an important node in satiety circuits, continues to increase through 5–6 weeks54,65,69–71. Visceral signaling to limbic regions (CeA) declines markedly after P14, concomitant with extensive synaptic remodeling and pruning72,73. Although physical connectivity between the NTS and PVH is established at birth, there is a delay in the transmission of visceral signals until the second postnatal week70,74. Visceral signaling in the PVH reaches adult levels by 2 weeks70, and there is no evidence of pruning or remodeling of these inputs75. The maturation of the electrophysiological properties of NTS neurons occurs at 3 weeks76. In summary, direct negative feedback systems promoting meal termination operate from birth, but indirect systems form and mature across lactation. More detailed information about the ontogeny of visceral inputs can be obtained from several excellent reviews7,77.
Reward Control Systems
Maternal exposures that increase preference for high fat foods13,14 are likely conveyed via effects on the developing mesolimbic dopamine reward circuits. Connectivity between the LHA and VTA is established by within the first week of life in rats (Figure 4)78,79. Projections from dopaminergic neurons in the VTA to the NAc are formed and remodeled through axon pruning by the end of gestation80. Varicosities on dopaminergic fibers are first detected at the end of gestation. The expansion of varicosities across the first two postnatal weeks is paralleled by the expression of dopamine and opioid receptors in the NAc81–83. Expression levels of the dopamine 2 receptor and opioid receptors declines after lactation (3–4 weeks), consistent with remodeling in these signaling pathways84,85. Synaptic connectivity between neurons in the VTA and their primary targets in the NAc, the medium spiny neurons, does not form until the end of the third postnatal week82,86. There is evidence of extensive pruning and remodeling within the dopaminergic system in the post-weaning period87. Thus, although reward circuits are physically formed by the end of lactation, processes that control learning-based aspects of feeding behavior are not developed until post-ingestive consequences can be reinforced by the action of cortico-limbic circuits, which mature in the post-weaning period88. More detailed information about the ontogeny of reward circuits can be obtained from83.
Figure 4. Ontogeny of reward control systems in rodents.
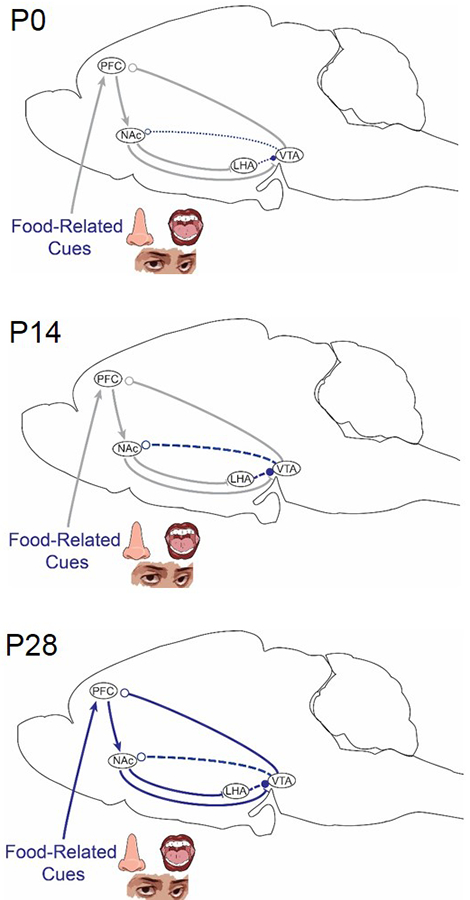
Information about the sensory properties of food is relayed from the prefrontal cortex (PFC) to key nodes in circuits that regulate reward. Projections between some nodes in the mesolimbic dopamine system can be detected at birth (lateral hypothalamic area (LHA)→ ventral tegmental area (VTA) and VTA→ nucleus accumbens (NAc)). Dopamine and opioid signaling pathways develop across lactation and are not fully functional until a week after weaning (postnatal day 28 (P28)). Thin dotted red lines indicate the presence of a physical connection, but weak or immature signal transmission. Bold dashed blue lines represent signal transmission that is robust, but immature. Solid blue lines represent mature connections. Gray lines represent projections for which developmental datapoints are missing. Arrowheads represent excitatory inputs, whereas lines represent inhibitory inputs. Open circles represent dopamine signaling.
Homeostatic Control Systems
Subpopulations of neurons in the ARH directly sense a wide range of circulating factors and thus serve as a conduit for transmitting maternal signals to feeding circuits in the brain. The majority of newly-born ARH neurons initially express pro-opiomelancortin (POMC) (Figure 5)89. The transmitters and neuropeptides that comprise the signaling outputs in adults (NPY, GABA and AgRP) are progressively turned on in the latter half of gestation and the early postnatal period89. During the early postnatal period, AgRP neurons that project to pre-autonomic neurons in the PVH that regulate feeding transiently express leptin receptor (LepR); the onset of expression in other AgRP neurons takes an additional two weeks to complete37,90–92. Throughout lactation, leptin activates and ghrelin inhibits AgRP neurons to regulate axonal outgrowth to downstream targets that control food intake75,92–95. In the latter half of lactation, AgRP neurons are also activated by excitatory inputs, but inhibitory inputs do not arrive until the post-weaning period96. Projections from AgRP neurons reach pre-autonomic components of the feeding circuitry in the PVH between at P14 in mice75,93,94 and P15–16 in rats93. AgRP→PVH projections reach adult levels by weaning (at P21), with no evidence of pruning75. In mice, AgRP projections to the reward circuits via the LHA appear at P12, while projections to cortico-limbic circuits via the BNST appear at P1675. Intrinsic (ion channels) and extrinsic (synaptic inputs) inhibitory regulation of AgRP neurons emerges in post-weaning period92,97. Less is known about the onset of homeostatic modulation of visceral and reward systems by direct signaling via leptin and ghrelin in these circuits. Leptin receptor expression and signaling in the rat VTA emerges in the second week78,79. In summary, although connectivity with homeostatic circuits is established by the second half of lactation, regulation of feeding behaviors in response to the availability of short- and long-term energy stores does not emerge until one week after weaning (P28)92,98–101. Before this stage, food intake is primarily driven by energetic requirements for temperature regulation and growth102. Thus, genetic disruptions in homeostatic circuits in the hypothalamus lead to increased growth103–105. More detailed information about the ontogeny of homeostatic circuits can be obtained from7,106.
Feeding circuit development in humans
As mentioned above, the ontogeny of human feeding circuit development has parallels with rodents. Human neonates have the ability to respond to maternal odors, mediated by olfactory control systems. At birth they exhibit a preference for amniotic fluid, but within the first week, this transitions to a preference for breast odor107. Tactile and social bonding are sufficient to positively reinforce the preference for breast odor, but orosensory reward likely contributes as well108,109. Infants are also capable of basic reactions to sweet or bitter taste. These responses are generated within the brainstem and do not require connectivity to higher brain structures110.
The ontogeny of indirect control systems in humans can be approximated by the onset of appetitive traits that distinguish between satiety, reward, homeostatic and emotional aspects of feeding behavior. The validation of the Baby and Child Eating Behavior Questionnaires as standardized methods to quantify distinct appetitive traits in infants and children111,112 made it possible to conduct prospective analyses of changes in their relative contributions to feeding behavior across the developmental continuum, an issue that has not been assessed in rodent models.
Within the first six to eight weeks of life, infants regulate food intake in response to nutrient and hormonal cues to maintain consistent levels of caloric intake over a 24 hour period – which involves input to the brain from the viscera. They compensate for variation in the frequency of meals or in the caloric density of a milk-based formula by adjusting the amount they consume113–116. This precise regulation of caloric intake is maintained through one year of age and the initial introduction of solid foods with different caloric densities113–116. The emergence of basic feeding regulation systems as assessed in the laboratory, is also reflected by the acquisition of quantifiable traits related to “satiety responsiveness” and “food responsiveness” that are associated with specific patterns of food intake117. High “satiety responsiveness” scores are associated with a smaller meal size but not with meal frequency, while high “food responsiveness” scores are associated with more-frequent meals but not with meal size118. In the first year of life, regulation of feeding behavior is largely driven by control systems that modulate food seeking versus meal terminating behaviors119, consistent with the maturation of orosensory and visceral control systems.
Basic patterns of connectivity are established within homeostatic circuits during gestation120. During the first year of life, there is no evidence of homeostatic regulation of food intake in response to signals of energy stores. Instead, nutrient intake is tightly correlated with and predictive of growth, not adiposity121–123. As observed in rodents, mutations that disrupt homeostatic systems exhibit increased growth in humans124. During the early childhood years, the association between intake and growth is gradually lost125.
Similar to rodents, mesolimbic reward circuits are formed during gestation126, but reward-based learning and motivation do not develop until the transition to independent feeding. As variety is increasingly introduced into the diet, toddlers learn to form associations between foods and post-ingestive consequences of eating and develop aversions to new foods127,128. These learned behaviors are strongly influenced by child-feeding practices and other environmental factors115,127. Between 1–4 years of age, compensatory reductions in portion sizes when consuming energy dense foods are progressively weakened, and individual differences in responsiveness to foods emerge115,116,127,129. Thus, circuits regulating reward-based aspects of feeding behavior develop in early childhood and gradually supersede regulation by orosensory and visceral systems.
Comparisons of rodent and human feeding circuits
Crucial to understanding how experimental manipulations of maternal nutrition in rat and mouse relate to humans is an appreciation for the comparative developmental biology of the species. The step-wise ontogeny of different feeding control systems progresses similarly in rodents and humans (Figure 6). Direct orosensory systems operate soon after birth in both species. During the period when milk or formula is the primary source of food, the balance between orosensory and visceral systems maintains a stable level of caloric intake. Although reward circuits are formed during gestation in rodents and humans, reward-based control of food intake is not a primary determinant of feeding behavior until more variety is introduced into the diet and learned associations can be drawn. At this point, visceral satiety systems become less prominent. Connectivity with homeostatic feeding circuits is established within the second postnatal week in rodents and within the third trimester in humans. Initially the function of homeostatic circuits is tightly linked to requirements for thermogenesis and growth, and the regulation of feeding behavior in response to circulating signals of energy status emerges after puberty. As children enter adolescence, traits related to “emotional eating” emerge as the principal determinants of eating behavior119,128,130,131. Little is known about the ontogeny of emotional control systems in rodent models.
Figure 6. Step-wise progression of ontogeny of feeding control systems is conserved between humans and rodents.
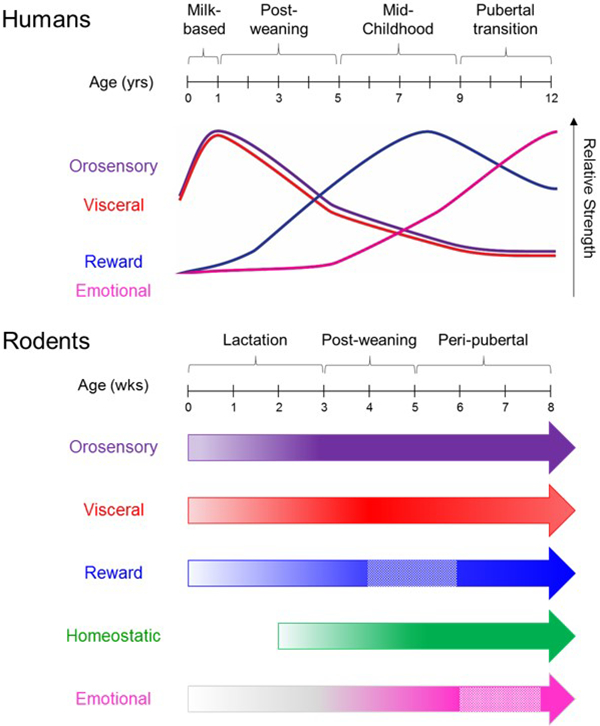
In humans (top), population-based studies of feeding behavior demonstrate that the relative balance between different control systems changes across the developmental continuum. In rodents (bottom), the ontogeny of control systems has been defined at the neuroanatomical level. The start position of each arrow reflects the onset of a physical connection. Gradations of color intensity reflect increased maturation. Boxes outlined with dashed lines in reward and emotional systems represent periods of remodeling. Circuits are most sensitive to the effects of external influences during periods of maturation and remodeling.
While the absolute timing of developmental processes is necessarily different, the emergence of different control systems in relation to major developmental milestones is highly conserved. Orosensory (purple) and visceral (red) control systems are present at birth, but are superseded by other controls systems. Reward control systems (blue) gain in importance after weaning and are influenced by environmental factors. Emotional control systems (pink) are the primary driver of weight gain in adolescence. Homeostatic control systems (green) have not been assessed in studies in humans.
Maternal Programming of Feeding Behaviors
Studies in humans and animal models consistently find a link between maternal metabolic status and long-term impact on susceptibility to obesity. Experiments in animal models provide strong evidence of pronounced maternal influences on the formation of hypothalamic feeding circuits and on feeding behavior at weaning. However, more detailed analysis reveals a more complex picture, with two main areas of contention: whether maternal influences on feeding behaviors persist to adulthood; and whether influences on feeding behavior contribute to obesity risk, or whether effects on energy expenditure are responsible. Resolving these issues is critical to elucidating the underlying mechanism.
Maternal Influences in Humans
A large body of epidemiological evidence supports the idea that exposure to maternal undernutrition and obesity programs increased obesity risk (for excellent reviews of this literature, see132–134). Because maternal nutritional and metabolic status are usually similar across all of human gestation, it is difficult to determine the time window for these effects. Two types of studies have been used to parse the contribution of early vs. late gestational influences to programming of obesity risk. Epidemiologists took advantage of the fact that famine conditions during the Dutch Hunger Winter of 1944–45 was limited in duration to evaluate the consequences of exposure to maternal undernutrition in the first vs. third trimester135. Temporal considerations were incorporated into studies of maternal obesity by comparing the influence of pre-gravid maternal BMI vs. gestational weight gain on later adiposity in offspring136. In both situations, the data point toward the first trimester as the critical period for programming susceptibility to obesity, while the third trimester is most important for birth weight. There is a growing appreciation that a rapid growth rate during infancy, and not initial birth weight, is the critical determinant of obesity risk137–141.
After birth, there is strong evidence to support the idea that maternal factors influence the development of orosensory positive feedback systems regulating infant feeding behavior. The responsiveness of olfactory and gustatory sensory systems to maternal odors and flavors is positively reinforced by tactile stimuli and social bonding. Whereas preferences for sweet and avoidance of bitter flavors are strongly influenced by innate factors, exposure to certain volatile flavors in amniotic fluid as well as breast milk shapes flavor preferences later in life142. In addition, offspring preference for fat and protein at 10 years of age is correlated with maternal consumption of these macronutrients during gestation, but not with postnatal maternal diet or paternal diet50. Studies in non-human primates further support the idea that maternal pre-gravid obesity and high fat diet consumption during pregnancy synergize to promote fat consumption in offspring at weaning, without effects on overall calorie intake51.
Development of gustatory systems in pre-term infants exposed to severe IUGR may be delayed143, but the ability to detect sweet or fat tastes is not impaired in adulthood15,144. Similar to observations with maternal obesity, exposure to maternal undernutrition or intrauterine growth restriction (IUGR) is also consistently associated with increased preference for palatable foods in the first few years of life, without effects on total caloric intake. However, there are differences between studies in the preferred macronutrient as well as the persistence of the effect. Some groups reported increased consumption of carbohydrates144, while others observed increased fat consumption15,17,145,146. Some studies reported that the preference for palatable food is diminished with age17, while others could detect effects in adults15,144,146. There also differences with regard to whether the primary impact is on females144, males145, or both genders15. Apparent inconsistencies between effects could stem from differences in the severity and duration of gestational restriction, as well as conditions in the postnatal environment.
The major limitation of epidemiological studies of maternal programming in the context of this article is that the main outcome measure is BMI, and rarely feeding behavior. Questionnaire-based quantification of appetitive traits has not yet been applied to investigate possible influences of maternal metabolic or nutritional status on specific appetitive traits. However, observations that satiety-related scores in infancy are more strongly correlated in monozygotic vs. dizygotic twins are consistent with the possibility that the gestational environment influences the development of visceral control systems147.
Maternal effects on obesity risk?
Prospective studies of appetitive traits in two independent cohorts in Europe and Asia found that low “satiety-responsiveness” trait scores at 3 months are associated with increased meal size, and are the largest determinants of excess weight gain in the first two years of life148–151,52. Importantly, the inverse relationship between satiety-related appetite scores and weight gain in infancy is observed across the BMI spectrum and is independent of initial body weight. However, early appetitive traits do not continue as far as adolescence and the tight association with BMI is lost by 5–8 years of age119,152. This observation raises the possibility that maternal influences on orosensory and visceral control systems are not the primary determinants of obesity risk in adulthood.
The gradual shift in strength from satiety- to reward-based regulation of food intake that occurs during early childhood (1–5 years) is reflected in the relationship between appetitive traits and subsequent weight gain115,116,119,127,128,130,153. Satiety-related appetitive scores are no longer predictive of later weight gain, while scores related to emotional and motivated aspects of feeding are associated with higher BMI119,152,154,155. Prospective twin studies support the idea that feeding behavior in middle childhood is strongly influenced by environmental factors, including the quality and quantity of available food, parenting styles and psychosocial stress128,131,156. During adolescence, influences of environmental factors on feeding behavior decrease while genetic (and shared placental) contributions increase156. Emotional eating in the absence of hunger, but not deficits in satiety or homeostatic regulation, has been implicated as a primary driver of weight gain in adolescence119,128.
In summary, studies in humans identified influences of gestational exposures on early appetitive traits that do not persist or predict weight gain in adulthood, yet long-lasting impacts on food preferences and obesity risk can be observed. To begin to understand the mechanism underlying these apparently paradoxical observations, I provide an overview of studies maternal influences on neurobiological and behavioral correlates of feeding control systems in rodents.
Maternal Influences in Rodents
A wide variety of developmental exposures have been used to investigate the timing and persistence of maternal influences on food intake in offspring. Despite some inconsistencies between findings in individual studies, there general principles that emerge from this body of research. These are discussed in the next section followed by a brief overview of impacts of maternal influences on reward and homeostatic circuits, where they are best characterized. For more comprehensive reviews of maternal influences on feeding circuits see7,106,157–159.
Although in humans, the formation of circuits regulating basic feeding behaviors is largely complete during gestation, in rodents, connectivity is not established until the suckling period160. By targeting experimental manipulations to gestation and/or lactation, researchers identified periods, and therefore processes, that are sensitive to maternal influences. The most common strategies to study developmental programming of obesity risk involve manipulations of the maternal diet (i.e. caloric or macronutrient restriction or high fat diet (HFD)) or reducing or increasing postnatal litter size (to achieve over- or under-nutrition, respectively). These experimental paradigms consistently produce significant effects on offspring body weight and food intake at weaning. However, there are disagreements about the degree to which effects on feeding behavior are permanently “programmed”. A major limitation of studies in rodents is that caloric intake of a single diet provided ad libitum is usually examined over a period of 1 to 7 days, which does not distinguish between different types of feeding control systems. In addition, a major confound that has rarely been addressed in rodent studies is the effect of rearing below the thermoneutral zone, which leads to increased metabolism and food intake161–164.
Impacts of Maternal Undernutrition
Reward Control Systems
Exposure to undernutrition exclusively during rodent gestation has been used to model maternal influences during the first trimester in humans, the time frame implicated in epidemiological studies of the Dutch Hunger Winter135. A meta-analysis of 89 effect sizes from 13 studies in 4 strains of mice and 40 studies in 3 strains of rats found weak, non-significant effects of gestational undernutrition on offspring caloric intake12. However, exposure to a low-protein diet during gestation (but not caloric restriction per se) is associated with mild hyperphagia in adult females (12 weeks), but not males12. Consistent with observations in humans15,165, exposure to undernutrition during gestation is associated with an increased preference consumption of high fat foods in male and female rats13 and increased motivation for palatable food reward in male rats166,167 and mice168. However, preferences for fat are only linked to increased adiposity in females, with no effect on body weight in either gender13. With age, effects on food preference are no longer apparent (30 weeks)13.
Despite the robust link between gestational restriction and fat preference in rodents and humans, consistent neurobiological correlates have not been identified. Some groups reported increased responsiveness to a palatable food reward in the NAc of adult rats166,167, but other groups did not169,170. Although several groups reported changes in the expression of key components of the dopamine signaling pathway in the NAc, findings were not consistent across the groups168–171.
Homeostatic Control Systems
The most pronounced effects on growth and food intake are achieved by manipulations during the lactation period in rodents and the third trimester in humans136. Increasing litter sizes to cause undernutrition by limiting the milk supply to an individual pup results in marked reductions in caloric intake at weaning60,61, but these often do not persist to adulthood. Exposure to postnatal undernutrition has been linked to increased weight gain and adiposity when challenged with a HFD. However, higher weight gain is not due to increased caloric intake172–177. The only large-scale study to study the impact of litter size across the spectrum revealed that the main determinant of HFD-induced weight gain in adulthood is post-weaning growth rate and adiposity, and not food intake177.
The maturation of circuits that provide pre-synaptic and post-synaptic inhibitory signals to AgRP neurons is delayed by postnatal undernutrition in rodents97. The increased time window for leptin-induced activation of AgRP neurons likely underlies the persistent increase in orexigenic AgRP→PVH projections in models of undernutrition97,178–181. The delay in the onset of negative feedback from leptin signaling also promotes catch-up growth97, a significant predictor of obesity risk137–141.
Impacts of Maternal Obesity
Reward Control Systems
Maternal intake of an obesogenic diet has been used to model developmental exposure to obesity. Exposure throughout gestation and lactation to maternal consumption of an obesogenic HFD is correlated with increases preference for fat in offspring in young adult rats (10–12 weeks)14,182. Although some groups report that this effect is permanent194, others found that it diminished with age14,182. As reported in early childhood in humans128,131,156, environmental influences in the rodent post-weaning period are important determinants of food preferences. Exposure to a low fat/sugar diet from 3–6 weeks can reverse food preferences associated with gestational exposure to high fat/sugar diets183, and conversely, exposure to high fat/sugar diets from 3–4 weeks is sufficient to program persistent preferences for dietary fat in adulthood16.
Several groups assessed the impact of exposure to an obesogenic maternal diet on the expression of opioid and dopamine pathway components in mesolimbic reward circuits. Among these studies, the most consistent finding is that mu opioid receptor (MOR) expression is elevated in the NAc182,184–187. Increased MOR expression is associated with hypomethylation184 and is reversed with methyl donor supplementation188. However, normalization of expression levels by treatment with a methyl donor188 or naloxone189 is not sufficient to reverse the preference for fat. Another complexity in transcriptional analyses of dopamine and opioid circuits is that they undergo extensive remodeling in the peri-weaning period. For example, MOR expression is positively correlated with fat intake in the post-weaning period (6 weeks)183, but this relationship is reversed at 3 months182. This plasticity likely underlies observations that the post-weaning diet can override earlier exposures183,16.
Homeostatic Control Systems
As seen with undernutrition, the largest impact of exposure to overnutrition on caloric intake and growth is observed during rodent lactation. Reductions in litter size to produce “overnutrition” due to less suckling competition or maternal HFD consumption during lactation lead to increased caloric intake and adiposity at weaning60,61,190–194. Although findings of programmed increases in body weight are consistent, observations about the impacts on offspring food intake are not. A meta-analysis of approximately 2500 unique individuals for food intake and body weight measurements from 53 studies in 8 laboratory strains of rats and mice identified the main source of variability in reported outcomes – whether food intake is scaled allometrically to body mass194. There are small and statistically non-significant difference in food intake, indicating that apparent increases in food intake likely reflect increased body mass of offspring. Notably, offspring diet did not interact with maternal HFD exposure to increase weight gain. Thus, overnutrition models in rodents recapitulate the observation that accelerated postnatal growth is the strongest predictor of obesity risk in humans139,195. However, the data support the idea that influences of early growth rates on later obesity risk are primarily mediated via effects on systems regulating energy expenditure and not food intake106,177,196.
AgRP neuronal maturation extends from mid-gestation through the post-weaning period. Although maternal factors can alter the number of NPY+ neurons during gestation and lactation, these differences rarely persist to adulthood197,198. Exposure to maternal obesity or over-nutrition during lactation can also reduce the number of neurons that express leptin receptors, with lasting decreases in responsiveness to negative homeostatic feedback by circulating leptin199,200. In parallel, genetic or nutritional manipulations that reduce leptin signaling during lactation (i.e. HFD, diabetes) are also associated with a permanent reduction in the number of orexigenic AgRP→PVH inputs193,199,201,202. These counterbalanced impacts of maternal obesity on the homeostatic system are reflected in the absence of persistent impact on caloric intake.
Maternal Programming: a framework
There are strong parallels between rodent and human studies with respect to the ontogeny of feeding control systems and key periods of susceptibility to maternal influences on feeding behaviors and obesity risk. Behavioral and epidemiological studies in humans provide insights into changes in the relative strength of different feeding control systems across the developmental continuum and the relative importance of environmental factors during each stage. Studies in rodent models identified critical periods of developmental when circuits regulating each type of control system are remodeled, and are thus sensitive to maternal and/or environmental influences. In this section, I develop a generalizable framework for interpreting observations across a range of species and experimental manipulations by exploiting the strengths of each experimental system.
Orosensory and Visceral Control Systems
Maternal nutritional and metabolic status influences early patterns of food intake regulated by direct orosensory and visceral systems. These do not continue into adulthood and do not predict obesity risk, because reward and emotional control systems are the primary determinants of feeding behavior in the post-weaning period (early childhood).
Reward Control Systems
Exposure to maternal undernutrition13,15,203 and consumption of high fat/sugar foods14,50,51,182 are associated with increased preference for fat in the post-weaning period, but this behavior often diminishes with time. Across species, early gestation is a critical window for programming increased preference for high fat foods in response to maternal undernutrition13,15,203. However, in response to a maternal obesogenic diet, exposure during lactation is sufficient to program increased reward signaling in response to fat. Although the underlying neurobiology remains to be elucidated, it does not require changes in expression levels of critical components of the mesolimbic dopamine system188,189,204. Evidence from humans115,127 and rodents16,183 supports the idea that post-weaning diet and other environmental factors continue to influence pruning and remodeling in reward circuits during throughout childhood87, which overrides earlier effects.
Homeostatic Control Systems
Physical connectivity within homeostatic control circuits forms later than other systems (Figure 6B) and across rodent species and experimental paradigms, maternal influences during lactation produce marked and persistent effects on the structure of homeostatic feeding circuits. The onset of homeostatic control of feeding occurs 3–4 weeks after the circuits can sense and transmit leptin and ghrelin signals. The function of homeostatic circuits during lactation and the peri-weaning period in humans and rodents is linked to growth rate and not food intake. Thus, maternal influences on homeostatic systems are well-positioned to mediate the impacts of catch-up growth on later obesity risk. Because the impact of catch-up growth is thought to be mediated via effects on energy expenditure and not on food intake106,139,152, it would seem to contradict the idea that maternal influences on homeostatic circuits are responsible for persistent impacts on feeding behavior.
Homeostatic Circuits and Reward Systems
Recent studies present another possible avenue by which maternal influences on hypothalamic homeostatic circuits during lactation might program lasting impacts on feeding behavior – through indirect effects on the development of reward control systems204. Although the focus of neuroanatomical studies of homeostatic feeding circuits has been on projections to the PVH, innervation of key nodes mediating reward (LHA) is also reduced in mice exposed to an obesogenic diet during lactation193. During lactation, transient projections from AgRP neurons to the VTA provide another means by which nascent homeostatic circuits can communicate with reward circuits205. Projections from AgRP neurons are well-positioned to influence the maturation of opioid and dopamine signaling systems that spans the second to fourth postnatal weeks84,85 (Figure 6B).
Two different experimental paradigms that diminish the formation of AgRP projections to downstream targets in lactation – neonatal AgRP neuronal ablation and/or dysfunction205,206 and exposure to overnutrition/obesogenic diet193,199,201,202 – increase the strength function of reward control systems in response to high fat diet184,191,204,207. These effects do not require changes in the expression of dopamine pathway components189,204, but are dependent on direct ghrelin signaling within the VTA204. Direct ghrelin signaling in dopamine neurons has also been linked to increased sensitivity of feeding behavior to social stress204,208. Thus, reductions in the function of neural homeostatic inputs during development may lead to an enhanced influence of the action of neuroendocrine signals of energy status (i.e. leptin and ghrelin) on the activity of reward systems.
Summary and Future Directions
Feeding behavior reflects the integrated output of orosensory, visceral, reward, homeostatic and emotional control systems. In adults, the balance between these system shifts in response to metabolic status (i.e. fasting vs. fed) and type of food (palatable vs. aversive). This review presents evidence that the balance between these systems changes across the developmental continuum as additional control systems emerge and mature. Studies in rodent models have provided insight into how maternal influences during the formation of reward and homeostatic circuits determine fat preference and growth. These effects are later overridden by environmental influences during the post-weaning period, a time of synaptic remodeling in reward circuits. Investigating how interactions between developing homeostatic and reward circuits regulate susceptibility to nutritional and environmental stressors in an important area for future research. In addition, we need to define neurobiological substrates of emotional control systems that emerge in adolescence as primary drivers of feeding behavior and weight gain. The application of advanced techniques to trace neuronal projections and synaptic architecture in the intact brain209 and systems to record activation in genetically-defined subsets of neurons in free-behaving animals210,211 will accelerate these efforts. To increase the likelihood that experimental paradigms in rodents are addressing physiologically relevant phenomena, studies to assess the influence of maternal factors on different types of feeding behavior across the developmental continuum in humans are critical.
Acknowledgements
This work was funded by the NIH R01 DK089038, the Klarman Family Foundation for Eating Disorders Research and the American Diabetes Association (1–17-IBS-208).
Glossary
- Metabolic status
the sum of short-term energy availability and long-term energy stores; this information is transmitted by a combination of nutrient (i.e. glucose), hormonal (i.e. insulin) and neural (i.e. vagus-mediated gastric distension) signals.
- Maternal undernutrition
insufficient food intake during pregnancy and/or lactation, usually resulting in growth restriction.
- Pre-gravid
relating to the period before pregnancy
- Emotional eating
eating to satisfy emotional needs rather than to satisfy hunger or homeostatic needs; a classic example is eating behavior in response to stress.
- Dutch Hunger Winter
a famine that took place in the Netherlands near the end of World War II. Epidemiological studies of children of pregnant women exposed to this famine provided some of the earliest evidence of maternal programming of disease risk.
- Orosensory inputs
information about food from sensory receptors in the oral cavity, such taste and texture
- Intrauterine growth restriction
a condition when a baby is smaller than expected for its gestational age because it is not growing at the normal rate inside the uterus.
References
- 1.Taylor PD & Poston L Developmental programming of obesity in mammals. Exp Physiol 92, 287–298, (2007). [DOI] [PubMed] [Google Scholar]
- 2.Spencer SJ Early life programming of obesity: the impact of the perinatal environment on the development of obesity and metabolic dysfunction in the offspring. Curr Diabetes Rev 8, 55–68, (2012). [DOI] [PubMed] [Google Scholar]
- 3.Hales CN & Barker DJ The thrifty phenotype hypothesis. British medical bulletin 60, 5–20, (2001).This article proposed the concept that exposure to environmental factors in utero, and poor maternal nutrition and restricted fetal growth in particular, influences later risk of metabolic dysfunction.
- 4.Ellis PJ et al. Thrifty metabolic programming in rats is induced by both maternal undernutrition and postnatal leptin treatment, but masked in the presence of both: implications for models of developmental programming. BMC Genomics 15, 49, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vickers MH Early life nutrition, epigenetics and programming of later life disease. Nutrients 6, 2165–2178, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholas LM et al. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes (Lond) 40, 229–238, (2016). [DOI] [PubMed] [Google Scholar]
- 7.Elson AE & Simerly RB Developmental specification of metabolic circuitry. Front Neuroendocrinol 39, 38–51, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dearden L & Ozanne SE Early life origins of metabolic disease: Developmental programming of hypothalamic pathways controlling energy homeostasis. Front Neuroendocrinol 39, 3–16, (2015). [DOI] [PubMed] [Google Scholar]
- 9.Bouret SG, Draper SJ & Simerly RB Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110, (2004).By demonstrating that leptin signals during a critical period of development influence the formation of hypothalamic circuits regulating body weight in rodents, this article paved the way for studies of the mechanism underlying maternal programming of obesity and diabetes risk.
- 10.Coupe B, Grit I, Darmaun D & Parnet P The timing of “catch-up growth” affects metabolism and appetite regulation in male rats born with intrauterine growth restriction. Am J Physiol-Reg I 297, R813–R824, (2009). [DOI] [PubMed] [Google Scholar]
- 11.Zhu S, Eclarinal J, Baker MS, Li G & Waterland RA Developmental programming of energy balance regulation: is physical activity more ‘programmable’ than food intake? Proc Nutr Soc 75, 73–77, (2016). [DOI] [PubMed] [Google Scholar]
- 12.Lagisz M et al. Transgenerational effects of caloric restriction on appetite: a meta-analysis. Obes Rev 15, 294–309, (2014). [DOI] [PubMed] [Google Scholar]
- 13.Bellinger L, Lilley C & Langley-Evans SC Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr 92, 513–520, (2004).This article presents the first evidence that exposure to maternal undernutrition exerts a long-lasting effect on feeding behavior in rats - increased preference for high fat foods, with no effect on total caloric intake.
- 14.Bayol SA, Farrington SJ & Stickland NC A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr 98, 843–851, (2007). [DOI] [PubMed] [Google Scholar]
- 15.Lussana F et al. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am J Clin Nutr 88, 1648–1652, (2008). [DOI] [PubMed] [Google Scholar]
- 16.Teegarden SL, Scott AN & Bale TL Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience 162, 924–932, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shultis WA et al. Does birth weight predict childhood diet in the Avon longitudinal study of parents and children? J Epidemiol Community Health 59, 955–960, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanson LW Cerebral hemisphere regulation of motivated behavior. Brain Res 886, 113–164, (2000). [DOI] [PubMed] [Google Scholar]
- 19.Berthoud H-R Multiple neural systems controlling food intake and body weight. Neuroscience & Biobehavioral Reviews 26, 393–428, (2002). [DOI] [PubMed] [Google Scholar]
- 20.Sternson SM & Eiselt AK Three Pillars for the Neural Control of Appetite. Annu Rev Physiol 79, 401–423, (2017). [DOI] [PubMed] [Google Scholar]
- 21.Smith GP The direct and indirect controls of meal size. Neurosci Biobehav Rev 20, 41–46, (1996).This article classified controls of meal size as “direct” or “indirect” on the basis of whether they involved direct stimulation of preabsorptive receptors with food stimuli.
- 22.Schwartz GJ & Zeltser LM Functional organization of neuronal and humoral signals regulating feeding behavior. Annu Rev Nutr 33, 1–21, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norgren R Gustatory responses in the hypothalamus. Brain Res 21, 63–77, (1970). [DOI] [PubMed] [Google Scholar]
- 24.Saper CB & Loewy AD Efferent connections of the parabrachial nucleus in the rat. Brain Res 197, 291–317, (1980). [DOI] [PubMed] [Google Scholar]
- 25.Tokita K, Armstrong WE, St John SJ & Boughter JD Jr. Activation of lateral hypothalamus-projecting parabrachial neurons by intraorally delivered gustatory stimuli. Front Neural Circuits 8, 86, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reilly S The parabrachial nucleus and conditioned taste aversion. Brain Res Bull 48, 239–254, (1999). [DOI] [PubMed] [Google Scholar]
- 27.Baird JP, Travers SP & Travers JB Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281, R1581–1593, (2001). [DOI] [PubMed] [Google Scholar]
- 28.Carter ME, Soden ME, Zweifel LS & Palmiter RD Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alhadeff AL, Baird JP, Swick JC, Hayes MR & Grill HJ Glucagon-like Peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology 39, 2233–2243, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinaman L Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350, 18–34, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CS, Cho YK & Smith DV Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol 93, 1183–1196, (2005). [DOI] [PubMed] [Google Scholar]
- 32.Wu Z et al. GABAergic projections from lateral hypothalamus to paraventricular hypothalamic nucleus promote feeding. J Neurosci 35, 3312–3318, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowley MA et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661, (2003). [DOI] [PubMed] [Google Scholar]
- 34.van den Top M, Lee K, Whyment AD, Blanks AM & Spanswick D Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7, 493–494, (2004). [DOI] [PubMed] [Google Scholar]
- 35.Aponte Y, Atasoy D & Sternson SM AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14, 351–355, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krashes MJ et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betley JN, Cao ZF, Ritola KD & Sternson SM Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naleid AM, Grace MK, Cummings DE & Levine AS Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 26, 2274–2279, (2005). [DOI] [PubMed] [Google Scholar]
- 39.Abizaid A et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116, 3229–3239, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hommel JD et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51, 801–810, (2006). [DOI] [PubMed] [Google Scholar]
- 41.Grill HJ et al. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143, 239–246, (2002). [DOI] [PubMed] [Google Scholar]
- 42.Hayes MR et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11, 77–83, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alhadeff AL, Hayes MR & Grill HJ Leptin receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake. Am J Physiol Regul Integr Comp Physiol 307, R1338–1344, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Lin YC, Kuo TW & Knight ZA Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malik S, McGlone F, Bedrossian D & Dagher A Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 7, 400–409, (2008). [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Zhang X, Muralidhar S, LeBlanc SA & Tonegawa S Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron 93, 1464–1479 e1465, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sah P & Lopez De Armentia M Excitatory synaptic transmission in the lateral and central amygdala. Ann N Y Acad Sci 985, 67–77, (2003). [DOI] [PubMed] [Google Scholar]
- 48.Beier KT et al. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell 162, 622–634, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jennings JH, Rizzi G, Stamatakis AM, Ung RL & Stuber GD The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brion MJ et al. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: exploring parental comparisons and prenatal effects. Am J Clin Nutr 91, 748–756, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivera HM et al. Maternal high-fat diet and obesity impact palatable food intake and dopamine signaling in nonhuman primate offspring. Obesity (Silver Spring) 23, 2157–2164, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johanson IB & Hall WG Appetitive learning in 1-day-old rat pups. Science 205, 419–421, (1979). [DOI] [PubMed] [Google Scholar]
- 53.Hall WG Feeding and behavioral activation in infant rats. Science 205, 206–209, (1979). [DOI] [PubMed] [Google Scholar]
- 54.Phifer CB, Browde JA Jr. & Hall WG Ontogeny of glucose inhibition of independent ingestion in preweanling rats. Brain Res Bull 17, 673–679, (1986). [DOI] [PubMed] [Google Scholar]
- 55.Ackerman SH, Albert M, Shindledecker RD, Gayle C & Smith GP Intake of different concentrations of sucrose and corn oil in preweanling rats. Am J Physiol 262, R624–627, (1992). [DOI] [PubMed] [Google Scholar]
- 56.Terry LM & Johanson IB Olfactory influences on the ingestive behavior of infant rats. Dev Psychobiol 20, 313–331, (1987). [DOI] [PubMed] [Google Scholar]
- 57.Myers KP, Ferris J & Sclafani A Flavor preferences conditioned by postingestive effects of nutrients in preweanling rats. Physiol Behav 84, 407–419, (2005). [DOI] [PubMed] [Google Scholar]
- 58.Myers KP & Sclafani A Development of learned flavor preferences. Dev Psychobiol 48, 380–388, (2006). [DOI] [PubMed] [Google Scholar]
- 59.Philopena J, Greenberg D & Smith GP Naloxone decreases intake of 10% sucrose in preweanling rats. Pharmacol Biochem Behav 54, 333–337, (1996). [DOI] [PubMed] [Google Scholar]
- 60.Widdowson EM & McCance RA The Effect of Finite Periods of Undernutrition at Different Ages on the Composition and Subsequent Development of the Rat. Proc R Soc Lond B Biol Sci 158, 329–342, (1963).This article provides the first evidence that the amount of milk consumed during rodent lactation can have lasting impacts on body weight and feeding behavior.
- 61.Fiorotto ML, Burrin DG, Perez M & Reeds PJ Intake and use of milk nutrients by rat pups suckled in small, medium, or large litters. Am J Physiol 260, R1104–1113, (1991). [DOI] [PubMed] [Google Scholar]
- 62.Zhang LL & Ashwell KW Development of the cyto- and chemoarchitectural organization of the rat nucleus of the solitary tract. Anat Embryol (Berl) 203, 265–282, (2001). [DOI] [PubMed] [Google Scholar]
- 63.Rinaman L & Levitt P Establishment of vagal sensorimotor circuits during fetal development in rats. J Neurobiol 24, 641–659, (1993). [DOI] [PubMed] [Google Scholar]
- 64.Rinaman L, Roesch MR & Card JP Retrograde transynaptic pseudorabies virus infection of central autonomic circuits in neonatal rats. Brain Res Dev Brain Res 114, 207–216, (1999). [DOI] [PubMed] [Google Scholar]
- 65.Swithers SE & Hall WG A nutritive control of independent ingestion in rat pups emerges by nine days of age. Physiol Behav 46, 873–879, (1989). [DOI] [PubMed] [Google Scholar]
- 66.Rinaman L Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiol Behav 79, 65–70, (2003). [DOI] [PubMed] [Google Scholar]
- 67.Grill HJ & Norgren R Chronically decerebrate rats demonstrate satiation but not bait shyness. Science 201, 267–269, (1978).This article demonsrates that the caudal brainstem is sufficient to mediate negative feedback on feeding behavior from visceral cues, but not learning-based controls.
- 68.Lorenz DN, Ellis SB & Epstein AN Differential effects of upper gastrointestinal fill on milk ingestion and nipple attachment in the suckling rat. Dev Psychobiol 15, 309–330, (1982). [DOI] [PubMed] [Google Scholar]
- 69.Koehnle TJ & Rinaman L Progressive postnatal increases in Fos immunoreactivity in the forebrain and brain stem of rats after viscerosensory stimulation with lithium chloride. Am J Physiol Regul Integr Comp Physiol 292, R1212–1223, (2007). [DOI] [PubMed] [Google Scholar]
- 70.Rinaman L, Hoffman GE, Stricker EM & Verbalis JG Exogenous cholecystokinin activates cFos expression in medullary but not hypothalamic neurons in neonatal rats. Brain Res Dev Brain Res 77, 140–145, (1994). [DOI] [PubMed] [Google Scholar]
- 71.Lasiter PS & Kachele DL Postnatal development of the parabrachial gustatory zone in rat: dendritic morphology and mitochondrial enzyme activity. Brain Res Bull 21, 79–94, (1988). [DOI] [PubMed] [Google Scholar]
- 72.Rao H, Jean A & Kessler JP Postnatal ontogeny of glutamate receptors in the rat nucleus tractus solitarii and ventrolateral medulla. J Auton Nerv Syst 65, 25–32, (1997). [DOI] [PubMed] [Google Scholar]
- 73.Yoshioka M & Kawai Y Activity-dependent reorganization of local circuitry in the developing visceral sensory system. Neuroscience 150, 905–914, (2007). [DOI] [PubMed] [Google Scholar]
- 74.Rinaman L Postnatal development of catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol 438, 411–422, (2001). [DOI] [PubMed] [Google Scholar]
- 75.Bouret SG, Draper SJ & Simerly RB Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24, 2797–2805, (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vincent A & Tell F Postnatal development of rat nucleus tractus solitarius neurons: morphological and electrophysiological evidence. Neuroscience 93, 293–305, (1999). [DOI] [PubMed] [Google Scholar]
- 77.Rinaman L Ontogeny of hypothalamic-hindbrain feeding control circuits. Dev Psychobiol 48, 389–396, (2006). [DOI] [PubMed] [Google Scholar]
- 78.Naef L, Gjerde E, Long H, Richard D & Walker CD Neonatal onset of leptin signaling in dopamine neurons of the ventral tegmental area in the rat J Neuroendocrinol, (2014). [DOI] [PubMed] [Google Scholar]
- 79.Gjerde E, Long H, Richard D & Walker CD Developmental Responses of the Lateral Hypothalamus to Leptin in Neonatal Rats, and its Implications for the Development of Functional Connections with the Ventral Tegmental Area. J Neuroendocrinol 28, 12354, (2016). [DOI] [PubMed] [Google Scholar]
- 80.Hu Z, Cooper M, Crockett DP & Zhou R Differentiation of the midbrain dopaminergic pathways during mouse development. J Comp Neurol 476, 301–311, (2004). [DOI] [PubMed] [Google Scholar]
- 81.Voorn P, Kalsbeek A, Jorritsma-Byham B & Groenewegen HJ The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience 25, 857–887, (1988). [DOI] [PubMed] [Google Scholar]
- 82.Antonopoulos J, Dori I, Dinopoulos A, Chiotelli M & Parnavelas JG Postnatal development of the dopaminergic system of the striatum in the rat. Neuroscience 110, 245–256, (2002). [DOI] [PubMed] [Google Scholar]
- 83.Van den Heuvel DM & Pasterkamp RJ Getting connected in the dopamine system. Prog Neurobiol 85, 75–93, (2008). [DOI] [PubMed] [Google Scholar]
- 84.Spain JW, Roth BL & Coscia CJ Differential ontogeny of multiple opioid receptors (mu, delta, and kappa). J Neurosci 5, 584–588, (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McDowell J & Kitchen I Development of opioid systems: peptides, receptors and pharmacology. Brain Res 434, 397–421, (1987). [DOI] [PubMed] [Google Scholar]
- 86.Tepper JM, Sharpe NA, Koos TZ & Trent F Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev Neurosci 20, 125–145, (1998). [DOI] [PubMed] [Google Scholar]
- 87.Tarazi FI & Baldessarini RJ Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci 18, 29–37, (2000). [DOI] [PubMed] [Google Scholar]
- 88.Ogawa H, Hasegawa K, Ohgushi M & Murayama N Changes in properties of neuronal responses in two cortical taste areas in rats of various ages. Neurosci Res 19, 407–417, (1994). [DOI] [PubMed] [Google Scholar]
- 89.Padilla SL, Carmody JS & Zeltser LM Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med 16, 403–405, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frontini A et al. Leptin-dependent STAT3 phosphorylation in postnatal mouse hypothalamus. Brain Res 1215, 105–115, (2008). [DOI] [PubMed] [Google Scholar]
- 91.Cottrell EC et al. Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat. Am J Physiol Regul Integr Comp Physiol 296, R631–639, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baquero AF et al. Developmental switch of leptin signaling in arcuate nucleus neurons. J Neurosci 34, 9982–9994, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grove KL & Smith MS Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav 79, 47–63, (2003). [DOI] [PubMed] [Google Scholar]
- 94.Bouyer K & Simerly RB Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. J Neurosci 33, 840–851, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steculorum SM et al. Neonatal ghrelin programs development of hypothalamic feeding circuits. J Clin Invest 125, 846–858, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baquero AF et al. Developmental changes in synaptic distribution in arcuate nucleus neurons. J Neurosci 35, 8558–8569, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Juan De Solis A, Baquero AF, Bennett CM, Grove KL & Zeltser LM Postnatal undernutrition delays a key step in the maturation of hypothalamic feeding circuits. Mol Metab 5, 198–209, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahima RS, Prabakaran D & Flier JS Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101, 1020–1027, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mistry AM, Swick A & Romsos DR Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol 277, R742–747, (1999). [DOI] [PubMed] [Google Scholar]
- 100.Ahima RS & Hileman SM Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul Pept 92, 1–7, (2000). [DOI] [PubMed] [Google Scholar]
- 101.Steculorum SM & Bouret SG Developmental effects of ghrelin. Peptides 32, 2362–2366, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kennedy GC The development with age of hypothalamic restraint upon the appetite of the rat. J Endocrinol 16, 9–17, (1957). [DOI] [PubMed] [Google Scholar]
- 103.Huszar D et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141, (1997). [DOI] [PubMed] [Google Scholar]
- 104.Ring LE & Zeltser LM Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest 120, 2931–2941, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bumaschny VF et al. Obesity-programmed mice are rescued by early genetic intervention. J Clin Invest 122, 4203–4212, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeltser LM Developmental influences on circuits programming susceptibility to obesity. Front Neuroendocrinol, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Porter RH & Winberg J Unique salience of maternal breast odors for newborn infants. Neurosci Biobehav Rev 23, 439–449, (1999). [DOI] [PubMed] [Google Scholar]
- 108.Porter RH, Makin JW, Davis LB & Christensen KM An assessment of the salient olfactory environment of formula-fed infants. Physiol Behav 50, 907–911, (1991). [DOI] [PubMed] [Google Scholar]
- 109.Aoyama S et al. Maternal breast milk odour induces frontal lobe activation in neonates: a NIRS study. Early Hum Dev 86, 541–545, (2010). [DOI] [PubMed] [Google Scholar]
- 110.Steiner JE The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept, 254–278, (1973). [PubMed] [Google Scholar]
- 111.Wardle J, Guthrie CA, Sanderson S & Rapoport L Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry 42, 963–970, (2001). [DOI] [PubMed] [Google Scholar]
- 112.Llewellyn CH, van Jaarsveld CH, Johnson L, Carnell S & Wardle J Development and factor structure of the Baby Eating Behaviour Questionnaire in the Gemini birth cohort. Appetite 57, 388–396, (2011).This article describes the development and validation of a parent-report psychometric questionare that quantitfies appetitive traits in infants during the period of exclusive milk-feeding. It opened the door to prospective studies that assess changes in the strengths of these traits across the lifespan.
- 113.Fomon SJ, Filer LJ Jr., Thomas LN, Rogers RR & Proksch AM Relationship between formula concentration and rate of growth of normal infants. J Nutr 98, 241–254, (1969). [DOI] [PubMed] [Google Scholar]
- 114.Adair LS The infant’s ability to self-regulate caloric intake: a case study. J Am Diet Assoc 84, 543–546, (1984). [PubMed] [Google Scholar]
- 115.Birch LL & Deysher M Caloric compensation and sensory specific satiety: evidence for self regulation of food intake by young children. Appetite 7, 323–331, (1986). [DOI] [PubMed] [Google Scholar]
- 116.Fox MK, Devaney B, Reidy K, Razafindrakoto C & Ziegler P Relationship between portion size and energy intake among infants and toddlers: evidence of self-regulation. J Am Diet Assoc 106, S77–83, (2006). [DOI] [PubMed] [Google Scholar]
- 117.Shepard DN & Chandler-Laney PC Prospective associations of eating behaviors with weight gain in infants. Obesity (Silver Spring) 23, 1881–1885, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Syrad H, Johnson L, Wardle J & Llewellyn CH Appetitive traits and food intake patterns in early life. Am J Clin Nutr 103, 231–235, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Parkinson KN, Drewett RF, Le Couteur AS, Adamson AJ & Gateshead Milennium Study Core, T. Do maternal ratings of appetite in infants predict later Child Eating Behaviour Questionnaire scores and body mass index? Appetite 54, 186–190, (2010).This article describes a longitudinal study from 6 weeks to 8 years that demonstrates that appetitive traits in infancy do not continue into later childhood, nor do they predict later BMI.
- 120.Koutcherov Y, Mai JK & Paxinos G Hypothalamus of the human fetus. J Chem Neuroanat 26, 253–270, (2003). [DOI] [PubMed] [Google Scholar]
- 121.Stunkard AJ, Berkowitz RI, Stallings VA & Schoeller DA Energy intake, not energy output, is a determinant of body size in infants. Am J Clin Nutr 69, 524–530, (1999). [DOI] [PubMed] [Google Scholar]
- 122.Stunkard AJ, Berkowitz RI, Schoeller D, Maislin G & Stallings VA Predictors of body size in the first 2 y of life: a high-risk study of human obesity. Int J Obes Relat Metab Disord 28, 503–513, (2004). [DOI] [PubMed] [Google Scholar]
- 123.Hoppe C, Molgaard C, Thomsen BL, Juul A & Michaelsen KF Protein intake at 9 mo of age is associated with body size but not with body fat in 10-y-old Danish children. Am J Clin Nutr 79, 494–501, (2004). [DOI] [PubMed] [Google Scholar]
- 124.Yeo GS et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 20, 111–112, (1998). [DOI] [PubMed] [Google Scholar]
- 125.Pimpin L, Jebb S, Johnson L, Wardle J & Ambrosini GL Dietary protein intake is associated with body mass index and weight up to 5 y of age in a prospective cohort of twins. Am J Clin Nutr 103, 389–397, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meng SZ, Ozawa Y, Itoh M & Takashima S Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Res 843, 136–144, (1999). [DOI] [PubMed] [Google Scholar]
- 127.Birch LL & Fisher JO Development of eating behaviors among children and adolescents. Pediatrics 101, 539–549, (1998). [PubMed] [Google Scholar]
- 128.Ashcroft J, Semmler C, Carnell S, van Jaarsveld CH & Wardle J Continuity and stability of eating behaviour traits in children. Eur J Clin Nutr 62, 985–990, (2008).his article describes a longitudinal study in children between 4 and 10 years that provides evidence that appetitive traits related to satiety tend to decrease with age, while hedonic responses to food tend to increase.
- 129.Shea S, Stein AD, Basch CE, Contento IR & Zybert P Variability and self-regulation of energy intake in young children in their everyday environment. Pediatrics 90, 542–546, (1992). [PubMed] [Google Scholar]
- 130.Pimpin L et al. Dietary intake of young twins: nature or nurture? Am J Clin Nutr 98, 1326–1334, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Llewellyn C & Wardle J Behavioral susceptibility to obesity: Gene-environment interplay in the development of weight. Physiol Behav 152, 494–501, (2015). [DOI] [PubMed] [Google Scholar]
- 132.Fernandez-Twinn DS & Ozanne SE Early life nutrition and metabolic programming. Ann N Y Acad Sci 1212, 78–96, (2010). [DOI] [PubMed] [Google Scholar]
- 133.Poston L Developmental programming and diabetes - The human experience and insight from animal models. Best Pract Res Clin Endocrinol Metab 24, 541–552, (2010). [DOI] [PubMed] [Google Scholar]
- 134.Li M, Sloboda DM & Vickers MH Maternal obesity and developmental programming of metabolic disorders in offspring: evidence from animal models. Exp Diabetes Res 2011, 592408, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ravelli GP, Stein ZA & Susser MW Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 295, 349–353, (1976). [DOI] [PubMed] [Google Scholar]
- 136.Catalano PM et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 90, 1303–1313, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stettler N, Kumanyika SK, Katz SH, Zemel BS & Stallings VA Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr 77, 1374–1378, (2003). [DOI] [PubMed] [Google Scholar]
- 138.Sachdev HS et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr 82, 456–466, (2005). [DOI] [PubMed] [Google Scholar]
- 139.Ong KK & Loos RJ Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr 95, 904–908, (2006).This article descfribes a meta-analysis of 21 studies that unifrmly demonstarte a positive asociation between rapid infancy weight gain, and not birth weight, with increased tisk of obesity.
- 140.Ekelund U et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab 92, 98–103, (2007). [DOI] [PubMed] [Google Scholar]
- 141.Taveras EM et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med 165, 993–998, (2011). [DOI] [PubMed] [Google Scholar]
- 142.Beauchamp GK & Mennella JA Flavor perception in human infants: development and functional significance. Digestion 83 Suppl 1, 1–6, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ayres C et al. Intrauterine growth restriction and the fetal programming of the hedonic response to sweet taste in newborn infants. Int J Pediatr 2012, 657379, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barbieri MA et al. Severe intrauterine growth restriction is associated with higher spontaneous carbohydrate intake in young women. Pediatr Res 65, 215–220, (2009). [DOI] [PubMed] [Google Scholar]
- 145.Stafford M & Lucas A Possible association between low birth weight and later heart disease needs to be investigated further. BMJ 316, 1247–1248, (1998).This article reports that environmental factors influence BMI in childhood, but in late adolescence contributions from genetic factors far outweigh contributions from environmental factors.
- 146.Stein AD, Rundle A, Wada N, Goldbohm RA & Lumey LH Associations of gestational exposure to famine with energy balance and macronutrient density of the diet at age 58 years differ according to the reference population used. J Nutr 139, 1555–1561, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Llewellyn CH, van Jaarsveld CH, Johnson L, Carnell S & Wardle J Nature and nurture in infant appetite: analysis of the Gemini twin birth cohort. Am J Clin Nutr 91, 1172–1179, (2010). [DOI] [PubMed] [Google Scholar]
- 148.van Jaarsveld CH, Llewellyn CH, Johnson L & Wardle J Prospective associations between appetitive traits and weight gain in infancy. Am J Clin Nutr 94, 1562–1567, (2011). [DOI] [PubMed] [Google Scholar]
- 149.van Jaarsveld CH, Boniface D, Llewellyn CH & Wardle J Appetite and growth: a longitudinal sibling analysis. JAMA Pediatr 168, 345–350, (2014). [DOI] [PubMed] [Google Scholar]
- 150.Quah PL et al. Prospective associations of appetitive traits at 3 and 12 months of age with body mass index and weight gain in the first 2 years of life. BMC Pediatr 15, 153, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van Deutekom AW, Chinapaw MJ, Vrijkotte TG & Gemke RJ The association of birth weight and postnatal growth with energy intake and eating behavior at 5 years of age - a birth cohort study. Int J Behav Nutr Phys Act 13, 15, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wright CM et al. To what extent do weight gain and eating avidity during infancy predict later adiposity? Public Health Nutr 15, 656–662, (2012). [DOI] [PubMed] [Google Scholar]
- 153.Carnell S & Wardle J Associations between multiple measures of parental feeding and children’s adiposity in United Kingdom preschoolers. Obesity (Silver Spring) 15, 137–144, (2007). [DOI] [PubMed] [Google Scholar]
- 154.Fuemmeler BF, Lovelady CA, Zucker NL & Ostbye T Parental obesity moderates the relationship between childhood appetitive traits and weight. Obesity (Silver Spring) 21, 815–823, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Syrad H et al. Meal size is a critical driver of weight gain in early childhood. Sci Rep 6, 28368, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Silventoinen K et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) study. Am J Clin Nutr 104, 371–379, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bedont JL, Newman EA & Blackshaw S Patterning, specification, and differentiation in the developing hypothalamus. Wiley Interdiscip Rev Dev Biol 4, 445–468, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gugusheff JR, Ong ZY & Muhlhausler BS The early origins of food ptargeting the critical windows of development. FASEB J 29, 365–373, (2015). [DOI] [PubMed] [Google Scholar]
- 159.Pitman KA & Borgland SL Changes in mu-opioid receptor expression and function in the mesolimbic system after long-term access to a palatable diet. Pharmacol Ther 154, 110–119, (2015). [DOI] [PubMed] [Google Scholar]
- 160.Symonds ME Brown adipose tissue growth and development. Scientifica 2013, 305763, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Young JB & Shimano Y Effects of rearing temperature on body weight and abdominal fat in male and female rats. Am J Physiol 274, R398–405, (1998). [DOI] [PubMed] [Google Scholar]
- 162.Swoap SJ et al. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol 294, H1581–1588, (2008). [DOI] [PubMed] [Google Scholar]
- 163.Cannon B & Nedergaard J Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214, 242–253, (2011). [DOI] [PubMed] [Google Scholar]
- 164.Xiao C, Goldgof M, Gavrilova O & Reitman ML Anti-obesity and metabolic efficacy of the beta3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22 degrees C. Obesity (Silver Spring) 23, 1450–1459, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stein AD et al. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr 85, 869–876, (2007). [DOI] [PubMed] [Google Scholar]
- 166.da Silva AA et al. Perinatal undernutrition stimulates seeking food reward. Int J Dev Neurosci 31, 334–341, (2013). [DOI] [PubMed] [Google Scholar]
- 167.Alves MB, Dalle Molle R, Desai M, Ross MG & Silveira PP Increased palatable food intake and response to food cues in intrauterine growth-restricted rats are related to tyrosine hydroxylase content in the orbitofrontal cortex and nucleus accumbens. Behav Brain Res 287, 73–81, (2015). [DOI] [PubMed] [Google Scholar]
- 168.de Melo Martimiano PH et al. Perinatal malnutrition stimulates motivation through reward and enhances drd(1a) receptor expression in the ventral striatum of adult mice. Pharmacol Biochem Behav 134, 106–114, (2015). [DOI] [PubMed] [Google Scholar]
- 169.Dalle Molle R et al. Intrauterine growth restriction increases the preference for palatable foods and affects sensitivity to food rewards in male and female adult rats. Brain Res 1618, 41–49, (2015). [DOI] [PubMed] [Google Scholar]
- 170.Laureano DP et al. Intrauterine growth restriction modifies the hedonic response to sweet taste in newborn pups - Role of the accumbal mu-opioid receptors. Neuroscience 322, 500–508, (2016). [DOI] [PubMed] [Google Scholar]
- 171.Thanos PK et al. Suboptimal maternal diets alter mu opioid receptor and dopamine type 1 receptor binding but exert no effect on dopamine transporters in the offspring brain. Int J Dev Neurosci, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Levin BE, Triscari J, Marquet E & Sullivan AC Dietary obesity and neonatal sympathectomy. I. Effects on body composition and brown adipose. Am J Physiol 247, R979–987, (1984). [DOI] [PubMed] [Google Scholar]
- 173.Plagemann A et al. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res 836, 146–155, (1999). [DOI] [PubMed] [Google Scholar]
- 174.Biddinger JE & Fox EA Meal parameters and vagal gastrointestinal afferents in mice that experienced early postnatal overnutrition. Physiol Behav 101, 184–191, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Velkoska E, Cole TJ & Morris MJ Early dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, and adiposity markers. Am J Physiol Endocrinol Metab 288, E1236–1243, (2005). [DOI] [PubMed] [Google Scholar]
- 176.Lopez M et al. Perinatal overfeeding in rats results in increased levels of plasma leptin but unchanged cerebrospinal leptin in adulthood. Int J Obes (Lond) 31, 371–377, (2007). [DOI] [PubMed] [Google Scholar]
- 177.Zhang LN, Morgan DG, Clapham JC & Speakman JR Factors predicting nongenetic variability in body weight gain induced by a high-fat diet in inbred C57BL/6J mice. Obesity (Silver Spring) 20, 1179–1188, (2012). [DOI] [PubMed] [Google Scholar]
- 178.Plagemann A et al. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol 11, 541–546, (1999). [DOI] [PubMed] [Google Scholar]
- 179.Plagemann A et al. Hypothalamic neuropeptide Y levels in weaning offspring of low-protein malnourished mother rats. Neuropeptides 34, 1–6, (2000). [DOI] [PubMed] [Google Scholar]
- 180.Yura S et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 1, 371–378, (2005). [DOI] [PubMed] [Google Scholar]
- 181.Patterson CM et al. Large litter rearing enhances leptin sensitivity and protects selectively bred diet-induced obese rats from becoming obese. Endocrinology 151, 4270–4279, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ong ZY & Muhlhausler BS Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J 25, 2167–2179, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ong ZY & Muhlhausler BS Consuming a low-fat diet from weaning to adulthood reverses the programming of food preferences in male, but not in female, offspring of ‘junk food’-fed rat dams. Acta Physiol (Oxf) 210, 127–141, (2014). [DOI] [PubMed] [Google Scholar]
- 184.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E & Reyes TM Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151, 4756–4764, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gugusheff JR et al. Sex and age-dependent effects of a maternal junk food diet on the mu-opioid receptor in rat offspring. Behav Brain Res 301, 124–131, (2016). [DOI] [PubMed] [Google Scholar]
- 186.Grissom NM et al. Obesity at conception programs the opioid system in the offspring brain. Neuropsychopharmacology 39, 801–810, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Paradis J et al. Perinatal Western Diet Consumption Leads to Profound Plasticity and GABAergic Phenotype Changes within Hypothalamus and Reward Pathway from Birth to Sexual Maturity in Rat. Front Endocrinol (Lausanne) 8, 216, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carlin J, George R & Reyes TM Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS One 8, e63549, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gugusheff JR, Ong ZY & Muhlhausler BS Naloxone treatment alters gene expression in the mesolimbic reward system in ‘junk food’ exposed offspring in a sex-specific manner but does not affect food preferences in adulthood. Physiol Behav 133, 14–21, (2014). [DOI] [PubMed] [Google Scholar]
- 190.Bayol SA, Simbi BH & Stickland NC A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 567, 951–961, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gorski JN, Dunn-Meynell AA, Hartman TG & Levin BE Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol 291, R768–778, (2006). [DOI] [PubMed] [Google Scholar]
- 192.Gugusheff JR, Vithayathil M, Ong ZY & Muhlhausler BS The effects of prenatal exposure to a ‘junk food’ diet on offspring food preferences and fat deposition can be mitigated by improved nutrition during lactation. Journal of developmental origins of health and disease 4, 348–357, (2013). [DOI] [PubMed] [Google Scholar]
- 193.Vogt MC et al. Neonatal Insulin Action Impairs Hypothalamic Neurocircuit Formation in Response to Maternal High-Fat Feeding. Cell, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lagisz M et al. Little appetite for obesity: meta-analysis of the effects of maternal obesogenic diets on offspring food intake and body mass in rodents. Int J Obes (Lond), (2015).This article describes a meta-analysis that concludes that feeding behavior is not the primary driver of increased obesity risk in offspring exposed to a maternal obesogenic diet.
- 195.Gillman MW The first months of life: a critical period for development of obesity. Am J Clin Nutr 87, 1587–1589, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Baker MS, Li G, Kohorst JJ & Waterland RA Fetal growth restriction promotes physical inactivity and obesity in female mice. Int J Obes (Lond) 39, 98–104, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Remmers F, Verhagen LA, Adan RA & Delemarre-van de Waal HA Hypothalamic neuropeptide expression of juvenile and middle-aged rats after early postnatal food restriction. Endocrinology 149, 3617–3625, (2008). [DOI] [PubMed] [Google Scholar]
- 198.Carmody JS, Wan P, Accili D, Zeltser LM & Leibel RL Respective Contributions of Maternal Insulin Resistance and Diet to Metabolic and Hypothalamic Phenotypes of Progeny Obesity (Silver Spring), (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bouret SG et al. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7, 179–185, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Glavas MM et al. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology 151, 1598–1610, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kirk SL et al. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One 4, e5870, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Steculorum SM & Bouret SG Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology 152, 4171–4179, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nakashima Y, Tsukita Y & Yokoyama M Preferential fat intake of pups nursed by dams fed low fat diet during pregnancy and lactation is higher than that of pups nursed by dams fed control diet and high fat diet. J Nutr Sci Vitaminol (Tokyo) 54, 215–222, (2008). [DOI] [PubMed] [Google Scholar]
- 204.Denis RG et al. Palatability Can Drive Feeding Independent of AgRP Neurons. Cell Metab 22, 646–657, (2015).This article provides evidence that circuits regulating reward-related feeding behavior can compensate for the loss of homeostatic inputs.
- 205.Dietrich MO et al. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors Nat Neurosci, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luquet S, Perez FA, Hnasko TS & Palmiter RD NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685, (2005). [DOI] [PubMed] [Google Scholar]
- 207.Naef L et al. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 176, 225–236, (2011). [DOI] [PubMed] [Google Scholar]
- 208.Chuang JC et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest 121, 2684–2692, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chung K et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ziv Y et al. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci 16, 264–266, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gunaydin LA et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


