Abstract
Cough-variant asthma is one of the most common reasons for chronic cough. It is important to treat appropriately cough-variant asthma because 30% to 40% of cough-variant asthma becomes a typical asthma. However, little is known about the treatment of cough-variant asthma except for inhaled corticosteroid (ICS). The aim of this study was to validate the additive efficacy of a leukotriene receptor antagonist (LTRA) on cough score and respiratory function in patients with cough-variant asthma being treated with ICS. A total 28 patients were randomly assigned to either an ICS + LTRA group or an ICS group. There were statistically significant improvements in cough scores in the ICS + LTRA group from 0 weeks (6.7 ± 4.4) to 2 weeks (2.9 ± 3.2) (P < 0.05), 4 weeks (0.7 ± 1.1) (P < 0.001), and 8 weeks (0.8 ± 1.2) (P < 0.001). However similar improvements were not evident in the ICS group from 0 weeks (6.7 ± 4.4) to 2 weeks (5.6 ± 10.0) (P = 0.59), 4 weeks (4.6 ± 7.6) (P = 0.32), and 8 weeks (2.9 ± 5.2) (P = 0.08). On the other hand, no significant changes were evident in the forced expiratory volume in 1 s (FEV1) and FEV1/forced vital capacity (FVC). In conclusion, the LTRA was useful in improving cough in patients with cough-variant asthma, even though it appeared to be ineffective in improving respiratory function.
Keywords: randomized study, cough-variant asthma, leukotriene receptor antagonist, inhaled corticosteroid, cough score
INTRODUCTION
In 1979, Corrao and colleagues reported a case series of patients who were affected by chronic cough without dyspnea or wheezing. This cough was characterized by normal respiratory function and a hyper-reactive airway, and was referred to as cough-variant asthma (CVA) [1]. The CVA was also characterized by sensitivity to a bronchodilator [2].
The treatment policy of CVA is generally the same as that of typical asthma. Approximately 30% to 40% of CVA reportedly becomes typical asthma, and inhaled corticosteroids (ICS) generally prevent the progression of CVA to asthma [3–5]. Therefore, ICS are considered the first-line treatment for CVA. If CVA is not adequately responsive to ICS, an increased dose of ICS, or its combination with a long-acting beta-agonist (LABA), leukotriene receptor antagonist (LTRA), or sustained release theophylline are generally considered.
The LTRAs are anti-asthmatic agents that alleviate the clinical symptoms of typical asthma, and improve pulmonary function and airway inflammation. Although the efficacy of an LTRA as a single agent against clinical symptoms, physiological functions, and airway inflammation in patients with CVA has been reported elsewhere [6], to our knowledge, no studies have been conducted on the clinical effects of combination treatment with ICS and LTRA against CVA. Therefore, the aim of this study was to validate the additive efficacy of an LTRA on cough score and respiratory function in patients with CVA being treated with ICS.
MATERIALS AND METHODS
Study design
This was an open-label, multi-institutional, randomized study conducted between November 2007 and March 2009 at four general hospitals in Japan: Kobe University Hospital, Akashi Medical Center, Takatsuki General Hospital, and Kobe Rosai Hospital. The study consisted of a one-week screening period and an eight-week treatment period. Eligible patients were consecutively randomized (1:1) to receive the LTRA (montelucast 10 mg), in addition to the ICS (fluticasone 400 μg, budesonide 800 μg, or beclomethasone 400 μg), or ICS. The study was approved by the Institutional Review Board of each hospital, and written informed consent was obtained before the commencement of treatment. This clinical trial was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) (Registration number: UMIN000033398).
Study population
The inclusion criteria were as follows: (1) age greater than 20 years; (2) dry cough for 3 weeks or more, without wheezing; (3) cough responsive to tulobuterol tapes; (4) normal respiratory function; (5) absence of rhinitis or sinusitis; (6) absence of symptoms associated with gastroesophageal reflux disease; (7) no onset of respiratory infection within 3 weeks; and (8) no remarkable radiological findings. All patients provided written informed consent. We excluded patients if they were diagnosed with bronchial asthma, atopic cough, sinobronchial syndrome, tuberculosis, or lung cancer.
Randomization
All eligible patients who were diagnosed with CVA during the study period were randomly allocated to the ICS + LTRA group or the ICS group in a 1:1 ratio. The order in which shuffled envelopes were opened by a third party determined the allocation of participants to the treatment groups. We did not set a limit on the size of each group.
Outcome measures
Primary outcomes
The primary outcome assessments included change in cough score and peak expiratory flow (PEF) from baseline. The cough score was canonical assessment methods of cough and calculated by first dividing the day into morning, noon, and night time periods, and scoring the cough frequency and intensity of each period to five levels from 0 to 4. The scores of each period were summed and the product of the frequency and strength was calculated (Table I).
Table I.
Cough Score
| Score | ||||
|---|---|---|---|---|
| Cough | Frequency | Too many | ≥ 21 | 4 |
| Many | 11–20 | 3 | ||
| A Few | 6–10 | 2 | ||
| Few | ≤ 5 | 1 | ||
| Nothing | 0 | 0 | ||
|
| ||||
| Strength | Too strong | 4 | ||
| Strong | 3 | |||
| Slightly strong | 2 | |||
| Weak | 1 | |||
| Nothing | 0 | |||
Secondary outcomes
The secondary efficacy outcomes included the change from baseline to week 8 in forced expiratory volume in 1 s (FEV1), FEV1/forced vital capacity (FVC), and usage of short-acting beta-agonist (SABA). Primary outcome assessments and confirmation of SABA usage were conducted during the screening period and at the beginning of the study, and at 2, 4, and 8 weeks after drug administration. A respiratory function test was performed at the beginning of the study, and at 8 weeks after drug administration.
Safety assessments
Adverse events were monitored throughout the study.
Statistical analysis
The SPSS version 20 software (SPSS Incorporated, Chicago, IL, USA) was used for statistical analysis, and the results were expressed as the mean ± standard deviation (SD). The Pearson’s chi-squared test was used to detect possible interactions between two categorical variables. The unpaired t-test was used to compare the baseline patient characteristics and the cough scores, PEF, FEV1, FEV1/FVC, and SABA usage with each baseline. P-values < 0.05 were considered statistically significant.
Patient and public involvement
The development of the research question and outcome measures was based on the fact that no studies had been previously conducted about the clinical effects of combination treatment with ICS and LTRA against CVA. Patients were not involved in the design, subject recruitment, or conduct of the study. The findings will be disseminated to the study participants on publication of the study as an original article.
RESULTS
Study participants
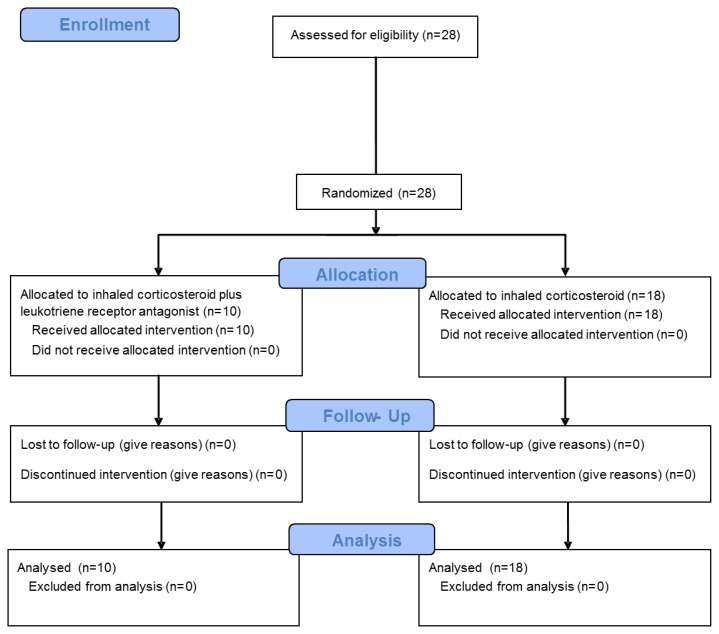
From November 2007 to March 2009, 28 patients were diagnosed with CVA and recruited for the study. Eighteen patients were allocated to the control group and 10 to the intervention group (Fig. 1). Demographics of the 28 consecutive patients are presented in Table II. Age, cough score, FEV1/FVC, and frequency of SABA usage were evenly distributed. The PEF tended to be higher in the ICS + LTRA group than in the ICS group.
Fig. 1.
The Consort Flow Diagram of this study. ICS, inhaled corticosteroid; LTRA, leukotriene receptor antagonist.
Table II.
Baseline patient characteristics
| Characteristic | ICS + LTRA (n = 10) | ICS (n = 18) | P-value |
|---|---|---|---|
| Age (years) | 45.8 ± 16.8 | 44.6 ± 14.5 | 0.85 |
| Gender (male/female) | 7/3 | 8/10 | 0.19 |
| Height (cm) | 168.9 ± 8.1 | 162.3 ± 10.2 | 0.09 |
| Weight (kg) | 65.1 ± 13.2 | 59.2 ± 10.2 | 0.19 |
| Cough Score | 6.7 ± 4.4 | 7.3 ± 8.9 | 0.84 |
| PEF (mL) | 479.3 ± 112.8 | 381.8 ± 137.0 | 0.07 |
| FEV1 (L) | 3.5 ± 0.6 | 3.0 ± 0.9 | 0.13 |
| FEV1/FVC (%) | 85.3 ± 8.7 | 84.8 ± 5.3 | 0.86 |
| SABA usage (/day) | 0.6 ± 0.9 | 0.6 ± 1.0 | 0.95 |
Data are expressed as the mean ± SD. For comparison of patient characteristics, the unpaired t-test and chi-squared test were used.
Key: ICS, inhaled corticosteroid; SABA, short-acting beta-agonist; LTRA, leukotriene receptor antagonist; PEF, peak expiratory flow; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Efficacy
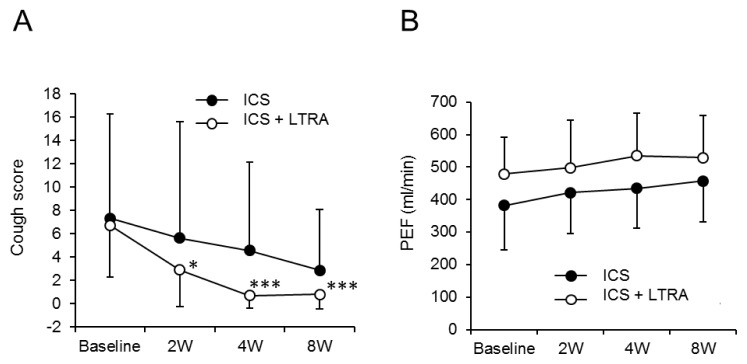
The cough scores in the ICS + LTRA group were significantly improved from 0 weeks (6.7 ± 4.4) to 2 weeks (2.9 ± 3.2) (P < 0.05), 4 weeks (0.7 ± 1.1) (P < 0.001), and 8 weeks (0.8 ± 1.2) (P < 0.001). However, those in the ICS group showed no significant improvement from 0 weeks (6.7 ± 4.4) to 2 weeks (5.6 ± 10.0) (P = 0.59), 4 weeks (4.6 ± 7.6) (P = 0.32), and 8 weeks (2.9 ± 5.2) (P = 0.08) (Fig. 2A).
Fig. 2.
Change in cough scores and PEF according to time and group. Figures demonstrate values of cough score (A) and PEF (B) at each time point in ICS group (●) and ICS + LTRA group (○). ICS, inhaled corticosteroid; LTRA, leukotriene receptor antagonist; PEF, peak expiratory flow. * P-value < 0.05; *** P-value < 0.001, compared to baseline.
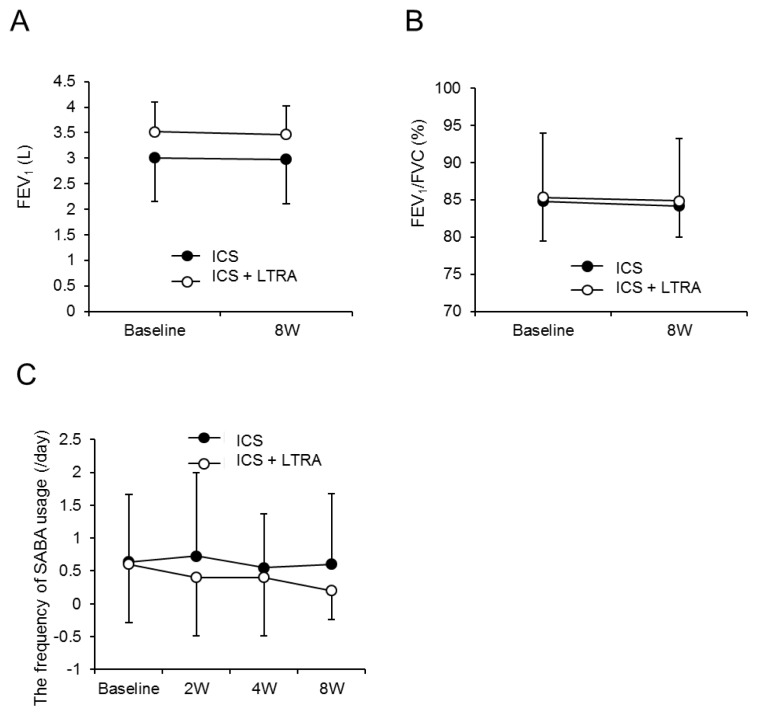
The PEFs in the ICS + LTRA group tended to improve from 0 weeks (479.3 ± 112.8 mL/min) to 2 weeks (498.2 ± 145.1 mL/min), 4 weeks (534.8 ± 130.6 mL/min), and 8 weeks (529.5 ± 128.7 mL/min). Those in the ICS group also tended to improve from 0 weeks (381.8 ± 137.0 mL/min) to 2 weeks (421.5 ± 126.3 mL/min), 4 weeks (434.2 ± 121.9 mL/min), and 8 weeks (457.0 ± 126.1 mL/min) (Fig. 2B). On the other hand, the FEV1 and FEV1/FVC showed no considerable differences from baseline (Fig. 3A and B).
Fig. 3.
Change in FEV1, FEV1/FVC, and SABA usage according to time and group. Figures demonstrate values of FEV1 (A), FEV1/FVC (B), and the frequency of SABA usage (C) at each time point in ICS group (●) and ICS + LTRA group (○).
FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroid; LTRA, leukotriene receptor antagonist; FVC, forced vital capacity; SABA, short-acting beta-agonist.
The frequency of SABA usage in the ICS + LTRA group tended to decline from 0 weeks (0.6 ± 0.9 /day) to 2 weeks (0.4 ± 0.9 /day), 4 weeks (0.4 ± 0.9 /day), and 8 weeks (0.2 ± 0.4 /day), whereas those in the ICS group showed no obvious decline from 0 weeks (0.6 ± 1.0 /day) to 2 weeks (0.7 ± 1.3 /day), 4 weeks (0.5 ± 0.8 /day), or 8 weeks (0.6 ± 1.1 /day) (Figure 3C).
Safety
No obvious adverse events were observed in this study.
DISCUSSION
In this study, we clarified the additive effect of an LTRA in patients with CVA. The ICS + LTRA group showed significant improvement in the cough score at all time points. The ICS group also showed improvement, but this change was not significant. In general, ICS is effective for CVA to some extent. However, it is noteworthy that half of the cough remains in ICS group, whereas the cough in ICS+LTRA group almost diminishes. Similarly, the ICS + LTRA group showed an improvement in symptoms, which was necessary before the SABA was administered. However, the ICS group showed no such improvement. In the case of PEFs, both groups showed a tendency to improve. On the other hand, the FEV1, and FEV1/FVC showed no response to either treatment. Interestingly, a previous study showed that LTRA monotherapy significantly reduced the cough visual analog scale (VAS), which is novel assessment method of cough (P = 0.0008), whereas pulmonary function, airway responsiveness, and sputum mediator levels remained unchanged [6]. The current study confirmed these results.
Kita et al [7]. reported that a 2-week treatment with LTRA plus clenbuterol significantly reduced cough scores, and treatment with LTRA plus clenbuterol was superior to that with LTRA monotherapy. In the LTRA plus clenbuterol group, PEF values in the morning and evening were significantly increased after 2 weeks compared to values measured before treatment. In the current study, PEF values showed no statistically significant increase after 2 weeks compared to values obtained before treatment. This is partly because ICS improve the PEF sufficiently, as shown in Figure 2.
The ICS + LTRA group showed significant improvement in the cough score from an early stage, and this improvement was evident even at 8 weeks. This observation period was comparatively longer than that of a previous study [6–9]. Therefore, the current study shows that LTRA contributes to an improvement in the quality of life in CVA patients from a relatively early to comparatively late stage.
In the guidelines of the European Respiratory Society, the use of the cough VAS is recommended to evaluate the severity of cough. Cough VAS scores are reportedly reproducible and have responded to interventions in a chronic cough clinical trial [10]. However, the standard evaluation method is controversial and has not yet been established [11, 12]. In the current study, we used the cough score instead of the cough VAS.
Although ICS-based therapy rapidly relieves cough in most patients with CVA, recurrence remains a challenge when treatment is discontinued [4]. The continuation of long-term treatment is important, especially in refractory cases and those with persistent symptoms. Based on the results of the current study, an LTRA in addition to an ICS is recommended for patients for whom the ICS alone is insufficiently effective.
The limitation of the current study is its relatively small sample size and that randomization by the envelope method probably biased in the number of cases. In addition, we lack the data of disease duration. However, the strong additive effect of the LTRA made it possible for the study to meet one of primary endpoints. This is partly because the LTRA has a unique antitussive effect via the tussive mediator, substance P [6]. In comparison to asthma patients, the expression of substance P in the airway epithelium is increased in CVA patients. The LTRA inhibits the release of substance P, which is induced by cysteinyl leukotrienes (Cys-LTs) through the stimulation of airway afferent nerve fibers [6]. Furthermore, the LTRA itself can attenuate eosinophilic inflammation [6]. These antitussive effects might enhance the anti-inflammatory effect of ICS in patients with CVA.
ACKNOWLEDGEMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med. 1979;300:633–637. doi: 10.1056/NEJM197903223001201. [DOI] [PubMed] [Google Scholar]
- 2.Niimi A, Matsumoto H, Mishima M. Eosinophilic airway disorders associated with chronic cough. Pulm Pharmacol Ther. 2009;22:114–120. doi: 10.1016/j.pupt.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Fujimura M, Ogawa H, Nishizawa Y, Nishi K. Comparison of atopic cough with cough variant asthma: is atopic cough a precursor of asthma? Thorax. 2003;58:14–18. doi: 10.1136/thorax.58.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto H, Niimi A, Takemura M, et al. Prognosis of cough variant asthma: a retrospective analysis. J Asthma. 2006;43:131–135. doi: 10.1080/02770900500498477. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima T, Nishimura Y, Nishiuma T, et al. Characteristics of patients with chronic cough who developed classic asthma during the course of cough variant asthma: a longitudinal study. Respiration. 2005;72:606–611. doi: 10.1159/000087459. [DOI] [PubMed] [Google Scholar]
- 6.Takemura M, Niimi A, Matsumoto H, et al. Clinical, physiological and anti-inflammatory effect of montelukast in patients with cough variant asthma. Respiration. 2012;83:308–315. doi: 10.1159/000332835. [DOI] [PubMed] [Google Scholar]
- 7.Kita T, Fujimura M, Ogawa H, et al. Antitussive effects of the leukotriene receptor antagonist montelukast in patients with cough variant asthma and atopic cough. Allergol Int. 59:185–192. doi: 10.2332/allergolint.09-OA-0112. [DOI] [PubMed] [Google Scholar]
- 8.Spector SL, Tan RA. Effectiveness of montelukast in the treatment of cough variant asthma. Ann Allergy Asthma Immunol. 2004;93:232–236. doi: 10.1016/S1081-1206(10)61493-7. [DOI] [PubMed] [Google Scholar]
- 9.Dicpinigaitis PV, Dobkin JB, Reichel J, et al. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough-variant asthma. J Asthma. 2002;39:291–297. doi: 10.1081/jas-120002285. [DOI] [PubMed] [Google Scholar]
- 10.Morice AH, Fontana GA, Belvisi MG, et al. European Respiratory Society (ERS): ERS guidelines on the assessment of cough. Eur Respir J. 2007;29:1256–1276. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- 11.Brightling CE, Monterio W, Green RH, et al. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: safety and repeatability. Respir Med. 2001;95:999–1002. doi: 10.1053/rmed.2001.1195. [DOI] [PubMed] [Google Scholar]
- 12.Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58:339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]