Abstract
Overtraining leads to an increase in IL-1β systemically due to muscle microtrauma, which affects the hippocampus, an important structure in spatial memory consolidation. The administration of Hibiscus sabdariffa Linn is expected to decrease IL-1β and increase IL-1ra, thereby potentially preventing impairments in spatial memory consolidation. This research was an experimental study using 20 male Wistar rats. The overtraining of Wistar rats altered the ratio of IL-1β/IL-1ra in the plasma and hippocampus. Moreover, this overtraining impaired spatial memory consolidation. The methanol extract of H. sabdariffa improved spatial memory consolidation in Wistar rats and prevented impairment in spatial memory consolidation by maintaining the ratio of IL-1β/IL-1ra in the plasma and hippocampus of Wistar rats who experienced overtraining. H. sabdariffa is a potent anti-inflammatory substance that prevents impairments in spatial memory consolidation in overtrained Wistar rats.
Keywords: overtraining, Hibiscus sabdariffa Linn, spatial memory consolidation, hippocampus, plasma, IL-1β, IL-1ra
Athletes generally undergo long training programs to prepare for important games to improve and optimize their performance. To improve performance, several aspects must be considered by athletes proportionally, such as intensity, duration, and total workload (4). If these aspects are excessive and accompanied by inadequate periods of rest and recovery, athletes may experience a condition called overtraining syndrome (OT). This syndrome is characterized by a decrease in performance accompanied by the emergence of complaints and maladaptive physiological aspects of the psychological, neurological, endocrine, and immune systems (16).
Overtraining leads to performance decreases when playing sports and could develop into memory impairments. Individuals with chronic fatigue syndrome usually show symptoms such as impaired concentration and memory, and these symptoms are also found in athletes who experience OT (7,19,30). In addition, experimental studies using a water E-maze with animals have also shown memory impairments in overtrained rats (24).
Systemic inflammation may cause the memory impairments that occur during overtraining (19). When physical exercises are performed intensively, repeatedly, and sustainably and then exacerbated by inadequate recovery, thus causing overtraining, a local acute inflammatory response that is profitable initially can become stronger, chronic, and even pathological (16, 30). One cytokine involved in systemic inflammatory responses is interleukin-1beta (IL-1β). IL-1β, its receptor and its antagonist receptor (IL-1ra) are found abundantly in the hippocampus, a brain structure known to play a role in spatial and contextual memory consolidation. These facts show that these molecules could modulate hippocampal-dependent memories. Supporting facts also prove that IL-1β signaling contributes to excessive deficits in hippocampus-dependent memory formation (21, 22, 25, 30).
The performance of IL-1β can be regulated by natural antagonists of the IL-1 receptor, namely, IL-1ra. IL-1ra appear to block the effects of IL-1β (8, 23). Increases of IL-1β and decreases in IL-1ra levels are associated with spatial memory impairments during chronic inflammation (22). Inflammation that occurs in the periphery can change the ratio of IL-1β/IL-1ra in the brain, and monitoring the ratio of plasma IL-1β/IL-1ra levels is important for predicting inflammation trends in the brain (23).
H. sabdariffa is a herbaceous plant of the family of Malvaceae. The petal extract of H. sabdariffa has anti-inflammatory effects because it contains polyphenol compounds that have anti-inflammatory properties (2). Meraiyebu et al. (2013) concluded that the methanol extract of H. sabdariffa reduced inflammation in an equivalent manner as diclofenac in carrageenan-induced rat paw edema (20). The polyphenol content in H. sabdariffa works as an anti-inflammatory by improving antioxidant conditions and regulating the expression of cyclooxygenase-2 [COX-2] (15). The molecular activity of flavonoids (polyphenols that are most commonly found in food) inhibits Nf-κB transcription factor, which plays a role in the expression of IL-1β (5–6, 11, 26, 27).
Recent studies have attempted to reveal the anti-inflammatory benefits of polyphenols from various plants, but the effects of H. sabdariffa, which contains a large number of polyphenols, on the ratio of IL-1β/IL-1ra in overtrained rats are uncertain (13–14). Therefore, this study was conducted to determine the mechanisms underlying spatial memory consolidation impairments due to overtraining and to observe the potential of H. sabdariffa in preventing a systemic inflammatory process and preventing malfunctioning in the consolidation of spatial memories in OT. This research is expected to provide new insights on the mechanisms underlying impairments in spatial memory consolidation as well as the anti-inflammatory effects of H. sabdariffa in preventing impairments of spatial memory consolidation due to systemic inflammation in OT.
MATERIAL AND METHODS
Materials
Methanolic extracts of H. sabdariffa were obtained from the Central Laboratory of Medicinal Studies - Institut Pertanian Bogor (Bogor, Indonesia) with 86.34% purity. Based on quantitative tests, the methanolic extract of H. sabdariffa contained phenolic, flavonoid, tannin, saponin, quinone, and steroid elements. The H. sabdariffa extract was administered orally by oral gavage, 3 hours before physical exercises, 5 times a week until the end of the study (11 weeks).
Animals
Twenty adult male Wistar rats (Rattus norvegicus), aged 8–11 weeks, were randomly divided into four groups: a control group treated with a placebo, aqua bidest (C), a control group given H. sabdariffa at 500 mg/kg (C-Hib), a group treated with physical exercise overtraining (OT), and a group treated with physical exercise overtraining and H. sabdariffa at 500 mg/kg (OT-Hib). Before and during treatment, rats were kept in disease-free environments. Rats were given ad libitum access to food and water. Cages were kept clean and set on a 12/12-h light/dark cycle. The ambient temperature was maintained at 23±1 °C. Prior to decapitation, the animals were given a total anesthesia using a combination of xylazine (0.01 ml/kg BW) and ketamine (0.05 ml/kg BW). The protocol of this study has been approved by the Ethical Committee of the Faculty of Medicine, Universitas Indonesia (No. 749/UN2.F1/ETIK/2016).
Physical exercise protocol
The overtraining of physical exercise was increased gradually, from mild to severe, and was provided to experimental animals using an animal treadmill; the physical exercise was given as much as five times a week. The duration, speed, and recovery of the physical exercise corresponded to a protocol by Hohl (12). To meet the inclusion criteria before physical activity, rats underwent an acclimatization and adaptation phase for two weeks. Rats that could run voluntarily on the treadmill met the inclusion criteria for the physical exercise protocol.
Measurement of the IL-1β/IL-1ra ratio
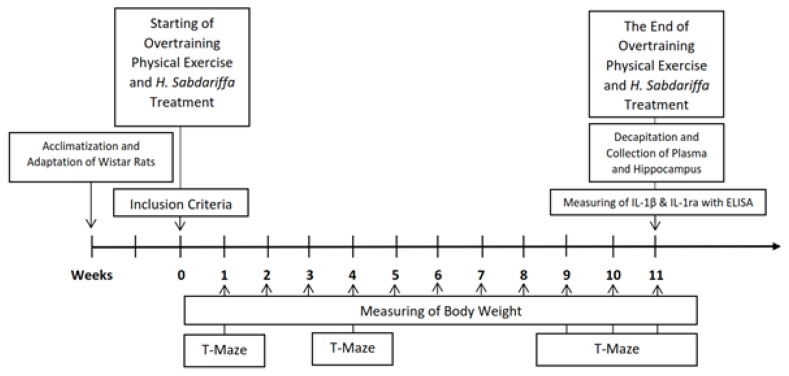
The IL-1β/IL-1ra ratio data in this study were obtained by calculating the ratio between the IL-1β/IL-1ra levels in the plasma and the homogenate of the hippocampus. Plasma was collected from the retro-orbital sinus blood and the homogenate of the hippocampus was collected after decapitation; both samples were collected at the last treatment in the eleventh week (Figure 1). The measurements of IL-1β and IL-1ra levels in the plasma and hippocampus were performed using an ELISA kit (Elabscience Biotechnology Co., Ltd®) for rats with a minimum detection range of 78.125–5000 pg/ml for IL-1ra and 31.25–2000 pg/ml for IL-1β. Optical density (OD) readings were performed with a microplate reader at 450 nm. All samples were tested in duplicate.
Figure 1.
Research Timeline
Assessment of spatial memory consolidation
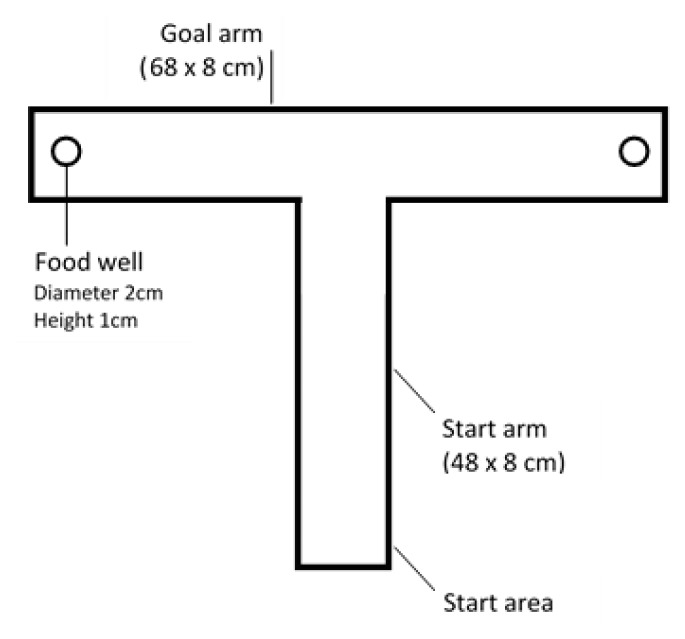
Spatial memory consolidation was assessed with a T-maze protocol adapted from Grzeda (2007) at the end of the first, fourth, ninth, tenth, and eleventh weeks for all groups (9). The spatial memory consolidation assessment procedure was divided into two phases: a learning phase and a memory testing phase. The learning phase consisted of 10 timed trials with food rewards. Food rewards were placed randomly at one of the ends of the T-maze arms, and then the position was recorded. Trials begun by placing each rat in the start area and then letting them voluntarily choose the arm to enter. Rats were correct if they reached the arm that contained food. Rats were allowed to eat the food as a reward, and then the rats were removed from the T-maze, and the procedure was repeated. The inclusion criterion was successfully choosing the correct arm as many as two times consecutively in the 10 trials. Next, rats were fasted from food for 24 hours. In the second stage, a memory test was conducted 24 hours after the learning phase. This phase consisted of 5 trials with no food rewards. The test began by placing each rat in the start area and letting them choose the arm that had previously contained food (during the learning stage); the rats had to choose voluntarily. Rats were declared correct if they reached the arm corresponding to the position of the food during the previous learning stage. Scores were calculated by dividing the number of correctly chosen arms by the number of trials (5) and multiplying by 100 (percent).
Data analysis
All data in this research were analyzed using one-way ANOVAs for independent data with normal distributions, paired t-tests for dependent data with normal distributions, Wilcoxon tests for independent data that were not normally distributed and Kruskal-Wallis tests for dependent data that were not normally distributed. Multiple comparisons were performed with the post hoc Bonferroni method for one-way ANOVAs and the post hoc Mann-Whitney method for Wilcoxon tests. A value of p<0.05 was taken as the limit of statistical significance.
RESULTS
Body weight (BW) loss in all overtraining groups
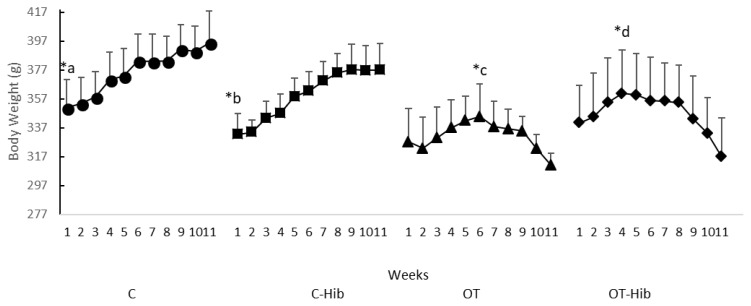
Weight loss or body mass loss could be used as a symptom that most likely is a marker of OT. Therefore, BW measurement in this study was used to ensure that OT had occurred. The changes in body weight (BW) in this study are presented in Figure 3; BW increased steadily in all control groups (C; p=0.008 and C-Hib; p=0.002), while BW decreased in all groups that underwent OT after specific weeks (OT; p=0.001 and OT-Hib; p=0.003).
Figure 3.
Rat Weekly Weight Average for Each Treatment Group
*Description: Paired t-test = a) BW C w-1 vs BW w-11 (p<0.05); b) BW C-Hib w-1 vs BW w-11 (p<0.05); c) BW OT w-6 vs BW w-11 (p<0.001); d) BW OT-Hib w-4 vs BW w-11 (p<0.05); BW = body weight; w = week. OT: overtraining; OT-Hib: overtraining with H. sabdariffa; C-Hib: control H. sabdariffa; C: control.
There was an increase in BW in the C group and C-Hib group from the first to eleventh weeks. However, the BW of the OT group from the sixth to the eleventh weeks decreased; this decreases also occurred in the OT-Hib group from the fourth to eleventh weeks.
The Levels of IL-1β Decreased in the C-Hib Group Plasma and Increased in the OT Group Hippocampus
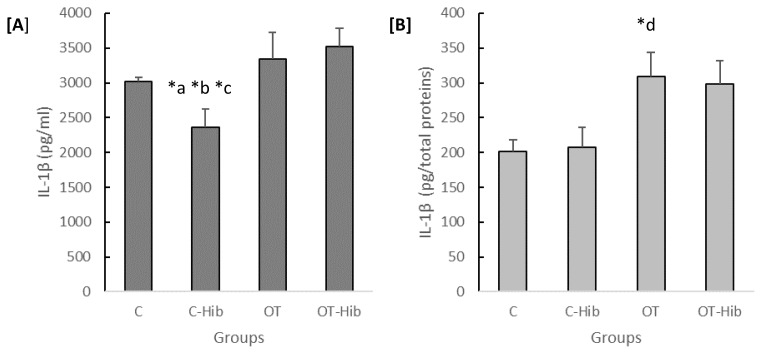
IL-1β is a proinflammatory cytokine that was investigated in this study. The concentration of IL-1β in both the plasma and hippocampus varied significantly by group (p=0.002 and p=0.014). The concentration of IL-1β in the C-Hib group plasma (Figure 4A) was lower than that in the other groups (OT-Hib; p=0.003, OT; p=0.012, and C; p=0.019). However, in the hippocampus, the IL-1β concentration in the OT group was higher than that in the C group (p=0.005) (Figure 4B).
Figure 4.
The levels of IL-1β in blood plasma [A] and the hippocampus [B]
*Description: One-way ANOVA ([A]: p<0.01; [B]: p<0.05) with post hoc Bonferroni tests: a) average level of plasma IL-1β C-Hib vs OT-Hib (p<0.01); b) average level of plasma IL-1β C-Hib vs OT (p<0.05); c) average level of plasma IL-1β C-Hib vs C (p<0.05). With post hoc Games-Howell test: d) average level of hippocampal IL-1β OT vs C (p<0.01). The data for the plasma IL-1β levels [A] are presented as quantities (pg) of plasma volume (ml), and the data for the hippocampal IL-1β levels [B] are presented as quantities (pg) of total protein content. OT: overtraining; OT-Hib: overtraining with H. sabdariffa; C-Hib: control with H. sabdariffa; C: control.
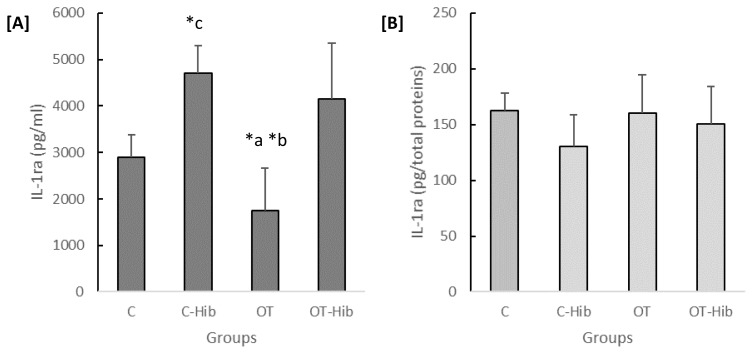
The anti-inflammatory cytokine investigated in this study was IL-1ra. The concentration of IL-1ra varied significantly in the plasma (p=0.0001) but not in the hippocampus. The concentration of plasma IL-1ra in the OT group (Figure 5A) was lower than that in the OT-Hib (p=0.002) and C-Hib (p=0.0001) groups. Furthermore, the plasma IL-1ra concentration in the C-Hib group was higher than that of the C group (p=0.002). There were no significant differences among the groups in Figure 5B.
Figure 5.
The levels of IL-1ra in the blood plasma [A] and hippocampus [B]
*Description: One-way ANOVA ([A]: p<0.001) with post hoc Bonferroni tests: a) average level of plasma IL-1ra OT vs OT-Hib (p<0.05); b) average level of plasma IL-1ra OT vs C-Hib (p<0.001); c) average level of plasma IL-1ra C-Hib vs C (p<0.05). The data for the plasma IL-1ra levels [A] are presented as quantities (pg) of plasma volume (ml), and the data for the hippocampal IL-1ra levels [B] are presented as quantities (pg) of total protein content. OT: overtraining; OT-Hib: overtraining with H. sabdariffa; C-Hib: control with H. sabdariffa; C: control.
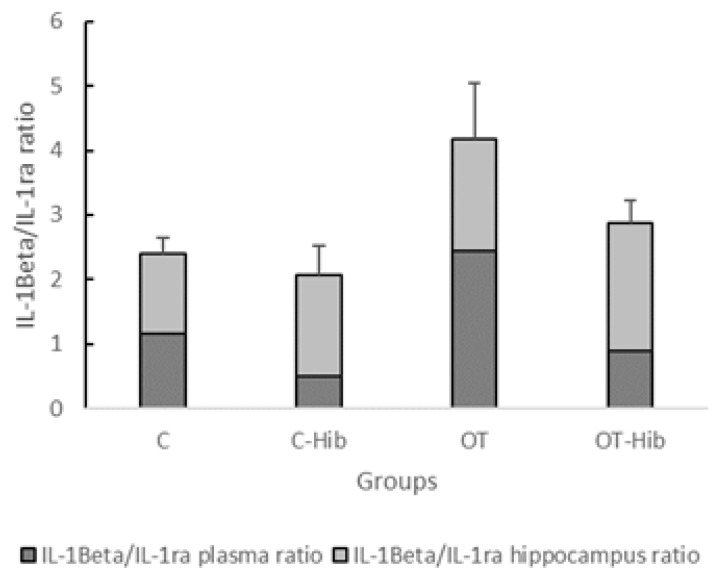
Measurement of IL-1β/IL-1ra Ratio as a Predictor of Inflammation Status
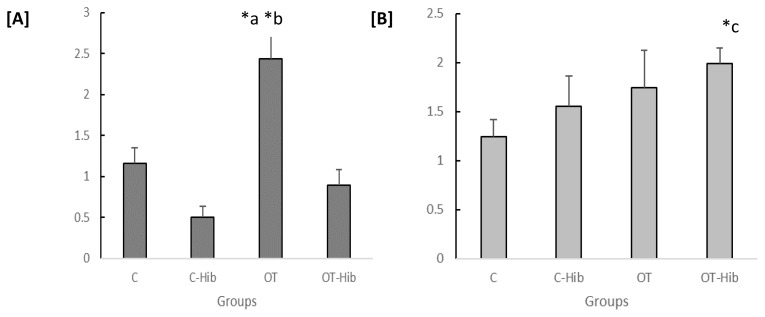
We measured the IL-1β/IL-1ra ratio as a predictor of inflammation status in this study. The ratio of plasma IL-1β/IL-1ra levels in the OT group was significantly higher than that in the C-Hib (p=0.003) and OT-Hib (p=0.02) groups, but in the hippocampus, the difference in the IL-1β/IL-1ra ratio was significant only between the OT-Hib and C groups (p=0.003). However, we could see the trend for the ratio of the hippocampal IL-1β/IL-1ra ratio to be higher in the OT group than in the C group, although this difference was not statistically significant (Figure 6).
Figure 6.
Ratio of IL-1β/IL-1ra levels in the blood plasma [A] and hippocampus [B]
*Description: One-way ANOVA [A] (p<0.005) with post hoc Bonferroni tests: *) OT vs C-Hib and OT vs OT-Hib (p<0.05); one-way ANOVA [B] (p<0.005) with post hoc Bonferroni test: *) OT-Hib vs C (p<0.005).
The calculations of plasma and hippocampal levels were performed as indicators of the circumstances that represent the occurrence of inflammation in the brain, especially hippocampus, caused by peripherally initiated inflammation. There was a significant difference in the ratios of IL-1β/IL-1ra levels in the plasma and hippocampi from each treatment group; p=0.001 (Fig. 6). Further testing also showed that there were differences in the average ratios of IL-1β/IL-1ra levels in the plasma and hippocampus between the OT group and the C-Hib group, p=0.002, and the C group, p=0.004; the average ratios of the IL-1β/IL-1ra levels in the plasma and hippocampus of the OT group were higher than those in the C-Hib and C groups.
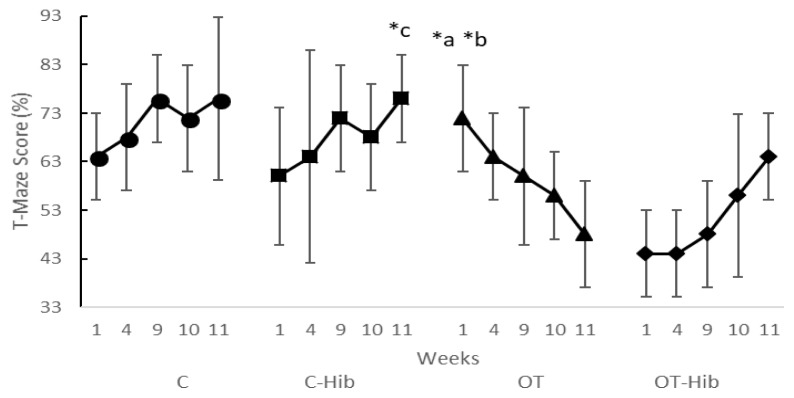
There were significant differences in the spatial memory consolidation T-maze scores in the OT group between the first week and the tenth, p=0.046, and between the first week and the eleventh, p=0.034. The spatial memory consolidation T-maze scores decreased from the first week to the eleventh week in the OT group (Figure 8). There was a difference in the spatial memory consolidation T-maze scores between the first and the eleventh weeks in the C-Hib group, p=0.046, and in this group, the spatial memory consolidation T-maze scores increased from the first week to eleventh week.
Figure 8.
Spatial Memory Consolidation T-Maze Scores Averaged for Each treatment group
*Description: Wilcoxon test = a) OT group, w-10 vs w-1 score (p<0.05); b) OT group, w-11 vs w-1 score (p<0.05); c) C-Hib group, w-11 vs w-1 score (p<0.05). w: the end of the week; OT: overtraining; OT-Hib: overtraining with H. sabdariffa; C-Hib: control with H. sabdariffa; C: control.
The next step was the calculation of the differences in the spatial memory consolidation T-maze scores between the eleventh and first weeks for each group (Δ T-maze score); then, the entire set of data was averaged for each group (Table 1). The results showed that there was a significant difference in the Δ T-maze scores in each group; p=0.011. Further tests summarized that there were significant differences in the change between the eleventh and first weeks between the OT and OT-Hib groups, p=0.006; between the OT and C-Hib groups, p=0.005; between the OT and C groups, p=0.019. The lowest Δ T-maze score was in the OT group.
Table 1.
Δ T-maze score between the Eleventh and First Weeks
Description: Kruskal-Wallis (p<0.05) with post hoc Mann-Whitney tests:
) OT vs OT-Hib groups (p<0.05);
) OT vs. C-Hib groups (p<0.05);
) OT vs C groups (p < 0.05).
OT: overtraining; OT-Hib: overtraining with H. sabdariffa; C-Hib: control with H. sabdariffa; C: control.
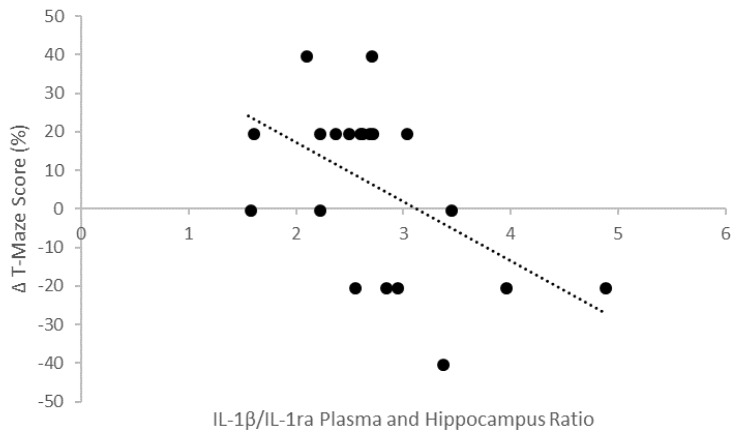
Correlation between the Average Ratio of IL-1β/IL-1ra Levels in the Plasma and Hippocampus and Δ T-maze Scores
The illustration in Figure 9 shows the correlation between the average ratio of IL-1β/IL-1ra levels in the plasma and hippocampus and Δ T-maze scores. The results showed that there was a strong negative correlation between the average ratio of IL-1β/IL-1ra levels in the plasma and hippocampus and Δ T-maze scores. Thus, the lower ratios of IL-1β/IL-1ra levels in the plasma and hippocampus in the experimental animals caused the differences in spatial memory consolidation T-maze scores to become bigger.
Figure 9.
Correlation between the ratio of IL-1β/IL-1ra levels in the plasma and hippocampus and Δ T-maze scores (r= −0.657; p<0.05).
DISCUSSION
There was a significant increase in body weight (BW) in the C-Hib and C groups from the first week to the eleventh week. This result is in line with Sengupta (2003), which mentioned that in the mature age range, a maturity of the musculoskeletal system will occur, continue, and lead to weight gain until the age of 210 days, or approximately the 30th week, in Wistar rats. Body weight gradually decreases after the age 30 weeks (27). Weight loss occurred in the OT group between the sixth week and eleventh week and in the OT-Hib group between the fourth week and eleventh week. These results are in line with Hohl (2009), indicating that the natural decline in BW that occurred in these two groups could be considered a positive adaptation to physical exercise (12). Some studies conclude that weight loss or a loss of body mass could be used as a symptom or marker of OT in humans (29) and that this weight loss is associated with the hypermetabolism and proteolysis caused by a persistent physical workload (12).
Even though the difference was not statistically significant, the levels of plasma IL-1β increased in the OT group compared with the control group. This result could be because overtraining causes systemic inflammation, and one cytokine that is involved in systemic inflammatory responses is IL-1β (16, 31). The systemic inflammation that occurred in the OT group also caused the levels of anti-inflammatory cytokines to decline, especially plasma IL-1ra; consequently, the levels of hippocampal IL-1β also increased (8, 23). Conversely, the levels of plasma IL-1ra in the C-Hib group increased, followed by a decrease in plasma IL-1β, which could be caused by the anti-inflammatory properties of H. sabdariffa. The levels of hippocampal IL-1ra did not differ significantly between the OT group and the other groups; this result indicated that IL-1ra was not expressed adequately in the OT group even when the levels of IL-1β were high. This result is also supported by the low levels of IL-1ra in the plasma.
Many studies have assessed the ratio of IL-1β/IL-1ra levels as a predictor of inflammatory status. According to Palin (2003), the ratio of the local concentration of IL-1β and IL-1ra has been known to influence the initiation and progression of various cases of inflammatory and autoimmune diseases in several peripheral systems. However, when the same measurements are conducted in the brain, particularly in the hippocampus, the research conducted by Palin (2003) also concluded that inflammation in the periphery of the body could change the ratio of IL-1β/IL-1ra levels in the brain. Therefore, the ratio of plasma IL-1β/IL-1ra levels is important in monitoring and predicting the predisposition of the brain to inflammation (23). Thus, when determining whether the occurrence of inflammation in the brain is caused by peripherally initiated inflammation, the levels of proinflammatory cytokines in the blood plasma should not be ignored because the determination of cytokine levels in the hippocampus is inadequate to indicate such an inflammation. Therefore, the summed ratio of IL-1β/IL-1ra levels in the plasma and hippocampus was used to represent the relation between OT and the status of brain inflammation in this study.
The increase in the overtraining IL-1β/IL-1ra ratio occurred not only in the plasma but also in the hippocampus, although this difference was not significant in the OT group (Figure 6). According to Skelly (2013), though the cytokines that are involved in the systemic inflammatory process (IL-1β, TNF-α, IL-6 and IL-1ra) affect the mRNA transcriptional regulation of the same cytokines elaborated in the brain inflammatory process, studies of brain disorders associated with systemic inflammatory mediators show that systemic inflammation has different significant effects on the brain that depend on the brain’s vulnerability (28). Systemic inflammation that is not sufficiently severe to induce CNS dysfunction in most individuals can be sufficient to impact individuals with cognitive vulnerabilities (28). Despite the IL-1β/IL-1ra ratio increase, H. Sabdariffa may reduce brain vulnerability through its polyphenol content, which has a neuroprotective effect (2), especially on maintaining memory (Figure 8). In this case, the effects of H. sabdariffa on the brain also work systemically; therefore, a fairly complex interaction occurs involving systemic inflammation, the action of H. Sabdariffa, and the inflammation of the brain. Consequently, the analysis of the ratio of IL-1β/IL-1ra levels in the plasma and in the hippocampus should not be performed separately because this could show a biased interpretation.
There was a significant increase in the IL-1β/IL-1ra plasma and hippocampus ratio in the OT group compared with the C-Hib and C groups. This finding could be associated with the inflammation that occurred in the OT group. Research that has attempted to reveal the relationship between the ratio of IL-1β/IL-1ra levels and overtraining is still not widely practiced despite several hypotheses that suggest that overtraining could increase proinflammatory cytokines, such as IL-1β, and decrease anti-inflammation cytokines, such as IL-1ra, due to immunosuppression caused by excessive inflammatory responses. Although anti-inflammatory effects are required by the body to fight proinflammatory effects, the end result of prolonged and intense antiregulation (hyperinflammation) is immunosuppression that is harmful to the body (12).
H. sabdariffa is rich with polyphenols including flavonoids. A recent study has proven that polyphenols and flavonoids are able to reach and cross the blood-brain barrier, and they can also gain access to the central nervous system. A study using a blood-brain barrier model (RBE4 cells, an immortal cell line of rat cerebral capillary endothelial cells) showed that flavonoids were able to cross the membranes of RBE4 cells. Based on this finding, H. sabdariffa was expected to maintain the ratio of IL-1β/IL-1ra levels as a marker of inflammation not only in the plasma but also in the hippocampus such that no excess ratio spike occurred, as was seen in the OT group (1,10,18). It is possible that the average ratio of the IL-1β/IL-1ra levels in the OT-Hib group was lower than that in the OT group although this difference was not statistically significant. A decline in the average ratio of IL-1β/IL-1ra levels in the OT-Hib group is supported by several studies, although research into the effects of H. sabdariffa on changes in markers of inflammation due to overtraining have not been found. A study conducted by Meraiyebu (2013) concluded that the methanol extract of H. sabdariffa has potential to act as an anti-inflammatory in the rat; in addition to flavonoids, phytonutrients within H. sabdariffa could inhibit the Nf-κB transcription factor, which plays a role in the expression of IL-1β (11, 21, 29). The polyphenol administration conducted by Cavalcanti also showed anti-inflammatory effects by increasing IL-1ra levels in rats with acute intestinal inflammation (5). A similar study by Chiva-Blanch also found that polyphenols increased the concentration of IL-1ra in plasma (6).
The Δ T-maze scores showed that the OT group had the lowest average score, which was significantly different from the OT-Hib, C-Hib, and C groups. The OT group had the lowest delta average score compared to the other groups as it reached a negative value (−). The spatial memory consolidation decline in the OT group is in line with a variety of studies; Parwata (2014) conducted an experimental study using a water E-maze with experimental animals and showed that there was a decline in memory in overtrained rats (24).
An increase of IL-1β levels in the overtrained rat hippocampus is one factor that plays a role in spatial memory disorders, and numerous studies have suggested this theory. Lynch (2010) attempted to inject IL-1β into the ventricles of rats and then tested their performance in a Morris water maze. The results showed that IL-1β did not affect memory retention but did negatively impact neuronal function (17). Subsequent experiments conducted by Barrientos (2010) demonstrated the same phenomenon in rats; there was a specific effect on context-dependent hippocampal memories following intraperitoneal and intrahippocampal injections of IL-1β (3). Lynch (2010) noted that the memory impairments caused by increased levels of IL-1β occurred through an inhibition of LTP in some parts of the hippocampus in young animals, as long-term potentiation (LTP) is generally considered to be the basis for hippocampal-dependent memories. Therefore, the memory deficits caused by excesses levels of IL-1β were also caused by parallel deficits in LTP (17).
There was no decline in the average spatial memory consolidation T-maze scores in the OT-Hib group. This result proves that there was no significant change in the average spatial memory consolidation T-maze score from the first week to the eleventh week. Even though the OT-Hib group showed lower scores than the OT group in the first week, these results indicate no negative or harmful effects of H. sabdariffa, and this difference may be caused by differences in the individual memory retention abilities of the rats in each group. This idea was proven by the fact that at the end of the study, a memory decline did not occur in the OT-Hib group.
The administration of H. sabdariffa at 500 mg/kgBW could prevent memory impairments. This effect could be caused by a significant increase in the average plasma levels of IL-1ra, which is a proinflammatory cytokine, in the OT-Hib group. Such improvements resulted in an average ratio of IL-1β/IL-1ra plasma and hippocampus levels in the OT-Hib group that was lower than that in the OT group, although this was not a significant difference. In other words, H. sabdariffa could maintain the IL-1β/IL-1ra plasma and hippocampus ratio during overtraining. Based on the Pearson analysis, there was a significantly strong negative correlation between the ratio of IL-1β/IL-1ra plasma and hippocampus levels and Δ T-maze scores. This finding could be interpreted as the lower the ratio of IL-1β/IL-1ra plasma and hippocampus levels, the larger the Δ T-maze score (no spatial memory consolidation impairments). Conversely, the higher the ratio of IL-1β/IL-1ra plasma and hippocampus levels, the lower the Δ T-maze score (impairments in spatial memory consolidation).
CONCLUSION
The administration of the methanolic extract of H. sabdariffa at 500 mg/kg body weight is indicated as a potential anti-inflammatory by maintaining the ratio of IL-1β/IL-1ra plasma and hippocampus levels in overtrained rats; therefore, the provision of H. sabdariffa at 500 mg/kg body weight could prevent impairments in spatial memory consolidation.
Figure 2.
T-maze used to assess spatial memory consolidation in rats.
Figure 7.
Ratio of IL-1β/IL-1ra Levels in the Blood Plasma and hippocampus
*Description: One-way ANOVA (p<0.05) with post hoc Bonferroni tests: a) average ratio of IL-1β/IL-1ra levels in the OT vs C-Hib groups (p<0.05); b) average ratio of IL-1β/IL-1ra levels in the OT vs C groups (p<0.05). OT: overtraining; OT-Hib: overtraining with H. sabdariffa; C-Hib: control with H. sabdariffa; C: control.
ACKNOWLEDGMENTS
This research was supported by the Directorate of Research and Community Service-Universitas Indonesia (DRPM UI) in the Grant of International Publications Indexed for the Final Assignment (PITTA) 2016.
REFERENCES
- 1.Faria A, Pestana D, Teixeira D, Azevedo J, De Freitas V, Mateus N, Calhau C. Flavonoid transport across rbe4 cells: a blood-brain barrier model. Cell Mol Biol Lett. 2010;15:234–241. doi: 10.2478/s11658-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anokwuru CP, Esiaba I, Ajibaye O, Adesuyi AO. Polyphenolic content and antioxidant activity of Hibiscus sabdariffa calyx. Res J Med plant. 2011;5:557–566. doi: 10.3923/rjmp.2011.557.566. [DOI] [Google Scholar]
- 3.Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. NIH Public Access. Brain Behav Immun. 2010;23:46–54. doi: 10.1016/j.bbi.2008.07.002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birrer D, Lienhard D, Williams CA, Röthlin P, Morgan G. Prevalence of non-functional overreaching and the overtraining syndrome in Swiss elite athletes. Schweizerische Zeitschrift fur Sport und Sport. 2013;61:23–29. [Google Scholar]
- 5.Cavalcanti E, Vadrucci E, Delvecchio FR, Addabbo F, Bettini S, Liou R, Monsurrò V, Huang AYC, Pizarro TT, Santino A, Chieppa M. Administration of reconstituted polyphenol oil bodies efficiently suppresses dendritic cell inflammatory pathways and acute intestinal inflammation. PLoS One. 2014 doi: 10.1371/journal.pone.0088898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiva-Blanch G, Magraner E, Condines X, Valderas-Martínez P, Roth I, Arranz S, Casas R, Navarro M, Hervas A, Sisó A, Martínez-Huélamo M, Vallverdú-Queralt A, Quifer-Rada P, Lamuela-Raventos RM, Estruch R. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: A randomized feeding trial. Nutr Metab Cardiovasc Dis. 2015;25:36–45. doi: 10.1016/j.numecd.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Fry RW, Morton AR, Keast D. Overtraining in athletes. An update. Sports Med. 1991;12:32–65. doi: 10.2165/00007256-199112010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Grzeda E, Wiśniewska RJ, Wiśniewski K. Effect of an NMDA receptor agonist on T-maze and passive avoidance test in 12-week streptozotocin-induced diabetic rats. Pharmacol Reports. 2007;59:656–663. [PubMed] [Google Scholar]
- 10.Guardiola S, Mach N. Therapeutic potential of Hibiscus sabdariffa: a review of the scientific evidence. Endocrinol y Nutr English Ed. 2014;61(5):229–98. doi: 10.1016/j.endoen.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Gupta C, Prakash D. Phytonutrients as therapeutic agents. J Complement Integr Med. 2014;11:151–169. doi: 10.1515/jcim-2013-0021. [DOI] [PubMed] [Google Scholar]
- 12.Hohl R, Ferraresso RLP, De Oliveira RB, Lucco R, Brenzikofer R, De Macedo DV. Development and characterization of an overtraining animal model. Med Sci Sports Exerc. 2009;41:1155–1163. doi: 10.1249/MSS.0b013e318191259c. [DOI] [PubMed] [Google Scholar]
- 13.Ilyas EII, Kartinah NT, Andraini T, Goenarjo RA, Kahandjak DN. Effects of Hibiscus sabdariffa Linn. on insulin-like growth factor binding protein 3 (IGFBP-3) to prevent overtraining syndrome. 2014;23:187–191. doi: 10.13181/mji.v23i4.991. [DOI] [Google Scholar]
- 14.Kahanjak DN. Efek Hibiscus sabdariffa Linn. dalam mencegah stres oksidatif pada tikus yang diberi latihan fisik aerobik overtraining: Pengamatan terhadap kadar MDA dan aktivitas glutation peroksidase. [Effect of Hibiscus sabdariffa Linn. in preventing oxidative stress]. Universitas Indonesia; 2014. [Google Scholar]
- 15.Kao ES, Hsu JD, Wang CJ, Yang SH, Cheng SY, Lee HJ. Polyphenols extracted from Hibiscus sabdariffa L. inhibited lipopolysaccharide-induced inflammation by improving antioxidative conditions and regulating cyclooxygenase-2 expression. Biosci Biotechnol Biochem. 2009;73:385–390. doi: 10.1271/bbb.80639. [DOI] [PubMed] [Google Scholar]
- 16.Kreher JB, Schwartz JB. Overtraining Syndrome: A Practical Guide. Sport Heal A Multidiscip Approach. 2012;4:128–138. doi: 10.1177/1941738111434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci. 2010;1:6. doi: 10.3389/neuro.24.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahadevan N, Kamboj S, Kamboj P. Hibiscus sabdariffa Linn.–An overview. Nat Prod Radiance. 2009;8:77–83. [Google Scholar]
- 19.Meeusen R, Duclos M, Foster C, Fry A, Gleeson M, Nieman D, Raglin J, Rietjens G, Steinacker J, Urhausen A. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the european college of sport science and the American College of Sports Medicine. Med Sci Sports Exerc. 2013;45:186–205. doi: 10.1249/MSS.0b013e318279a10a. [DOI] [PubMed] [Google Scholar]
- 20.Meraiyebu AB, Olaniyan OT, Eneze C, Anjorin YD, Dare JB. Anti-inflammatory Activity of Methanolic Extract of Hibiscus sabdariffa on Carrageenan Induced Inflammation in Wistar Rat. Int J Pharm Sci Invent. 2013;2:22–24. [Google Scholar]
- 21.Oitzl MS, van Oers H, Schöbitz B, Ron de Kloet E. Interleukin-1β, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- 22.Palin K, Bluthé RM, Verrier D, Tridon V, Dantzer R, Lestage J. Interleukin-1beta mediates the memory impairment associated with a delayed type hypersensitivity response to bacillus Calmette-Guérin in the rat hippocampus. Brain Behav Immun. 2004;18:223–30. doi: 10.1016/j.bbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Palin K, Verrier D, Tridon V, Hurst J, Perry VH, Dantzer R, Lestage J. Influence of the course of brain inflammation on the endogenous IL-1Beta/IL-1Ra balance in the model of brain delayed-type hypersensitivity response to bacillus Calmette-Guérin in Lewis rats. J Neuroimmunol. 2004;149:22–30. doi: 10.1016/j.jneuroim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Parwata NMRN. Effect of aerobik exercise overtraining on levels of brain derived neurotrophic factor (BDNF) and memory in the rat brain (Rattus norvegicus) Universitas Indonesia 2014 [Google Scholar]
- 25.Patterson SL. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology. 2015;96:11–18. doi: 10.1016/j.neuropharm.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santangelo C, Varì R, Scazzocchio B, Di Benedetto R, Filesi C, Masella R. Polyphenols, intracellular signalling and inflammation. Ann Ist Super Sanita. 2007;43:394–405. [PubMed] [Google Scholar]
- 27.Sengupta P. The Laboratory Rat: Relating Its Age With Human’s. Am J Obstet Gynecol. 2014;4:624–630. doi: 23930179. [PMC free article] [PubMed] [Google Scholar]
- 28.Skelly DT, Hennessy E, Dansereau MA, Cunningham C. A Systematic Analysis of the Peripheral and CNS Effects of Systemic LPS, IL-1B, TNF-α and IL-6 Challenges in C57BL/6 Mice. PLoS One. 2013;8:1–20. doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 2010;69:273–278. doi: 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- 30.Smith LL. Cytokine hypothesis of overtraining: a physiological adaptation to excessive stress? Med Sci Sports Exerc. 2000;32:317–31. doi: 10.1097/00005768-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Tong L, Prieto GA, Kramar EA, Smith ED, Cribbs DH, Lynch G, Cotman CW. Brain-Derived Neurotrophic Factor-Dependent Synaptic Plasticity Is Suppressed by Interleukin-1 via p38 Mitogen-Activated Protein Kinase. J Neurosci. 2012;32:17714–17724. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]