Abstract
Ala55Val and 45 basepair (bp) insertion/deletion (I/D) of UCP2 gene polymorphisms cause a decrease in resting energy expenditure, decreasing fatty acid oxidation and influencing mRNA transcription and stability, thereby increasing the risk of obesity. The purpose of this study was to determine the role of Ala55Val and 45 bp I/D polymorphisms of the UCP2 gene as a risk factor for obesity. This study consisted of 200 Indonesian subjects of Javanese ethnicity consisting of 100 obese and 100 non-obese participants. Examination of Ala55Val (C > T) UCP2 genotype used Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) methods and 45 bp I/D genotype used PCR methods. Polymorphism of Ala55Val UCP2 genotype in the male group showed that TT genotype and T allele significantly lowers the risk of obesity. Insertion/deletion of 45 bp UCP2 gene in the male group showed that II genotype and I allele significantly increase the risk of obesity whereas for women it showed that the DI genotype and I allele lower the risk of obesity. The results of this study demonstrate that Ala55Val and 45 bp I/D UCP2 polymorphisms play a role in the risk of obesity in Javanese ethnicity of Indonesia after gender stratification.
Keywords: Deletion, Insertion, Obesity, Polymorphism, UCP2
INTRODUCTION
Obesity is a major health problem for most countries in the world and is influenced by environmental and some genetic factors (1). Most of the overweight populations live in countries where there is increasing incidence of mortality caused by complications of obesity (2). Uncoupling proteins (UCP) are a group of mitochondrial anion carrier proteins (MACP) located in the inner mitochondrial membrane and are associated with energy homeostasis (3). There are three types of uncoupling proteins: UCP1, UCP2 and UCP3. One of the genes that is important for regulating intracellular adenosine tri-phosphate (ATP) is UCP2.
The UCP2 gene is widely expressed throughout the body tissues and acts as an uncoupler in oxidative phosphorylation including regulation of lipid metabolism and the production of ATP (4–6). Homo sapiens’ UCP2 gene is located on chromosome 11q13, consisting of 8 exons and 7 introns, with 8174 base pair (bp) length and has 309 amino acids. Disturbance of UCP2 expression in adipose tissue can lead to obesity (7). UCP2 gene polymorphisms that are widely studied include Ala55Val in exon 4, −866G/A in the promoter region and insertion/deletion in the 45 bp of 3′UTR region in exon 8. Ala55Val polymorphism is a missense mutation C to T that occurs in exon 4. Ala55Val polymorphism is also associated with a reduction in 24-hour energy expenditure and decreases in the rate of fat oxidation, thereby increasing the risk of obesity (8–10).
Until now there are limited information about the biological effects of I/D 45 bp polymorphism of the UCP2 gene, and it is thought this polymorphism influences the process of mRNA transcription and stability (8,11). Insertion/deletion of the 45 bp polymorphism at 3′untranslated region (3′UTR) in the exon 8 UCP2 gene plays an important role in some metabolic diseases, causing changes in the rate of metabolism and increased body mass index (BMI) (3). This polymorphism leads to the decrease of UCP2 protein expression and lower energy expenditure. It causes an imbalance of the ratio between intake and expenditure of energy that can lead to obesity and the polymorphism depends on the population (12).
In Indonesia, especially in the Javanese ethnic group, research on Ala55Val and 45 bp I/D polymorphisms UCP2 gene is necessary to determine the effect of this polymorphism as a risk factor for obesity.
MATERIALS AND METHODS
Blood sample collection
This study was a case control study. Subjects were unrelated from students of Universitas Gadjah Mada Yogyakarta and Universitas Muhammadiyah Purwokerto who were eligible according to the inclusion criteria with consecutive sampling. All subjects agreed and signed an informed consent form prior to participating. The approval for the study was obtained from the Ethics Committee of the Faculty of Medicine, Public Health, and Nursing Universitas Gadjah Mada, Yogyakarta, Indonesia. Subjects consisted of obese (BMI > 25) as case group (n = 100) and non-obese participants with BMI > 18.5 – 24.9 as the control group (n = 100) with ages 18 – 30 years old. Subjects were excluded if they were breast-feeding mothers, pregnant women, had infectious diseases, or taking medications such as anti-hypertensive drugs, medications for diabetes, ketosteroids and hypolipidemic agents. Five mL fasting blood were taken and inserted into a tube containing EDTA and then centrifuged to obtain buffy coat. DNA isolation was done using the Promega kit reagent.
Genotyping
Genotyping of Ala55Val UCP2 gene was performed using PCR-RFLP with forward primer 5′-GGCCAGTGCGCGCTACGG-3′ and reverse primer 5′-ATT GTA GAG GCT TCG GGG GCC C-3′ (13). The steps of the PCR cycle included initial denaturation at 95°C for 3 min, followed by 35 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 15 s, extension at 72°C 15 s, final extension at 72°C for 1 min and storage at 4°C. Products of PCR underwent electrophoresis with 3% agarose resulting in 95 bp length and then digested by HaeIII enzyme giving fragments of CC genotype (95 bp), CT genotype (95, 77 and 18 bp), and TT genotype (77 bp and 18 bp) length.
Genotyping of 45 bp I/D UCP2 polymorphism was performed using PCR methods with the forward primer 5′-TCTGGCTGAACTTTCCAA-3′ and reverse primer 5′-TTCATGCCCTCCTTTCTC-3′ (14). The steps of the PCR cycle included initial denaturation at 95°C for 3 min, followed by 35 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 15 s, extension at 72°C 15 s, final extension at 72°C for 1 min and storage at 4°C. Electrophoresis of product PCR for DD genotype was 383 bp, II genotype was 428 bp and DI genotype was 383 bp and 428 bp.
Statistical analysis
Characteristics of subjects were compared by T-test analysis. Differences between genotype and allele frequencies of each obese and control groups were analyzed by chi-square test and odds ratio analysis with P < 0.05 considered as significant.
RESULTS
Characteristics of subjects were listed in Table I and electrophoretic products of Ala55Val and 45 bp I/D polymorphism of UCP2 gene were shown in Figures 1 and 2.
Table I.
Characteristics of subjects
| Characteristics of Subjects | Obese (n = 100) | Control (n = 100) | P |
|---|---|---|---|
| Gender | |||
| Men | 55 | 57 | 0.776 |
| Women | 45 | 43 | |
| Age (year) | 22.35 ± 4.81 | 22.03 ± 4.61 | 0.559 |
| Height (cm) | 164.96 ± 9.88 | 163.35 ± 9.03 | 0.232 |
| Weight (kg) | 89.09 ± 15.55 | 57.28 ± 7.85 | < 0.001 |
| BMI (kg/m2) | 32.65 ± 4.38 | 21.40 ± 1.76 | < 0.001 |
Data were reported as mean ± SD. Significant if P < 0.05.
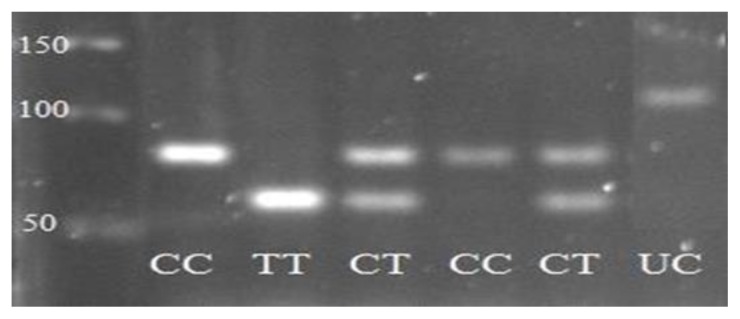
Figure 1.
Product of RFLP Ala55Val UCP2 gene polymorphism. CC genotype shown with a 95 bp, CT genotype with 95, 77 and 18 bp (not shown), TT genotype with 77 and 18 bp (not shown). UC was un-cut PCR product.
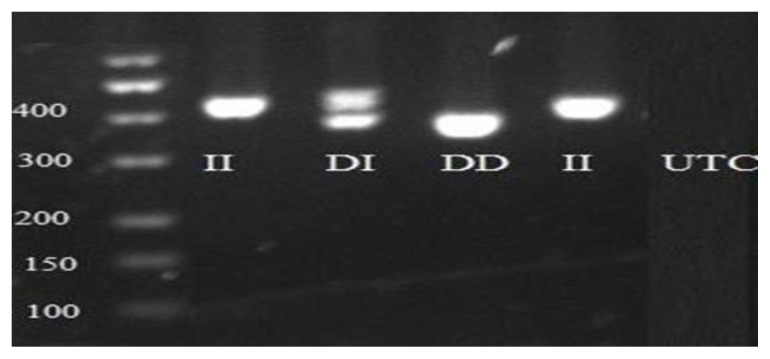
Figure 2.
Insertion/deletion (I/D) of 45 bp PCR product of UCP2 polymorphism. DD genotype was shown with 383 bp fragment, DI genotype indicated by 428 and 383 bp, II genotype was shown with 428 bp fragment. UTC was negative water control. Table II and III showed the frequency of Ala55Val UCP2 genotype and allele and 45 bp D/I UCP2 genotype and allele in the obese and control groups.
Table II and III show there were no significant differences in frequency of Ala55Val UCP2 genotypes and allele also in frequency of 45 bp D/I UCP2 genotypes and allele in obese and control groups and these polymorphisms were not risk factors for obesity.
Table II.
The frequency of Ala55Val UCP2 genotypes and allele in obese and control groups.
| Obese (%) | Control (%) | OR (95% CI) | P | |
|---|---|---|---|---|
| Genotype | ||||
| CC | 16 (16) | 14 (14) | 1.00 | 1.00 |
| CT | 52 (52) | 41 (41) | 1.11 (0.48–2.53) | 0.80 |
| TT | 32 (32) | 45 (45) | 0.6 (0.27–1.45) | 0.27 |
| CC | 16 (16) | 14 (14) | 1.00 | 1.00 |
| CT+TT | 84 (84) | 86 (86) | 0.85 (0.39–1.86) | 0.69 |
| CC+CT | 68 (68) | 55 (55) | 1.00 | 1.00 |
| TT | 32 (32) | 45 (45) | 0.57 (0.32–1.02) | 0.06 |
| Allele | ||||
| C | 84 (42) | 69 (34.5) | 1.00 | 1.00 |
| T | 116 (58) | 131 (65.5) | 0.73 (0.48–1.09) | 0.12 |
Table III.
The frequency of 45 bp D/I UCP2 genotypes and allele in obese and control groups
| Obese (%) | Control (%) | OR (95% CI) | P | |
|---|---|---|---|---|
| Genotype | ||||
| DD | 68 (68) | 68 (68) | 1.00 | 1.00 |
| DI | 25 (25) | 29 (29) | 0.86 (0.46–1.62) | 0.64 |
| II | 7 (7) | 3 (3) | 2.33 (0.58–9.40) | 0.22 |
| DD | 68 (68) | 68 (68) | 1.00 | 1.00 |
| DI+II | 32 (32) | 32 (32) | 1 (0.55–1.81) | 0.88 |
| DD+DI | 93 (93) | 97 (97) | 1.00 | 1.00 |
| II | 7 (7) | 3 (3) | 2.43 (0.61–9.67) | 0.19 |
| Allele | ||||
| D | 161 (80.5) | 165 (82.5) | 1.00 | 1.00 |
| I | 39 (19.5) | 35 (17.5) | 1.14 (0.69–1.89) | 0.61 |
Results after being stratified with gender in these two UCP2 polymorphism were shown in Tables IV and V.
Table IV.
The frequency of Ala 55Val UCP2 genotypes and allele in male groups
| Obese (n = 55) (%) | Control (n = 57) (%) | OR (95% CI) | P | |
|---|---|---|---|---|
| Genotype | ||||
| CC | 11 (20) | 8 (14.0) | 1.00 | 1.00 |
| CT | 28 (50.9) | 20 (35.1) | 1.01 (0.35–2.99) | 0.97 |
| TT | 16 (29.1) | 29 (50.9) | 0.4 (0.13–1.20) | 0.09 |
| CC | 11 (20) | 8 (14.0) | 1.00 | 1.00 |
| CT+TT | 44 (80) | 49 (86.0) | 0.65 (0.24–1.77) | 0.40 |
| CC+CT | 39 (70.9) | 28 (49.1) | 1.00 | 1.00 |
| TT | 16 (29.1) | 29 (50.9) | 0.40 (0.18–0.86) | 0.02 |
| Allele | ||||
| C | 50 (45.5) | 36 (31.6) | 1.00 | 1.00 |
| T | 60 (54.5) | 78 (68.4) | 0.55 (0.32–0.95) | 0.03 |
Table V.
The frequency of Ala 55Val UCP2 genotypes and allele in female groups
| Obese n = 45 (%) | Control n = 43 (%) | OR (95% CI) | P | |
|---|---|---|---|---|
| Genotype | ||||
| CC | 5 (11.1) | 6 (14.0) | 1.00 | 1.00 |
| CT | 24 (53.3) | 21 (48.8) | 1.37 (0.36–5.15) | 0.64 |
| TT | 16 (35.6) | 16 (37.2) | 1.2 (0.30–4.74) | 0.79 |
| CC | 5 (11.1) | 6 (14) | 1.00 | 1.00 |
| CT+TT | 40 (88.9) | 37 (86) | 1.30 (0.36–4.61) | 0.69 |
| CC+CT | 29 (64.4) | 27 (62.8) | 1.00 | 1.00 |
| TT | 16 (35.6) | 16 (37.2) | 0.93 (0.39–2.22) | 0.87 |
| Allele | ||||
| C | 34 (37.8) | 33 (38.4) | 1.00 | 1.00 |
| T | 56 (62.2) | 53 (61.6) | 1.02 (0.56–1.88) | 0.93 |
Table IV shows TT genotype and T allele in the male group had OR 0.40 and 0.55, respectively, and were significantly different compared to CC+CT genotype and C allele. This result showed TT genotype and T allele were risk protective for obesity in males, but in the female group this genotype was not a risk factor for obesity (Table V).
Tables VI and VII show the 45 bp I/D genotype and allele frequency of UCP2 gene in the male and female groups.
Table VI.
The frequency of 45 bp I/D genotypes and allele in male groups
| Obese n = 55 (%) | Control n = 57 (%) | OR (CI 95%) | P | |
|---|---|---|---|---|
| Genotype | ||||
| DD | 33 (60.0) | 46 (80.7) | 1.00 | 1.00 |
| DI | 15 (27.3) | 10 (17.5) | 2.1 (0.83–5.22) | 0.11 |
| II | 7 (12.7) | 1 (1.8) | 9.76 (1.14–83.14) | 0.01 |
| DD | 33 | 46 (80.7) | 1.00 | 1.00 |
| DI+II | 22 | 11 (19.3) | 2.79 (1.19–6.53) | 0.02 |
| DD+DI | 48 (87.2) | 56 (98.2) | 1.00 | 1.00 |
| II | 7 (12.7) | 1 (1.8) | 8.17 (0.97–68.76) | 0.02 |
| Allele | ||||
| D | 81 (73.6) | 102 (89.5) | 1.00 | 1.00 |
| I | 29 (26.4) | 12 (10.5) | 3.04 (1.46–6.33) | 0.002 |
Table VII.
The frequency of 45 bp I/D genotypes and allele in female groups
| Obese n = 45 (%) | Control n = 43 (%) | OR (CI 95%) | P | |
|---|---|---|---|---|
| Genotype | ||||
| DD | 35 (77.8) | 22 (51.2) | 1.00 | 1.00 |
| DI | 10 (22.2) | 19 (44.2) | 0.33 (0.13–0.84) | 0.02 |
| II | 0 (0) | 2 (4.6) | 0 | 0.08 |
| DD | 35 (77.8) | 22 (51.2) | 1.00 | 1.00 |
| DI+II | 10 (22.2) | 21 (48.8) | 0.33 (0.12–0.75) | 0.009 |
| DD+DI | 45 (100) | 41 (95.3) | 1.00 | 1.00 |
| II | 0 (0) | 2 (4.7) | 0 | 0.14 |
| Allele | ||||
| D | 80 (88.9) | 63 (73.3) | 1.00 | 1.00 |
| I | 10 (11.1) | 23 (26.7) | 0.34 (0.15–0.77) | 0.008 |
Based on Table VI, males with II or DI genotypes and I allele were significantly different and these genotypes or allele were risk factors for obesity. In the female group (Table VII), there were no subjects found with the II genotype in the obese group. DI +II genotype and I allele were lower in the obese group compared to control and this genotype or allele were risk protective for obesity with OR 0.33 and 0.34, respectively. This result showed 45 bp I/D UCP2 gene in males and females had contrary risk for obesity.
DISCUSSION
In this study we found the frequency of Ala55Val UCP2 gene polymorphism was not significantly different in the obese and control groups and the polymorphism was not a risk factor for obesity. Results showed the C allele frequency was 0.42 in the obese and 0.35 in the control group. This frequency was almost the same as in a Swedish population (15). This result differs from previous meta-analysis study in Asian populations, which found the C allele frequency range from 0.51 in the Japanese population and 0.81 in the Taiwan population (16).
Frequency of C allele in this study was significantly lower than in the Oktavianthi study with a Balinese population that found the C allele was 0.657 and 0.606 in obese and control groups, respectively (17). Bali is east of Indonesia while our sample came from Javanese populations belonging to the West of Indonesia. From our previous study examining the gene polymorphism in three populations of Indonesia showed that the frequency of gene differed between western, central and the eastern of Indonesia (18). In this study, Ala55Val UCP2 genotype polymorphism was not a risk factor for obesity and this result was similar to Italian and Swedish women populations which showed that polymorphism of Ala55Val UCP2 gene was unrelated to the characteristics of clinical, metabolic and anthropometric obesity (15,19). In a French Caucasian population, polymorphism of UCP2 gene was also not correlated with obesity (20). Other studies in an Asian population showed Ala55Val polymorphism in the UCP2 gene was a risk factor for obesity in a recessive model (21). UCP2 polymorphism was also found to be a risk factor for type 2 diabetes mellitus in Asian individuals but had no effect on European individuals (22). This variant is considered a predisposing factor of obesity and increased insulin secretion in aboriginal populations in Taiwan (23). Polymorphism of Ala55Val UCP2 gene correlated with higher weight loss 12 and 24 months after laparoscopic adjustable gastric banding (LAGB) and 12 months after Roux-en-Y-gastric bypass (RYGB) intervention in obese patients carrying TT genotype and T allele compared to other genotypes and alleles (21,24).
Polymorphism of Ala55Val UCP2 gene in this study, after grouped by gender, showed results which were different. Analysis of Ala55Val, TT genotype and T allele had protective risk of obesity compared to CC + CT genotype or C allele in male group. In the female group, Ala55Val UCP2 gene polymorphism was not different in obese and control groups and this polymorphism was not associated with obesity. Similarly results found in an Indian population showed this gene polymorphism reduced the risk of type 2 diabetes (25). In the Japanese population, it was found that Ala55Val UCP2 mutation may serve as a marker of obesity in women but not in men (26). Different results in a variety of different populations and gender are due to genetic variation of Ala55Val UCP2 genes which are influenced by ethnic, gender and geographic differences. Effects of Ala55Val polymorphism include decreasing 24 hour-resting energy expenditure and decreasing fat oxidation rate so that it can influence the risk of obesity (10). Susmiarsih et al. reported from the reconstruction of the SNP Ala55Val position, determining it was adjacent to the phosphorylation site of Protein Kinase C (PKC) and proposed that this polymorphism possibly interferes with the phosphorylation of PKC which influences the UCP2 function as a controller of body heat (27).
Polymorphism of 45 bp I/D UCP2 gene in this study found the frequency of II genotype was 0.07 in the obese group and 0.03 in the control group, similar to that of research in the Japanese population (28). Meta-analysis study in the Asian population found the frequency of II genotype and I allele ranged from 0.005 – 0.02 (22). The study on three populations of Malaysia found the II genotype frequency was 0.02 (29) and another did not find the II genotype in the Tongan (30) nor French populations (20). The polymorphism of 45 bp I /D UCP2 gene in this study showed this polymorphism was not a risk factor for obesity. Similarly, a meta-analysis that studied 42 research results did not show any association of 45 bp I/D UCP2 gene polymorphism with obesity, as well as research on an Italian population (19, 22). Other studies found no association between DI genotype of 45 bp I/D UCP2 gene polymorphism with obesity, rest energy expenditure, BMI and insulin secretion (3, 31–33).
Contrasting results from other studies showed II genotype and I allele were risk factors for obesity compared to the other genotypes or allele (29, 34). Result study by Ochoa, showed the haplotype of the carrier −866G rs659336, −55T rs1800849 and the D allele UCP2 gene increased 9 times higher the risk of obesity (35). Research by Hashemi et al. in an Iranian population suggests that individuals with DI genotype may have reduced risk of metabolic syndrome, and individuals who were carriers of II genotype and I allele had higher risk for obesity compared with individuals who had DD genotype (14). This finding in line with research by Oguzkan-Balci et al. showed that individuals with II genotype and I allele were associated with obesity and other metabolic diseases. This research found II genotype and I allele were associated with obesity in children and metabolic syndrome (36). Meta-analysis research by Brondani et al. found I/D polymorphism was associated with obesity in a European population (37). Analysis of 45 bp I/D UCP2 polymorphism based on gender stratification in this research showed the gene polymorphisms influence both male and female groups with opposite effect. In the male group, II and DI+II as a dominant and recessive model had a significant effect in the increasing risk of obesity compared to the DD genotype. In the female group, 45 bp I/D UCP2 polymorphisms have significant associations with obesity as a recessive model. One research with the same result by Papazoglou et al. showed II genotype and I allele lead to decreased UCP2 expression. In male individuals, II genotype and I allele 45 bp I/D polymorphism lead to decreased UCP2 expression, and causes weight gain (38). One study on three populations of Malaysia; Malay, Chinese and Indian populations showed that 45 bp I/D UCP2 gene polymorphism was a risk factor for obesity, but when grouped by ethnicity and gender the results were different. In men, the polymorphism of 45 bp UCP2 gene was not associated with the risk of obesity among ethnic groups. In women I allele of 45 bp UCP2 allele was a risk factor for obesity, especially in subjects with Indian ethnicity (29).
The biological effects of the 45 bp I/D polymorphism are not widely understood. The location of the polymorphism in the 3′UTR in exon 8 is thought to be related with the mRNA transcription process or affects its stability. This polymorphism is thought to affect UCP2 protein expression and cause obesity (8). Differences between the role of UCP2 gene polymorphism in the incidence of obesity in some populations were likely to be due to several factors, including: 1) The gene pool for each population had a different genetic variation; 2) The genes associated with obesity are numerous; 3) There was variability of the adjacent loci of UCP2 gene that may also play a role in the risk of obesity; and 4) Environmental factors and eating habits in each population/ethnic were different. There were several weaknesses in this research due to the limited sample population: one, we could not find the II genotype of 45 bp I/D UCP2 gene polymorphism in females, and second as a result, this condition caused identification of gene polymorphism to have different risks in men and women for obesity. Moreover, in this study we did not control the environmental factors that could interfere with the results such as considering the variables of diet, nutrition, lifestyle, and thermogenesis as possible confounding factors of obesity.
CONCLUSION
In conclusion, our results suggest that UCP2 gene polymorphisms of Ala55Val and 45 bp insertion/deletion are associated with the risk of obesity in the Javanese ethnic group of Indonesia with gender stratification.
ACKNOWLEDGMENTS
This study was supported in part by a grant from National Nuclear Energy Agency of Indonesia and Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada through Research and Public funding no. 632/UN11-P.III/DIT-LIT/2015. None of the authors have any conflict of interest or any financial ties to disclose.
REFERENCES
- 1.Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard AM, et al. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl 1):S130–5. doi: 10.2337/diabetes.53.2007.s130. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Noncommunicable diseases prematurely take 16 million lives annually, WHO urges more action. WHO Media Centre; Jan 19, 2015. Retrieved from http://www.who.int/mediacentre/news/releases/2015/noncommunicable-diseases/en/ [Google Scholar]
- 3.Dalgaard LT, Andersen G, Larsen LH, Sorensen TI, Andersen T, et al. Mutational analysis of the UCP2 core promoter and relationships of variants with obesity. Obes Res. 2003;11:1420–1427. doi: 10.1038/oby.2003.191. [DOI] [PubMed] [Google Scholar]
- 4.Diano S, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med. 2012;18:52–58. doi: 10.1016/j.molmed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 6.Arsenijevic D, Onuma H, Pecqueur C. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26(4):435–9. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 7.Oberkofler H, Liu YM, Esterbauer H, Hell E, Krempler F, et al. Uncoupling protein-2 gene: reduced mRNA expression in intraperitoneal adipose tissue of obese humans. Diabetologia. 1998;41:940–946. doi: 10.1007/s001250051011. [DOI] [PubMed] [Google Scholar]
- 8.Esterbauer H, Schneitler C, Oberkofler H, Ebenbichler C, Paulweber B, et al. A common polymorphism in the promoter of UCP2 is associated with decreased risk of obesity in middle aged humans. Nat Genet. 2001;28:178–183. doi: 10.1038/88911. [DOI] [PubMed] [Google Scholar]
- 9.Krempler F. A functional polymorphism in the promoter of UCP2 enhances obesity risk but reduces type 2 diabetes risk in obese middle-aged humans. Diabetes. 2002;51:3331–3335. doi: 10.2337/diabetes.51.11.3331. [DOI] [PubMed] [Google Scholar]
- 10.Astrup A, Toubro S, Dalgaard LT, Urhammer SA, Sorensen TI, et al. Impact of the v/v 55 polymorphism of the uncoupling protein 2 gene on 24-h energy expenditure and substrate oxidation. Int J Obes Relat Metab Disord. 1999;23:1030–1034. doi: 10.1038/sj.ijo.0801040. [DOI] [PubMed] [Google Scholar]
- 11.Walder K, Norman RA, Hanson RL, Schrauwen P, Neverova M, et al. Association between uncoupling protein polymorphisms (UCP2–UCP3) and energy metabolism/obesity in Pima Indians. Hum Mol Genet. 1998;7:1431–1435. doi: 10.1093/hmg/7.9.1431. [DOI] [PubMed] [Google Scholar]
- 12.Jiffri EH. Association of the UCP2 45 bp insertion/deletion polymorphism with diabetes type 2 and obesity in Saudi population. Egypt J Med Hum Gen. 2012;13:257–262. [Google Scholar]
- 13.Qin LJ, Wen Y, Qu YL, Huang QY. Lack of association of functional UCP2 −866G/A and Ala55Val polymorphisms and type 2 diabetes in the Chinese population based on a case-control study and a meta-analysis. Genet Mol Res. 2013;12(3):3324–3334. doi: 10.4238/2013.September.3.9. [DOI] [PubMed] [Google Scholar]
- 14.Hashemi M, Rezaei H, Kaykhaei MA, Taheri MA. 45 bp insertion/deletion polymorphism of UCP2 gene is associated with metabolic syndrome. J Diab Met Disord. 2014;13:1–5. doi: 10.1186/2251-6581-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mottagui-Tabar S, Hoffstedt J, Brookes AJ, Jiao H, Arner P, et al. Association of ADRB1 and UCP3 gene polymorphisms with insulin sensitivity but not obesity. Horm Res. 2008;69:31–36. doi: 10.1159/000111793. [DOI] [PubMed] [Google Scholar]
- 16.Qian L, Xu K, Xu X, Gu R, Liu X, et al. UCP2 −866G/A, Ala55Val and UCP3 −55C/T polymorphisms in association with obesity susceptibility — a meta-analysis study. PLoS ONE. 2013;8(4):e58939. doi: 10.1371/journal.pone.0058939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oktavianthi S, Trimarsanto H, Febinia CA, Suastika K, Saraswati MR, et al. Uncoupling protein 2 gene polymorphisms are associated with obesity. Card Diab. 2012;11:41. doi: 10.1186/1475-2840-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hastuti P, Sofro ASM, Asdie AH, Sadewa AH. Genetic variation of Apolipoprotein E (ApoE) in Surabaya, Palu and Alor populations of Indonesia. Indonesian J Biotech. 2011;16(2):118–125. [Google Scholar]
- 19.Maestrini S, Podestà F, Di Blasio AM, Savia G, Brunani A, et al. Lack of association between UCP2 gene polymorphisms and obesity phenotype in Italian Caucasians. J Endocrinol Invest. 2003;26:985–990. doi: 10.1007/BF03348196. [DOI] [PubMed] [Google Scholar]
- 20.Otabe S, Clement K, Rich N, Warden C, Pecqueur C, et al. Mutation screening of the human UCP 2 gene in normoglycemic and IDDM morbidly obese patients: lack of association between new UCP 2 polymorphisms and obesity in French Caucasians. Diabetes. 1998;47:840–842. doi: 10.2337/diabetes.47.5.840. [DOI] [PubMed] [Google Scholar]
- 21.Chen HH, Lee WJ, Wang W, Huang MT, Lee YC, et al. Ala55Val polymorphism on UCP2 gene predicts greater weight loss in morbidly obese patients undergoing gastric banding. Obesity Surgery. 2007;17:926–933. doi: 10.1007/s11695-007-9171-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Wang M, Zhao ZT. Uncoupling protein 2 gene polymorphisms in, association with overweight and obesity, susceptibility: A meta-analysis. Meta Gene. 2014;2:143–159. doi: 10.1016/j.mgene.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu K, Zhang M, Cui D, Fu Y, Qian L, et al. UCP2 -866G/A and Ala55Val, and UCP3-55C/T polymorphisms in association with type 2 diabetes susceptibility: a meta-analysis study. Diabetologia. 2011;54(9):2315–24. doi: 10.1007/s00125-011-2245-y. [DOI] [PubMed] [Google Scholar]
- 24.Nicoletti CF, de Oliveira APRP, Brochado MJF, Pinhel MAS, de Oliveira BAP, et al. The Ala55Val and −866G>A polymorphisms of the UCP2 gene could be biomarkers for weight loss in patients who had Roux-en-Y gastric bypass. Nutrition. 2017;33:326–330. doi: 10.1016/j.nut.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Vimaleswaran KS, Radha V, Ghosh S, Majumder PP, Sathyanarayana Rao MR, et al. Uncoupling protein 2 and 3 gene and their association with type 2 diabetes in Asian Indians. Diab Tech Ther. 2011;13(1):19–25. doi: 10.1089/dia.2010.0091. [DOI] [PubMed] [Google Scholar]
- 26.Kosuge K, Soma M, Nakayama T, Aoi N, Sato M, et al. Human uncoupling protein 2 and 3 genes are associated with obesity in Japanese. Endocrine. 2008;34(1–3):87–95. doi: 10.1007/s12020-008-9111-9. [DOI] [PubMed] [Google Scholar]
- 27.Susmiarsih T, Trimarsanto H. Kajian Bioinformatika Uncoupling Protein 2 (UCP2) dan Mutasi Ala55Val UCP2 Pada Obesitas dan Diabetes Melitus Tipe 2 (DMT2) Majalah Kesehatan Pharmamedika. 2013;5:1. [Google Scholar]
- 28.Shiinoki T, Suehiro T, Ikeda Y, Inoue M, Nakamura T, et al. Screening for variants of the uncoupling protein 2 gene in Japanese patients with non-insulin-dependent diabetes mellitus. Metabolism. 1999;48(5):581–584. doi: 10.1016/s0026-0495(99)90054-9. [DOI] [PubMed] [Google Scholar]
- 29.Say YH, Ban ZL, Arumugam Y, Kaur T, Tan ML, et al. Uncoupling protein 2 gene (UCP2) 45-bp I/D polymorphism is associated with adiposity among Malaysian women. J Biosci. 2014;39:867–875. doi: 10.1007/s12038-014-9488-y. [DOI] [PubMed] [Google Scholar]
- 30.Duarte NL, Colagiuri S, Palu T, Wang XL, Wilcken D. A 45-bp insertion/deletion polymorphism of uncoupling protein 2 in relation to obesity in Tongans. Obes Res. 2003;11:512–517. doi: 10.1038/oby.2003.72. [DOI] [PubMed] [Google Scholar]
- 31.Avesani CM, Kamimura MA, Utaka S, Pecoits-Filho R, Nordfors L, et al. Is UCP2 gene polymorphism associated with decreased resting energy expenditure in nondialyzed chronic kidney disease patients? J Ren Nutr. 2008;18:489–494. doi: 10.1053/j.jrn.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Berentzen T, Dalgaard LT, Petersen L, Pedersen O, Sorensen TI. Interactions between physical activity and variants of the genes encoding uncoupling protein-2 and −3 in relation to body weight changes during a 10-y follow up. Int J Obes. 2005;29:93–99. doi: 10.1038/sj.ijo.0802841. [DOI] [PubMed] [Google Scholar]
- 33.Lee YH, Kim W, Yu BC, Park BL, Kim LH, et al. Association of the ins/del polymorphisms of uncoupling protein 2 (UCP2) with BMI in a Korean population. Biochem Biophys Res Commun. 2008;371:767–771. doi: 10.1016/j.bbrc.2008.04.144. [DOI] [PubMed] [Google Scholar]
- 34.Kaabi YA. The deletion polymorphism in Exon 8 of Uncoupling Protein 2 is associated with severe obesity in a Saudi Arabian case-control study. Biomed J Sci &Tech Res. 2018;2(2) doi: 10.4103/ijem.IJEM_655_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochoa MC, Santos JL, Azcona C, Moreno-Aliaga MJ, Martinez-Gonzalez MA, et al. Association between obesity and insulin resistance with UCP2–UCP3 gene variants in Spanish children and adolescents. Mol Genet Metab. 2007;92:351–358. doi: 10.1016/j.ymgme.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Oguzkan-Balci S, Col-Araz N, Nacak M, Araz M, Sabanci H, et al. Mitochondrial uncoupling protein 2 (UCP2) gene polymorphisms are associated with childhood obesity and related metabolic disorders. J Ped Endocrinol Met. 2013;26:277–283. doi: 10.1515/jpem-2012-0267. [DOI] [PubMed] [Google Scholar]
- 37.Brondani LA, Assmann TS, de Souza BM, Bouças AP, Canani LH, et al. Meta-analysis reveals the association of common variants in the uncoupling protein (UCP) 1–3 genes with body mass index variability. PLoS One. 2014;9:e96411. doi: 10.1371/journal.pone.0096411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papazoglou D, Papathanasiou P, Papanas N, Papatheodorou K, Chatziangeli E, et al. Uncoupling protein-2 45-base pair insertion/deletion polymorphism: is there an association with severe obesity and weight loss in morbidly obese subjects? Metab Syndr Relat Disord. 2012;10:307–311. doi: 10.1089/met.2012.0003. [DOI] [PubMed] [Google Scholar]