Abstract
Many animals that use acoustic communication synchronize their mate attraction signals: individuals precisely time their calls to overlap those of their neighbors. In contrast, synchrony in the mate attraction displays of species with visual/motion-based signals is rare. It has only been documented in five species of fiddler crabs. In all of them, small groups of males wave their single large claw in close synchrony. Here, I review what we know about synchrony in fiddler crabs, comparing the five species with each other to determine whether similar mechanisms and functions are common to all. I also propose future research questions that, if answered, would shed light on synchronous behavior in both visual and acoustic signallers.
Keywords: fiddler crab, synchrony, waving, mate choice, wave timing, visual signal
Introduction
Synchrony is integral to the lives of many animals. Some flocks of birds and fish schools move in a swirl of turns and twists involving individuals coordinating their complicated movements without planning or practice. How and why individuals coordinate their movements and other behaviors, such as courtship, is a growing field of research. One particularly interesting and active area of research is the synchrony of mate attraction signals (Greenfield et al. 2017). In some species of frogs (Grafe 1999), katydids (Greenfield and Roizen 1993), bush crickets (Barbosa et al. 2016), and fireflies (Moiseff and Copeland 2010), males gather in groups and signal to females in synchrony. This is an unusual context to observed synchrony because each male is competing with the others to catch the female’s attention: why would they time their signals to overlap with each other?
There are two (non-mutually exclusive) categories of signal synchrony: adaptive and incidental synchrony. First, in adaptive synchrony, the synchrony itself is beneficial to the group members. They co-operate with each other to produce very precisely overlapping signals, with the aim of each individual being to not ‘stand out’. The functions of adaptive synchrony are usually to attract attention (e.g., mate attraction) from a distance as the precisely overlapping signals combine to produce a higher intensity group signal with a preserved sound envelope (Hartbauer et al. 2014; Greenfield 1994); or to avoid predators as precisely overlapping signals can confuse a predator (Nityananda and Balakrishnan 2009). The mechanisms thought to underlie adaptive synchrony are phase-locking, in which individuals accelerate or decelerate their own intrinsic rhythms to match those of their neighbors (Murphy et al. 2016); or chirp-by-chirp adjustments, whereby individuals adjust the timing of each signal to match that of its neighbur (Hartbauer et al. 2005).
The second main category is incidental synchrony. Here, there is no advantage to synchrony in itself, and the pattern is an incidental by-product of competition between individual group members (Greenfield et al. 2017). Unlike adaptive synchrony, the aim of each individual is to stand out. Individuals compete with each other to produce leading signals because there is an advantage to signaling first as receivers preferentially approach leading signals (Hartbauer et al. 2005; Greenfield and Schul 2008; Party et al. 2015; Greenfield et al. 2017). The proximate mechanism generating the synchrony is most likely to be inhibitory resetting in which a male is inhibited from signaling when his neighbor is signaling (Greenfield et al. 2017). This leads to a less perfect form of synchrony than that found in adaptive scenarios.
The synchrony of mate attraction signals is most commonly studied in acoustic species, and it is in the field of sound/hearing where most of the recent advances have therefore been made. The bioluminescent flashes of fireflies have also been well studied (Moiseff and Copeland 2010). It is very rare for visual/motion-based species to display in synchrony: it is known from only five species of fiddler crabs where males wave their single enlarged claw to attract females for mating, with each wave timed to overlap those of his neighbors (Backwell et al. 1998; Backwell et al. 2006; Reaney et al. 2008a; Rorato et al. 2017). Here, I review what we know about synchronous waving in fiddler crabs in the hope of spurring further research, particularly in translating what we know about acoustic synchrony into the visual context. I end the review with suggested topics for future research.
Fiddler Crabs
Fiddler crabs are inter-tidal animals that live on mudflats in and around mangrove forests. In all of the 103 currently recognized species, males have a single greatly enlarged major claw that can contribute up to half of his total mass (Crane 1975). The major claw is critical in territory defence where it is a formidable weapon that is used in male-male fights (Jennions and Backwell 1996). In many species, however, the major claw is also used in mate attraction (Crane 1975).
Fiddler crabs can be divided into two mating categories: male-searchers and female-searchers (deRivera et al. 2003). In male-searching species, a male will approach a neighboring female and, with no apparent courtship, the pair will mate on the surface and both will then return to their own territories (Crane 1975). Little is known about this mating system. We know a lot, however, about female-searching species (e.g., Crane 1975; Backwell and Passmore 1996; Zeil et al. 2006; Dyson and Backwell 2016). Males wave their enlarged major claw to attract females. When a female is ready to mate, she leaves her territory and moves through the population of courting males. Males wave their enlarged claws in a species-specific pattern to attract them (Crane 1975). Females can visit up to 106 males before choosing a mate (Reaney and Backwell 2007; deRivera 2005). Mate choice is based on multiple male traits (e.g., wave rate, claw size; Backwell and Passmore 1996; Reaney and Backwell 2007) that initially determine whether a female approaches a male, as well as multiple burrow characteristics (e.g., burrow depth, internal temperature; Reaney and Backwell 2007) that influence whether or not she stays and mates. Once a female selects a male, the pair enters his burrow and mating occurs underground.
This group of fiddler crabs have surprisingly complex social lives: they live in stable neighborhoods (Booksmythe et al. 2012); form coalitions to fight off intruders (Backwell and Jennions 2004); and deceive each other about their strength and signaling abilities (Backwell et al. 2000). In these species, females have a vast array of sensory biases and preferences for specific male traits such as claw size, speed of movement, wave rate and wave timing (Backwell and Passmore 1996; Murai and Backwell 2005; Reaney et al. 2008b and others). And it is within the group of female-searching fiddler crabs that synchronized waving has evolved (Backwell et al. 1998; Figure 1).
Figure 1.

Photograph of Austruca mjoebergi males waving in synchrony.
These crabs are ideal study animals. They occur in huge populations with thousands of individuals living in small, abutting territories. Males and females live intermixed. Their small size means you can watch a whole neighborhood simultaneously. The flat, two-dimensional environment allows you to track individuals with a clear, unimpeded view. They are easy to catch and mark. They are ideal animals for manipulative experiments because they are relatively unaffected by handling and return to their natural behaviors almost immediately after being placed back on the sediment. We also know a great deal about their visual system and how its peripheral information processing works (Zeil et al. 1986; Zeil and Hemmi 2006; How et al. 2015).
Synchronous Waving in Fiddler Crabs: What We Know so Far
The first scientific report of synchronous waving in a fiddler crab was made by Helen Gordon in southern Africa in 1958 (Gordon 1958). She reported that small groups of 5–7 neighboring male Uca annulipes (now called Austruca occidentalis) moved their bodies and waved their claws with a remarkable degree of synchrony. Although there was near-perfect timing, occasionally a leader would be evident. She suggested that synchrony “could be best described as a physical expression of abundant energy in a sun-loving species”.
A second paper on synchrony in fiddler crabs (on the same southern African species) showed that synchronous waving was always initiated by a mate-searching female (Backwell et al. 1998). A cluster of 2–6 neighboring males formed around the female and waved in close synchrony, with some males falling out and new males joining in as the female moved across the mudflat. We made video recordings of 45 females as they selected mates. By analyzing the videos frame-by-frame, we documented the start and end of every wave of every male within the clusters of wavers surrounding each female.
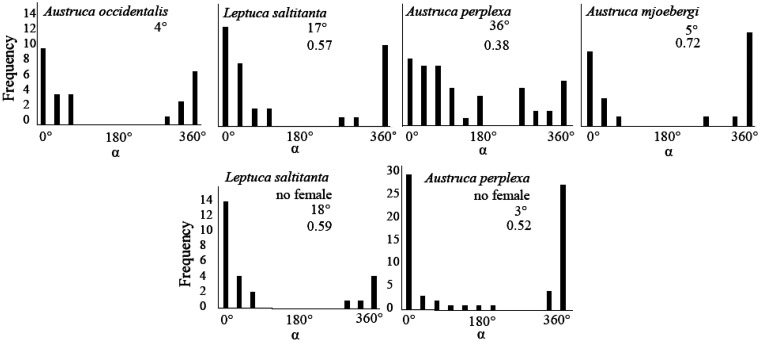
Quantifying synchrony can be challenging: how do you pair males up with each other, or do you compare every possible combination of male pairs? We took the approach of selecting the target male as the one chosen by the female, and compared each male in the cluster with the target male (but did not compare non-chosen males with each other). We defined a “wave cycle” as the interval between the onset of successive waves of the target male (wave cycle duration = Tt). Each wave of each of the neighbors was then assigned to the wave cycle of the target male in which the wave began. We calculated the difference in the onset times of the target male (tt) and the neighboring male (tn) and calculated the phase angle, α = [(tn−tt)/Tt] × 360°. The phase angle is a measure of synchrony: if α = 0° or 360°, the waves of the neighbor and the target male are in perfect synchrony; if α = 180°, waves are perfectly alternating. Using circular statistics, we calculated the mean α per group of waving males and tested whether they were uniformly distributed using Rayleigh’s test. We found that the phase angles were not uniformly distributed, but were significantly concentrated around an α of 5° (Figure 2).
Figure 2.
The mean phase angles (α) for synchronous groups of males when females were present (top row) and when females were absent (bottom row) in four species of fiddler crabs. An α of 0° or 360° represents precise synchrony; an α of 180° represents complete alternation. The average phase angle and the average length of the mean vector (for all synchronous groups combined) is given below the species name. Vector length is a measure of data clumping around the mean α: a vector length of 0 is uniformly distributed; a vector of 1 is tightly clumped. The vector length data for Austruca occidentalis is not presented because it is currently stored on “floppy disks”.
We also found that males chosen by females had higher wave rates than non-chosen males (Backwell et al. 1999). This is because they added extra (unsynchronized) waves between the waves that were given in synchrony with their neighbors. Furthermore, chosen males were more likely to produce leading waves, given shortly before the waves of the neighboring males. It is therefore possible that females select males because they have fast wave rates, or because they produce leading waves, or because of another factor correlated with one or both of these traits. It is not possible to separate these alternatives with observational data.
In Austruca occidentalis, synchronized signaling does not appear to be a cooperative behavior. There are no visual predators that would be confused by group synchrony; and synchrony does not attract females from a distance since it is a localized phenomenon that only occurs once a female is within 10 cm of a cluster of males. Distant females would not have the opportunity to approach a synchronously waving cluster without being surrounded by their own cohort of synchronously waving males. We therefore concluded that synchrony is more likely to be an incidental byproduct of males competing to produce leading waves (Backwell et al. 1998). We could not, however, confirm this with our correlational data.
In 2006, we discovered two other fiddler crab species that waved in synchrony: Austruca perplexa from Japan and Leptuca saltitanta from Panama (Backwell et al. 2006). Synchronous waving fiddler crabs might be rare, but they are geographically wide-spread! Unlike A. occidentalis, both of these species waved in synchrony when a female was present as well as when there was no mate-searching female in the vicinity (Figure 2). In A. perplexa, chosen males had higher wave rates and produced more leading waves than their neighbors. In L. saltitanta, chosen males had higher wave rates but did not produce more leading waves than non-chosen males.
Our new findings raised an important question: if synchrony was the result of males competing to produce leading waves (because females preferred leading waves), then why was there synchrony in a non-mating context? In both A. perplexa and L. saltitanta, males form small clusters around mate-searching females and wave in synchrony, with very similar patterns of behavior to those previously observed in Austruca occidentalis. In A. perplexa, chosen males produced more leading waves than non-chosen males. This classic pattern of behavior strongly suggests that synchrony is a byproduct of male competition to produce leading waves. But why are males competing to lead when there are no females to compete for? Austruca perplexa is indeed perplexing! Is it reasonable to think that the synchrony that occurs in clusters around a female is an incidental byproduct of males competing to produce leading waves, but that patches of synchrony that occur in the absence of a female are due to males cooperating to attract females from a distance?
Recently, a fourth synchronously waving fiddler crab was reported from Brazil, Leptuca leptodactyla (Rorato et al. 2017). This study was the first to experimentally examine the effect of female presence and male density on synchronous waving. They found that female presence induced previously inactive males to start waving; stimulated higher wave rates; and triggered synchronous waving. Waves were not synchronized when females were absent. As in the other studied species, males formed a small cluster (2–6 males) around a female. Although the authors claim that male density had a negligible effect on synchronization, there was, in fact, a strong effect of male density: synchrony was more precise when males were at low density. Under natural mate-searching conditions in this species, a maximum of 5–6 males form a cluster around a female and engage in male–male combat in order to establish and maintain their position (Rorato et al. 2017). This is very unusual for a fiddler crab; males usually form synchronous clusters for a very brief amount of time since the mate-searching female quickly moves away. The extended period of synchronous waving at a female (at least 24 min!) and male combat to maintain his position within a cluster, provides an excellent opportunity to make fine-scaled measurements of temporal changes in the level of synchrony within a cluster.
The fifth and final synchronously waving fiddler crab was found in Australia: Austruca mjoebergi (Reaney et al. 2008a). The patterns of behavior are identical to those in Austruca occidentalis: small clusters of synchronously waving males form around mate-searching females (Figure 1). There is no synchrony when females are absent and the synchrony is strong and precise (a mean α of 5° compared to 7° in A. occidentalis; Figure 2).
In A. mjoebergi, we have been able to overcome the limitations of correlational data and experimentally test female mating preferences. We designed and constructed a set of robotic fiddler crabs, each consisting of a metal arm driven by a twin-cam motor to exactly mimic the wave form. Each motor is housed in a small container that sits below a sediment-covered arena on which the female is released. Only the metal arm protrudes above the sediment surface. A plaster cast of a real claw, painted to match the natural claw color, can be attached to the metal arm. A central motor powers up to eight robotic crabs simultaneously, giving control over the exact timing (wave duration and inter-wave interval) of every wave of each robot. We can control the: spacing of males by selecting the placement of each robot on the arena; the claw size, color, and shape of the plaster claw that we attach to the metal arms; and the physical environment (e.g., shade or sun; background) the female experiences.
Using this robotic system, we are able to test female preferences for particular male traits by giving them a choice between alternative signals under a fairly well-controlled environment, but still within the natural habitat of A. mjoebergi. We can capture a mate-searching female, test her immediately, and release her, whereupon she will continue natural mate-searching within a few minutes. We are able to determine, with great precision, what traits are attractive to females, how repeatable their choices are, how variable the preferences are between different females, and how accurately females approach different stimuli.
We first gave a female the option of approaching one of four males, each with the same wave rate, the same wave structure and identical claws. The four robotic crabs were set up in pairs on either side of the female. One pair waved in synchrony, the other pair waved in alternation. We found that females were not attracted to the synchronously waving pair of males: they were equally likely to approach a synchronous pair as an alternating pair. We then tested females’ preferences for leading waves and found that they strongly preferred the leader, both when there was a 0.9 s delay (α = 30°) and a 1.8 s delay (α = 60°) between the starts of the two overlapping waves.
Females are not preferentially attracted to synchronously waving males, but they have a strong preference for leading wavers. This, again, suggests that synchrony is an incidental byproduct of males competing to lead rather than being a cooperative behavior from which all members of a synchronous group of males benefit.
We then further tested female preferences for wave timing by presenting them with a choice between a group of three synchronously waving robotic males and a single robot waving in alternation with the group (Kahn et al. 2014). We found that females preferentially approached the alternating waver, and that the preference for alternation was equally as strong as the preference for leader. We also gave females a choice between three synchronously waving male robots and one that lagged (in the first experiment, the laggard’s wave started half-way through the wave of the synchronous group; in the second experiment, the laggard’s wave started at the end of the synchronous wave). In both these cases, we found that females showed no preference for synchrony or lagging, and they were equally likely to approach any one of the four robotic crabs. From these results we concluded that there is no disadvantage in lagging; but there is a strong advantage in being a leader or waving in complete alternation with the rest of your neighbors. Since females did not discriminate against the solitary males whose waves started immediately after the synchronous group, it is not the overlapping of waves that females avoid. We proposed an explanation for this pattern of female choice, which is that females prefer waves that are (i) immediately preceded by a period of no waving; and (ii) have a unique starting point (only one male initiates a wave at that time). Both alternators (Kahn et al. 2014) and leaders (Backwell et al. 1998) satisfy both of these conditions.
Why would females prefer waves with a unique starting point (i.e., that “stand out” from other signallers)? It could be an adaptive response in that producing such waves may be correlated with male quality. It is equally likely that the female response is due to a deeper constraint generating a sensory bias: when a female detects the start of a male’s wave, movement is triggered towards that male. If the female receives a cluttered signal (multiple waves start at the same time), then the sensory bias does not “kick in” and she uses another signal trait to select a male (e.g., claw size). [Note: adaptive explanations and those based on sensory biases are not mutually exclusive, for example, a sensory bias may arise adaptively].
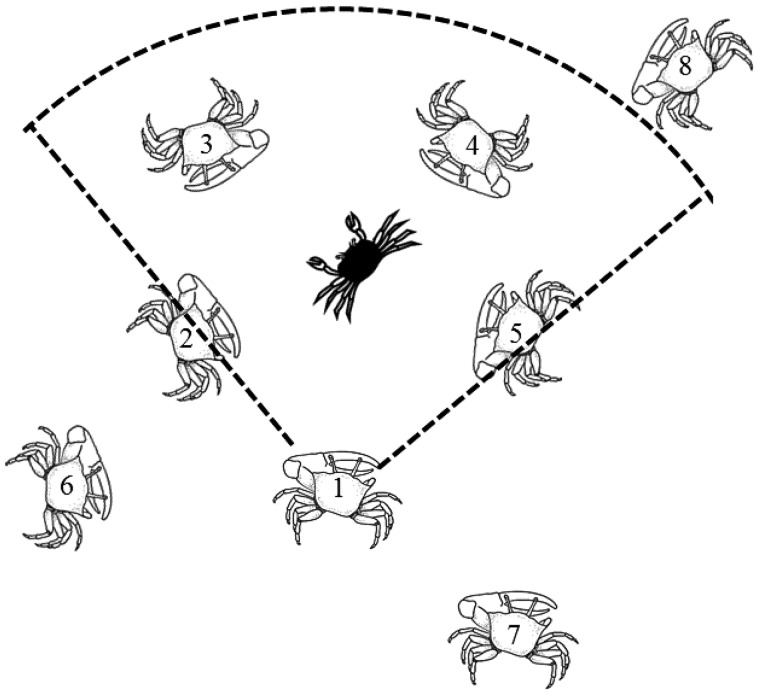
The final study published on synchrony in fiddler crabs produced a simple model that is able to successfully account for the emergence of local synchrony in small neighborhoods (Araujo et al. 2013). It shows that synchrony is more likely to evolve if males have selective attention (Figure 3). Fiddler crabs have compound eyes on stalks with 360° vision. They are excellent motion detectors, but they have poor visual resolution (Zeil et al. 1986; Zeil and Hemmi 2006). In all the synchronous fiddler crabs, males form small clusters around a mate-searching female and wave in synchrony. The males that fall within the cone of selective attention of a particular male will trigger his synchronous waving. Males that fall outside of his field of selective attention would not trigger synchrony (Figure 3).
Figure 3.
A cluster of males around a female (black). The males that fall within the cone of selective attention of male # 1 (males 2, 3, 4, and 5) would be the neighbors that trigger his synchronous waving. Males that fall outside of his field of selective attention (males 6, 7, and 8) would not trigger synchrony.
Phylogeny
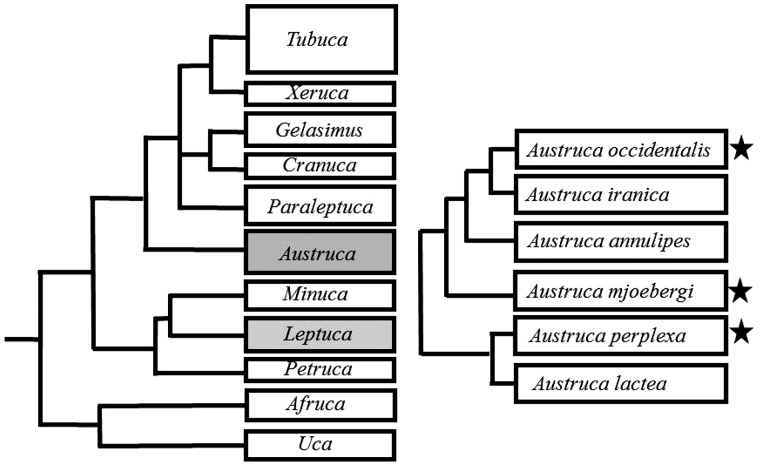
As noted, we currently know of five synchronously waving fiddler crab species. Using a recent molecular phylogeny of this taxon (Shih et al. 2016), it is clear that synchrony must have evolved at least twice: once in the genus Leptuca and once in the genus Austruca (Figure 4). The A. lactea complex is a group of six closely related species, three of which are known to have synchronous waving (A. occidentalis; A. mjoebergi; and A. perplexa;Figure 4). One species, A. lactea, does not appear to have synchronous waving. It is unknown whether the other two species wave in synchrony, but it seems likely given the phylogenetic relationships between these six species.
Figure 4.
Relationship between the seven genera of fiddler crabs (left) with the two genera that contain synchronously waving species marked in grey. The relationship between the Austruca lactea complex (right): species with synchronous waving are marked with an asterisk. Based on Shih et al. (2016).
The phylogenetic relationship of the two synchrony Leptuca species is not yet resolved since only 14 of the 30 Leptuca species have been included in the genetic phylogeny (Shih et al. 2016). It is clear, however, that L. uruguayensis is closely related to L. leptodactyla and may, therefore, have synchronous waving. Of the other six related species, three are known to have no synchronous waving (L. deichmani; L. stenodactylus; L. terpsichores; Backwell, pers. comm.) and there is no information available for the other three (L. speciosa; L. dorothea; L. cumulanta).
Similarities between the Synchronous Species
All five of the synchronously waving fiddler crab species are similar to each other: they are all small species with simple, circular waving movements. They also all live at high densities but the synchrony does not spread through the population. Instead, it occurs in small groups, when 2–10 males cluster around a mate-searching female and wave in synchrony with each other. There are, of course, also some differences between the five species. In three species (A. occidentalis; A, mjoebergi and A. perplexa), there are low levels of predation and no predators that would be confused by synchronous waves. In L. saltitanta, however, there are high levels of predation (mostly by grackles: Quiscalus mexicanus;Koga et al. 1998). Predation levels in the L. leptodactyla populations is unknown.
In three of the five species, synchrony only occurs when a mate-searching female is close (usually <10 cm) so synchrony could not be a long-distance attractant for females. In A. perplexa and L. saltitanta, however, males wave in synchrony even when no female is present. It is therefore possible that distant females are attracted to synchronous groups in these two species.
Where to from Here?
There are four questions that emerge from studies of fiddler crab synchrony. Each of them is likely to shed light not only on vision-based synchrony, but also on the broader topic of synchronous signaling in all taxa.
Why is synchrony so rare in visual communication?
There are fundamental reasons that synchrony should be favored in acoustic (rather than visual) signaling systems: 1) sound waves interfere with each other (e.g., superposition of sound waves; “jamming”, combined sound fields) while image-forming eyes are easily able to resolve multiple simultaneous movements. 2) Sound travels long distances so long-range communication is possible, while visual signals are usually restricted to short-range communication. 3) Acoustic signallers have the ability to precisely control the timing of their calls, while the adjustment of movements may be less precise (see Greenfield 2015). Visual signallers can, however, still form synchronous groups. Understanding the contexts in which visual synchrony arises, and comparing these contexts with acoustic synchrony, is likely to be a fruitful area of research.
Why would females prefer leading waves?
What male traits correlate with wave leadership? Are leading males larger, or faster wavers, or do they own better resources? Are they somehow fitter? An alternative to this adaptive explanation, although not mutually exclusive, is that a female preference for leadership arises as a sensory bias (Ryan and Keddy-Hector 1992). The first signal that a female sees might be the most stimulating and therefore more likely to elicit a response. If so, the sensory bias that drives females to prefer leading signals should be evident in closely related species that lack synchrony. The ideal study species of fiddler crab would be A. lactea: males do not display in synchrony, but the species is closely related to three synchronously waving species (Figure 4). The two other species in this small, closely related group of fiddler crabs also deserve greater attention: do A. iranica and A. annulipes also wave in synchrony? If not, do females exhibit a preference for leading signals?
Why is there synchronous waving in a non-mating context in two fiddler crab species?
If fiddler crab synchrony is an incidental by-product of competition between males to produce leading signals, then why do A. perplexa and L. saltitanta wave in synchrony when no females are present? Do females of these two species have a preference for synchronous groups per se? Do they also have a preference for leading signals?
What is the most appropriate way to analyse synchrony in groups?
Statistical analysis of group events has progressed rapidly in the past decade, along with the exponential increase in studies of synchrony in music, heart pacemaker cells, mass migration, the physics of oscillator, and multiple other aspects of animal (including human) behaviors. Earlier statistical analyses of synchrony were based on the coordination of dyads within the group, calculating differences in phase angles (Greenfield and Roizen 1993; Backwell et al. 1998; Reaney et al. 2008a; Nityananda and Balakrishnan 2009). This approach has many limitations: if all possible combinations of males are compared, there would be a high level of pseudo-replication; if males within the group are compared to a single target male (e.g., the male that was chosen by the female), there could be a statistical artefact due to the multiple use of one male against successive neighbors; if the target male is compared to only his nearest neighboring male, there may not be pseudo-replication but much of the information would be excluded from the analysis. A multivariate analysis that overcomes these limitations is required. Richardson et al. (2012) has proposed a ‘cluster phase’ analysis that is able to directly quantify phase synchronization in noisy experimental multivariate data. It would be illuminating to apply the cluster phase analysis to our current data on synchrony in fiddler crabs in order to compare the results with those obtained by dyadic phase angle analyses.
There is still much to be done before we can fully understand the synchronous signaling of animals. Combining knowledge from the acoustic and visual modalities is a start to developing a broad and multidisciplinary approach to this fascinating behavior.
Acknowledgments
I thank all the researchers on whose work this review is based.
Funding
The work was supported by an Australian Research Council Discovery Grant: DP160100316.
Conflict of interest statement
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Araujo SBL, Rorato AC, Perez DM, Pie MR, 2013. A spatially explicit model of synchrony in fiddler crab waving displays. PLoS One 8:e57362.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backwell PRY, Jennions MD, 2004. Coalition among male fiddler crabs. Nature 430:417.. [DOI] [PubMed] [Google Scholar]
- Backwell PRY, Passmore NI, 1996. Time constraints and multiple choice criteria in the sampling behaviour and mate choice of the fiddler crab, Uca annulipes. Behav Ecol Sociobiol 38:407–416. [Google Scholar]
- Backwell PRY, Jennions MD, Passmore NI, Christy JH, 1998. Synchronised courtship in a fiddler crab. Nature 391:31e32. [Google Scholar]
- Backwell PRY, Jennions MD, Christy JH, Passmore NI, 1999. Female choice in the synchronously waving fiddler crab Uca annulipes. Ethology 105:415–421. [Google Scholar]
- Backwell PRY, Jennions MD, Wada K, Murai M, Christy JH, 2006. Synchronous waving in two species of fiddler crabs. Acta Ethol 9:22–25. [Google Scholar]
- Backwell PRY, Christy JH, Telford SR, Jennions MD, Passmore NI, 2000. Dishonest signalling in a fiddler crab. Proc Roy Soc B 267:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa F, Rebar D, Greenfield MD, 2016. Female preference functions drive inter-population divergence in male signalling: call diversity in the bushcricket Ephippiger diurnus. J Evol Biol 29:2219–2228. [DOI] [PubMed] [Google Scholar]
- Booksmythe I, Hayes C, Jennions MD, Backwell PRY, 2012. The effects of neighbor familiarity and size on cooperative defence of fiddler crab territories. Behav Ecol 23:285–289. [Google Scholar]
- Crane J, 1975. Fiddler Crabs of the World (Ocypodidae: Genus Uca). New Jesrsey: Princeton University Press. [Google Scholar]
- deRivera CE, 2005. Long searches for male-defended breeding burrows allow female fiddler crabs Uca crenulata to release larvae on time. Anim Behav 70:289–297. [Google Scholar]
- deRivera CE, Backwell PRY, Christy JH, Vehrencamp SL, 2003. Density affects female and male mate searching in the fiddler crab. Uca Beebei Behav Ecol Sociobiol 53:72–83. [Google Scholar]
- Dyson ML, Backwell PRY, 2016. Alternative mating tactics and male mating success in two species of fiddler crab. Behaviour 153:1403–1418. [Google Scholar]
- Gordon HRS, 1958. Synchronous claw–waving of fiddler crabs. Anim Behav 134:238–241. [Google Scholar]
- Grafe TU, 1999. A function of synchronous chorusing and a novel female preference shift in an anuran. Proc Roy Soc B 266:2331e2336. [Google Scholar]
- Greenfield MD, 2015. Signal interactions and interference in insect choruses: singing and listening in the social environment. J Comp Physiol A 201:143–154. [DOI] [PubMed] [Google Scholar]
- Greenfield MD, Marin–Cudraz T, Party V, 2017. Evolution of synchronies in insect choruses. Biol J Linn Soc 122:487–504. [Google Scholar]
- Greenfield MD, Roizen I, 1993. Katydid synchronous chorusing is an evolutionarily stable outcome of female choice. Nature 364:618–620. [Google Scholar]
- Greenfield MD, Schul J, 2008. Mechanisms and evolution of synchronous chorusing: emergent properties and adaptive functions in Neoconocephalus katydids (Orthoptera: tettigoniidae). J. Comp. Psychol 122:289–297. [DOI] [PubMed] [Google Scholar]
- Greenfield MD, 1994. Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Am Zool 34:605e615. [Google Scholar]
- Hartbauer M, Haitzinger L, Römer H, 2014. Competition and cooperation in a synchronous bushcricket chorus. R Soc Open Sci 1:140167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartbauer M, Kratzer S, Steiner K, Römer H, 2005. Mechanisms for synchrony and alternation in song interactions of the bushcricket Mecopoda elongate (Tettigoniidae: orthoptera). J Comp Physiol A 191:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How MJ, Christy JH, Temple SE, Hemmi JM, Marshall NJ, Roberts NW, 2015. Target detection is enhanced by polarization vision in a fiddler crab. Curr Biol 25:3069–3073. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Backwell PRY, 1996. Residency and size affect fight duration and outcome in the fiddler crab Uca annulipes. Biol J Linn Soc 57:293–306. [Google Scholar]
- Kahn AT, Holman L, Backwell PRY, 2014. Female preferences for timing in a fiddler crab with synchronous courtship waving displays. Anim Behav 98:35–39. [Google Scholar]
- Koga T, Backwell PRY, Jennions MD, Christy JH, 1998. Elevated predation risk changes mating behaviour and courtship in a fiddler crab. Proc Roy Soc B 265:1385–1390. [Google Scholar]
- Moiseff A, Copeland J, 2010. Firefly synchrony: a behavioral strategy to minimize visual clutter. Science 329:181.. [DOI] [PubMed] [Google Scholar]
- Murai M, Backwell PRY, 2005. More signalling for earlier mating: conspicuous male claw waving in the fiddler crab Uca perplexa. Anim Behav 70:1093–1097. [Google Scholar]
- Murphy MA, Thompson NL, Schul J, 2016. Keeping up with the neighbor: a novel mechanism of call synchrony in Neoconocephalus ensiger katydids. J Comp Physiol A 202:225–234. [DOI] [PubMed] [Google Scholar]
- Nityananda V, Balakrishnan R, 2009. Modelling the role of competition and cooperation in the evolution of katydid acoustic synchrony. Behav Ecol 20:484–489. [Google Scholar]
- Party V, Streiff R, Marin-Cudraz T, Greenfield MD, 2015. Group synchrony and alternation as an emergent property: elaborate chorus structure in a bushcricket is an incidental by-product of female preference for leading calls. Behav Ecol Sociobiol 69:1957–1973. [Google Scholar]
- Reaney LT, Backwell PRY, 2007. Temporal constraints and female preference for burrow width in the fiddler crab Uca mjoebergi. Behav Ecol Sociobiol 61:1515–1521. [Google Scholar]
- Reaney LT, Sims RA, Sims SWM, Jennions MD, Backwell PRY, 2008a. Experiments with robots explain synchronized courtship in fiddler crabs. Curr Biol 18:R62–R63. [DOI] [PubMed] [Google Scholar]
- Reaney LT, Milner RN, Detto T, Backwell PRY, 2008b. The effects of claw regeneration on territory ownership and mating success in the fiddler crab Uca mjoebergi. Anim Behav 75:1473–1478. [Google Scholar]
- Richardson MJ, Garcia RL, Frank TD, Gergor M, Marsh KL, 2012. Measuring group synchrony: a cluster-phase method for analysing multivariate movement time-series. Front Psychol 3:405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorato AC, Araujo SB, Perez DM, Pie MR, 2017. Social cues affect synchronization of male waving displays in a fiddler crab (Crustacea: ocypodidae). Anim Behav 126:293–300. [Google Scholar]
- Ryan MJ, Keddy-Hector A, 1992. Directional patterns of female mate choice and the role of sensory biases. Am Nat 139:S4eS35. [Google Scholar]
- Shih H, Ng PKL, Davie PJF, Schubart CD, Turkay M. et al. 2016. Systematics of the family Ocypodidae Rafinesque, 1815 (Crustacea: brachyura), based on phylogenetic relationships, with a reorganisation of subfamily ranking and a review of the taxonomic status of Uca Leach, 1814, sensu lato and its subgenera. Raffles Bull Zool 64:139–175. [Google Scholar]
- Zeil J, Nalbach G, Nalbach H-O, 1986. Eyes, eye-stalks and the visual world of semi-terrestrial crabs. J Comp Physiol A 159:801–811. [Google Scholar]
- Zeil J, Hemmi JM, 2006. The visual ecology of fiddler crabs. J Comp Physiol A 192:1–25. [DOI] [PubMed] [Google Scholar]
- Zeil J, Hemmi JM, Backwell PRY, 2006. Fiddler crabs. Curr Biol 16:R40–R41. [DOI] [PubMed] [Google Scholar]