Abstract
Functional asymmetries, for example, the preferential involvement of 1 brain hemisphere to process stimuli, may increase brain efficiency and the capacity to carry out tasks simultaneously. We investigated which hemisphere was primarily involved in processing acoustic stimuli in goats using a head-orienting paradigm. Three playbacks using goat vocalizations recorded in different contexts: food anticipation (positive), isolation (negative), food frustration (negative), as well as 1 playback involving dog barks (negative) were presented on the left and right sides of the test subjects simultaneously. The head-orienting response (left or right) and latency to resume feeding were recorded. The direction of the head-orienting response did not differ between the various playbacks. However, when the head-orienting response was tested against chance level, goats showed a right bias regardless of the stimuli presented. Goats responded more to dog barks than to food frustration calls, whereas responses to food anticipation and isolation calls were intermediate. In addition, the latency to resume feeding, an indicator of fear reaction, was not affected by the kind of vocalization presented. These results provide evidence for asymmetries in goat vocal perception of emotional-linked conspecific and heterospecific calls. They also suggest involvement of the left brain hemisphere for processing acoustic stimuli, which might have been perceived as familiar and non-threatening.
Keywords: auditory processing, brain asymmetry, emotions, lateralization, social cognition, vocal communication
Behavioral lateralization refers to how behaviors are performed predominantly using either the right or the left side of the body (Rogers and Andrew 2002; Baruzzi et al. 2017). When an individual shows a right or left preference, it indicates asymmetry at an individual level (e.g., being left- or right-handed; Rogers and Andrew 2002). When the majority of individuals show the same side preference, this suggests asymmetry at the population level (Vallortigara and Rogers 2005). In humans, population-level asymmetries are represented by the predominance of the left hemisphere in processing syntactic and semantic information, and by the prevalence of the right hemisphere in processing information about prosody, novelty, and emotional content (Fitch et al. 1997; Friederici and Alter 2004).
The experimental procedure usually applied to test functional auditory asymmetries in response to vocalizations of conspecifics and heterospecifics is based on a major assumption (Teufel et al. 2007; Siniscalchi et al. 2008). It is assumed that when a sound is perceived simultaneously in both ears, the head orientation to either the left or right side is an indicator of the side of the hemisphere that is primarily involved in the response to the stimulus presented. There is strong evidence that this is the case in humans; auditory input in humans is processed by the contralateral hemisphere when 2 auditory stimuli are presented simultaneously from both sides (dichotic paradigm). The assumption is also supported by the neuroanatomic evidence of the contralateral connection of the auditory pathways in the mammalian brain (Rogers and Andrew 2002; Ocklenburg et al. 2011).
In animals, brain lateralization seems to underline the different response to conspecific versus heterospecific calls. Japanese macaques Macaca fuscata, rhesus monkeys Macaca mulatta, California sea lions Zalophus californianus and dogs Canis lupus familiaris displayed a left hemisphere asymmetry when processing calls from conspecifics (Petersen et al. 1978; Heffner HE and Heffner RS 1984; Hauser and Andersson 1994; Poremba et al. 2004; Böye et al. 2005). By contrast, mouse lemurs Microcebus myoxinus and Barbary macaques Macaca sylvanus showed no orientation preferences in response to conspecific or heterospecific vocalizations (Scheumann and Zimmermann 2005; Teufel et al. 2007). Vervet monkeys Cercopithecus aethiops displayed a right hemisphere asymmetry for conspecific vocalizations regardless of their familiarity with these vocalizations (Gil-da-Costa and Hauser 2006). Horses Equus caballus showed a left hemisphere (right ear turn) processing for calls emitted by a familiar neighbor (familiar horse housed in a close field or stall), but no preference for group members (also familiar) or strangers (Basile et al. 2009). The lack of consistency between species regarding which hemisphere processes specific types of acoustic stimuli shows that further investigations are needed to explore the mechanisms underlying the variation in the direction of auditory asymmetry across species.
Emotional content could account for the variation observed between species in auditory asymmetries. Historically, 2 main theories of brain lateralization have been proposed for the cortical lateralization of emotional processing (Demaree et al. 2005). The “right-hemisphere model” proposes right hemisphere dominance for expression and perception of emotionally loaded signals, regardless of valence. By contrast, the “valence model,” suggests a dominance of the right hemisphere in processing negative emotions and a dominance of the left hemisphere in processing positive emotions (Tucker 1981; Silberman and Weingartner 1986; Ehrlichman 1987; Demaree et al. 2005). In dogs, a left hemisphere preference has been observed when processing different types of vocalizations from a conspecific and a right hemisphere preference (head turning to the left side) when processing thunderstorm sounds (Siniscalchi et al. 2008). In addition, a right hemisphere preference was linked with conspecific calls produced in a context eliciting intense arousal, like isolation and play (Siniscalchi et al. 2008). The involvement of the right side of the brain for processing emotional signals was also confirmed by later research showing a left turning bias in response to the visual presentation of threatening (silhouette of snake) and alarming (silhouette of cat) stimuli (Siniscalchi et al. 2010), and also in response to broadcasted dog barks (Reinholz-Trojan et al. 2012). This left turning bias was claimed to result from the emotional content of the barks used, which were recorded when an unknown dog appeared (Reinholz-Trojan et al. 2012; Andics et al. 2017). Dogs also exhibit a right hemisphere asymmetry (left head-orienting bias) in response to a meaningless human voice (phonemic components removed) with positive intonation (Ratcliffe and Reby 2014). In addition, fMRI in dogs found a left hemisphere bias for processing human and dog sounds with positive valence (Andics et al. 2014, 2016, 2017; Andics 2017; Reinholz-Trojan et al. 2012). These findings indicate that both the familiarity with the stimulus, whether it is produced by a conspecific or heterospecific, and its emotional arousal and valence, could interact to affect lateralized behavioral responses in non-univocal ways.
Goats display different behavioral, neural, and physiological reactions to situations inducing positive (i.e., food anticipation) and negative emotions (i.e., isolation or food frustration, in which food was inaccessible; Gygax et al. 2013; Briefer et al. 2015). When goats were expected to receive food reward after 3 days of habituation and when they experienced food frustration, they had high physiological and behavioral activation compared with a control and isolation situation, and also high activation in the prefrontal cortex (Gygax et al. 2013; Briefer et al. 2015). Bilateral prefrontal cortex activation was found in the negative condition, whereas in the positive situation, the activation was mainly revealed in the left hemisphere (Gygax et al. 2013). This suggests that situations that elicit positive emotions preferentially engaged one side of the brain (i.e., left hemisphere). Remarkably, goat vocalizations also vary according to the emotional arousal and valence experienced by the animals (Briefer et al. 2015). However, to date, hemispheric lateralization in goats in response to emotional vocalizations from conspecifics, and how this compares to processing heterospecific vocalizations, remains to be investigated.
Potential auditory processing asymmetries in goats were investigated in this study. A head-orienting paradigm was used to examine perceptual asymmetry in response to playbacks of conspecifics emitted under positive high arousal (food anticipation), negative low arousal (isolation) and negative high arousal (food frustration) emotional states, and to dog barks (i.e., stimuli potentially perceived as negative). According to previous findings (Petersen et al. 1978; Hauser and Andersson 1994; Siniscalchi et al. 2008), it was predicted that goats would turn their heads toward the right (left hemisphere processing) in response to conspecific calls, and to the left in response to dog barks (right hemisphere processing). Alternatively, if the right hemisphere processes only high arousal sounds (“right-hemisphere model”; Demaree et al. 2005), we would expect a right hemisphere bias to process food anticipation calls, food frustration calls, and dog barks, because they are all produced under high arousal and likely elicit high arousal in receivers (Briefer et al. 2015). A right hemisphere (left side) bias for processing all tested acoustic stimuli could also be expected, because this hemisphere is involved in processing novel stimuli and/or stimuli with emotional content. Finally, according to the “valence model” (Demaree et al. 2005), we would expect the right hemisphere to process negative sounds (dog barks, food frustration, and isolation calls), and the left hemisphere to process positive sounds (food anticipation calls).
Materials and Methods
Subjects and management conditions
The study was carried out at a goat sanctuary (Buttercups Sanctuary for Goats, http://www.buttercups.org.uk; Kent, UK). Employees and volunteers at the sanctuary provided routine care for the animals (120 animals housed at the time of testing), and therefore the goats were fully habituated to human presence and handling (Briefer et al. 2015). During the day, goats were released together into 1 or 2 large fields where shelters are provided. During the night, goats were kept indoors either in individual or shared pens (average size = 3.5 m2) with straw bedding. Goats had ad libitum access to hay, grass, and water, and were also fed with a commercial concentrate in quantities that vary according to their size, health, and age. In total, 18 adult goats (9 females and 9 castrated males) of different breeds and ages (age range: 2–16 years old) housed at the sanctuary for at least 1 year were randomly selected and tested from September to October 2016.
Playback test
Sound recordings
The goat vocalizations used in the playback test were obtained from a previous study (Briefer et al. 2015) at the same location. Vocalizations were recorded at distances of 3–5 m from the focal animal using a Sennheiser MKH-70 directional microphone (frequency response 50–20, 000 Hz, max SPL 124 dB at 1 kHz) connected to a Marantz PMD-660 numeric recorder (sampling rate: 44.1 kHz with amplitude resolution of 16 bits in WAV format), and were then edited and rescaled to the same maximum amplitude using PRAAT software (Boersma and Weenink 2009). The vocalizations for the playback test were recorded during 3 different contexts: 1) food anticipation (positive, high arousal), in which the animals, tested in pairs in 2 adjacent pens, learned to anticipate a food reward after 3 days of training (1 session per day to the situation), and were recorded on the 4th day when the experimenter approached them with the food; 2) food frustration (negative, high arousal), in which only 1 of 2 goats tested in adjacent pens received food from the experimenter (duration: 4 min); 3) isolation (negative, low arousal), in which goats were left alone for 5 min in an outdoor isolated pen, after 3 days of habituation (1 exposition per day to the situation; Briefer et al. 2015). The arousal and valence of the situations during which the calls were recorded were validated using physiological and behavioral indicators of emotions. The arousal was established based on the heart rate elicited by the various situations and revealed that the food anticipation and food frustration triggered emotions of similar high arousal, whereas the isolation situation triggered emotions of low arousal that did not differ from the control situation (by pair, undisturbed, with hay in the feeders). Analyses revealed that the high arousal situations (food anticipation and food frustration), compared with the low arousal ones (isolation and control), were also associated with lower heart-rate variability, higher respiration rate, more movements, more calls, more time spent with ears pointing forward and less time with ears on the side. In the positive situation (food anticipation), compared with the neutral (control) and negative situations (isolation and food frustration), goats had their ears oriented backward less often and spent more time with their tails up (Briefer et al. 2015). These indicators of positive situations are similar to those found in other studies (e.g., Reimert et al. 2013, 2015). The detailed acoustic vocal parameter analysis identified 6 acoustic parameters affected by the arousal. F0 contour over time and energy quartile increased with arousal, whereas the 1st formant decreased. F0 variation within the call was influenced by valence and decreased from negative to positive valence (for more details see Briefer et al. 2015). In addition, a 4th kind of vocalization (heterospecific) was played back: dog barks (obtained from sounddog.com), with a sampling rate of 44.1 kHz and amplitude resolution of 16 bits in WAV format.
The audio stimuli used in the playback test consisted of one single vocalization each (mean duration: 0.74 ± 0.12 s). In total, 4 treatments were prepared: food anticipation, food frustration, isolation, and dog bark (Figure 1). For each treatment, 3 unique stimuli, produced by 3 different individuals (for both goats and dogs) were selected to reduce pseudoreplication (Waller et al. 2013). The goat calls used were recorded in 2011 at the same location. The calls selected belonged to goats that did not share a pen with the subjects during the night, or to goats that were no longer at the sanctuary at the time of testing. Therefore, we expected all goat calls used in our experiment to be equally familiar for the subjects (Pitcher et al. 2017).
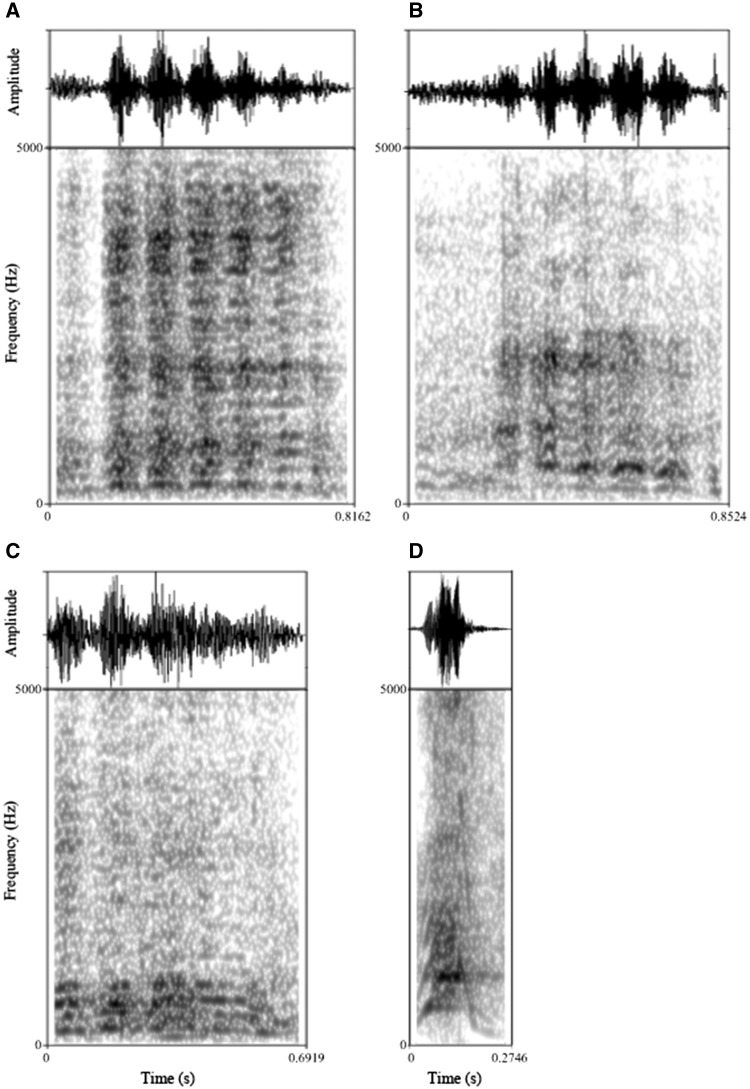
Figure 1.
Examples of calls used in the experiment. Oscillograms (above) and spectrograms (below) of (A) goat food anticipation call, (B) goat isolation call, (C) goat food frustration call and (D) dog bark used in the playback experiment.
Test procedure
Figure 2 illustrates the experimental setup (7 m × 5 m), which was placed in the usual daytime range of the goats. A feeding bowl filled with a mixture of dry pasta and hay and familiar to the goats was fixed in the center, on the opposite side of the entrance of the arena. Each vocalization was broadcasted from 2 Mackie Thump TH-12 A loudspeakers (LOUD Technologies Inc., Woodinville, WA; frequency response: 57–20 kHz ± 3 dB) connected to an active box to boost the sound (Active Box DI-100 Fame) and an Mp4 player (Technika MP111), at approximately natural amplitude commonly used in previous studies (Briefer and McElligott 2011a, 80.08 ± 0.90 dB measured at 2 m using an ASL-8851 sound level meter). Both speakers were set at the same, constant volume. The speakers were positioned at equal distance (2 m) from the right and left side of the bowl, and were aligned to it. In addition, the speakers were concealed using camouflage netting.
Figure 2.
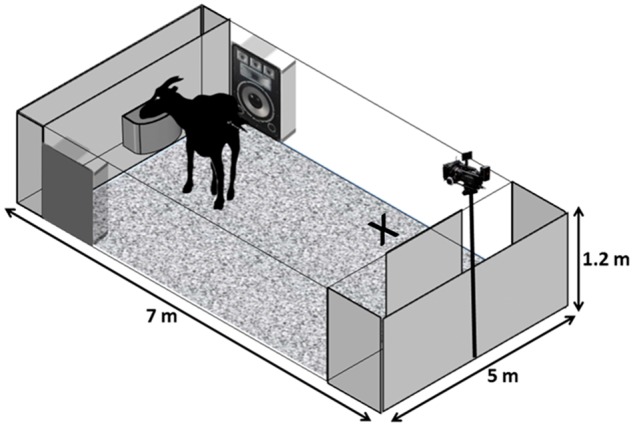
Experimental enclosure. The experimental apparatus (7 m × 5 m) consisted of a door that allowed access to a central arena. A familiar feeding bowl was fixed at the center of the opposite side of the arena. The speakers were positioned at a distance of 2 m from the right and left side of the bowl and were aligned to it. X indicates the position of Experimenter 2.
Each subject received 3 sessions, with 1 session being administered per day. Each session consisted of 8 consecutive trials, that is, 2 repetitions of each treatment (same stimulus was repeated within the session but changed across the sessions), adding up to 6 repetitions per treatment over the 3 sessions. The order in which the treatments were tested within each session was counterbalanced between subjects and sessions. As soon as the goat started to feed from the bowl, 1 of 4 treatment vocalizations was played from the 2 speakers simultaneously. The minimum time between each playback trial was 10 s. The maximum time to resume feeding (i.e., the subject moved the head inside the bucket) was set at 30 s (average time to resume feeding after the offset of the playback: 3.70 ± 0.21 s). Playbacks were initiated only if the test subject’s body was positioned orthogonally to the speakers. In cases where the subject was in an incorrect position, a 2nd experimenter adjusted the body position of the goat before the next trial started. During the test, this 2nd experimenter was standing still, behind the goat, close to the gate inside the testing arena (Figure 2).
All trials were video recorded using a digital video camera placed behind the subject (Sony HDR-CX190E). The head-orienting responses of goats toward the speakers were recorded, from the time the sound started to 30 s after. Four possible responses were considered and scored: head oriented right (head toward the right side when the body of the goat was orthogonal to the speaker), head oriented left (head toward the left side when the body of the goat was orthogonal to the speaker), head up (no turning to either the left or right sides and head raised toward the horizon from the initial position), and no response (i.e., the subject did not move its head within 30 s from the start of the sound). The latency to resume feeding from the bowl (measure of fear reaction) was scored directly during the testing. The maximum time to resume feeding was set at 30 s after the offset of the sound. If the subject did not resume feeding within the 30 s time window, they were gently moved toward the bucket and all goats tested continued feeding.
Statistical analyses
In order to determine if the strength of the responses differed between treatments, we tested the effect of the sound treatment on the proportion of head movement response and on the time to resume feeding. The proportion of head movement response was treated as a binary choice (head oriented right, head oriented left or head up = 1, and no response = 0) and was analyzed with a generalized mixed-effects model (GLMM) fit with binomial family distribution and logit link function (GLMM; glmer function, lme4 library; Pinheiro 2000) in R v.3.2.2 (R Core 2013). The time to resume feeding was analyzed with a linear mixed-effects model (LMM) fit with Gaussian family distribution and identity link function (lmer function, lme4 library). Both models included the treatment (Food Anticipation, Isolation, Food Frustration, and Dog) and the order of presentations of stimuli for each treatment over the 3 sessions as fixed factors. Including the presentation order allowed us to control for any potential habituation effect over the 3 sessions. The session nested within the identity of the goats was included as a random factor to control for repeated measurements.
We also analyzed the effect of the treatment on the head-orienting response of the goats. Head orientation was treated as a binary choice variable (head oriented right = 1, head oriented left = 0, head up and no response = NA) and was analyzed using a GLMM fit with binomial family distribution and logit link function (glmer function). This model included the same fixed effects (treatment and presentation order) and random effect structure (session nested within goat identity) as the models described above.
For all models (GLMM and LMM), we checked the residuals of the models graphically for normal distribution and homoscedasticity (simulateResiduals function, DHARMa library). In order to meet the model assumptions, the latency to resume feeding was log-transformed. P-values (PBmodcomp function, pbkrtest library) were calculated using parametric bootstrap methods (1,000 bootstrap samples). To this aim, models were fitted with maximum likelihood. P-values calculated with parametric bootstrap tests give the fraction of simulated likelihood ratio test (LRT) statistic values that are larger or equal to the observed LRT value. This test is more adequate than the raw LRT because it does not rely on large-sample asymptotic analysis and correctly takes the random-effects structure into account (Halekoh and Højsgaard 2014). When a significant treatment effect was detected, we carried out Tukey post hoc tests for 2-by-2 comparison (glht function, multcomp library in R).
In addition, we investigated whether the head-orienting response showed a deviation from chance level. This was done by comparing the average head-orienting response for each goat (ranging from 0 to 0.5 for a left bias, and from 0.5 to 1 for a right bias) to a hypothetic mean of 0.5 (absence of laterality) using a 1-sample t-test. The average head-orienting response was logit-transformed beforehand in order to approximate a normal distribution.
Results
Proportion of head movement responses and latency to resume feeding
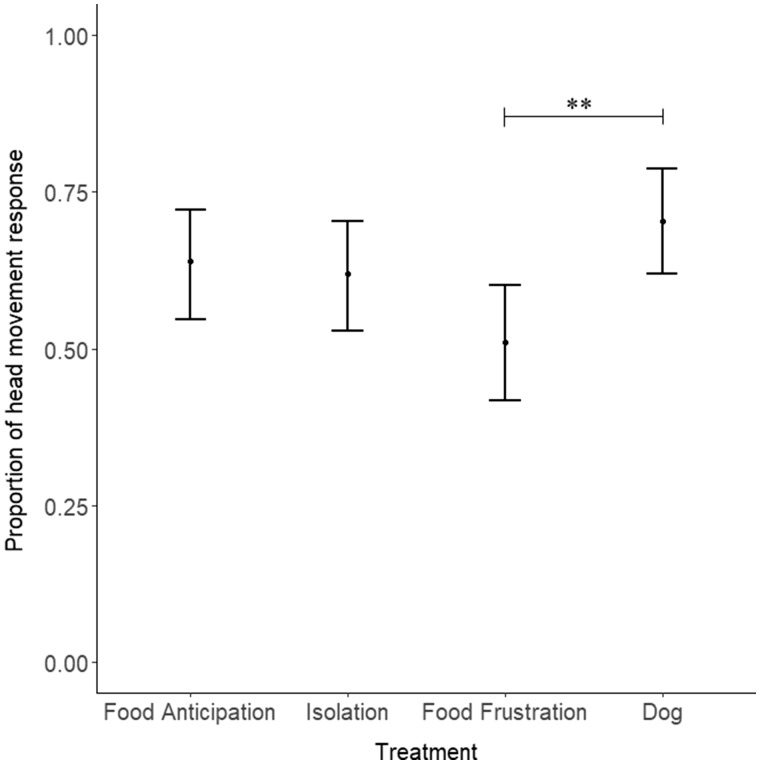
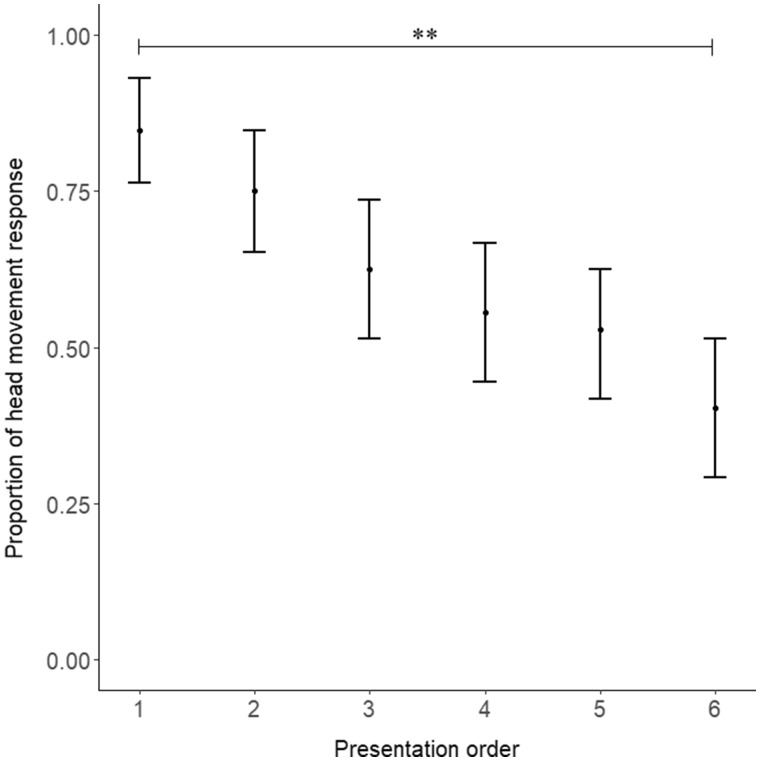
The kind of vocalization presented during the playback (food anticipation, food frustration, isolation, and dog bark) affected the proportion of head movement responses of the goats (GLMM: n = 432 trials, 18 goats; P = 0.005; Figure 3). Post hoc comparisons revealed that goats moved their heads more often after dog barks compared with food frustration calls (z = −3.36, P = 0.005; Figure 3). The other 2-by-2 comparisons were not significant (P ≥ 0.11). An effect of the order of stimulus presentation was also found (P = 0.001; Figure 4), with goats gradually habituated to the vocalizations during the 6 presentations.
Figure 3.
Proportion of head movement responses for each of the 4 treatments (mean and 0.025 and 0.975 quantiles). The vocalizations presented during the playback (Food Anticipation, Isolation, Food Frustration, and Dog) affected the response pattern of the goats (P = 0.005). Post hoc comparisons revealed that goats responded less when a food frustration compared with dog call was presented (**P < 0.01).
Figure 4.
Proportion of head movement response over the 6 repetitions of the stimuli (mean 0.025 and 0.975 quantiles). Goats gradually habituated to the vocalizations during the 6 presentations of each treatment (P = 0.001).
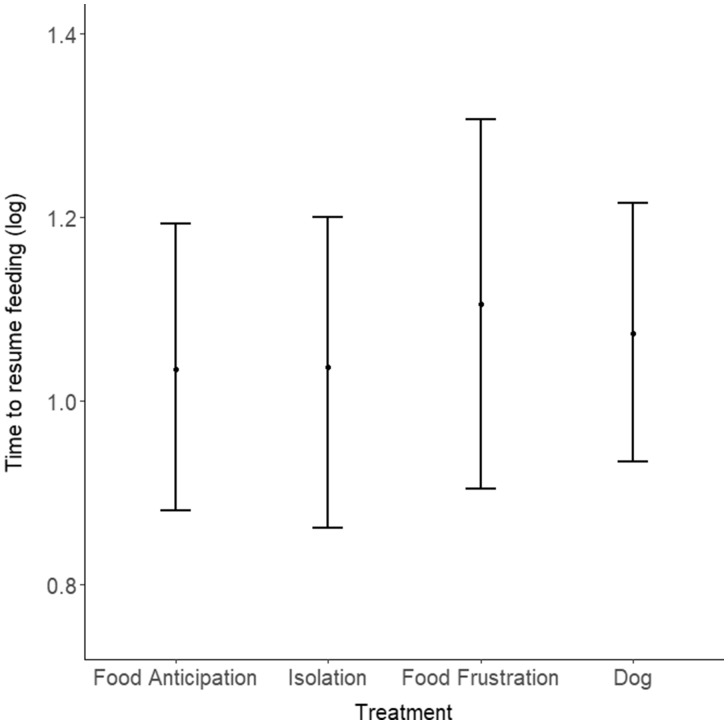
The time to resume feeding was affected neither by the treatments (LMM: n = 267 trials, 18 goats; , P = 0.89; Figure 5) nor by the presentation order (P = 0.43). Overall, this suggests that goats were more alert when hearing dog barks compared with food frustration calls, and that they habituated to the sound treatments during the 6 trials.
Figure 5.
Time to resume feeding (log-transformed) for each treatment (mean 0.025 and 0.975 quantiles). The latency to resume feeding(s) was not affected by the kind of vocalizations presented (Food Anticipation, Isolation, Food Frustration, and Dog; P = 0.89).
Head-orienting response and head-orienting bias
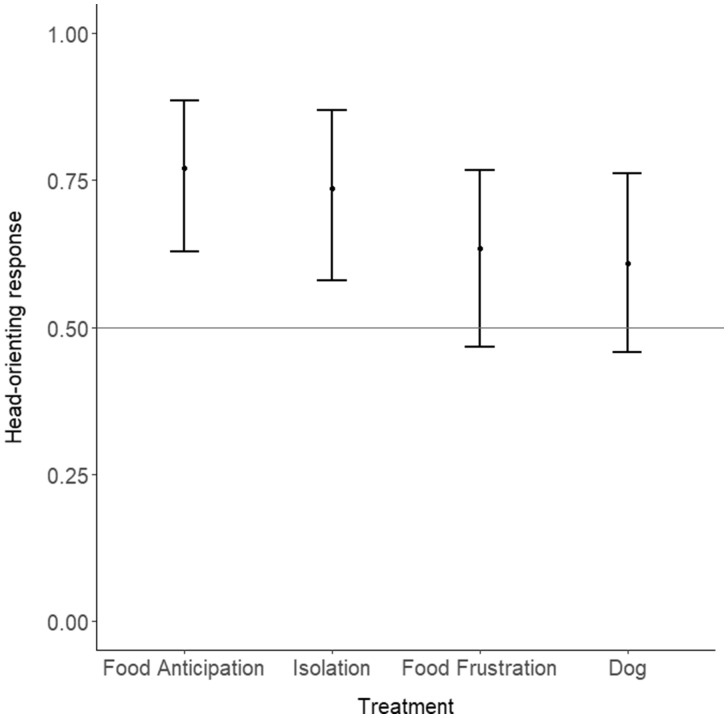
The head-orienting response was not affected by the kind of vocalizations presented (GLMM: n = 149 trials, 18 goats; P = 0.26; Figure 6). This parameter was also not affected by the order of stimulus presentations (P = 0.29), suggesting that the direction of the head-orienting response did not differ between treatments.
Figure 6.
Head-orienting response toward the various vocalizations presented during the playbacks (mean 0.025 and 0.975 quantiles). The head-orienting response was not affected by the kind of vocalizations presented (P = 0.26). Values from 0 to 0.5 indicate a left bias, whereas values from 0.5 to 1 indicate a right bias.
The 1-sample t-test performed on all treatments combined revealed a significant deviation of head-orienting response toward the right side (mean [95% confidence interval] head-orienting response = 0.74 [0.50, 0.89]); 1-sample t-test: feeding, t17 = 2.15, P = 0.046) compared with chance level (i.e., 0.50). Goats thus showed a general right bias in their head-orienting responses.
Discussion
Auditory asymmetries were investigated in goats in response to vocalizations of conspecifics produced in situations eliciting positive high arousal (food anticipation) or negative low and high arousal emotions (isolation and food frustration, respectively), as well as dog barks. The direction of the head-orienting response did not differ between treatments. However, goats showed a general right bias toward the presented acoustic stimuli. These results suggest the involvement of the left hemisphere in response to both conspecific and heterospecific acoustic stimuli in this species. Brain asymmetries provide neural advantages and a general increase in brain efficiency, and therefore have been selected and favored over the course of evolution (Rogers et al. 2004; Vallortigara 2007). However, brain asymmetry direction (e.g., left or right side) could vary across species due to genetics or environmental constraints (Rogers et al. 2004; Gil-da-Costa and Hauser 2006; Vallortigara 2007; Ocklenburg et al. 2011). For example, head rotation in vertebrate embryos is determined by several genes (e.g., Nodal, Lefty; Schier 2003). Furthermore, steroid hormones can reduce the degree of visual lateralization in chicks leaving the direction of lateralization unaltered (Rogers and Deng 2005).
Goats showed a head-orienting response to the right side when conspecific vocalizations were played back regardless of the context on which the calls were recorded. Our findings could thus be in line with the general interpretation that the left hemisphere (right side bias) is specialized to process vocalizations that are familiar and/or positive/non-threatening (Craig 2005; Demaree et al. 2005). However, these findings have not been replicated consistently in response to vocalizations of conspecifics in species such as Vervet monkeys and dogs (Gil-da-Costa and Hauser 2006; Siniscalchi et al. 2008; Ratcliffe and Reby 2014). Vervet monkeys show a left orienting response (i.e., right hemisphere asymmetry) when processing conspecifics calls, but no side bias for heterospecific calls (Gil-da-Costa and Hauser 2006). In dogs, the vocalizations emitted from conspecifics are normally processed by the left hemisphere, whereas the right hemisphere seems to be involved in processing auditory cues eliciting intense emotions, for example, a thunderstorm (Siniscalchi et al. 2008). In horses, a right head-orienting bias (i.e., left hemisphere asymmetry) is associated with a non-group member (i.e., neighbors or strangers, thus the bias is affected by level of familiarity; Basile et al. 2009). In contrast to the head-orienting response, the ears-orienting response is biased to the right side for of familiar neighbor individuals, and to the left side for calls of strangers (Basile et al. 2009). In addition, a positive correlation between the right head-ears orienting response is associated with hearing a known whinny (familiar neighbor and group member; Basile et al. 2009). Conclusions on which hemisphere is involved (left vs. right direction across species) in specific stimuli processing are difficult to draw because factors such as ontogeny, genetics or environmental constraints interact to generate varying patterns of hemispheric preference (Vallortigara and Rogers 2005; Ocklenburg et al. 2011).
According to the “right-hemisphere model,” a left head-orienting response to calls eliciting high arousal would have been expected. Indeed, the use of the right hemisphere has been linked with the expression of intense emotions (Quaranta et al. 2007; Siniscalchi et al. 2008; Ratcliffe and Reby 2014). By contrast, according to the “valence model,” we would expect the right hemisphere to process negative sounds (isolation, food frustration calls, and dog barks) and the left hemisphere to process positive sounds (food anticipation calls). The vocalizations used in our experiment have been analyzed previously and were shown to be associated with different patterns of behavioral and physiological responses in the caller (Briefer et al. 2015). However, the behavioral and physiological reactions on hearing these vocalizations and whether a conspecific is able to discriminate between calls with different valence and arousal have not been tested yet. Therefore, it is not known if the calls produced under high arousal (food anticipation and food frustration) also elicit high arousal emotions in receivers, and if the calls produced under positive emotional state (food anticipation) also elicited positive emotions in receivers (and vice versa for negative calls). State matching between producer and receiver of a signal is termed emotional contagion, and is predicted to be widespread in the animal kingdom (de Waal 2008; Briefer 2018). Such information would have been beneficial to disentangle the results predicted according to the “right-hemisphere model” and “the valence model” of brain asymmetries. If emotional contagion indeed had occurred in our study, according to the “right-hemisphere model,” we would have expected an involvement of the right hemisphere to process food anticipation and food frustration calls (i.e., high-arousal calls). By contrast, according to the “valence model,” we would have expected an involvement of the right hemisphere to process food frustration and, isolation calls and possibly dog barks (i.e., negative calls), and an involvement of the left hemisphere to process food anticipation calls (i.e., positive calls). The lack of positive calls of low arousal in this study represents a methodological limitation that has to be taken into account when considering the involvement of the right hemisphere and the valence model of brain asymmetries. Recent evidence has shown that contact calls in goats convey information about size, sex, age, and individuality (Briefer and McElligott 2011b, 2012; Pitcher et al. 2017), but the ability to extract emotional information from vocalizations had not been experimentally tested yet. Overall, our study suggests that the spontaneous response in the head-orienting paradigm might be under the control of the left hemisphere (Basile et al. 2009; Ghazanfar and Hauser 1999; Siniscalchi et al. 2008, 2010).
Our results do not confirm the hypothesis of a left head-orienting response (i.e., right hemisphere asymmetry) toward heterospecific calls or calls eliciting intense emotions (dog barks). Dogs are potential predators of small ruminants and hearing a dog barking from a close distance may induce a fear reaction and a more attentive response (Beausoleil et al. 2005). Although goats were more alert when hearing dog barks than conspecific food frustration calls, responses to dog barks did not differ from those to conspecific food anticipation and isolation calls. In addition, the time to resume feeding (a measure of fear), in our study did not differ between dog barks and the vocalizations of conspecifics. This suggests that goats at our study site may have been habituated to dog barks and that they did not perceive dog barks as a serious threat.
To summarize, goats showed a general head-orienting bias to the right side, providing evidence for perceptual lateralization of both conspecific and heterospecific acoustic stimuli, which might have been perceived as familiar and non-threatening. The overall findings of the study suggest that the head responses are potentially mediated by general acoustic features rather than specific information conveyed (Teufel et al. 2007). The results also indicate the need to control for the characteristics of the stimuli employed, such as degree of familiarity, emotional valence, and arousal, and the importance to use appropriate controls (e.g., non-biological sound) in order to disentangle the involvement of each brain hemisphere.
Acknowledgments
The authors are grateful to Carolina Baruzzi, Monica Padilla de la Torre, Caroline Spence, Luca Tommasi and Claudia Wascher for helpful comments on the manuscript. They also thank Bob Hitch, Gower McCarthy, Samantha Taylor, and all the volunteers at Buttercups Sanctuary for Goats (http://www.buttercups.org.uk) for their excellent help and free access to the animals
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Note
Animal care and all experimental procedures were conducted in accordance with the Association for the Study of Animal Behaviour guidelines (Association for the Study of Animal Behaviour, 2016). The study was approved by the Animal Welfare and Ethical Review Board of Queen Mary University of London (002/2016AWERBqmul). The tests were non-invasive and behaviors indicating stress (e.g., vocalizations and strong reaction to the sounds) were monitored throughout the exposure to the playbacks. If any signs of distress had occurred, the procedure would have been stopped and the subject removed. None of the goats were removed from the experiment.
References
- Andics A, 2017. Erratum for the report “Neural mechanisms for lexical processing in dogs” by A. Andics, A. Gábor, M. Gácsi, T. Faragó, D. Szabó, A. Miklósi. Science 356:eaan3276. [DOI] [PubMed] [Google Scholar]
- Andics A, Gácsi M, Faragó T, Kis A, Miklósi A, 2014. Report voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Curr Biol 24:574–578. [DOI] [PubMed] [Google Scholar]
- Andics A, Gábor A, Gácsi M, Faragó T, Szabó D. et al. , 2016. Neural mechanisms for lexical processing in dogs. Science 353:1030–1032. [DOI] [PubMed] [Google Scholar]
- Andics A, Gácsi M, Faragó T, Kis A, Miklósi A, 2017. Corrections voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Curr Biol 27:1248–1249. [DOI] [PubMed] [Google Scholar]
- Association for the Study of Animal Behaviour, 2016. Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 111:I–IX. [Google Scholar]
- Baruzzi C, Nawroth C, McElligott AG, Baciadonna L, 2017. Motor asymmetry in goats during a stepping task. Laterality, doi:10.1080/1357650X.2017.1417993. [DOI] [PubMed] [Google Scholar]
- Basile M, Boivin S, Boutin A, Blois-Heulin C, Hausberger M. et al. , 2009. Socially dependent auditory laterality in domestic horses Equus caballus. Anim Cogn 12:611–619. [DOI] [PubMed] [Google Scholar]
- Beausoleil NJ, Stafford KJ, Mellor DJ, 2005. Sheep show more aversion to a dog than to a human in an arena test. Appl Anim Behav Sci 91:219–232. [Google Scholar]
- Boersma P, Weenink D, 2009. Praat: doing phonetics by computer [cited 2018 February 24]. Available from: http://www.praat.org/.
- Böye M, Güntürkün O, Vauclair J, 2005. Right ear advantage for conspecific calls in adults and subadults, but not infants, California sea lions Zalophus californianus: hemispheric specialization for communication? Eur J Neurosci 21:1727–1732. [DOI] [PubMed] [Google Scholar]
- Briefer EF, 2018. Vocal contagion of emotions in non-human animals. Proc R Soc B 285:20172783.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briefer E, McElligott AG, 2011a. Mutual mother-offspring vocal recognition in an ungulate hider species Capra hircus. Anim Cogn 14:585–598. [DOI] [PubMed] [Google Scholar]
- Briefer E, McElligott AG, 2011b. Indicators of age, body size and sex in goat kid calls revealed using the source-filter theory. Appl Anim Behav Sci 133:175–185. [Google Scholar]
- Briefer EF, McElligott AG, 2012. Social effects on vocal ontogeny in an ungulate, the goat Capra hircus. Anim Behav 83:991–1000. [Google Scholar]
- Briefer EF, Tettamanti F, McElligott AG, 2015. Emotions in goats: mapping physiological, behavioural and vocal profiles. Anim Behav 99:131–143. [Google Scholar]
- Craig AD, 2005. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci 9:566–571. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, 2008. Putting the altruism back into altruism: the evolution of empathy. Annu Rev Psychol 59:279–300. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Everhart DE, Youngstrom EA, Harrison DW, 2005. Brain lateralization of emotional processing: historical roots and a future incorporating “dominance.” Behav Cogn Neurosci Rev 4:3–20. [DOI] [PubMed] [Google Scholar]
- Ehrlichman H, 1987. Hemispheric asymmetry and positive-negative affect In: Ottoson D, editor. Duality and Unity of the Brain. Boston (MA): Springer US, 194–206. [Google Scholar]
- Fitch RH, Miller S, Tallal P, 1997. Neurobiology of speech perception. Annu Rev Neurosci 20:331–353. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Alter K, 2004. Lateralization of auditory language functions: a dynamic dual pathway model. Brain Lang 89:267–276. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Hauser MD, 1999. The neuroethology of primate vocal communication: substrates for the evolution of speech. Trends Cogn Sci 3:377–384. [DOI] [PubMed] [Google Scholar]
- Gil-da-Costa R, Hauser MD, 2006. Vervet monkeys and humans show brain asymmetries for processing conspecific vocalizations, but with opposite patterns of laterality. Proc R Soc B 273:2313–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygax L, Reefmann N, Wolf M, Langbein J, 2013. Prefrontal cortex activity, sympatho-vagal reaction and behaviour distinguish between situations of feed reward and frustration in dwarf goats. Behav Brain Res 239:104–114. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Andersson K, 1994. Left hemisphere dominance for processing vocalizations in adult, but not infant, rhesus monkeys: field experiments. Proc Natl Acad Sci USA 91:3946–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS, 1984. Temporal lobe lesions and perception of species-specific vocalizations by macaques. Science 226:75–76. [DOI] [PubMed] [Google Scholar]
- Halekoh U, Højsgaard S, 2014. A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models the R package pbkrtest. J Stat Softw 59:1–32.26917999 [Google Scholar]
- Ocklenburg S, Ströckens F, Güntürkün O, 2011. Lateralisation of conspecific vocalisation in non-human vertebrates. Laterality 18:1–31. [DOI] [PubMed] [Google Scholar]
- Petersen MR, Beecher MD, Zoloth SR, Moody DB, Stebbins WC, 1978. Neural lateralization of species-specific vocalizations by Japanese macaques Macaca fuscata. Science 202:324–327. [DOI] [PubMed] [Google Scholar]
- Pitcher BJ, Briefer EF, Baciadonna L, Mcelligott AG, 2017. Cross-modal recognition of familiar conspecifics in goats. R Soc Open Sci 4:160346.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Malloy M, Saunders RC, Carson RE, Herscovitch P. et al. , 2004. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Nature 427:448–451. [DOI] [PubMed] [Google Scholar]
- Quaranta A, Siniscalchi M, Vallortigara G, 2007. Asymmetric tail-wagging responses by dogs to different emotive stimuli. Curr Biol 17:R199–R201. [DOI] [PubMed] [Google Scholar]
- Ratcliffe VF, Reby D, 2014. Orienting asymmetries in dogs’ responses to different communicatory components of human speech. Curr Biol 24:2908–2912. [DOI] [PubMed] [Google Scholar]
- Reimert I, Bolhuis JE, Kemp B, Rodenburg TB, 2013. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol Behav 109:42–50. [DOI] [PubMed] [Google Scholar]
- Reimert I, Bolhuis JE, Kemp B, Rodenburg BT, 2015. Emotions on the loose: emotional contagion and the role of oxytocin in pigs. Anim Cogn 18:517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholz-Trojan A, Włodarczyk E, Trojan M, Kulcz NA, Stefá NJ, 2012. Hemispheric specialization in domestic dogs Canis familiaris for processing different types of acoustic stimuli. Behav Processes 91:202–205. [DOI] [PubMed] [Google Scholar]
- Rogers L, Andrew R, 2002. Comparative Vertebrate Lateralization. Cambridge: (UK): Cambridge University Press. [Google Scholar]
- Rogers LJ, Deng C, 2005. Corticosterone treatment of the chick embryo affects light-stimulated development of the thalamofugal visual pathway. Behav Brain Res 159:63–71. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Zucca P, Vallortigara G, 2004. Advantages of having a lateralized brain. Proc R Soc B 271:S420–S422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheumann M, Zimmermann E, 2005. Do mouse lemurs show asymmetries in handedness and the perception of communication calls? Primate Rep 72:84–85. [Google Scholar]
- Schier AF, 2003. Nodal Signaling in vertebrate development. Annu Rev Cell Dev Biol 19:589–621. [DOI] [PubMed] [Google Scholar]
- Silberman EK, Weingartner H, 1986. Hemispheric lateralization of functions related to emotion. Brain Cogn 5:322–353. [DOI] [PubMed] [Google Scholar]
- Siniscalchi M, Quaranta A, Rogers LJ, 2008. Hemispheric specialization in dogs for processing different acoustic stimuli. PLoS ONE 3:e3349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalchi M, Sasso R, Pepe AM, Vallortigara G, Quaranta A, 2010. Dogs turn left to emotional stimuli. Behav Brain Res 208:516–521. [DOI] [PubMed] [Google Scholar]
- Teufel C, Hammerschmidt K, Fischer J, 2007. Lack of orienting asymmetries in Barbary macaques: implications for studies of lateralized auditory processing. Anim Behav 73:249–255. [Google Scholar]
- Tucker DM, 1981. Lateral brain function, emotion, and conceptualization. Psychol Bull 89:19–46. [PubMed] [Google Scholar]
- Vallortigara G, 2007. The evolutionary psychology of left and right: costs and benefits of lateralization. Dev Psychobiol 48:832–840. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ, 2005. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci 28:575–589. [DOI] [PubMed] [Google Scholar]
- Waller BM, Warmelink L, Liebal K, Micheletta J, Slocombe KE, 2013. Pseudoreplication: a widespread problem in primate communication research. Anim Behav 86:483–488. [Google Scholar]