Abstract
Diabetic kidney disease (DKD) remains the leading cause of end-stage renal disease. A major challenge in preventing DKD is the difficulty in identifying high-risk patients at a preclinical stage. Existing methods that are used to assess renal function, including albuminuria and eGFR, do not give detailed insight into the location of the renal hemodynamic effects of pharmacological agents at the segmental level. To gain additional information about the intrarenal circulation in vivo in humans, equations were developed by Gomez et al. in the 1950s. These equations used measurements of glomerular filtration rate, renal blood flow, effective renal plasma flow, renal vascular resistance, hematocrit, and serum protein to calculate afferent and efferent arteriolar resistances, glomerular hydrostatic pressure, and filtration pressure. The Gomez equations are, however, indirect and based on physiological assumptions derived from animal models, which may not hold true in human pathophysiology, including the assumption of a normal gross filtration coefficient and not considering changes in intratubular pressure that may affect the pressure gradient across the glomerular capillaries. Nevertheless, the equations have the potential to improve researchers' ability to identify early preclinical changes in renal hemodynamic function in patients with a variety of conditions, including DKD, thereby offering potential in mechanistic human research studies. In this review, we focus on the application of Gomez' equations and summarize the potential and limitations of these techniques in DKD research. We also summarize illustrative data derived from Gomez' equations in patients with type 1 and type 2 diabetes and hypertension.
Keywords: diabetic kidney disease, glomerular filtration rate, renal blood flow, renal hemodynamics
diabetic kidney disease (DKD) is a public health burden that costs society billions of dollars annually (22, 54, 66). The 2015 United States Renal Data System Report listed DKD as the leading cause of end-stage renal disease (ESRD) in the United States (79). With the exception of the EMPA-REG OUTCOME trial, where the sodium-glucose cotransport-2 (SGLT2) inhibitor empagliflozin reduced the composite renal end points of doubling of serum creatinine, ESRD, or renal death in adults with type 2 diabetes (T2D) with high cardiovascular risk, clinical trials in DKD have yielded disappointing results for the past two decades (15, 24, 46, 55, 57, 93). The underlying explanation for this trend can be explained partly by: 1) the lack of valid early markers to identify and monitor high-risk individuals; and 2) the lack of interventions at an early stage of disease when benefit is most likely to be attained.
Current screening for DKD consists of measuring urinary albumin excretion and/or estimating glomerular filtration rate (eGFR) (69, 77). About one-third of patients with microalbuminuria regress to normoalbuminuria, making microalbuminuria an unreliable predictor (37, 70, 71), and, by the time macroalbuminuria occurs, histological damage is extensive, and the opportunity to successfully intervene may have passed (30). For that reason, glomerular filtration rate (GFR) remains the most clinically relevant measure of kidney function in diabetes (2, 43a, 85). While renal hyperfiltration (GFR ≥135 ml·min−1·1.73 m−2) and rapid GFR decline (>3 ml·min−1·1.73 m−2·yr−1) are promising early markers of progressive DKD (80), serum creatinine or cystatin C-based eGFR measurements are inaccurate in subjects with normal or elevated GFR (23, 32, 51–53). While accurate measurements of GFR, such as inulin or iohexol clearances, are available, these methods remain expensive, labor intensive, and limited to specialized research centers. Thus, it would be ideal to identify a way to accurately measure eGFR, as well as the hemodynamic counterparts, such as renal plasma flow, filtration pressure, glomerular hydrostatic pressure, oncotic pressure, and afferent and efferent resistances. With such information one might better identify risk factors and potentially preventive strategies.
The renal physiologist and mathematician Dr. Domingo M. Gomez developed a series of equations based on measurement of GFR, effective renal plasma flow (ERPF), and renal blood flow (RBF) that enables the determination of a complete renal hemodynamic profile, including calculation of filtration fraction (FF), glomerular capillary pressure, and renal vascular resistance (34).
Here we review the application of Gomez' equations and summarize its potential and limitations in kidney disease research. We also summarize novel data derived from Gomez' equations in patients with type 1 diabetes (T1D), T2D, and hypertension.
Estimates of Renal Hemodynamic Profiles with Gomez' Equations
While direct measurements of glomerular hemodynamic variables such as afferent and efferent arteriolar resistance values and glomerular hydrostatic pressure are too invasive in humans, Dr. Domingo M. Gomez (34) published equations to estimate renal hemodynamics in humans based on clinical ERPF and GFR data in 1951. Gomez' equations were derived from simplifying assumptions and canine data (98) and necessitate the following: 1) that intrarenal vascular resistances be divided into afferent, postglomerular, and efferent resistance; 2) that hydrostatic pressures within the renal tubules, venules, Bowman's space, and interstitium are in equilibrium and given a value of 10 mmHg; 3) that filtration disequilibrium in the glomerulus is assumed; and 4) that the gross filtration coefficient is assumed to be 0.0867 ml·s−1·mmHg−1.
The assumption of the filtration coefficient being 0.0867 ml·s−1·mmHg−1 is based on normal kidney physiology (GFR 130 ml/min, glomerular hydrostatic pressure 60 mmHg) and Frank R. Winton's indirect estimates in a canine model that glomerular pressure is roughly two-thirds of mean arterial pressure (MAP) (34), and normal glomerular oncotic pressure is 25 mmHg. The limitations of these assumptions are discussed later in the manuscript.
Clinical variables, including MAP (mmHg), FF, ERPF (ml/s), GFR (ml/s), RBF (ml/s), hematocrit (Hct, %), and total protein (TP) (g/dl), are used to calculate efferent and afferent renal arteriolar resistance values (dyne/s/cm5), glomerular hydrostatic pressure (mmHg), glomerular filtration pressure (mmHg), and glomerular oncotic pressure (mmHg):
The filtration pressure across glomerular capillaries (ΔPF) is given by:
ΔPF = GFR/KFG, where KFG is the gross filtration coefficient. The glomerular oncotic pressure (πG) is calculated from the plasma protein mean concentration (CM) within the capillaries:
By substituting ΔPF, πG, and given that hydrostatic pressure in Bowman's space (PBow) is assumed to be 10 mmHg, glomerular hydrostatic pressure (PGLO) is calculated:
To calculate efferent (RE) and afferent (RA) renal arteriolar resistance values using principles of Ohm's law, where 1,328 is the conversion factor to dynes per second per centimeter to the fifth power (34):
Although the Gomez' equations were established and published six decades ago, their use in human research has been limited by the need for direct GFR and RBF measurements. Data on renal hemodynamics do, however, exist for several conditions, including hypertension, endocrine-related disease, and diabetic kidney disease (68, 91, 92) (Tables 1 and 2).
Table 1.
Renal hemodynamic responses to risk factors and therapies
| Exposure Variable | Ref. No. | Cohort | Duration of Therapy | GFR | RBF | FF | RA | RE | PGLO |
|---|---|---|---|---|---|---|---|---|---|
| SGLT2 inhibition | 18 | Adults with T1D | 8 wk | ↓ | / | / | ↑ | → | ↓ |
| Exenatide | 62 | Overweight adults without T2D | 120 min | ↑ | ↑ | / | ↓ | → | ↑ |
| Exenatide | 89 | Overweight adults with T2D | 150 min | → | → | → | ↑ | → | → |
| Manidipine | 67 | Adults with hypertension | 4 wk | / | / | / | ↓ | ↓ | ↓ |
| Plasma UA | 48 | Adults with T1D | N/A | ↓ | ↓ | → | ↓ | → | ↓ |
| Poor glycemic control | 92 | Adults with T2D | N/A | / | / | ↑ | / | / | ↑ |
| Hyperfiltration vs. normofiltration | 81 | Adults with T1D | N/A | ↑ | ↑ | ↑ | ↓ | → | ↑ |
GFR, glomerular filtration rate; RBF, renal blood flow; FF, filtration fraction; RA, afferent renal arteriolar resistance; RE, efferent renal arteriolar resistance; PGLO, glomerular hydrostatic pressure; SGLT2, sodium-glucose cotransport-2; T1d, type 1 diabetes; T2d, type 2 diabetes; UA, ; uric acid; ↑, increase; ↓, decrease; →, unchanged; /, not reported.
Table 2.
Published renal hemodynamic values in T1D, T2D, and nondiabetic controls
| Nondiabetic Controls |
T1D |
||||||
|---|---|---|---|---|---|---|---|
| Younger | Older | Euglycemia | Hyperglycemia | Normofiltration | Hyperfiltration | T2D Overweight | |
| Cohorts | |||||||
| Ref. no. | 81 | 99 | 81 | 81 | 81 | 81 | 89 |
| Size, n | 25 | 26 | N/A/73 | N/A/73 | 35/73 | 38/73 | 52 |
| Cohort characteristics | |||||||
| Age, yr | 27 ± 6 | 50 ± 13 | / | / | 24 ± 5 | 23 ± 4 | 63 ± 7 |
| Female, % | 56 | 50 | / | / | 40 | 55 | 25 |
| Renal hemodynamic parameters | |||||||
| GFR | 117 ± 11 | 95 ± 24 | 144 ± 32.9 | 156 ± 30.7 | 116 ± 10 | 177 ± 35 | 82 ± 4 |
| ERPF, ml·min−1·1.73 m−2 | 628 ± 162 | 497 ± 172 | 810 ± 269 | 831 ± 236 | 642 ± 93 | 970 ± 268 | 345 ± 18 |
| RBF, ml·min−1·1.73 m−2 | 1,039 ± 287 | 810 ± 279 | 1,299 ± 439 | 1,309 ± 399 | 1,031 ± 171 | 1,552 ± 466 | 605 ± 32 |
| RVR, mmHg/min | 0.081 ± 0.019 | 0.068 ± 0.02 | 0.067 ± 0.017 | 0.080 ± 0.014 | 0.057 ± 0.017 | 0.184 ± 0.012 | |
| FF | 0.19 ± 0.04 | 0.20 ± 0.06 | 0.18 ± 0.04 | 0.19 ± 0.05 | 0.18 ± 0.03 | 0.18 ± 0.06 | 0.24 ± 0.01 |
| Filtration pressure, mmHg | 22.4 ± 2.2 | 28 ± 6 | 30 ± 7 | 22 ± 2 | 32 ± 5 | ||
| RA, dyn·s−1·cm−5 | 1,676 ± 706 | 3,778 ± 2856 | 1,473 ± 798 | 1,325 ± 825 | 2,065 ± 597 | 914 ± 494 | 6,176 ± 607 |
| RE, dyn·s−1·cm−5 | 2,043 ± 463 | 1,596 ± 1,195 | 2,021 ± 590 | 2,205 ± 716 | 1,993 ± 367 | 2,047 ± 747 | 3,957 ± 105 |
| PGLO, mmHg | 60 ± 3 | 52 ± 8 | 61 ± 8 | 62 ± 8 | 55 ± 3 | 66 ± 6 | 60 ± 1 |
| Oncotic pressure, mmHg | 27 ± 3 | 23 ± 3 | 22 ± 3 | 22 ± 3 | 24 ± 3 | ||
Data are means ± SD; n, no. of subjects. ERPF, effective renal plasma flow; RVR, renal vascular resistance.
Renal Hemodynamic Function in Diabetes Mellitus
Historically, most of our knowledge about renal hemodynamic function in diabetes mellitus (DM) was derived from animal models, which may not be reflective of human pathophysiology. Over the past decade important human research has made significant contributions to our understanding of the underlying renal pathophysiology. Renal hemodynamic function is controlled by intrarenal autoregulatory mechanisms mediated by a variety of factors, including tubuloglomerular feedback (TGF), myogenic responses, and neurohormonal mediators (14, 21). TGF, for example, is a feedback mechanism whereby changes in sodium chloride delivery are sensed by the cells of the macula densa, leading to responses at the single nephron GFR level that directly influence afferent arteriolar tone (21, 95). TGF is thought to be an important contributor leading to characteristic renal hemodynamic function changes, including increased PGLO and renal hyperfiltration (21), which contribute to the development of DKD (3, 21, 74). In the setting of DM, hyperglycemia results in augmented proximal renal tubular sodium reabsorption via the SGLT2 with consequent diminished distal sodium chloride delivery, leading to TGF-mediated afferent vasodilatation and hyperfiltration (21). While glomerular hypertension and hyperfiltration have been demonstrated in both T1D and T2D (21, 43, 74), there is paucity of human data about the underlying renal hemodynamic function abnormalities that are responsible. The next two sections will focus on the limited data related to the renal hemodynamic function profiles that have been described in patients with either T1D or T2D.
Renal Hemodynamic Profiles in Type 1 Diabetes Mellitus
Effect of SGLT2 inhibition on renal segmental resistances in patients with T1D.
When examining renal hemodynamic function in patients with T1D, it is important to separate patients based on renal hyperfiltration status since there are a variety of physiological differences in those with baseline hyperfiltration compared with individuals with normofiltration (15, 17, 20, 39, 83). By directly measuring GFR and RBF by inulin and para-aminohippurate (PAH) clearances, respectively, and calculating the glomerular hemodynamic profiles on 98 participants (25 healthy control subjects, 35 patients with T1D and normofiltration, and 38 patients with T1D and hyperfiltration), Škrtić et al. demonstrated that renal hyperfiltration in T1D is primarily associated with changes in afferent arteriolar resistance rather than efferent arteriolar resistance (81). Furthermore, the same group demonstrated that young adults with T1D and renal hyperfiltration had greater RBF, FF, PGLO and lower RA pressure compared with those with T1D and normofiltration (81). These data suggest that, apart from a traditional focus on the RE with agents that block the renin-angiotensin-aldosterone system (RAAS), renal protective therapies should focus on RA mechanisms through the use of pharmacological agents that target TGF, including SGLT2 inhibitors and possibly incretin-based therapies that increase natriuresis (47).
For example, in a stratified, open-label, single-arm 8-wk clinical trial, Cherney et al. demonstrated that SGLT2 inhibition was associated with attenuation of renal hyperfiltration in adults with T1D by decreasing GFR by 33 ml·min−1·1.73 m−2, or 19% (18). This observation suggests that TGF plays a role in glomerular hyperfiltration and that SGLT2 inhibition can decrease renal hyperfiltration. A subsequent post hoc analysis from the same group applying Gomez' equations showed that 8 wk treatment with empagliflozin in participants with T1D and hyperfiltration increased RA, decreased ΔPF, and decreased PGLO [67.4 ± 5.4 vs 61.0 ± 5.2 mmHg, P < 0.0001 (during clamped euglycemia)] with no effect on RE (82). The effect of SGLT2 inhibition is proposed to be due to modulation of TGF via increased distal tubular sodium delivery (18). The preferential effect of SGLT2 inhibition on RA, rather than RE, is important, since it offers the opportunity of further renal protection by combining these agents with RAAS inhibitors that act at the efferent arteriole.
Effect of hyperglycemia on renal hemodynamic function in patients with T1D.
Acute hyperglycemia is recognized to influence renal hemodynamic function in T1D (7, 8, 11, 81). Škrtić et al. demonstrated that GFR increased in adults with T1D and normofiltration in response to clamped hyperglycemia (81). However, a similar increase in GFR was not observed in participants with T1D and hyperfiltration (81). While RA tended to decrease and RE increase in response to clamped hyperglycemia, these differences did not reach statistical significance (81). Therefore, while induction of clamped hyperglycemia has been shown to impact GFR, the Gomez' equations may not be sensitive enough to detect changes in the pre- or postglomerular circulations in response to hyperglycemia or may require larger cohort sample sizes. Alternatively, changes in GFR in response to clamped hyperglycemia may instead work at the level of the glomerulus rather than afferent or efferent arteriole. As a consequence, the Gomez' equations may simply not be useful to assess this type of physiological change, at least in response to modest acute hyperglycemia. In contrast, in the chronic setting, poor glycemic control, as reflected by elevated levels of HbA1c, were associated with higher efferent resistance and increased glomerular pressure in 31 patients with T2D (92), as discussed below.
Association between plasma uric acid concentrations and renal segmental resistances in T1D.
Serum uric acid (UA) is recognized to be associated with DKD in patients with T1D and T2D (9, 10). The mechanisms responsible for renal risk are poorly understood, especially in patients with T1D, since serum UA tends to be lower compared with healthy age- and gender-matched controls (12). In murine models, acute elevation of plasma UA by means of uricase inhibition resulted in acute hypertension, which over time can promote vascular and glomerular injury resulting in thickening and vasoconstriction of the afferent arteriole and reduction in renal blood flow, an observation thought to be mediated by RAAS activation (58, 59, 72). Furthermore, higher plasma UA levels are associated with a lower RBF response to an exogenous infusion of ANG II, suggesting a state of inappropriate intrarenal RAAS activation with higher plasma UA concentrations (72). Until recently, less was known about plasma UA and the impact on renal function in humans. Accordingly, the relationship between plasma UA and renal hemodynamic function in adults with T1D was recently examined by Lytvyn et al., who demonstrated that higher plasma UA was associated with lower GFR, RBF, and PGLO, which appeared to be mediated by higher RA (48) (Table 1). In contrast, no associations between plasma UA and renal hemodynamic parameters were demonstrated in nondiabetic healthy controls (48). A similar relationship between serum UA and RA has been observed in a heterogeneous cohort of patients with T2D with and without proteinuria (94). These observations suggest that plasma UA-associated DKD in patients with T1D may be mediated by impaired GFR and RBF secondary to increased RA, which might potentiate renal injury by causing ischemia to the renal microcirculation (48).
Sex-related differences in renal hemodynamics in T1D.
It is widely recognized that women with kidney disease progress to ESRD at a much slower rate than their male counterparts (38, 42). In fact, compared with healthy men, GFR decline is delayed and attenuated in women (38, 42). While the sex-related differences are attenuated in DM, male sex still appears to be a risk factor for DKD (56). The mechanisms underlying these sex differences remain unclear, but Ahmed et al. demonstrated that the renal vasculature of men becomes more dependent on nitric oxide (NO) with age compared with women (1). DKD is associated with reduced availability of NO (16, 19), which may in part explain why renal injury progresses more rapidly in men compared with women.
In addition to the influence of NO, sex-based differences in renal physiological responses to RAAS inhibition in humans have been well described (60). However, the impact of these RAAS-related sex-based differences on renal segmental resistances is incompletely understood. Our recent analysis may shed some light on the observed clinical and experimental differences in sex-based responses to RAAS blockade. We demonstrated that women with T1D and hyperfiltration had higher RE and lower RBF compared with men with T1D and hyperfiltration. Although the specific mechanisms responsible for the relatively higher RE and lower RBF in women with T1D and hyperfiltration compared with their male counterparts are still unclear, intrarenal RAAS activation is a plausible mediator (unpublished observations). This observation might explain the greater humoral, renal, and systemic responsiveness of RAAS inhibition in women compared with men (60). If RE is elevated in women with T1D secondary to RAAS activation, then the greater responsiveness to RAAS inhibition may be on the basis of augmented blockade of the intrarenal RAAS leading to enhanced efferent vasodilatation. Calculating RE by Gomez' equations in a clinical trial with RAAS inhibition in women with T1D may elucidate whether this hypothesis holds true.
Renal Hemodynamic Profiles in T2D
Similar to research in T1D, studies on T2D have also focused on the effects of poor glycemic control on renal hemodynamic function. Yasumoto et al. (99) and Tsuda et al. (92) demonstrated a significant association between higher HbA1c, PGLO, and RE in healthy nondiabetic subjects (99), nondiabetic subjects with proteinuria (99), and participants with poorly controlled T2D (92). Moreover, Tsuda et al. also showed that participants with poorly controlled T2D had increased FF and PGLO (92). The mechanisms underlying these glomerular hemodynamic changes are unclear but may be secondary to hyperglycemia-induced RAAS activation (74).
The burden of chronic hyperglycemia seen in patients with DM is associated with augmented renal proximal tubular sodium reabsorption, thereby reducing distal sodium delivery. A diminished sodium delivery to macula densa has two important effects, including: 1) TGF-mediated decrease in afferent arteriolar resistance that raises PGLO and GFR and 2) a decrease in renin release from the juxtaglomerular cells of the afferent and efferent arterioles. Glucagon-like receptor 1 agonists (GLP-1RA) have been proposed to restore the maladaptive response of hyperglycemia by reducing proximal sodium reabsorption (40) and thereby increasing sodium delivery to macula densa and consequent renin activity (62). Animal studies have demonstrated renoprotective effects of GLP-1RA by reducing PGLO and glomerular hyperfiltration (87). However, it remained unclear until recently whether similar effects on renal hemodynamic function are associated with GLP-1RA in humans. By applying Gomez' equations, Muskiet et al. were able to examine the acute effects of intravenous exenatide infusion on renal hemodynamic function in healthy overweight men in a randomized double-blind placebo-controlled trial (62). They demonstrated that exenatide infusion acutely increased GFR, ERPF, and PGLO by reducing RA. The same group repeated the same study in a cohort of overweight patients with T2D and, in contrast to their prior study, demonstrated an increase in RA and no effects on GFR, ERPF, FF, PGLO, or RE following exenatide infusion (89). The authors speculate that the contradictory effects of GLP-1RAs on renal hemodynamic function could be explained by the presence of suppressed TGF-mediated effects in the T2D cohort. Alternatively, differences in the healthy vs. T2D studies may be explained by the extensive use of RAAS inhibitors in the latter, which may have impeded the ability to detect changes in renal hemodynamic function (89).
Glomerular Hemodynamic Profiles in the Setting of Hypertension
Kimura et al. calculated renal hemodynamic function by Gomez' equations and by analyzing renal function curves (pressure-natriuresis relationship) in 30 patients with essential hypertension (44). They concluded that renal hemodynamics calculated by analyzing the renal function curve were comparable to those derived by Gomez' equations, suggesting that Gomez' equations can be applied to calculate renal hemodynamics in essential hypertension (44). Nagai et al. performed a similar study, demonstrating that renal hemodynamic function calculated by Gomez' equations were consistent with those obtained by analyzing the renal function curve in a small cohort of patients with primary aldosteronism (63).
Gomez' equations have also been used to examine the renal hemodynamic effects of antihypertensive agents such as Ca2+ channel blockers (CCB). CCBs are widely used to treat hypertension (4, 33). While high-voltage-activated L-type Ca2+ channels are predominantly found in the heart and peripheral vasculature, the low-voltage-activated T-type Ca2+ channels are expressed in various cell types, including vascular smooth muscle cells and endocrine cells (4, 33). The novel dihydropyridine Ca2+ CCB (e.g., manidipine) are thought to block both L- and T-type Ca2+ channels, which may explain their additional renoprotective properties compared with conventional CCB (e.g., amlodipine) (27, 28, 33). The renoprotective properties of combined L- and T-type CCB are thought to be related to their direct effects on renal hemodynamic function (27, 28). The vasodilatory activity of amlodipine acts primarily on RA with only a modest effect on the RE (35, 36). This preferential afferent arteriolar vasodilation is thought to lead to increased PGLO and hyperfiltration (35, 36). Conversely, manidipine has been proposed to decrease PGLO in people with essential hypertension by causing vasodilation of both afferent and efferent arterioles (35, 36). Ott et al. confirmed these findings by measuring GFR and RBF, and applying Gomez' equations to demonstrate that PGLO was significantly lower in patients with hypertension treated with manidipine compared with those treated with amlodipine (67). This is an example of how the application of the Gomez' equations was used to elucidate differences in action of CCBs on dilatory capacity on efferent and afferent arterioles.
Potential in Research and Clinical Practice
Despite recent advances in medical therapy for the treatment of DKD, a quarter of patients continue to progress to ESRD (45), and survival has not improved for patients after the onset of ESRD (31, 78). There is a desperate need for measurements of renal health that will enable us to identify patients at high risk of early DKD and intervene at a time when the renal injury may be responsive to therapy.
The application of Gomez' equations to accurate measurements of GFR and RBF potentially provides a complete renal hemodynamic profile, thereby greatly enhancing the opportunity to identify early abnormalities of renal health in patients with diabetes and also evaluate longitudinal changes in renal hemodynamic function over time. Rather than relying on changes in eGFR and albuminuria to evaluate responsiveness to pharmacological therapies, one can track specific changes in GFR, RBF, FF, PGLO, RA, and RE (Fig. 1). Data derived from the Gomez' equations may also allow researchers to identify important abnormalities in renal hemodynamic parameters in DKD, which may differ in patients with early, established, and advanced DKD. The opportunity to examine renal hemodynamic function may also allow better characterization and differentiation of early DKD phenotypes, including those with hyperfiltration, rapid GFR decline, and impaired GFR. Moreover, by identifying patients with preferential afferent or efferent renal arteriolar abnormalities, it may be possible to further personalize medical therapies for patients with subclinical renal hemodynamic abnormalities.
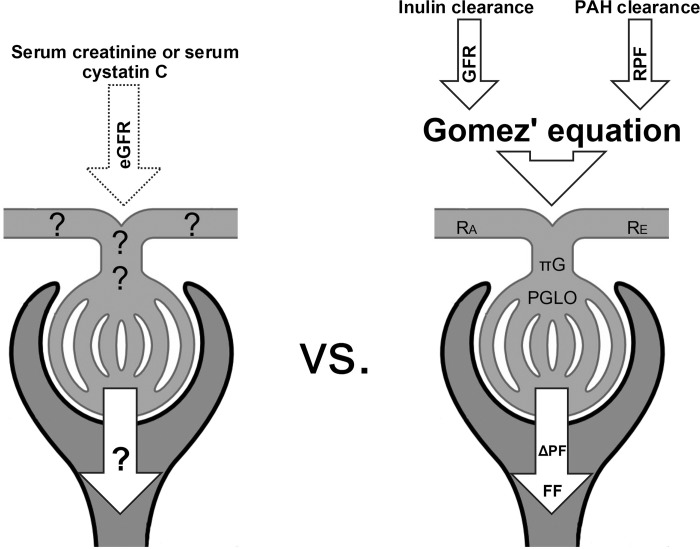
Fig. 1.
Renal hemodynamic profile vs. estimated glomerular filtration rate (GFR). While endogenous filtration markers (serum creatinine and cystatin C) can estimate GFR (eGFR), the application of Gomez' equations to measurements of insulin and para-aminohippurate (PAH) clearances can generate a complete intrarenal hemodynamic profile. RPF, renal plasma flow; RA, afferent arteriolar resistance; RE, efferent arteriolar resistance; πG, glomerular oncotic pressure; FF, filtration fraction; PGLO, intraglomerular pressure; ΔPF, filtration pressure across glomerular capillaries.
Limitations of Gomez' Equations
Gomez' equations are estimates of renal hemodynamic parameters, since direct measurements of glomerular hemodynamic variables such as afferent and efferent arteriolar resistance values and glomerular hydrostatic pressure are too invasive in humans. The gross filtration coefficient 0.0867 ml·s−1·mmHg−1) required for the Gomez' equations is based on the assumption of normal kidney physiology (GFR 130 ml/min, glomerular hydrostatic pressure 60 mmHg), on Winton's indirect estimates of PGLO in a dog model of roughly two-thirds of MAP (23), and on normal glomerular oncotic pressure of 25 mmHg. The lack of human data means these assumptions cannot be validated, and other assumptions will provide a different KFG value. Furthermore, the assumption of a static KFG (of 0.0867 ml·s−1·mmHg−1) is a significant limitation of the Gomez' equations, especially since KFG has been shown to be lower in diabetic rodent models compared with controls (41). KFG has also been shown to be reduced in patients with DKD using dextran sieving curves techniques (88) that instead used an estimate of the effective filtration pressure. Changes in intratubular pressure that relate to factors, including changes in tubular reabsorption of electrolytes and fluid, may also be observed in the setting of DM, a variable also not accounted for in the Gomez equations (73). For example, in early DM, increased proximal tubular reabsorption of glucose and sodium through the SGLT2 cotransporter is associated with reduced proximal tubular pressure, thereby increasing the pressure gradient across the glomerular capillaries, a potentially dynamic variable not accounted for in the Gomez equations (73, 96).
In theory, the accuracy of the output of the Gomez' equations can be improved by using individually calculated gross filtration coefficients. The glomerular capillary wall clearly screens solutes according to size, and large plasma proteins are screened almost entirely. The filtration of a macromolecule is usually quantified in terms of its sieving coefficient, which is the ratio of solute concentration in the filtrate relative to the retained solution. Sieving curves can be used to determine pore size and properties of ultrafiltration. The tracer used most often to generate sieving curves data is dextran, a polymer of anhydroglycose that is neither reabsorbed nor secreted by tubular cells, and its fractional clearance equals the glomerular sieving coefficient (concentration in plasma/concentration in Bowman's space) (25, 26). While different mathematical models exist to calculate ultrafiltration parameters, all of the models provide estimates of changes in glomerular capillary oncotic pressure, volume flows, and fluxes along the glomerular capillary, permitting computation of a series of theoretical sieving dextran curves each with values for capillary wall porosity and glomerular capillary ultrafiltration coefficient (25, 29, 61, 76). However, dextran-sieving data may not reflect non-dextran-sieving (e.g., proteins) data, since the dextran molecule is flexible (86). A flexible molecule (e.g., dextran) is far less hindered at crossing the filtration barrier than a rigid spherical molecule (e.g., albumin) (86). Ficoll, an inert spherical sucrose polymer with some intrinsic rigidity, provides lower sieving coefficients than size-matched dextran and may be more accurate in determining ultrafiltration, but its use in humans is limited due to potential toxicity (65). The estimation of ultrafiltration is further complicated by the function of the endothelial glycocalyx (84), a polysaccharide gel that covers the luminal surface of the endothelium, acts as a filtration barrier, and regulates endothelial vascular functions (13, 64, 75, 97). Detailed representation of sieving by glycocalyx remains elusive. While these methods have their own limitations, they may provide the opportunity to characterize subtle changes in the glomerular filtration barrier in kidney disease research and also examine barrier effects of medical therapies.
To limit the signal-to-noise ratio with Gomez' equations we recommend obtaining accurate measurements of GFR and RBF. GFR is measured indirectly as the urinary or plasma clearance of exogenous filtration markers that are eliminated exclusively by glomerular filtration, e.g., inulin, iohexol, iothalamate, technetium 99m-diethylenetriamine pentaacetic acid, and chromium 51Cr-ethylenediaminetetraacetic acid. Historically these methods were expensive, time consuming, and not suitable for the ambulatory setting, but the availability of calculating GFR by iohexol clearance on dried blood spots may offer some promise for translation of the Gomez' equations in clinical practice (5, 49). The Prevent Early Renal Loss study, a multicenter clinical trial of allopurinol to prevent kidney function loss in T1D, measured GFR by iohexol clearance, demonstrating the feasibility of such methods in larger studies (50).
In humans, ERPF is measured as the plasma clearance of PAH, based on the principle that renal clearance can be used to determine RPF if a substance is completely extracted in a single transit through the kidney. PAH is freely filtered, not reabsorbed and secreted by the proximal tubule (90). Consequently, PAH is almost completely cleared from the blood during a single pass through the kidney. At low concentrations, renal clearance of PAH approximates RPF, but with higher PAH concentrations the tubular transport mechanism becomes saturated, and extraction of PAH reduced with a consequent underestimation of RPF (90). RBF is measured from a patient's RPF and hematocrit by the following equation: RBF = RPF/(1 − Hct). Typically, clearance of PAH and an exogenous filtration marker (e.g., inulin) are measured simultaneously to determine RPF and GFR, respectively. While gold-standard GFR measurements have been piloted for the ambulatory setting by calculating GFR, practical and valid methods of measuring RBF in the clinical setting are to our knowledge not available.
Conclusions
This review summarizes existing data on renal hemodynamic function that used the Gomez' equations to further elucidate hemodynamic abnormalities that potentially contribute to the progression of DKD. There remains a large unmet need for more accurate and targeted measurements of renal health in DM that will enable the identification of patients at high risk of early DKD at a time when the renal injury may be responsive to therapy. Accurate measurements of GFR and RBF, and application of Gomez' equations, provide specific and accurate data on FF, PGLO, RA, and RE. Early changes in these renal hemodynamic parameters may increase individual risk of future renal disease, explain differences in response to renal protective therapies, and identify novel therapeutic targets.
GRANTS
D. Z. Cherney was also supported by a Canadian Diabetes Association-KRESCENT Program Joint New Investigator Award. P. Bjornstad is supported by a training grant from the National Institutes of Health (T32 DK063687).
DISCLOSURES
Petter Bjornstad, Marko Škrtić, and Yuliya Lytvyn declare that they have no conflict of interest.
David Z. Cherney has received speaker honoraria from Janssen, AstraZeneca, Boehringer-Ingelheim, Lilly and Merck and has received research grant support from AstraZeneca, Merck, Astellas, and Boehringer-Ingelheim. David M. Maahs is on the advisory board for Insulet and his institution has received research support from Dexcom and Medtronic. Richard J. Johnson holds a patent related to lowering uric acid in the treatment of diabetic nephropathy and has shares with XORT therapeutics.
AUTHOR CONTRIBUTIONS
P.B. and D.Z.I.C. conception and design of research; P.B. analyzed data; P.B. prepared figures; P.B., M.S., Y.L., D.M.M., R.J.J., and D.Z.I.C. drafted manuscript; P.B., M.S., Y.L., D.M.M., R.J.J., and D.Z.I.C. edited and revised manuscript; P.B., M.S., Y.L., D.M.M., R.J.J., and D.Z.I.C. approved final version of manuscript.
REFERENCES
- 1.Ahmed SB, Fisher ND, Hollenberg NK. Gender and the renal nitric oxide synthase system in healthy humans. Clin J Am Soc Nephrol : 926–931, 2007. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes–2013. Diabetes Care , Suppl 1: S11–S66, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arima S, Ito S. The mechanisms underlying altered vascular resistance of glomerular afferent and efferent arterioles in diabetic nephropathy. Nephrol Dial Trans : 1966–1969, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Baylis C, Qiu C, Engels K. Comparison of L-type and mixed L- and T-type calcium channel blockers on kidney injury caused by deoxycorticosterone-salt hypertension in rats. Am J Kidney Dis : 1292–1297, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Bjornstad P, Anderson PL, Maahs DM. Measuring glomerular filtration rate by iohexol clearance on filter paper is feasible in adolescents with type 1 diabetes in the ambulatory setting. Acta Diabetol : 331–333, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes : 279–286, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjornstad P, Cherney DZ, Maahs DM. Update on estimation of kidney function in diabetic kidney disease. Curr Diabetes Rep : 57, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornstad P, Lanaspa MA, Ishimoto T, Kosugi T, Kume S, Jalal D, Maahs DM, Snell-Bergeon JK, Johnson RJ, Nakagawa T. Fructose and uric acid in diabetic nephropathy. Diabetologia : 1993–2002, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjornstad P, Maahs DM, Rivard CJ, Pyle L, Rewers M, Johnson RJ, Snell-Bergeon JK. Serum uric acid predicts vascular complications in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes study. Acta Diabetol : 783–791, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjornstad P, McQueen RB, Snell-Bergeon JK, Cherney D, Pyle L, Perkins B, Rewers M, Maahs DM. Fasting blood glucose-a missing variable for GFR-estimation in type 1 diabetes? PLoS One : e96264, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjornstad P, Roncal C, Milagres T, Pyle L, Lanaspa MA, Bishop FK, Snell-Bergeon JK, Johnson RJ, Wadwa RP, Maahs DM. Hyperfiltration and uricosuria in adolescents with type 1 diabetes. Pediatr Nephrol : 787–793, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boels MG, Avramut MC, Koudijs A, Dane MJ, Lee DH, van der Vlag J, Koster AJ, Jan van Zonneveld A, van Faassen E, Grone HJ, van den Berg BM, Rabelink TJ. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes : 2429–2439, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev : 405–511, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherney DZ, Konvalinka A, Zinman B, Diamandis EP, Soosaipillai A, Reich H, Lorraine J, Lai V, Scholey JW, Miller JA. Effect of protein kinase Cbeta inhibition on renal hemodynamic function and urinary biomarkers in humans with type 1 diabetes: a pilot study. Diabetes Care : 91–93, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hebert RL, Sochett EB. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes : 688–695, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Cherney DZ, Miller JA, Scholey JW, Nasrallah R, Hebert RL, Dekker MG, Slorach C, Sochett EB, Bradley TJ. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care : 1344–1346, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. The renal hemodynamic effect of SGLT2 inhibition in patients with type 1 diabetes. Circulation : 587–597, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Cherney DZ, Reich HN, Jiang S, Har R, Nasrallah R, Hebert RL, Lai V, Scholey JW, Sochett EB. Hyperfiltration and effect of nitric oxide inhibition on renal and endothelial function in humans with uncomplicated type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol : R710–R718, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherney DZ, Reich HN, Jiang S, Har R, Nasrallah R, Hebert RL, Lai V, Scholey JW, Sochett EB. Hyperfiltration and the effect of nitric oxide inhibition on renal and endothelial function in humans with uncomplicated type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol : R710–R718, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherney DZ, Scholey JW, Miller JA. Insights into the regulation of renal hemodynamic function in diabetic mellitus. Curr Diabetes Rev : 280–290, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis : A7, e1–e420, 2012. [DOI] [PubMed] [Google Scholar]
- 23.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Zinman B, Steffes MW. Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol : 810–818, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med : 2492–2503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deen WM, Bridges CR, Brenner BM, Myers BD. Heteroporous model of glomerular size selectivity: application to normal and nephrotic humans. Am J Physiol Renal Fluid Electrolyte Physiol : F374–F389, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Deen WM, Robertson CR, Brenner BM. A model of glomerular ultrafiltration in the rat. Am J Physiol : 1178–1183, 1972. [DOI] [PubMed] [Google Scholar]
- 27.Dworkin LD. Effects of calcium antagonists on glomerular hemodynamics and structure in experimental hypertension. Am J Kidney Dis : 89–93, 1991. [PubMed] [Google Scholar]
- 28.Dworkin LD. Effects of calcium channel blockers on experimental glomerular injury. J Am Soc Nephrol : S21–S27, 1990. [PubMed] [Google Scholar]
- 29.Edwards A, Deen WM. Error propagation in the estimation of glomerular pressure from macromolecule sieving data. Am J Physiol Renal Fluid Electrolyte Physiol : F736–F745, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Ekinci EI, Jerums G, Skene A, Crammer P, Power D, Cheong KY, Panagiotopoulos S, McNeil K, Baker ST, Fioretto P, Macisaac RJ. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care : 3620–3626, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forsblom C, Harjutsalo V, Thorn LM, Waden J, Tolonen N, Saraheimo M, Gordin D, Moran JL, Thomas MC, Groop PH. Competing-risk analysis of ESRD and death among patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol : 537–544, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaspari F, Ruggenenti P, Porrini E, Motterlini N, Cannata A, Carrara F, Jimenez Sosa A, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Trevisan R, Bossi A, Zaletel J, Remuzzi G. The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int : 164–173, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Ge W, Ren J. Combined L-/T-type calcium channel blockers: ready for prime time. Hypertension : 592–594, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Gomez DM. Evaluation of renal resistances, with special reference to changes in essential hypertension. J Clin Invest : 1143–1155, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin KA, Picken M, Bakris GL, Bidani AK. Comparative effects of selective T- and L-type calcium channel blockers in the remnant kidney model. Hypertension : 1268–1272, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Griffin KA, Picken MM, Bakris GL, Bidani AK. Class differences in the effects of calcium channel blockers in the rat remnant kidney model. Kidney Int : 1849–1860, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, treatment. Diabetes Care : 164–176, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Hannedouche T, Chauveau P, Kalou F, Albouze G, Lacour B, Jungers P. Factors affecting progression in advanced chronic renal failure. Clin Nephrol : 312–320, 1993. [PubMed] [Google Scholar]
- 39.Har R, Scholey JW, Daneman D, Mahmud FH, Dekker R, Lai V, Elia Y, Fritzler ML, Sochett EB, Reich HN, Cherney DZ. The effect of renal hyperfiltration on urinary inflammatory cytokines/chemokines in patients with uncomplicated type 1 diabetes mellitus. Diabetologia : 1166–1173, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev : 1409–1439, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Hostetter TH, Troy JL, Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int : 410–415, 1981. [DOI] [PubMed] [Google Scholar]
- 42.Jungers P, Chauveau P, Descamps-Latscha B, Labrunie M, Giraud E, Man NK, Grunfeld JP, Jacobs C. Age and gender-related incidence of chronic renal failure in a French urban area: a prospective epidemiologic study. Nephrol Dial Trans : 1542–1546, 1996. [PubMed] [Google Scholar]
- 43.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med : 4–11, 2008. [DOI] [PubMed] [Google Scholar]
- 43a.Kidney Disease Outcomes Quality Initiative. KDOQI Clinical Practice Guidelines, and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis : S12–S154, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Kimura G, Imanishi M, Sanai T, Kawano Y, Kojima S, Yoshida K, Abe H, Ashida T, Yoshimi H, Kawamura M, Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, Pohl MA, Rohde RD, Raz I, Yerushalmy Y, Yagil Y, Herskovits T, Atkins RC, Reutens AT, Packham DK, Lewis JB, Collaborative Study Group. Intrarenal hemodynamics in patients with essential hypertension. Circ Res : 421–428, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med : 785–794, 1985. [DOI] [PubMed] [Google Scholar]
- 46.Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, Pohl MA, Rohde RD, Raz I, Yerushalmy Y, Yagil Y, Herskovits T, Atkins RC, Reutens AT, Packham DK, Lewis JB. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol : 131–136, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care : 132–139, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Lytvyn Y, Skrtic M, Yang GK, Lai V, Scholey JW, Yip PM, Perkins BA, Cherney DZ. Plasma uric acid effects on glomerular haemodynamic profile of patients with uncomplicated Type 1 diabetes mellitus. Diabetic Med : 1102–1111, 2016. [DOI] [PubMed] [Google Scholar]
- 49.Maahs DM, Bushman L, Kerr B, Ellis SL, Pyle L, McFann K, Bouffard A, Bishop FK, Nguyen N, Anderson PL. A practical method to measure GFR in people with type 1 diabetes. J Diabetes Comp : 667–673, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Maahs DM, Caramori L, Cherney DZ, Galecki AT, Gao C, Jalal D, Perkins BA, Pop-Busui R, Rossing P, Mauer M, Doria A, Consortium P. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diabetes Rep : 550–559, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maahs DM, Jalal D, Chonchol M, Johnson RJ, Rewers M, Snell-Bergeon JK. Impaired renal function further increases odds of 6-year coronary artery calcification progression in adults with type 1 diabetes: the CACTI study. Diabetes Care : 2607–2614, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maahs DM, Jalal D, McFann K, Rewers M, Snell-Bergeon JK. Systematic shifts in cystatin C between 2006 and 2010. Clin J Am Soc Nephrol : 1952–1955, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maahs DM, Prentice N, McFann K, Snell-Bergeon JK, Jalal D, Bishop FK, Aragon B, Wadwa RP. Age and sex influence cystatin C in adolescents with and without type 1 diabetes. Diabetes Care : 2360–2362, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maahs DM, Rewers M. Editorial: mortality and renal disease in type 1 diabetes mellitus–progress made, more to be done. J Clin Endocrinol Metab : 3757–3759, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Ryden L, Sleight P, Teo KK, Yusuf S. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens : 414–421, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Maric C. Sex, diabetes and the kidney. Am J Physiol Renal Physiol : F680–F688, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med : 40–51, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol : F991–F997, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Messerli FH, Frohlich ED, Dreslinski GR, Suarez DH, Aristimuno GG. Serum uric acid in essential hypertension: an indicator of renal vascular involvement. Ann Intern Med : 817–821, 1980. [DOI] [PubMed] [Google Scholar]
- 60.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimpelmann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol : 2554–2560, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Moran P, Baylis PH, Lindheimer MD, Davison JM. Glomerular ultrafiltration in normal and preeclamptic pregnancy. J Am Soc Nephrol : 648–652, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Muskiet MH, Tonneijck L, Smits MM, Kramer MH, Diamant M, Joles JA, van Raalte DH. Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes Metab : 178–185, 2016. [DOI] [PubMed] [Google Scholar]
- 63.Nagai T, Kimura G, Matsuoka H, Sanai T, Imanishi M, Kawano Y, Kojima S, Yoshida K, Abe H, Ashida T, Orchard TJ, Secrest AM, Miller RG, Costacou T. Estimation of the intrarenal hemodynamics in patients with primary aldosteronism. Nihon Jinzo Gakkai Shi : 235–241, 1989. [PubMed] [Google Scholar]
- 64.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes : 1127–1132, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Oliver JD 3rd Anderson S, Troy JL, Brenner BM, Deen WH. Determination of glomerular size-selectivity in the normal rat with Ficoll. J Am Soc Nephrol : 214–228, 1992. [DOI] [PubMed] [Google Scholar]
- 66.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia : 2312–2319, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ott C, Schneider MP, Raff U, Ritt M, Striepe K, Alberici M, Schmieder RE. Effects of manidipine vs. amlodipine on intrarenal haemodynamics in patients with arterial hypertension. Br J Clin Pharmacol : 129–135, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ott C, Schneider MP, Raff U, Ritt M, Striepe K, Alberici M, Schmieder RE. Effects of manidipine vs. amlodipine on intrarenal haemodynamics in patients with arterial hypertension. Br J Clin Pharmacol : 129–135, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. J Am Med Assoc : 1545–1552, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol : 1353–1361, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med : 2285–2293, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Perlstein TS, Gumieniak O, Hopkins PN, Murphey LJ, Brown NJ, Williams GH, Hollenberg NK, Fisher ND. Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int : 1465–1470, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Persson P, Hansell P, Palm F. Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf) : 3–10, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peti-Peterdi J, Kang JJ, Toma I. Activation of the renal renin-angiotensin system in diabetes–new concepts. Nephrol Dial Trans : 3047–3049, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rabelink TJ, de Zeeuw D. The glycocalyx–linking albuminuria with renal and cardiovascular disease. Nat Rev Nephrol : 667–676, 2015. [DOI] [PubMed] [Google Scholar]
- 76.Remuzzi A, Remuzzi G. Glomerular perm-selective function. Kidney Int : 398–402, 1994. [DOI] [PubMed] [Google Scholar]
- 77.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: UK Prospective Diabetes Study 74. Diabetes : 1832–1839, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS. Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol : 545–553, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O'Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis : A7–A8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes : 1–6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Škrtić M, Lytvyn Y, Yang GK, Yip P, Lai V, Silverman M, Cherney DZ. Glomerular haemodynamic profile of patients with Type 1 diabetes compared with healthy control subjects. Diabetic Med : 972–979, 2015. [DOI] [PubMed] [Google Scholar]
- 82.Škrtić M, Yang GK, Perkins BA, Soleymanlou N, Lytvyn Y, von Eynatten M, Woerle HJ, Johansen OE, Broedl UC, Hach T, Silverman M, Cherney DZ. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia : 2599–2602, 2014. [DOI] [PubMed] [Google Scholar]
- 83.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol : 1703–1709, 2006. [DOI] [PubMed] [Google Scholar]
- 84.Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol : 239–255, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med : 825–830, 2013. [DOI] [PubMed] [Google Scholar]
- 86.Thomson SC, Blantz RC. Biophysics of glomerular filtration. Compr Physiol : 1671–1699, 2012. [DOI] [PubMed] [Google Scholar]
- 87.Thomson SC, Kashkouli A, Singh P. Glucagon-like peptide-1 receptor stimulation increases GFR and suppresses proximal reabsorption in the rat. Am J Physiol Renal Physiol : F137–F144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomlanovich S, Deen WM, Jones HW 3rd Schwartz HC, Myers BD. Functional nature of glomerular injury in progressive diabetic glomerulopathy. Diabetes : 556–565, 1987. [DOI] [PubMed] [Google Scholar]
- 89.Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser AH, Diamant M, Joles JA, van Raalte DH. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia : 315–322, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toto RD. Conventional measurement of renal function utilizing serum creatinine, creatinine clearance, inulin and para-aminohippuric acid clearance. Curr Opin Nephrol Hypertens : 505–509, 1995. [DOI] [PubMed] [Google Scholar]
- 91.Tsuda A, Inaba M, Ichii M, Ochi A, Ohno Y, Nakatani S, Yamada S, Mori K, Tahara H, Ishimura E. Relationship between serum TSH levels and intrarenal hemodynamic parameters in euthyroid subjects. Eur J Endocrinol : 45–50, 2013. [DOI] [PubMed] [Google Scholar]
- 92.Tsuda A, Ishimura E, Ohno Y, Ichii M, Nakatani S, Mori K, Fukumoto S, Emoto M, Inaba M. Significant association of poor glycemic control with increased resistance in efferent arterioles–study of inulin and para-aminohippuric acid clearance in humans. Diabetes Res Clin Pract : 234–240, 2014. [DOI] [PubMed] [Google Scholar]
- 93.Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW. Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol : 631–636, 2007. [DOI] [PubMed] [Google Scholar]
- 94.Uedono H, Tsuda A, Ishimura E, Yasumoto M, Ichii M, Ochi A, Ohno Y, Nakatani S, Mori K, Uchida J, Nakatani T, Inaba M. Relationship between serum uric acid levels and intrarenal hemodynamic parameters. Kidney Blood Press Res : 315–322, 2015. [DOI] [PubMed] [Google Scholar]
- 95.Vallon V. Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol Sci : 169–174, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol : 2569–2576, 1999. [DOI] [PubMed] [Google Scholar]
- 97.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol : 902–910, 2005. [DOI] [PubMed] [Google Scholar]
- 98.Winton FR. Physical factors involved in the activities of the mammalian kidney. Physiol Rev : 408–435, 1937. [Google Scholar]
- 99.Yasumoto M, Tsuda A, Ishimura E, Uedono H, Ohno Y, Ichii M, Ochi A, Nakatani S, Mori K, Uchida J, Emoto M, Nakatani T, Inaba M. Significant association between glycemic status and increased estimated postglomerular resistance in nondiabetic subjects: study of inulin and para-aminohippuric acid clearance in humans. Physiol Rep : 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]