Abstract
Heart failure (HF) is characterized by decreased exercise capacity, attributable to neurocirculatory and skeletal muscle factors. Cardiac resynchronization therapy (CRT) and exercise training have each been shown to decrease muscle sympathetic nerve activity (MSNA) and increase exercise capacity in patients with HF. We hypothesized that exercise training in the setting of CRT would further reduce MSNA and vasoconstriction and would increase Ca2+-handling gene expression in skeletal muscle in patients with chronic systolic HF. Thirty patients with HF, ejection fraction <35% and CRT for 1 mo, were randomized into two groups: exercise-trained (ET, n = 14) and untrained (NoET, n = 16) groups. The following parameters were compared at baseline and after 4 mo in each group: V̇o2 peak, MSNA (microneurography), forearm blood flow, and Ca2+-handling gene expression in vastus lateralis muscle. After 4 mo, exercise duration and V̇o2 peak were significantly increased in the ET group (P = 0.04 and P = 0.01, respectively), but not in the NoET group. MSNA was significantly reduced in the ET (P = 0.001), but not in NoET, group. Similarly, forearm vascular conductance significantly increased in the ET (P = 0.0004), but not in the NoET, group. The expression of the Na+/Ca2+ exchanger (P = 0.01) was increased, and ryanodine receptor expression was preserved in ET compared with NoET. In conclusion, the exercise training in the setting of CRT improves exercise tolerance and neurovascular control and alters Ca2+-handling gene expression in the skeletal muscle of patients with systolic HF. These findings highlight the importance of including exercise training in the treatment of patients with HF even following CRT.
Keywords: heart failure, cardiac resynchronization, exercise, neurovascular control, skeletal muscle
NEW & NOTEWORTHY
Cardiac resynchronization therapy improves neurovascular control and exercise capacity in patients with heart failure (HF) with intraventricular conduction block. Our study shows that, in patients with HF on guideline-recommended pharmacological therapy in whom a biventricular pacemaker has recently been implanted, exercise training further improves exercise tolerance, neurovascular control, and Ca2+-handling gene expression in skeletal muscle.
chronic systolic heart failure (HF) has reached epidemic proportions in the United States, with ∼5 million Americans living with the disease and another 600,000 diagnosed each year (33).
Guideline-recommended treatment strategies include pharmacological therapies to interrupt neuro-humoral activation and device therapy to treat life-threatening rhythm disorders and to restore synchronous ventricular contraction. In fact, according to the most recent HF guidelines (33), the recommendations for cardiac resynchronization therapy (CRT) have been broadened to include even asymptomatic patients with cardiomyopathy with a left bundle branch. CRT has been shown to improve ejection fraction (EF), exercise capacity, and even survival in patients with HF with reduced EF and electrocardiographic intraventricular conduction delay (10, 27). CRT also decreases neuro-humoral activation (2, 7, 15). Studies in animal models of HF have shown that exercise improves several features of the skeletal myopathy of HF, including the muscular atrophy and capillary rarefaction (6) and expression of proteins involved in sarcoplasmic Ca2+ handling (9).
Exercise therapy is a mainstay of the present HF guidelines (33) and has been shown to improve exercise capacity, decrease neuro-humoral activation, and perhaps even increase EF as well (11, 14). However, these benefits of exercise training were reported in studies performed largely before CRT became widespread. Recent studies (12, 24) have demonstrated that exercise training in the setting of CRT improves cardiac function, exercise capacity, and quality of life, but the impact on neuro-humoral activation remains unexplored. Likewise, the impact of exercise training on skeletal myopathy in patients with HF and CRT is virtually unknown.
In the present study of patients with advanced HF following recent CRT, the effects of exercise training on muscle sympathetic nerve activity (MSNA) and peripheral vasoconstriction, as well as on skeletal muscle Ca2+-handling protein expression, were investigated. Calcium-handling protein expression should be investigated because Ca2+ is essential for fundamental muscle function. For example, tension during twitches and maximal tetany depends on Ca2+ release and intracellular Ca2+ transients (25). Thus changes in Ca2+-handling gene expression might give us more insights regarding the benefits of exercise training on skeletal myopathy in patients with HF and CRT.
We hypothesized that the benefits of exercise training would include a reduction in MSNA and peripheral vasoconstriction, an increase in Ca2+-handling gene expression in skeletal muscle, and improved cardiac function and exercise duration even in the setting of CRT in these patients with advanced HF on stable, guideline-recommended pharmacological therapy.
METHODS
Study Population
Patients with advanced HF, on stable HF medications for >3 mo, who underwent recent (∼1 mo) CRT, meeting the following criteria were eligible for the study: New York Heart Association (NYHA) functional class I to III, EF <35%, and without atrial fibrillation, unstable angina or recent myocardial infarction (<3 mo), severe chronic obstructive pulmonary disease, neurological or orthopedic disabilities, and/or obesity (or body mass index ≥30 kg/m2). Patients were randomized to exercise-trained (ET) or untrained (NoET) groups. Patients allocated to the NoET group were advised to maintain their normal daily routine, which included the recommendation of regular physical exercise, for the duration of the study. To make sure that they did not change the daily activity during the study, patients with HF were contacted by phone or seen in the clinic every 2 wk. Supervised exercise in the Heart Institute for the same period (4 mo) was offered to the patients selected to the untrained group when they finished their participation in the study. The pacemaker was programmed to optimize CRT. The study protocol is summarized in Fig. 1. Patients who required medication changes during the study were withdrawn. The protocol was not with the intention to treat. The patients who did not meet a minimum of 80% of the attendance in the exercise regimen were excluded. The atrioventricular interval was echocardiographically optimized using the Ritter method (13) with simultaneous biventricular pacing. The study was approved by the Human Subject Protection Committee of the Clinical Hospital, University of São Paulo, Medical School, and the participants gave their written, informed consent.
Fig. 1.
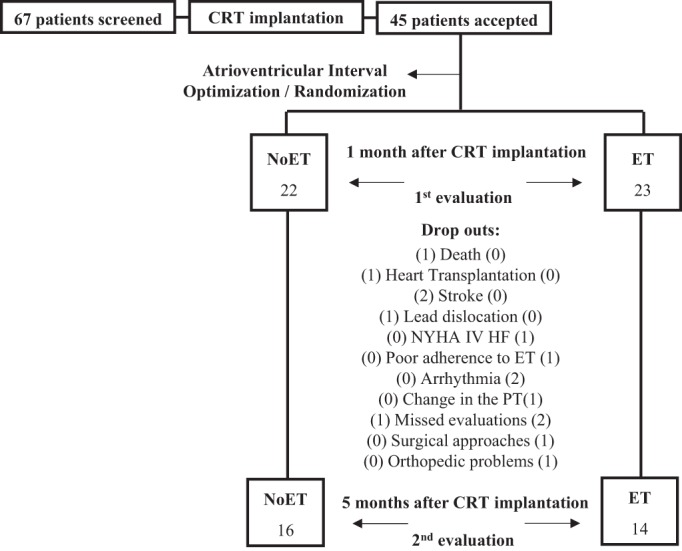
Study protocol. CRT, cardiac resynchronization therapy; NoET, untrained group; ET, exercise-trained group; HF, heart failure; PT, pharmacological therapy; NYHA IV, New York Heart Association functional class IV.
Measurements and Procedures
MSNA.
MSNA was recorded directly from the peroneal nerve using the technique of microneurography, as described in Fraga et al. (14). MSNA was determined by investigators blinded to randomization.
Forearm blood flow.
Forearm blood flow (FBF) was measured by the noninvasive technique of venous occlusion plethysmography, as previously described (14). Forearm vascular conductance (FVC) was calculated by dividing FBF (ml·min−1·100 ml−1) by mean arterial pressure (mmHg) and expressed in units. FBF was measured by blinded randomization.
Cardiopulmonary exercise test.
Maximal exercise capacity was determined by means of a maximal progressive exercise test on a treadmill (T-2100 Treadmill; GE Medical Systems Information Technologies, El Paso, TX) with the modified Balke protocol with rate increments of 2.0 to 3.4 mph, 2% slope every minute until exhaustion. Oxygen uptake (V̇o2) and carbon dioxide production were determined by means of gas exchange on a breath-by-breath basis in a computerized system (Vmax 229; SensorMedics, Buena Vista, CA). V̇o2 peak was defined as the maximum attained V̇o2 at the end of the exercise period. Anaerobic threshold (AT) was determined to occur at the breakpoint between the increase in the carbon dioxide output and V̇o2 (V-slope) (32) or the point at which the ventilatory equivalent for oxygen and end-tidal oxygen partial pressure curves reached their respective minimum values and began to rise (32). Respiratory compensation was determined to occur at the point at which the ventilatory equivalent for carbon dioxide was lowest before a systematic increase and when end-tidal carbon dioxide partial pressure reaches a maximum and begins to decrease. V̇o2 peak and AT were measured by a cardiologist blinded to randomization. The V̇e/V̇co2 slope was measured by linear regression, incorporating all exercise data.
Echocardiography.
Left ventricular EF (LVEF) was determined by two-dimensional echocardiography by Simpson's biplane method (30). All parameters, LVEF, LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV) were evaluated by an echocardiographer blinded to randomization.
Other measurements.
Blood pressure was measured noninvasively on a beat-to-beat basis using a finger device (Finometer; Finapres Medical System, Amsterdam, The Netherlands) as previously described (8). Heart rate was monitored continuously through lead II of the electrocardiogram.
Exercise training.
Subjects underwent 4 mo of exercise training, which consisted of three 60-min exercise sessions/week of intensive, supervised exercise training at the Heart Institute. Each exercise session consisted of 5 min of stretching exercises, 40 min of treadmill walking, 10 min of local strengthening exercises (sit-ups, push-ups, and pull-ups), and 5 min of cool down with stretching exercises. The exercise intensity was established by heart rate levels that corresponded to anaerobic threshold up to 10% below the respiratory compensation point obtained in the cardiopulmonary exercise test. Aerobic exercise training duration increased progressively so that all patients could perform 40 min of treadmill exercise at the established intensity.
Skeletal muscle biopsy.
Patients underwent percutaneous muscle biopsy in the vastus lateralis (VL), as previously described (5). In brief, percutaneous muscle biopsy procedures were performed in VL, approximately at the midway point between the top edge of the patella and the greater trochanter. With a sterile technique, and after adequate local anesthesia was ensured, a small incision was made in the skin and subcutaneous tissue. A 5-mm modified Allendale-Bergstrom needle was then inserted through the fascia, and an assistant immediately applied suction by using a syringe connected to a canister and attached to the top of the needle. The muscle sample was immediately frozen in liquid nitrogen and subsequently stored in a freezer at −80°C.
Total RNA was extracted from frozen VL muscle using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Evaluation of the total RNA concentration and purity was performed by measuring the absorbance at 260 and 280 nm using NanoDrop Spectrophotometer (NanoDrop Technologies, Rockland, DE), and 260/280 ratios ranging from 1.8 to 2.0 were considered satisfactory for purity standards. Denaturing agarose gel electrophoresis was used to assess the quality of the samples. A conventional reverse transcription reaction was performed to yield single-stranded cDNA. First-strand cDNA was synthesized from 1 μg of total RNA using the High-Capacity RNA-to-cDNA kit according to the manufacturer's recommendations (Life Technologies, Carlsbad, CA). The resulting cDNA was stored at −20°C until the expression analysis.
The relative expression levels of selected genes in sample tissues were analyzed by qRT-PCR; the assays were performed in triplicate using SYBR Green I with an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA). The genes analyzed were as follows: Ca2+ channel, voltage-dependent, L-type (CACNA1C); Na+/Ca2+ exchanger (NCX); phospholamban (PLN); ryanodine receptor (RyR); sarco (endo)plasmic reticulum Ca2+-ATPase 1 (SERCA1); and sarco (endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2). The relative expression data were calculated based on the geometric mean of cyclophilin as a housekeeping gene. All primers were designed based on different exons to avoid DNA amplification and were synthesized by Exxtend (Exxtend, Campinas, Brazil). The specific primer sequences used for qRT-PCR were as follows: CACNA1C (sense: 5′-TGA CTG CTT ATG GGG CTT TCT-3′, antisense: 5′-ACT GGA CTG GAT GCC AAA GG-3′); NCX (sense: 5′-GGG ATT TCA GCT CTG CTA CTC A-3′, antisense: 5′- GGC TTG CCC ATC TCT GCT AT-3′); PLN (sense: 5′- GCT GCC AAG GCT ACC TAA AAG-3′, antisense: 5′- CAG CAG GAC AGG AAG TCT GAA-3′); RyR (sense: 5′- TTG GAC AGA GTT CGC ACA GT-3′, antisense: 5′- TGC TGC GTT TGA TGC TTT CA-3′); SERCA1 (sense: 5′- GGA ACT ACC TAG AGG ATC CAG AA-3′, antisense: 5′- CCA CAG CTC TGC CTG AAG AT-3′); SERCA2 (sense: 5′- CTC CTT GCC CGT GAT TCT CA-3′, antisense: 5′- CCA GTA TTG CAG GTT CCA GGT A-3′). SYBR Green I amplification mixtures (10 μl) contained 1 μl of cDNA, 5 μl of 2× SYBR Green I Master Mix (Life Technologies), and forward and reverse primers. The cycle conditions included incubation at 50°C for 5 min, initial denaturation at 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min. A DNA melting curve analysis showed a single peak for all genes. Results were expressed using the comparative cycle threshold (Ct) method (21). The ΔCt obtained from the subtraction of target gene and the reference gene (cyclophilin) Cts was used to calculate de ΔΔCt from other groups in relation to the control group. The relative expression values were calculated with the equation 2−ΔΔCt. Control group levels were arbitrarily set to 1.
Study protocol.
Patients underwent echocardiography, cardiopulmonary exercise testing, FBF, microneurography testing, and muscle biopsy ∼1 mo following CRT and then 4 mo later following completion of exercise training or time control period.
Statistical Analysis
The data are presented as means ± SE. The sample size was calculated based on the effects of CRT on MSNA in patients with chronic HF (15). The online software (http://www.openepi.com/Menu/OE_Menu.htm) was used for this purpose with 95% confidence interval (bidirectional) and statistical power of 95%. According to this calculation, 11 patients should be included in each group. Chi-squared tests were used for discrete variables. One-way ANOVA for repeated measures was used to compare baseline (at 1 mo) differences between groups. In the case of significant difference, Tukey's post hoc test was employed. Paired Student's t-test was used to test within-group differences (before and after intervention) and unpaired Student's t-test to test between-group differences, as previously reported (29). A P value of <0.05 was considered statistically significant.
RESULTS
Study Population Characteristics
Forty-five consecutive patients with HF meeting the study criteria were enrolled in the study; however, 15 dropped out (ET, n = 9 and NoET, n = 6) during the study (Fig. 1). Thirty patients with HF completed the study: ET (n = 14) and NoET (n = 16). Study population characteristics (ET, NoET, and Dropouts) are displayed in Table 1. There were no statistically significantly baseline differences among the groups in any parameter. The medications were unchanged during the study period.
Table 1.
Baseline characteristics
| NoET (n = 16) | ET (n = 14) | Dropouts (n = 15) | |
|---|---|---|---|
| Age, yr | 55.0 ± 2.0 | 54.0 ± 4.0 | 54.0 ± 3.0 |
| Sex, M/F | 9/7 | 7/7 | 8/7 |
| BMI, kg/m2 | 26.9 ± 1.2 | 25.2 ± 0.9 | 28.0 ± 2.0 |
| LVEF, % | 27.0 ± 1.0 | 28.0 ± 3.0 | 28.0 ± 2.0 |
| Peak VO2, ml·kg−1·min−1 | 19.2 ± 1.1 | 17.9 ± 1.0 | 17.4 ± 1.4 |
| Heart rate, beats/min | 67.0 ± 2.0 | 67.0 ± 2.0 | 66.0 ± 4.0 |
| MAP, mmHg | 90.0 ± 2.0 | 89.0 ± 4.0 | 86.0 ± 6.0 |
| MSNA, bursts/min | 39.0 ± 4.0 | 39.0 ± 5.0 | 44.0 ± 4.0 |
| MSNA, bursts/100 heart beats | 66.0 ± 7.0 | 65.0 ± 7.0 | 68.0 ± 9.0 |
| FBF, ml·min−1·100 ml−1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| FVC, units | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.9 ± 0.2 |
| HF etiology | |||
| Idiopathic | 9 | 6 | 7 |
| Chagasic | 4 | 5 | 5 |
| Ischemic | 1 | 2 | 3 |
| Hypertensive | 2 | 1 | 0 |
| Medications | |||
| ACE-I/ARB | 14 | 14 | 10 |
| Digoxin | 5 | 4 | 4 |
| Diuretics | 8 | 12 | 8 |
| Spironolactone | 14 | 13 | 7 |
| β-Adrenergic blocker | 16 | 14 | 15 |
| NYHA functional class | |||
| I | 4 | 2 | 1 |
| II | 8 | 11 | 5 |
| III | 4 | 1 | 7 |
| IV | 0 | 0 | 1 |
Applicable values are means ± SE. ACE-I, angiotensin-converting enzyme; ARB, angiotensin receptor; BMI, body mass index; ET, exercise-trained group; FBF, forearm blood flow; FVC, forearm vascular conductance; HF, heart failure; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; MSNA, muscle sympathetic nerve activity; NoET, untrained group; NYHA, New York Heart Association; VO2, oxygen consumption.
Effects of Exercise Training
Effects of exercise training are displayed in Tables 2, 3 and 4. The exercise training program attendance was over 80% of the scheduled sessions.
Table 3.
Neurovascular control at 1 mo (baseline) and after 5 mo of CRT device implantation
| 1M | 5M | |
|---|---|---|
| MSNA, bursts/min | ||
| NoET | 39.0 ± 4.0 | 36.0 ± 4.0 |
| ET | 39.0 ± 5.0 | 27.0 ± 5.0* |
| MSNA, bursts/100 HR | ||
| NoET | 66.0 ± 7.0 | 59.0 ± 5.0 |
| ET | 65.0 ± 7.0 | 43.0 ± 8.0* |
| FBF, ml·kg−1·100 ml−1 | ||
| NoET | 1.5 ± 0.1 | 1.4 ± 0.1 |
| ET | 1.6 ± 0.1 | 1.8 ± 0.1* |
| FVC, units | ||
| NoET | 1.7 ± 0.1 | 1.6 ± 0.1 |
| ET | 1.6 ± 0.1 | 2.0 ± 0.1* |
Values are means ± SE.
P < 0.05 within-group comparisons.
Table 4.
Calcium cycling gene expressions in skeletal muscle at 1 mo (baseline) and after 5 mo of CRT device implantation
| 1M | 5M | |
|---|---|---|
| L-type Ca2+ channel | ||
| NoET | 1.6 ± 1.0 | 0.3 ± 0.1 |
| ET | 0.6 ± 0.5 | 4.4 ± 2.6 |
| NCX relative expression | ||
| NoET | 3.2 ± 1.2 | 0.9 ± 0.3 |
| ET | 1.2 ± 0.5 | 8.3 ± 2.4* |
| Phospholamban relative expression | ||
| NoET | 1.1 ± 0.5 | 0.3 ± 0.2 |
| ET | 0.2 ± 0.1 | 6.4 ± 2.5 |
| Ryanodine relative expression | ||
| NoET | 4.3 ± 1.1 | 0.9 ± 0.5* |
| ET | 2.9 ± 0.8 | 3.7 ± 1.1 |
| Serca 1 relative expression | ||
| NoET | 2.2 ± 1.3 | 0.7 ± 0.2 |
| ET | 0.9 ± 0.4 | 3.4 ± 0.8 |
| Serca 2 relative expression | ||
| NoET | 1.3 ± 0.5 | 0.5 ± 0.3 |
| ET | 2.4 ± 0.9 | 5.6 ± 1.9 |
Values are means ± SE. NCX, Na+-Ca2+ exchanger.
P < 0.05 within-group comparison.
Cardiac function and exercise capacity effects.
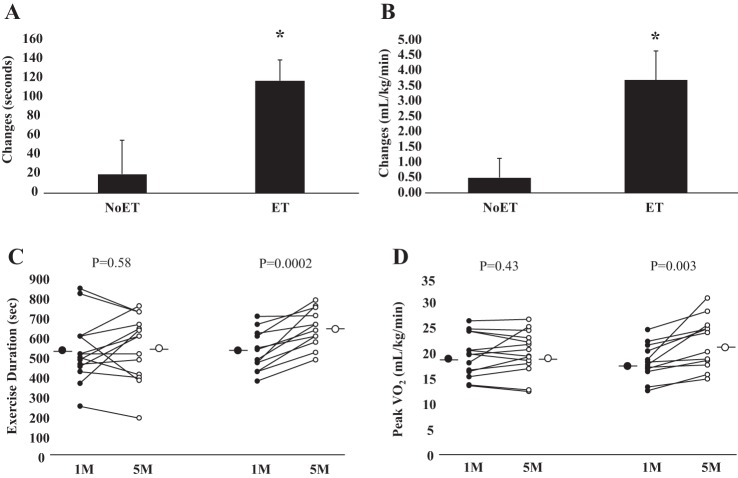
LVEF was significantly increased in patients in the ET group (P = 0.04, Table 2). This parameter did not change in the NoET group (P = 0.33). LVEDD, LVESD, LVEDV, and LVESV were not significantly changed in either group (Table 2). Similarly, heart rate and mean arterial pressure were not significantly changed in ET and NoET groups (Table 2). Exercise training significantly increased workload, exercise duration, and V̇o2 peak (P = 0.0003, P = 0.0002, and P = 0.003, respectively, Table 2). These parameters were unchanged in the NoET group. The comparison between groups showed that the changes in exercise duration and V̇o2 peak were higher (P = 0.04 and P = 0.01, respectively, Fig. 2, A and B) in ET group. V̇e/V̇co2 slope and respiratory exchange ratio were not changed in NoET and ET groups.
Table 2.
Cardiovascular and functional parameters at 1 mo (baseline) and after 5 mo of CRT device implantation
| Parameters | 1M | 5M |
|---|---|---|
| Cardiovascular Parameters | ||
| LVEF, % | ||
| NoET | 27.00 ± 1.00 | 29.00 ± 2.00 |
| ET | 28.00 ± 3.00 | 33.00 ± 4.00* |
| LVEDD, mm | ||
| NoET | 69.00 ± 2.00 | 69.00 ± 1.00 |
| ET | 67.00 ± 3.00 | 67.00 ± 3.00 |
| LVESD, mm | ||
| NoET | 59.00 ± 2.00 | 58.00 ± 2.00 |
| ET | 59.00 ± 2.00 | 57.00 ± 4.00 |
| LVESV, ml | ||
| NoET | 173.00 ± 15.00 | 151.00 ± 11.00 |
| ET | 159.00 ± 21.00 | 166.00 ± 28.00 |
| LVEDV, ml | ||
| NoET | 237.00 ± 18.00 | 211.00 ± 13.00 |
| ET | 215.00 ± 24.00 | 232.00 ± 31.00 |
| HR, beats/min | ||
| NoET | 67.00 ± 2.00 | 65.00 ± 2.00 |
| ET | 67.00 ± 2.00 | 65.00 ± 2.00 |
| MAP, mmHg | ||
| NoET | 90.00 ± 2.00 | 91.00 ± 2.00 |
| ET | 89.00 ± 4.00 | 92.00 ± 4.00 |
| Functional Parameters | ||
| RER | ||
| NoET | 1.08 ± 0.03 | 1.05 ± 0.03 |
| ET | 1.10 ± 0.03 | 1.09 ± 0.02 |
| VE/VCO2 | ||
| NoET | 29.80 ± 1.50 | 30.60 ± 1.40 |
| ET | 35.90 ± 2.00 | 33.30 ± 2.20 |
| Workload, W | ||
| NoET | 90.10 ± 13.90 | 102.80 ± 16.70 |
| ET | 93.10 ± 9.20 | 128.70 ± 9.90* |
| Exercise duration, s | ||
| NoET | 527.00 ± 43.00 | 547.00 ± 43.00 |
| ET | 523.00 ± 30.00 | 641.00 ± 27.00* |
| Peak VO2, ml·kg−1·min−1 | ||
| NoET | 19.20 ± 1.10 | 19.70 ± 1.20 |
| ET | 17.90 ± 1.00 | 21.60 ± 1.50* |
Values are means ± SE. CRT, cardiac resynchronization therapy; LVEDD, left ventricular end-diastolic diameter; LVESV, left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume; RER, respiratory exchange ratio.
P < 0.05 within-group comparison.
Fig. 2.
Exercise capacity. Cardiac resynchronization group before (1 mo) and after (5 mo) period of study is shown. Exercise (ET) and untrained (NoET) periods quantified as changes in exercise duration (seconds, A) and peak oxygen consumption (ml·kg−1·min−1, B). *Note that increase in exercise duration and peak oxygen consumption was significantly increased in the ET group.
Neurovascular effects.
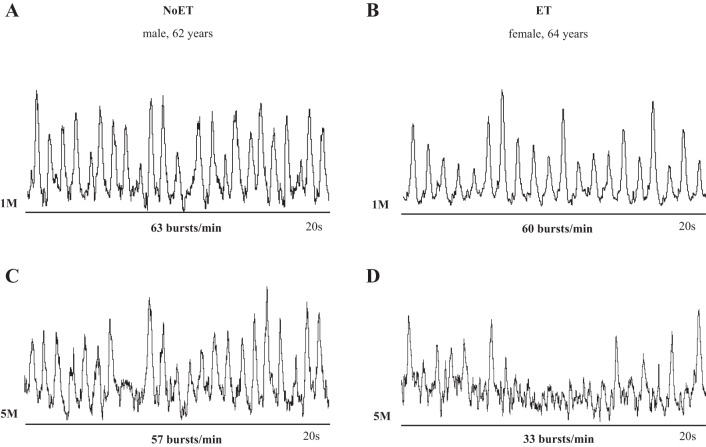
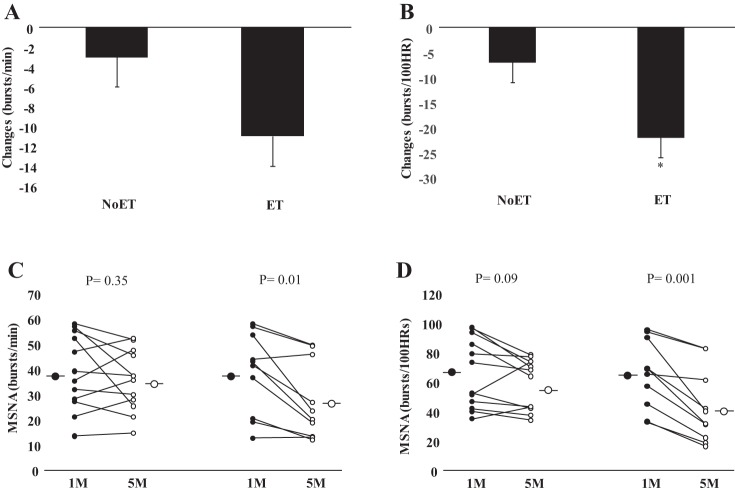
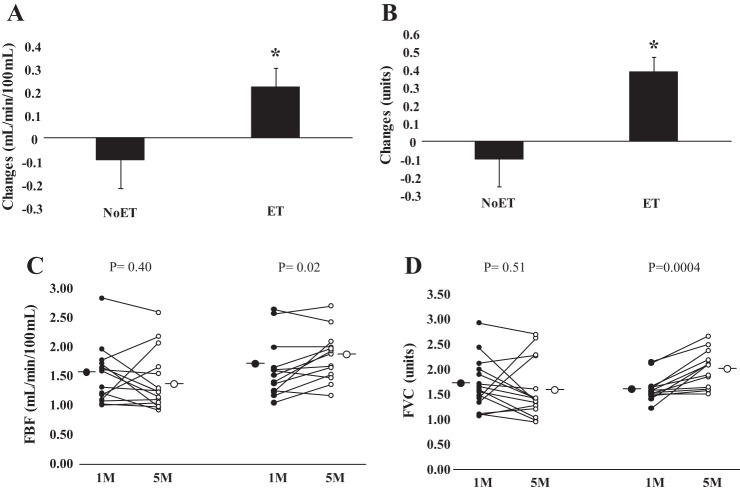
Examples of resting MSNA during study are shown in Fig. 3. In patients with HF randomized to the ET group, MSNA burst frequency and burst incidence (P = 0.01 and P = 0.001, respectively, Table 3) were significantly decreased following the training period. No changes were found in the NoET (P = 0.35 and P = 0.09, respectively). The comparison between groups showed that the changes in MSNA burst incidence were greater (P = 0.02, Fig. 4B) in the ET group. FBF and FVC (P = 0.02, P = 0.0004, respectively, Table 3) were significantly increased following the training period. No significant changes were found in the NoET group. The comparison between groups showed that the changes in FBF and FVC (P = 0.04 and P = 0.01, respectively, Fig. 5, A and B) were greater in the ET group.
Fig. 3.
Recordings of muscle sympathetic nerve activity (MSNA) in 1 nontrained patient with chronic HF (NoET, A and C) and 1 exercise-trained patient with chronic HF (ET, B and D). Note that the reduction in MSNA was much more pronounced in the patient in the ET group. 1M, 1 mo after CRT implantation; 5M, 5 mo after CRT implantation.
Fig. 4.
MSNA. Cardiac resynchronization group before (1 mo) and after (5 mo) period of study. Exercise (ET) and untrained (NoET) periods quantified as changes in burst frequency (bursts/min, A) and burst incidence (bursts/100 HR, B). Note that the reduction in MSNA burst incidence (C and D) was much more pronounced in the patient in the ET group.
Fig. 5.
Forearm blood flow (FBF). Patients in the cardiac resynchronization group before (1 mo) and after (5 mo) period of study are shown (A and B). Exercise (ET) and untrained (NoET) periods quantified as changes in (FBF, C) and forearm vascular conductance (FVC, D). *Note that the increase in FBF and FVC was markedly greater in the ET patients.
Expression of Ca+2-handling proteins in the skeletal muscle.
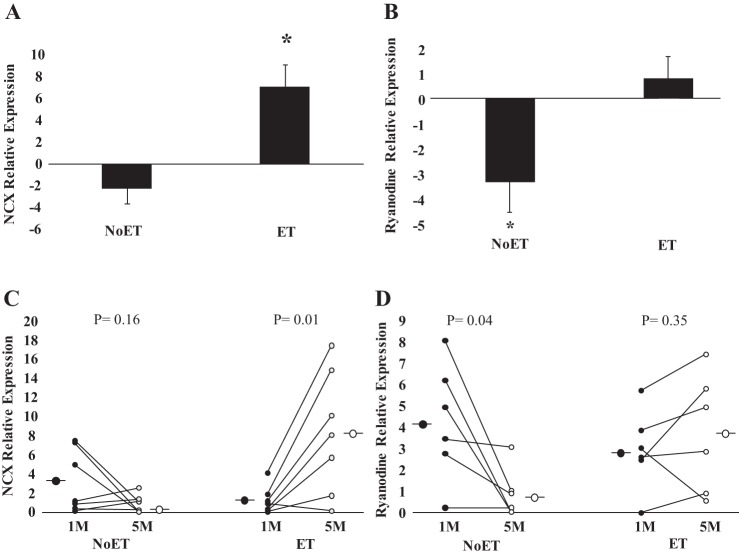
The expression of the Na+/Ca2+ exchanger was significantly increased in the ET (P = 0.01, Table 4). No changes were found in the NoET group (P = 0.16). Thus the comparison between groups showed that the expression of the Na/Ca2+ exchanger was significantly greater in the ET group (P = 0.003, Fig. 6A). Ryanodine receptor expression was significantly decreased in the NoET group (P = 0.04, Table 4). In contrast, no changes were found in the ET group (P = 0.35). Thus the comparisons between groups showed that ryanodine receptor expression was significantly greater (P = 0.02, Fig. 6B) in the ET group. The expression of the L-type Ca2+ channel, SERCA-1, SERCA-2a, and phospholamban was not changed in either the ET or NoET groups (Table 4). The expression of the single housekeeping gene (cyclophilin) did not change between groups.
Fig. 6.
Calcium cycling gene expression. Cardiac resynchronization patients before (1 mo) and after (5 mo) period of study (A and B). Exercise (ET) and untrained (NoET) periods quantified as changes in expression of the Na/Ca2+ exchanger (NCX, C) and ryanodine (D). *Note that the change in expression of the NCX was significantly greater in the ET group. In addition, the expression of ryanodine, in contrast to NoET group, was preserved in the ET group.
Further analysis showed no significant association between the functional outcomes (ΔEF, exercise duration, V̇o2 peak) and neurovascular control/skeletal muscle changes.
DISCUSSION
CRT is recommended in patients with HF with intraventricular conduction block and has been reported to improve neurovascular control, EF, and exercise capacity (1, 10, 33). Exercise training is also recommended in patients with HF and has similarly been reported to improve neurovascular control and exercise capacity (11, 14, 33). The major new findings of the present study are that, in patients with advanced HF on stable, guideline-recommended pharmacological therapy and in whom a biventricular pacemaker has recently been implanted, exercise training is associated with improvements in exercise tolerance and Ca2+-handling gene expression in skeletal muscle.
Exercise training duration for as short as 2 mo has been shown to reduce MSNA in previous studies (11). This nonpharmacological therapy has been shown to reduce MSNA significantly regardless of sex and age (3, 4). CRT has also been reported to decrease sympathetic activation in HF. When plasma norepinephrine levels have been used to estimate sympathetic activity in patients following CRT implantation, some studies (7), but not all (2), have shown a decrease in sympathetic activation. When MSNA is recorded directly using microneurography, MSNA is consistently lower in patients with CRT (15, 16, 23). In fact, when CRT is applied acutely, resynchronization is accompanied by an immediate and significant reduction in MSNA (16, 17). Baroreceptors modulate MSNA in response to acute changes in hemodynamics; thus it is likely the acute increases in blood pressure and cardiac output mediated by resynchronization that mediate this acute attenuation of MSNA.
In the first longitudinal study of CRT, Grassi et al. (15) reported a significant and sustained reduction in MSNA at 2 mo after CRT implantation in patients with advanced HF compared with controls. Furthermore, Najem and colleagues (23) demonstrated that CRT reduces MSNA in responders to CRT, defined as those experiencing a clinical improvement in NYHA functional class following CRT, but not in nonresponders, potentially providing a mechanistic explanation for the significant clinical improvement. More recently, Kuniyoshi et al. (19) showed that MSNA was reduced at rest and during moderate static exercise after 3 mo of CRT. Our study shows that the neurovascular benefits of exercise training persist in the setting of recent CRT. MSNA was lower and muscle blood flow higher after 4 mo of ET. In addition, these changes in neurovascular control were not seen in the NoET group. Although prior studies have shown a decline in MSNA following CRT, this was compared with pre-CRT MSNA levels. We compared CRT levels to 1 mo post-CRT levels, and it is possible that part of the benefits provoked by CRT alone occur within the first month of implantation, i.e., a period that was not included in our study.
The mechanisms involved in the reduction in MSNA and increase in FVC are beyond the scope of our study but potentially are mediated by an exercise-induced improvement in baroreflex (28), chemoreflex (20), and/or cardiopulmonary reflex (26) sensitivity. Alternatively, the sympatholytic effect of exercise may be centrally mediated because, in animal models of HF, exercise training has been shown to reduce AT1 receptor expression, thereby decreasing angiotensin II-mediated central sympathetic nerve outflow (34). Not surprisingly, exercise training increased muscle blood flow. Of course, SNA reduction is one potential mechanism to explain this training adaptation, but a number of other factors such as increased NO release and vasculature changes might have also contributed to the increase in FVC in our patients.
The effects of exercise training on cardiac function in HF have been a matter of interest and controversy. In some studies (18), but not all (31), exercise training improves LVEF and coronary blood flow. Similarly, CRT has been shown to increase the LVEF, a change that is detectable almost immediately; LVEF continues to increase further for at least 1 yr (10). In the present study, we found that exercise training on the background of CRT increased the LVEF even further in patients with HF. The mechanism of this increase is unknown. The increase in LVEF was not associated with changes in ventricular volumes, which were unchanged by exercise on the top of CRT. Similarly, it is unlikely that the amelioration in LVEF was due to changes in total peripheral resistance because no changes were observed in mean blood pressure. This is an interesting issue for future investigations. Previous studies have demonstrated that CRT resulted in a slight increase in exercise tolerance. Similar results were found in the present study. However, the increase in functional capacity is more pronounced when patients with CRT undergo exercise training. Workload and exercise duration were all improved in the ET group. Other investigators who have studied exercise training in patients with HF with CRT have reported similar findings (12, 24).
Alterations in Ca2+-handling proteins in the skeletal muscle have been reported in patients with systolic HF (22). Exercise training has been shown to increase muscle mass, which was accompanied by improvement in sarcoplasmic reticulum Ca2+ uptake in an animal model of heart failure (9). In addition, in sympathetic hyperactivity-induced heart failure mice, exercise training has been shown to increase ryanodine receptors, SERCA1 and SERCA2a and the Na+/Ca2+ exchanger (9). Our study shows for the first time that exercise training increases Ca2+-handling gene expression in the skeletal muscle in patients with treated advanced HF on stable, guideline-recommended pharmacological therapy and CRT. Exercise training restores Na/Ca2+ exchanger and prevents the reduction of ryanodine receptor expression in the skeletal muscle, which is consistent with the amelioration skeletal myopathy. The functional impact of these opposing alterations in Ca2+ handling gene expression remains to be tested. However, we can anticipate that these changes contribute to the amelioration in skeletal myopathy, exercise tolerance, and quality of life in patients with chronic HF with CRT. Moreover, they highlight the importance of including exercise training in the treatment of patients with HF even following CRT.
Exercise training has been shown to decrease MSNA and to increase muscle blood flow accompanied by an increase in exercise tolerance in patients with HF (4, 5, 30). Thus a significant association between the changes in neurovascular control and the changes in exercise should be expected. Surprisingly, this was not the case in our study. There were no significant associations between the changes in V̇o2 peak or exercise duration and the changes in MSNA and FBF. Likewise, there were no associations between functional outcomes (ΔEF, exercise duration, V̇o2 peak) and neurovascular control/skeletal muscle changes. We have no definitive explanation for these findings. Although all these improvements work to better the clinical outcomes in patients with chronic HF, they do not necessarily change at the same rate or degree.
Limitations
Only one patient was excluded (Fig. 1) because of the unexpected onset of psychological problems during the study, which resulted in the inability to achieve the minimum exercise adherence criteria of 80%. In the present study, responsiveness to CRT was not taken into consideration. It has been reported that not all patients with HF respond to CRT; in fact, ∼30% of the patients with HF do not have a clinical improvement with CRT (27). It is possible, but unlikely, that more CRT “responders” were included in the ET group, which then confounded the improvement attributed to exercise training.
It would have been of interest to have neurovascular and skeletal muscle data before CRT implantation to compare the relative neurovascular benefits of CRT and ET in these patients. However, such a study design would have been hampered by a potential order effect because randomizing the order of therapies (CRT vs. ET) would have necessarily resulted in a 4–5-mo delay in the clinically indicated, survival-enhancing CRT in the half of study patients undergoing ET first. More importantly, this decision would include the ethical problems associated with delaying treatment for a medically indicated device in very sick patients with HF.
We cannot rule out the possibility that the effects of exercise training would be more pronounced if more patients were involved in the study. In fact, 33% patient dropout might have effects on generalizability of the results of the present study.
Finally, there was insufficient tissue to confirm that the change in gene expression translated to change in protein expression.
Perspective
Exercise training remains a mainstay of therapy in patients with advanced HF even in this era of device therapy. The benefits of ET on exercise tolerance, neurovascular control, and skeletal muscle Ca2+ handling are significant even in the presence of recently placed CRT. Although not specifically examined in this study, CRT may enable a subgroup of patients with HF, who were otherwise too debilitated and deconditioned to participate in exercise training, now to enroll in an ET program post-CRT. The results from this study provide evidence that further improvements in exercise tolerance, neurovascular control, and skeletal muscle can be expected as a result of this participation.
GRANTS
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-2010/50048-1) and, in part, by Fundação Zerbini. T. Nobre, L. Antunes-Correa, A. Sarmento, M. Rondon, P. Brum, and C. Negrao were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). R. Groehs was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). H. Middlekauff was supported by NIH-RO1-HL084525.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.S.N., L.M.A.-C., P.C.B., M.M., H.R.M., and C.E.N. conception and design of research; T.S.N., L.M.A.-C., R.V.G., M.-J.N.A., A.V.N.B., G.B.A., M.U.P.B.R., and C.E.N. performed experiments; T.S.N., L.M.A.-C., R.V.G., M.-J.N.A., A.O.S., A.V.N.B., U.U., M.U.P.B.R., and C.E.N. analyzed data; T.S.N., L.M.A.-C., R.V.G., M.-J.N.A., A.O.S., A.V.N.B., U.U., G.B.A., M.U.P.B.R., P.C.B., H.R.M., and C.E.N. interpreted results of experiments; T.S.N., A.O.S., A.V.N.B., U.U., and C.E.N. prepared figures; T.S.N., L.M.A.-C., R.V.G., G.B.A., H.R.M., and C.E.N. drafted manuscript; T.S.N., A.V.N.B., M.U.P.B.R., P.C.B., M.M., H.R.M., and C.E.N. edited and revised manuscript; T.S.N., L.M.A.-C., R.V.G., M.-J.N.A., A.O.S., A.V.N.B., U.U., G.B.A., M.U.P.B.R., P.C.B., M.M., H.R.M., and C.E.N. approved final version of manuscript.
REFERENCES
- 1.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med : 1845–1853, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Adamson PB, Kleckner KJ, VanHout WL, Srinivasan S, Abraham WT. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation : 266–269, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Antunes-Correa LM, Melo RC, Nobre TS, Ueno LM, Franco FG, Braga AM, Rondon MU, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Impact of gender on benefits of exercise training on sympathetic nerve activity and muscle blood flow in heart failure. Eur J Heart Fail : 58–65, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antunes-Correa LM, Kanamura BY, Melo RC, Nobre TS, Ueno LM, Franco FG, Roveda F, Braga AM, Rondon MU, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training improves neurovascular control and functional capacity in heart failure patients regardless of age. Eur J Cardiovasc Prev Cardiol : 822–829, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Antunes-Correa LM, Nobre TS, Groehs RV, Alves MJ, Fernandes T, Couto GK, Rondon MU, Oliveira P, Lima M, Mathias W, Brum PC, Mady C, Almeida DR, Rossoni LV, Oliveira EM, Middlekauff HR, Negrao CE. Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am J Physiol Heart Circ Physiol : H1655–H1666, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacurau AV, Jardim MA, Ferreira JC, Bechara LR, Bueno CR, Alba-Loureiro TC, Negrao CE, Casarini DE, Curi R, Ramires PR, Moriscot AS, Brum PC. Sympathetic hyperactivity differentially affects skeletal muscle mass in developing heart failure: Role of exercise training. J Appl Physiol : 1631–1640, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Blanc JJ, Bertault-Valls V, Fatemi M, Gilard M, Pennec PY, Etienne Y. Midterm benefits of left univentricular pacing in patients with congestive heart failure. Circulation : 1741–1744, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol : 437–446, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Bueno CR, Ferreira JC, Pereira MG, Bacurau AV, Brum PC. Aerobic exercise training improves skeletal muscle function and Ca2+ handling-related protein expression in sympathetic hyperactivity-induced heart failure. J Appl Physiol : 702–709, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med : 1539–1549, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation : 2119–2131, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Conraads VM, Vanderheyden M, Paelinck B, Verstreken S, Blankoff I, Miljoen H, De Sutter J, Beckers P. The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: A pilot trial. Eur J Cardiovasc Prev Rehabil : 99–106, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Dupuis JM, Kobeissi A, Vitali L, Gaggini G, Merheb M, Rouleau F, Leftheriotis G, Ritter P, Victor J. Programming optimal atrioventricular delay in dual chamber pacing using peak endocardial acceleration: Comparison with a standard echocardiographic procedure. Pacing Clin Electrophysiol : 210–213, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail : 630–636, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Grassi G, Vincenti A, Brambilla R, Trevano FQ, Dell'Oro R, Ciro A, Trocino G, Vincenzi A, Mancia G. Sustained sympathoinhibitory effects of cardiac resynchronization therapy in severe heart failure. Hypertension : 727–731, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Hamdan MH, Barbera S, Kowal RC, Page RL, Ramaswamy K, Joglar JA, Karimkhani V, Smith ML. Effects of resynchronization therapy on sympathetic activity in patients with depressed ejection fraction and intraventricular conduction delay due to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol : 1047–1051, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hamdan MH, Zagrodzky JD, Joglar JA, Sheehan CJ, Ramaswamy K, Erdner JF, Page RL, Smith ML. Biventricular pacing decreases sympathetic activity compared with right ventricular pacing in patients with depressed ejection fraction. Circulation : 1027–1032, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: The benefit depends on the type of training performed. J Am Coll Cardiol : 2329–2336, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Kuniyoshi RR, Martinelli M, Negrão CE, Siqueira SF, Rondon MU, Trombetta IC, Kuniyoshi FH, Laterza MC, Nishioka SA, Costa R, Tamaki WT, Crevelari ES, Peixoto GD, Ramires JA, Kalil R. Effects of cardiac resynchronization therapy on muscle sympathetic nerve activity. Pacing Clin Electrophysiol : 11–18, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Li YL, Ding Y, Agnew C, Schultz HD. Exercise training improves peripheral chemoreflex function in heart failure rabbits. J Appl Physiol : 782–790, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-delta delta C (T)) method. Methods : 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Middlekauff HR, Vigna C, Verity MA, Fonarow GC, Horwich TB, Hamilton MA, Shieh P, Tupling AR. Abnormalities of calcium handling proteins in skeletal muscle mirror those of the heart in humans with heart failure: a shared mechanism? J Card Fail : 724–733, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najem B, Unger P, Preumont N, Jansens JL, Houssière A, Pathak A, Xhaet O, Gabriel L, Friart A, De Roy L, Vandenbossche JL, van de Borne P. Sympathetic control after cardiac resynchronization therapy: Responders versus nonresponders. Am J Physiol Heart Circ Physiol : H2647–H2652, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Patwala AY, Woods PR, Sharp L, Goldspink DF, Tan LB, Wright DJ. Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: A randomized controlled study. J Am Coll Cardiol : 2332–2339, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Perreault CL, Gonzalez-Serratos H, Litwin SE, Sun X, Franzini-Armstrong C, Morgan JP. Alterations in contractility and intracellular Ca2+ transients in isolated bundles of skeletal muscle fibers from rats with chronic heart failure. Circ Res : 405–412, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Pliquett RU, Cornish KG, Patel KP, Schultz HD, Peuler JD, Zucker IH. Amelioration of depressed cardiopulmonary reflex control of sympathetic nerve activity by short-term exercise training in male rabbits with heart failure. J Appl Physiol : 1883–1888, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Reuter S, Garrigue S, Barold SS, Jais P, Hocini M, Haissaguerre M, Clementy J. Comparison of characteristics in responders versus nonresponders with biventricular pacing for drug-resistant congestive heart failure. Am J Cardiol : 346–350, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Rondon E, Brasileiro-Santos MS, Moreira ED, Rondon MU, Mattos KC, Coelho MA, Silva GJ, Brum PC, Fiorino P, Irigoyen MC, Krieger EM, Middlekauff HR, Negrao CE. Exercise training improves aortic depressor nerve sensitivity in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol : H2801–H2806, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: A randomized controlled trial. J Am Coll Cardiol : 854–860, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr : 358–367, 1989. [DOI] [PubMed] [Google Scholar]
- 31.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: A meta-analysis. Eur J Heart Fail : 841–850, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Wasserman K, Whipp BJ, Koyl SN, Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol : 236–243, 1973. [DOI] [PubMed] [Google Scholar]
- 33.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation : e240–e327, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Zucker IH, Patel KP, Schultz HD, Li YF, Wang W, Pliquett RU. Exercise training and sympathetic regulation in experimental heart failure. Exerc Sport Sci Rev : 107–111, 2004. [DOI] [PubMed] [Google Scholar]