Abstract
Coccidioidomycosis is a human fungal disease cause by inhalation of aerosol spores produced by Coccidioides posadasii or Coccidioides immitis. This disease is a common cause of community-acquired pneumonia in the endemic areas of the Southwestern United States. It also can present as a life-threatening disease as the fungal cells disseminate to skin, bone, and central nervous system. The outcome of coccidioidomycosis is largely determined by the nature of host immune response to the infection. Escalation of symptomatic infections and increased cost of long-term antifungal treatment warrant a concerted effort to better understand the innate and adaptive immune responses and the genetics associated with coccidioidomycosis susceptibility. This knowledge can be harnessed for development of a human vaccine against Coccidioides and advance clinic management of this disease. This review discusses recently reported studies on innate and adaptive immunity to Coccidioides infection, Mendelian susceptibility to disseminated disease and progress toward a human vaccine against this formidable disease.
Keywords: innate immunity, Coccidioides, Dectin-1, MyD88, interleukin-1, fungal susceptibility, Mendelian genetics, adaptive immunity, T-cell response, adjuvants, and fungal vaccines
This paper represents the work of three separate laboratories conducting basic research into the host immune response to coccidioidomycosis. In the first section, the work from the laboratory of Dr. Joshua Fierer on mouse genetics that affects the host immune response to coccidioidomycosis is presented. In the second part, Dr. Amy Hsu presents the work she and Dr. Steve Holland have performed on identifying genetic variations in humans with disseminated coccidioidomycosis that correlate with susceptibility to coccidioidal infection. Finally, Dr. Chiung-Yu Hung outlines her studies on vaccine development and protective immunity against Coccidioides infection.
Immune response to coccidioidomycosis
Interplay of Innate and adaptive immunity to Coccidioides infection
The innate immune system consisting of a network of immune cells and molecules provides the first line of defense against Coccidioides infection. Innate immune cells express pattern recognition receptors (PRRs) that recognize unique and conserved pathogen-associated molecular patterns (PAMPs) such as bacterial flagellin, lipopolysaccharide, viral ssRNA, and fungal β-glucan.1 Many components of the innate immune system are highly conserved in evolution from invertebrates to vertebrates.2 PRRs have been divided into four families: Toll-like receptors (TLRs), nucleotide-oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-1 (RIG-1)-like receptors (RLRs), and the C-type lectin-like receptors (CLRs). Recent studies using a murine model of coccidioidomycosis have shown that several PRRs are important for the control of Coccidioides infection.3–9 These results suggest that TLRs and CLRs may orchestrate the recognition of spherule wall components by host cells to initiate innate immunity and subsequently guide the development of adaptive response against this fungus.
Both CD4+ T-helper (Th) and CD8+ cytotoxic (Tc) cells have been reported to contribute to adaptive immunity against coccidioidomycosis.10 Reported CD4+ T-cell subsets that contribute to protective and pathogenic immune response upon Coccidioides infection include Th1, Th2, Th17, and perhaps interleukin (IL)-10-producing cells that play roles in cellular, humoral, inflammatory, and regulatory immunity, respectively.11,12Bidirectional interplay between the innate and adaptive immune systems during coccidioidal infection is plausible. For example, Th17 cells, which are normally present in mucosal surfaces and are programmed in response to the normal microflora that colonizes our epithelial surfaces, can secrete cytokines and chemokines to attract polymorphonuclear neutrophils (PMNs) into infected tissues.13
People vary greatly in their susceptibility to disseminated coccidioidomycosis. African Americans and Asian/Pacific Islanders have higher rates of disseminated disease compared to European-Americans.14 Several human genetic factors associate with their susceptibility to disseminated coccidioidomycosis have been discovered and reviewed in the second section. Inbred strains of mice also vary greatly in their susceptibility to both C. immitis and C. posadasii infection.15,16 Resistance to Coccidioides infection in inbred strains of mice is a polygenic trait.17 A correlation has been revealed between susceptibility to Coccidioides infection and the amount of IL-10 produced.18,19 IL-10 is a pleiotropic cytokine with anti-inflammatory and immunosuppressive functions and ability to impact both innate and adaptive immunity to microbial infections. IL-10 KO mice significantly improves the outcome of coccidioidomycosis compared to their wild type counterparts.18 The major IL-10 producing leucocytes are T cells, neutrophils and macrophages in lungs of Coccidioides-infected B6 mice.20 The role of each types of IL-10-producing cells in response to Coccidioides infection require further examination. In this review we highlight several critical PRRs and immune modulators that are also essential for the recognition and defense against Coccidioides.
Role of Dectin-1 and Card9-mediated immunity to coccidioidomycosis
Clec7a, which encodes the C-type lectin receptor Dectin-1, has been implicated in host resistance to Coccidioides infection in mice.4,6,8 Dectin-1 is a membrane spanning β-glucan receptor containing an extra-cellular, carbohydrate binding domain separated from the cell membrane by a stalk region, a transmembrane domain, and an intracellular signaling region. Since β-glucans are major structural components of nearly all fungal cell walls, this receptor is an important mediator of innate immunity against fungal infections.21 Upon binding of β-glucans, Dectin-1 can induce a vast array of cellular effects including phagocytosis, activation of the respiratory burst, and increase of cytokine and chemokine secretion such as tumor necrosis factor α (TNF-α), IL-1α, IL-1β, IL-6, CXCL2 (IL-8), and GM-CSF. There are two major splicing variants of Dectin-1 in humans and mice. We found that DBA/2 mice, which are genetically resistant, express a full length Dectin-1, while susceptible B6 mice contain a splice variant which results in the skipping of exon 3 encoding the stalk region.7 In a collection of recombinant inbred mice made by B6 × DBA/2 mating, the expression of a full-length Dectin-1 was strongly associated with resistance to this infection.7 At 37°C, macrophages and dendritic cells expressing the shorter splice form of Dectin-1 do not bind spherules as efficiently4; however, binding to C. albicans does not significantly different.22 Dectin-1 has to be clustered to maximize binding and activation of the receptor-mediated signal response. We postulate that the truncated receptor is too short to reach and interact with the surface carbohydrate components of mature spherules (∼40–100 μm; much larger than yeast) and thus cannot initiate phagocytosis of Coccidioides cells, a necessary step for antigen presentation. We have found that Dectin-1 KO mice produce much less IL-17a and IFN- in their bronchoalveolar lavage fluid (BALF) post Coccidioides infection.4 Furthermore, we have discovered that caspase recruitment domain-containing protein 9 (Card9), an intracellular immune adaptor downstream of Dectin-1, is essential for activation of protective Th1 and Th17 immunity against Coccidioides infection.3,6,23 Card9 KO mice were significantly more susceptible to both subcutaneous and pulmonary Coccidioides infection compared to wild-type mice.6,23 Moreover, Card9 KO mice vaccinated with formalin-killed spherules or a live attenuated vaccine failed to acquire protective immunity to coccidioidomycosis.3,6 These results support the hypothesis that interaction of Dectin-1 with β-glucan can promote Th1 and Th17 immune responses against Coccidioides infection as well as other fungal diseases.4,6,21,23,24
in their bronchoalveolar lavage fluid (BALF) post Coccidioides infection.4 Furthermore, we have discovered that caspase recruitment domain-containing protein 9 (Card9), an intracellular immune adaptor downstream of Dectin-1, is essential for activation of protective Th1 and Th17 immunity against Coccidioides infection.3,6,23 Card9 KO mice were significantly more susceptible to both subcutaneous and pulmonary Coccidioides infection compared to wild-type mice.6,23 Moreover, Card9 KO mice vaccinated with formalin-killed spherules or a live attenuated vaccine failed to acquire protective immunity to coccidioidomycosis.3,6 These results support the hypothesis that interaction of Dectin-1 with β-glucan can promote Th1 and Th17 immune responses against Coccidioides infection as well as other fungal diseases.4,6,21,23,24
IL-1 receptor and MyD88-mediated response to coccidioidomycosis
MyD88 is the adapter protein for all Toll-like receptors except TLR3, but it is also the adapter protein for many IL-1 family receptors.25 Early on we learned that MyD88 deficiency severely impaired cytokine production by macrophages in response to spherules,5 and that TLR2 was the receptor that was needed. However, the importance of MyD88-dependent signaling molecules in resistance to infection was not clearly established. Interestingly, B6 mice that vaccinated with a live attenuated vaccine produced significantly higher amount of IL-1β (an proinflammatory cytokine) in their lungs after a pulmonary challenge compared to nonvaccinated mice.26 Furthermore, MyD88 and the IL-1 receptor (IL-1R1) were required for vaccine-induced resistance to infection but not TLR2.3 We further investigated whether MyD88 is required for nonvaccinated mice to defense against primary Coccidioides infection.27 We found that MyD88 KO mice were more susceptible to Coccidioides infection than control B6 mice as evidenced by increased numbers of colony-forming units (cfu) recovered from lungs and spleens.27
We postulated that the importance of MyD88 may lie in its role as the adapter protein for IL-1 and IL-18 receptors, because TLR2 was not required and no other TLR is known to interact with spherules (at least in vitro). IL-18 in combination with IL-12 promotes the development of interferon γ (IFN-γ) producing CD4+ T cells,28 and IFN-γ is known to be critical for immunity to coccidioidomycosis in mice and humans.16,29 To our surprise IL-18R KO mice were not more susceptible to coccidioidal infection, perhaps because B6 mice are inherently susceptible to this infection and when infected, they make very little IL-18 and low amounts of IFN-γ. This is a limitation of using KO mice to study immune response to this fungal infection because almost most KO mice are on the B6 genetic background. Even so, IL-1R KO mice on B6 genetic background were more susceptible, but not as much as MyD88 KO mice.27 We have observed that IL-1R KO mice had similar numbers of lung fungal colony-forming units (cfu), but 100-fold more cfu in the spleen compared to wild-type mice at 14 days post Coccidioides infection, suggesting more fungal dissemination at the same severity of pneumonia or possibly less ability to control growth in the spleen once it was seeded.
Role of IL-1Ra, a circulating antagonist of IL-1 receptor
We also measured the concentration of IL-1β cytokine, an important cytokine signaling via IL-1 receptor, in the BALF of infected B6 mice. Our data showed that the concentration was <50 pg/ml, indicating that B6 mice do not make much of this cytokine. In contrast, they made a large amount of IL-1Ra that competes with IL-1β for binding to IL-1 receptor. As a consequence, there could be essentially no IL-1R1 signaling in the lungs of B6 mice, which could explain why B6 and IL-1R1 KO mice had the same severity of infection in their lungs. IL-1β is synthesized in cells as an inactive cytokine (pro-IL-1β) that is enzymatically cleaved by caspase 1 to release the active form (IL-1β). Caspase 1 needs to be activated by an intracellular complex of proteins called inflammasomes. One would speculate that IL-1R KO and caspase 1 KO mice should have a comparable phenotype to microbial infection due to that their deficiency in the same IL-1R1 signaling pathway. However, we observed that caspase-1 KO were not more susceptible to coccidioidomycosis compared to wild-type littermates.27 Taken together, these results suggest that there may be unrevealed mechanisms of regulation and cleavage of pro-IL-1 β in these mice. Others have shown that caspase 8 is stimulated by Dectin-1 activation by β-glucans, which could be happening in this infection.30 Collectively, these results all point to IL-1 signaling as a key component of innate immunity in coccidioidomycosis, not just inflammation. IL-1β is known to be important for promoting the development of Th17 and Th1 cells.31 Furthermore, IL-1 receptor signaling is essential for adaptive immunity in vaccinated mice against Coccidioides infection.3,26 We suggest that polymorphisms of human and murine molecules in Dectin-1-Card9- and IL-1R1-MyD88-mediated pathways may be in part responsible for the differences in protective and nonprotective responses to this infection.
Mendelian susceptibility to disseminated coccidioidomycosis
Given the highly virulent nature of Coccidioides spp. and its prevalence in endemic areas, it is not surprising that infection rates are estimated at up to 150000 individuals per year in the United States.14 However, of the roughly 1% of infected individuals who go on to develop disseminated disease there is a marked overrepresentation of African Americans,32 suggesting possible genetic components to the risk of dissemination. Further, immunosuppressed or immunocompromised individuals, including pregnant women, have a lower survival rate after dissemination.14 Coupling these two observations, we looked for genetic variations in immune related genes in patients with disseminated coccidioidomycosis (DCM). We used high throughput next-generation sequencing (NGS), filtered the identified variants by frequency, predictions of deleteriousness and potential relatedness to the DCM phenotype and tabulated the results. We were able to demonstrate causative mutations in genes in innate signaling pathways involved in other opportunistic infections. Additionally, we found deleterious variants in other genes with increased constraints against missense changes, supporting the hypothesis that reduced innate recognition and signaling may be important in allowing dissemination of this pathogen.
Mutations of molecules in the IL-12-IFN-γ signaling pathway
The first Mendelian mutation identified in DCM was a dominant IFNGR1 mutation in a patient with refractory DCM and mycobacteriosis.29 This was followed in 2009 by a report of a STAT3 deficient hyper-immunoglobulin E (IgE) patient with Coccidioides immitis meningitis.33 and a pair of consanguineous siblings with DCM and Salmonella infections, who were homozygous for a deleterious IL12RB1 mutation.34 The most recent gene implicated in DCM was found in two patients with dominant gain-of-function mutations in STAT1.35 These four genes are part of the IL-12-IFN-γ signaling pathway known to be mutated in some patients with non-tuberculous mycobacterial infections.
To continue our search for deleterious mutations, additional patients were enrolled in an Institutional Review Board approved study protocol at the National Institutes of Health. Written informed consent was provided by all study participants. We enrolled 58 patients with DCM, performed next gene sequence analysis (NGS) using either a targeted panel of immune related genes or whole exome capture, filtered the variants for ExAC frequency <1%,36 CADD score >10 and predicted to be directly or indirectly related to the key words, “disseminated, fungal, infection” using VarElect.37 The resulting filtered list contained 103 variants within genes that clustered in recognized innate pathways (Table 1). One patient was identified with an IL12RB2 c.302G>A, p.C101Y mutation. Similarly, three variants were identified in STAT4: p.E626G, a novel change occurring in a proband within a three generation family with a history of DCM; p.I115V in two probands; and p.R240Q in one proband. IL12 signaling through the heterodimeric IL12RB1-IL12RB2 receptor leads to phosphorylation of STAT4 (pSTAT4) and target gene transcription including upregulation of IFN-γ. Functional studies were carried out using cDNA constructs of selected variants and comparing signaling responses to wild-type and empty vectors. Transfection assays demonstrated both decreased pSTAT4 and decreased STAT4-mediated transcriptional activity for the STAT4 p.E626G and p.I115V variants, while the p.R240Q had activity equivalent to wild-type STAT4. These functional assays suggest that reduced IL-12 driven signaling caused by IL12RB2-C101Y, STAT4-E626G and STAT4-I115V may impair immunity sufficient for dissemination of a high virulent pathogen like Coccidioides, but still prevent infections with other less virulent pathogens.
Table 1.
Genes with rare, deleterious variants in disseminated coccidioidomycosis.
| Pathway | Genes |
|---|---|
| IL-12-IFN-γ signaling | IFNGR1, IL12RB1 (AR), STAT3, STAT1, IL12RB2, STAT4, IFNAR2 (AR) |
| Innate immune sensing | DDX58, IFIH1, TLR4, TLR5 |
| Signaling from innate sensing | PIK3R3, PIK3R5, PIK3CG |
| NFκB signaling | IRF5, IKBKB, RIPK2, NFKBIA |
| IL-17 signaling | IL17RA, IL17RC, TRAF3IP2 |
(AR), autosomal recessive, indicates bi-allelic variants identified.
We also identified two additional patients with DCM and novel mutations in the C terminal portion of STAT3. The first, p.V713M, is near the tyrosine phosphorylation site (Y705), while the second is a single base insertion causing a frame shift after P728 leading to loss of the C terminus of the protein, including the serine phosphorylation site. STAT3 and STAT4 form the heterodimeric signal transducers of IL-23, produced by phagocytic cells in response to IFN-γ and leading to production of Th17 cells. (Fig. 1). Th17 cells have been implicated in host control of fungal pathogens and are known to be severely decreased in STAT3 deficient Hyper-IgE patients and STAT1 gain of function.38,39
Figure 1.
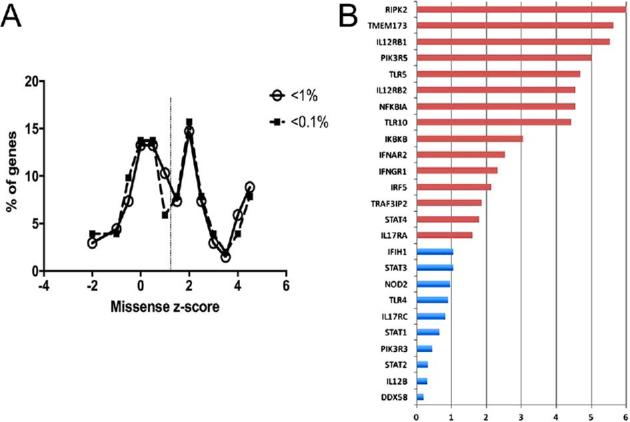
A. Missense z-scores from EXAC of genes identified in DCM. Z-scores for genes with deleterious variants in DCM patients and population frequency <1% (open circles, n = 68) or 0.1% (filled squares, n = 51) were obtained from ExAC. Scores were binned in 0.5 increments and plotted as number of genes in bin / total genes. Dotted vertical line is mean z-score of 1.3. B. Ratio of variant frequency in African / African American populations versus variant frequency across all populations. Data derived from ExAC, August, 2016 release. This Figure is reproduced in color in the online version of Medical Mycology.
Mutations in phosphoinositide-3-kinase pathway
An additional signaling pathway implicated in innate immunity is the PIK3CG (p110-γ) signal transduction occurring in response to chemokines. Three patients were identified with novel, heterozygous variants in PIK3CG (n = 1) and its regulatory subunit, PIK3R5 (n = 2). Western blot analysis of expanded T cells from the two PIK3R5 variant patients shows reduced PIK3R5 protein levels compared to loading controls, suggesting aberrant protein processing or stability as a result of the mutation.
Survey for additional genetic variants
Specific variants found in DCM patients were scored using CADD, an in silico measure of deleteriousness at either an amino acid or nucleotide level.40 CADD scores are ranked and scaled (CADD-Phred) to provide a reference across all changes in the genome with CADD-Phred score greater than 10 indicating a variant in the top 10% most deleterious variants in the genome, a score greater than 20, the top 1% of variants, while a score of 30 places the variant in the top 0.1%. The average CADD-Phred score of DCM variants is 24.1 placing them in the top 0.39% of possible variants.
While the variants identified in the IL-17, NFκB, innate sensing, and PI3K pathways require additional functional validation for their relationship to disseminated coccidioidomycosis, in silico analysis of those genes suggests that they are less tolerant to variation than many protein coding genes. Using the z-score metric from ExAC, an indication of observed versus expected numbers of amino acid substitutions, genes with variants identified in the DCM population had a higher mean z-score (1.33) than the mean of all genes in ExAC (0.10 calculated from Lek (Fig. 1A). In keeping with the observation that the dissemination rate is higher in African Americans, using the population data in ExAC, most of the genes identified in DCM patients had a higher proportion of variants in African and African American populations compared to the dataset as a whole (Fig. 1B).
Of the 58 DCM probands analyzed, 43 (78%) had variants that had a population frequency <1% and predicted damaging effects. More stringently, 39 (67%) had damaging variants with a frequency <0.1%. Demonstrated dominant or bi-allelic recessive mutations were identified in 13 probands (22%) including mutations in STAT1 (n = 2), STAT3 (n = 2), STAT4 (n = 3), IL12RB1 (n = 2), IL12RB2, IFNGR1, and PIK3R5 (n = 2). Whether these mutations contribute to dissemination or to primary infection or both needs to be established by comparing the variants identified in DCM patients to those from patients with isolated pulmonary Coccidioides infections.
Whether all the variants identified above turn out to be deleterious and responsible for DCM will take considerable work using bench biology and mouse modeling. However, the identification of numerous bona fide pathologic DNA variants in DCM confirms that there are critical genetic factors that underlie this serious infection. The challenges of identifying genetic susceptibility to a relatively virulent organism are different from identifying them for non-pathogenic organisms such as nontuberculous mycobacteria. Relatively minor defects are likely to allow predisposition to virulent organisms, whereas relatively major lesions are needed to disseminate a nonpathogen. The fascinating story of susceptibility to disseminated coccidioidomycosis is evolving rapidly and promises to teach us much about host-pathogen interactions and human genetics.
Progress toward a human vaccine against coccidioidomycosis Protective vaccine immunity against coccidioidomycosis
Patients recovering from coccidioidomycosis often develop lifelong protective immunity suggesting that intervention therapy against this fungal disease by vaccination is highly feasible.41,42 The successful development of an anti-Coccidioides vaccine relies on our understanding of host protective immunity, identification of microbial antigens and formulation with an adjuvant and delivery system.41,43 Early laboratory studies of host immune responses against coccidioidomycosis have reported IFN-γ production as a major correlate of vaccine-induced protection in mice.41,44–47 Recently we used a live attenuated vaccine (ΔT) strain with 2 disrupted chitinase genes (CTS2 and CTS3) to explore the nature of adaptive immunity in mice during the first 2 weeks of fungal infection following an intranasal challenge with Coccidioides spores.48 Both ΔT-vaccinated B6 and BALB/c mice could survive for a period over 50 days postchallenge. Profiles of cytokines detected in lung homogenates of ΔT-vaccinated mice were indicative of a mixed Th1, Th2, and Th17 immune response.26 Vaccination with ΔT was able to protect mice lacking receptors for IFN-γ or IL-4 against a pulmonary Coccidioides challenge to a degree equivalent to that of vaccinated wild-type counterparts. In contrast, mice lacking the IL-17 receptor had higher cfu in their lungs compared to wild-type mice.26,49 Collectively, these data suggest that vaccine-induced Th17 cells are essential for vaccine immunity against Coccidioides infection, and Th17 cells may work synergistically with Th1 cells to eliminate Coccidioides infection.26,43,49–51 Despite the apparent capacity of live, attenuated vaccines to elicit a highly protective immunity in mice, they may not be safe for individuals with underlying conditions of compromised cell-mediated immune systems.50,52 Generation of a subunit vaccine is an alternative strategy to develop a clinically safe vaccine against Coccidioides infection.
Multivalent antigens for developing a subunit vaccine
A dozen Coccidioides antigens have been identified and characterized in the past two decades. These spherule-expressed antigens include cell wall antigen 2 (Ag2/Pra), proline-rich protein 2 (Prp2), Coccidioides-specific antigen (Cs-Ag), proximal matrix protein 1 (Pmp1), urease (Ure), β-1,3-glucanosyltransferase (Gel1), aspartyl protease 1 (Pep1), α-mannosidase 1 (Amn1), and phospholipase B (Plb).46,53–59 Each of these antigens has been expressed using a bacterial or a yeast expression system and purified to homogeneity for evaluation as experimental vaccines against coccidioidomycosis. Protective efficacy of these vaccines were evaluated using a murine model of pulmonary coccidioidomycosis and all conferred significant protection at various degrees.46,53–59 Compelling evidence suggests that multivalent vaccines that contain 2 or more antigens are more potent against pulmonary Coccidioides infection than a vaccine containing a single antigen.53,55,57 Vaccination with a combination of rPep1, rPlb, and rAmn1 provided enhanced protection against pulmonary coccidioidomycosis, compared to immunization with each individual antigen.57 Mice vaccinated with a chimeric vaccine consisting of Ag2/Pra tandemly linked to Csa also showed better survival than littermates immunized with each antigen alone.53 Furthermore, the combination of Ag2/Pra with Prp2 also improved protective efficacy.55 Our recent efforts focus on the rational design of a multivalent vaccine that is composed of several promising coccidioidal antigens. Multivalent vaccines may stimulate a broader range of T-cell repertoire than single antigen and thereby may stimulate protection in the heterogeneous human population with many HLA variants.60,61 We have identified five peptides from Pep1, Amn1, and Plb using computational prediction of their ability to bind promiscuously to human major histocompatibility complex class II (MHC II) molecules.57,62 A recombinant epitope-based antigen (rEBV; GenBank no. JX103829) containing these five epitopes was created for developing a human vaccine.60,62 Although immunization with rEBV showed only moderate reduction in fungal burden in vaccinated mice, results of these studies provided insight into antigen design and formulation of an effective delivery system.60,61 Recently, we created a newly designed Coccidioides polypeptide antigen (rCpa1) that contains Ag2/Pra, Cs-Ag, Pmp1, and the five previously identified human epitopes (GenBank no. KY883768). This rCpa1 antigen can be processed and presented by both human and mouse MHC II molecules and provides vaccination mediated protection against pulmonary coccidioidal infection in a transgenic mouse expressing human HLA-DR4.63
Evaluation of vaccine immunity and efficacy using human HLA-DR transgenic mice
MHC II molecules are encoded by human leukocyte antigen (HLA) alleles and murine H2 gene clusters. Evaluation of human vaccines in rodent models solely based on conventional mouse strains may be problematic given the differences in the MHC II binding properties between murine and human antigen-presenting cells. To bridge the gap, a strain of human leukocyte antigen DR4 (HLA-DR4; DRB1*0401 allele) transgenic mice on B6 genetic background, expressing human MHC II molecules, has been used to evaluate vaccine immunity and efficacy.51,57,62,63 HLA-DR4 human haplotype occurs at high frequency amongst individuals of different racial backgrounds within North America.64 HLA-DR4 mice recognize T-cell-restricted epitopes and trigger T-cell responses similar to those observed in humans.65 We have shown previously that antigen presenting cells isolated from HLA-DR4 mice were able to present peptide antigens of Coccidioides to autologous CD4+ T cells and to activate them to produce IFN-γ and IL-17A.51,57,62,63 We compared protective efficacy of the ΔT vaccine in B6 mice and HLA-DR4 mice.51 Both B6 and HLA-DR4 mice that were vaccinate with the ΔT vaccine had significantly reduced fungal burden in their lungs and prolonged survival compared to the nonvaccinated corresponding strain of mice. While all vaccinated B6 mice could survive for a period of 50 days, vaccinated HLA-DR4 mice displayed three distinct manifestations of Coccidioides infection including 40% fatal acute, 30% disseminated and 30% pulmonary disease.51 It appears that HLA-DR4 mice are more susceptible to Coccidioides infection compared to B6 mice. While the potential mechanisms underlying the increased susceptibility of HLA-DR4 mice require further study, this strain of mice are useful for evaluation of vaccine efficacy and identification of immunological correlates against this mycosis.51
An innovative adjuvant and delivery platform for enhancing protective efficacy
Recombinant peptide antigens elicit a relatively weak immune response and require the use of adjuvants to induce robust and durable protective immunity.66 To develop a vaccine for clinical use, the success is critically depends on the choice of an appropriate adjuvant. Only a handful of adjuvants have been licensed for use in human vaccines (e.g., Alum, MF59, AS03, AS04) but numerous products are in the research pipeline.67 As discussed above, activation of Decin-1-Card9 and IL-1-MyD88 signaling pathways are essential for innate defense and stimulation of adaptive Th1 and Th17 response against Coccidioides infection. We postulate that immunostimulatory compounds, which can elicit a mixed Th1 and Th17 immunity, are attractive adjuvants for vaccine development against Coccidioides infections. Several types of purified, porous yeast cell-wall particles have been generated for vaccine development.68 Pure β-glucan particles (GPs) and glucan-mannan particles (GMPs) are derived from Saccharomyces cerevisiae, whereas glucan-chitin particles (GCPs) and glucan-chitin-mannan particles (GCMPs) are produced from Rhodotorula mucilaginosa, a non-pathogenic yeast.68 These particles are hollow and predominantly composed of β-1,3-glucan, which has been shown to stimulate both Th1 and Th17 immunity.69,70 Notably, these particles have shown to be safe in both preclinical and human trials.71 Vaccination of HLA-DR4 mice with rCpa1 encapsulated into GCP particles has shown to significantly reduce fungal burden compared to the same antigen formulated with GPs, GMPs, GCMPs and CpG-oligonucleotide adjuvants.63 We speculate that GCPs may interact with Dectin-1 and other pattern recognition receptors that synergistically activate Th1 and Th17 immunity. Studies are underway to delineate the mechanisms of Th1 and Th17 stimulation by the GCP adjuvant/delivery system in the vaccine development against coccidioidomycosis.
Reported studies to date have assumed that eliciting cell-mediated immunity will translate to protection against coccidioidomycosis. However, the role of innate and adaptive response to Coccidioides infection is still largely unexplored. Now we are beginning to understand the molecular mechanisms behind host immunity to pulmonary coccidioidomycosis, the genetics underlying host susceptibility to disseminated disease, and vaccine-induced immunity against Coccidioides infection. Most disseminated cases of coccidioidomycosis recorded in the United States occur in African Americans, patients with immunocompromising conditions, and individuals with genetic polymorphisms in certain genes involved in immune responses. Advancement of disease management of DCM and development of an effective coccidioidomycosis vaccine faces several major challenges. It may be possible to study murine immunity to Coccidioides infection and protect inbred mice with a vaccine that elicits cell-mediated immunity alone, but we cannot assume that these observations will translate to humans. A current research priority is to study similarities and differences between humans and mice in activation, response, and defense strategies of innate, adaptive immunity to coccidioidomycosis.
Presented at the International Coccidioidomycosis Symposium, Basic Science Session, Standard, California, August 12, 2017.
Funding
This work was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (APH, SMH), a research grant (R21 AI114762) to CYH from National Institute of Allergy and Infectious Diseases and VA Merit Review Funds to J.F. Additional support for Coccidioides research was donated by the Valley Fever of the Americas Foundation and community supporters.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and the writing of this manuscript.
References
- 1. Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011; 11: 275–288. [DOI] [PubMed] [Google Scholar]
- 2. Buchmann K. Evolution of innate immunity: clues from invertebrates via fish to mammals. Frontiers in Immunology. 2014; 5: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hung CY, Jimenez-Alzate Mdel P, Gonzalez A, Wuthrich M, Klein BS, Cole GT. Interleukin-1 receptor but not Toll-like receptor 2 is essential for MyD88-dependent Th17 immunity to Coccidioides infection. Infect Immun. 2014; 82: 2106–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viriyakosol S, Jimenez Mdel P, Gurney MA, Ashbaugh ME, Fierer J. Dectin-1 is required for resistance to coccidioidomycosis in mice. MBio. 2013; 4: e00597–00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Viriyakosol S, Fierer J, Brown GD, Kirkland TN. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect Immun. 2005; 73: 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang H, LeBert V, Hung CY et al. . C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America. J Immunol. 2014; 192: 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. del Pilar Jimenez AM, Viriyakosol S, Walls L et al. . Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of Dectin-1 (Clec7a). Genes Immun. 2008; 9: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Viriyakosol S, Jimenez Mdel P, Saijo S, Fierer J. Neither dectin-2 nor the mannose receptor is required for resistance to Coccidioides immitis in mice. Infect Immun. 2014; 82: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Awasthi S. Susceptibility of TLR4-defective C3H/HeJ mice to Coccidioides posadasii infection. Med Mycol. 2010; 48: 470–475. [DOI] [PubMed] [Google Scholar]
- 10. Fierer J, Waters C, Walls L. Both CD4+ and CD8+ T cells can mediate vaccine-induced protection against Coccidioides immitis infection in mice. J Infect Dis. 2006; 193: 1323–1331. [DOI] [PubMed] [Google Scholar]
- 11. Magee DM, Cox RA. Interleukin-12 regulation of host defenses against Coccidioides immitis. Infect Immun. 1996; 64: 3609–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cole GT, Hung CY, Sanderson SD et al. . Novel strategies to enhance vaccine immunity against coccidioidomycosis. PLoS Pathog. 2013; 9: e1003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang SC, Long AJ, Bennett F et al. . An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007; 179: 7791–7799. [DOI] [PubMed] [Google Scholar]
- 14. Odio CD, Marciano BE, Galgiani JN, Holland SM. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis. 2017; 23: 308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirkland TN, Fierer J. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect Immun. 1983; 40: 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cox RA, Kennell W, Boncyk L, Murphy JW. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect Immun. 1988; 56: 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fierer J, Walls L, Wright F, Kirkland TN. Genes influencing resistance to Coccidioides immitis and the interleukin-10 response map to chromosomes 4 and 6 in mice. Infect Immun. 1999; 67: 2916–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fierer J, Walls L, Eckmann L, Yamamoto T, Kirkland TN. Importance of interleukin-10 in genetic susceptibility of mice to Coccidioides immitis. Infect Immun. 1998; 66: 4397–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jimenez Mdel P, Walls L, Fierer J. High levels of interleukin-10 impair resistance to pulmonary coccidioidomycosis in mice in part through control of nitric oxide synthase 2 expression. Infect Immun. 2006; 74: 3387–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hung CY, Castro-Lopez N, Cole GT. Vaccinated C57BL/6 mice develop protective and memory T cell responses to Coccidioides posadasii infection in the absence of interleukin-10. Infect Immun. 2014; 82: 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012; 13: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heinsbroek SE, Taylor PR, Rosas M et al. . Expression of functionally different dectin-1 isoforms by murine macrophages. J Immunol. 2006; 176: 5513–5518. [DOI] [PubMed] [Google Scholar]
- 23. Hung CY, Castro-Lopez N, Cole GT. Card9- and MyD88-mediated gamma interferon and nitric oxide production is essential for resistance to subcutaneous Coccidioides posadasii infection. Infect Immun. 2016; 84: 1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LeibundGut-Landmann S, Gross O, Robinson MJ et al. . Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007; 8: 630–638. [DOI] [PubMed] [Google Scholar]
- 25. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013; 39: 1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hung CY, Gonzalez A, Wuthrich M, Klein BS, Cole GT. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect Immun. 2011; 79: 4511–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Viriyakosol S, Walls L, Okamoto S, Raz E, Williams DL, Fierer J. Myeloid differentiation factor 88 (MyD88) and IL-1R1 signaling contribute to resistance to Coccidioides immitis. Infect Immun. 2018; 86, doi: 10.1128/IAI.00028-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshimoto T, Takeda K, Tanaka T et al. . IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-g production. J Immunol. 1998; 161: 3400–3407. [PubMed] [Google Scholar]
- 29. Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2009; 49: e62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gringhuis SI, Kaptein TM, Wevers BA et al. . Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012; 13: 246–254. [DOI] [PubMed] [Google Scholar]
- 31. van de Veerdonk FL, Joosten LA, Shaw PJ et al. . The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol. 2011; 41: 2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seitz AE, Prevots DR, Holland SM. Hospitalizations associated with disseminated coccidioidomycosis, Arizona and California, USA. Emerg Infect Dis. 2012; 18: 1476–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Powers AE, Bender JM, Kumanovics A et al. . Coccidioides immitis meningitis in a patient with hyperimmunoglobulin E syndrome due to a novel mutation in signal transducer and activator of transcription. Pediatr Infect Dis J. 2009; 28: 664–666. [DOI] [PubMed] [Google Scholar]
- 34. Vinh DC, Schwartz B, Hsu AP et al. . Interleukin-12 receptor beta1 deficiency predisposing to disseminated coccidioidomycosis. Clin Infect Dis. 2011; 52: e99–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sampaio EP, Hsu AP, Pechacek J et al. . Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol. 2013; 131: 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lek M, Karczewski KJ, Minikel EV et al. . Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016; 536: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stelzer G, Plaschkes I, Oz-Levi D et al. . VarElect: the phenotype-based variation prioritizer of the GeneCards Suite. BMC Genomics. 2016; 17: 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Milner JD, Brenchley JM, Laurence A et al. . Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008; 452: 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu L, Okada S, Kong XF et al. . Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011; 208: 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014; 46: 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cole GT, Xue JM, Okeke CN et al. . A vaccine against coccidioidomycosis is justified and attainable. Med Mycol. 2004; 42: 189–216. [DOI] [PubMed] [Google Scholar]
- 42. Spinello IM, Munoz A, Johnson RH. Pulmonary coccidioidomycosis. Semin Respir Crit Care Med. 2008; 29: 166–173. [DOI] [PubMed] [Google Scholar]
- 43. Castro-Lopez N, Hung CY. Immune response to coccidioidomycosis and the development of a vaccine. Microorganisms. 2017; 5. doi: 10.3390/microorganisms5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cox RA, Magee DM. Coccidioidomycosis: host response and vaccine development. Clin Microbiol Rev. 2004; 17: 804–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xue J, Hung CY, Yu JJ, Cole GT. Immune response of vaccinated and non-vaccinated mice to Coccidioides posadasii infection. Vaccine. 2005; 23: 3535–3544. [DOI] [PubMed] [Google Scholar]
- 46. Li K, Yu JJ, Hung CY, Lehmann PF, Cole GT. Recombinant urease and urease DNA of Coccidioides immitis elicit an immunoprotective response against coccidioidomycosis in mice. Infect Immun. 2001; 69: 2878–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shubitz LF, Dial SM, Perrill R, Casement R, Galgiani JN. Vaccine-induced cellular immune responses differ from innate responses in susceptible and resistant strains of mice infected with Coccidioides posadasii. Infect Immun. 2008; 76: 5553–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009; 77: 3196–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wuthrich M, Hung CY, Gern BH et al. . A TCR transgenic mouse reactive with multiple systemic dimorphic fungi. J Immunol. 2011; 187: 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hung CY, Hurtgen BJ, Bellecourt M, Sanderson SD, Morgan EL, Cole GT. An agonist of human complement fragment C5a enhances vaccine immunity against Coccidioides infection. Vaccine. 2012; 30: 4681–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hurtgen BJ, Castro-Lopez N, Jimenez-Alzate MD, Cole GT, Hung CY. Preclinical identification of vaccine induced protective correlates in human leukocyte antigen expressing transgenic mice infected with Coccidioides posadasii. Vaccine. 2016; 34: 5336–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kirkland T. The quest for a vaccine against coccidioidomycosis: a neglected disease of the Americas. Journal of Fungi. 2016; 2: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shubitz LF, Yu JJ, Hung CY et al. . Improved protection of mice against lethal respiratory infection with Coccidioides posadasii using two recombinant antigens expressed as a single protein. Vaccine. 2006; 24: 5904–5911. [DOI] [PubMed] [Google Scholar]
- 54. Orsborn KI, Shubitz LF, Peng T et al. . Protein expression profiling of Coccidioides posadasii by two-dimensional differential in-gel electrophoresis and evaluation of a newly recognized peroxisomal matrix protein as a recombinant vaccine candidate. Infection and immunity. 2006; 74: 1865–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Herr RA, Hung CY, Cole GT. Evaluation of two homologous proline-rich proteins of Coccidioides posadasii as candidate vaccines against coccidioidomycosis. Infect Immun. 2007; 75: 5777–5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abuodeh RO, Shubitz LF, Siegel E et al. . Resistance to Coccidioides immitis in mice after immunization with recombinant protein or a DNA vaccine of a proline-rich antigen. Infect Immun. 1999; 67: 2935–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tarcha EJ, Basrur V, Hung CY, Gardner MJ, Cole GT. Multivalent recombinant protein vaccine against coccidioidomycosis. Infect Immun. 2006; 74: 5802–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Delgado N, Xue J, Yu JJ, Hung CY, Cole GT. A recombinant beta-1,3-glucanosyltransferase homolog of Coccidioides posadasii protects mice against coccidioidomycosis. Infect Immun. 2003; 71: 3010–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kirkland TN, Finley F, Orsborn KI, Galgiani JN. Evaluation of the proline-rich antigen of Coccidioides immitis as a vaccine candidate in mice. Infect Immun. 1998; 66: 3519–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hurtgen BJ, Hung CY. Rational design of T lymphocyte epitope-based vaccines against Coccidioides infection. Methods Mol Biol. 2017; 1625: 45–64. [DOI] [PubMed] [Google Scholar]
- 61. Cole GT, Hurtgen BJ, Hung CY. Pogress toward a human vaccine against coccidioidomycosis. Curr Fungal Infect Rep. 2012; 6: 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, Cole GT. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against coccidioidomycosis. Infect Immun. 2012; 80: 3960–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hung C-Y, Zhang H, Castro-Lopez N et al. . Glucan-chitin particles enhance Th17 response and improve protective efficacy of a multivalent antigen (rCpa1) against pulmonary Coccidioides posadasii infection. Infect Immun. 2018. doi: 10.1128/IAI.00070-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mori M, Beatty PG, Graves M, Boucher KM, Milford EL. HLA gene and haplotype frequencies in the North American population: the National Marrow Donor Program Donor Registry. Transplantation. 1997; 64: 1017–1027. [DOI] [PubMed] [Google Scholar]
- 65. Ito K, Bian HJ, Molina M et al. . HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J Exp Med. 1996; 183: 2635–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bonam SR, Partidos CD, Halmuthur SKM, Muller S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol Sci. 2017; 38: 771–793. [DOI] [PubMed] [Google Scholar]
- 67. De Gregorio E, Caproni E, Ulmer JB. Vaccine adjuvants: mode of action. Front Immunol. 2013; 4: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Young SH, Ostroff GR, Zeidler-Erdely PC, Roberts JR, Antonini JM, Castranova V. A comparison of the pulmonary inflammatory potential of different components of yeast cell wall. J Toxicol Environ Health A. 2007; 70: 1116–1124. [DOI] [PubMed] [Google Scholar]
- 69. Huang H, Ostroff GR, Lee CK et al. . Relative contributions of Dectin-1 and complement to immune responses to particulate b-glucans. J Immunol. 2012; 189: 312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Characterization and optimization of the glucan particle-based vaccine platform. Clin Vaccine Immunol. 2013; 20: 1585–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Weitberg AB. A phase I/II trial of b-(1,3)/(1,6) D-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J Exp Clin Cancer Res. 2008; 27: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]


