Abstract
The recorded history of coccidioidomycosis began in 1892 with the report of the illness of Domingo Escurra by Alejandro Posadas followed by a description of the first North American cases by Rixford and Gilchrist. Originally considered a protozoan, William Ophüls determined that Coccidioides was a fungus and that the lungs were the apparent initial site of infection. During the 1930s, both Gifford and Dickson determined that a self-limited illness, Valley Fever, was caused by the same fungus that caused the often fatal coccidioidal granuloma. Charles Smith, over a period of approximately 2 decades, comprehensively described the clinical and geographic epidemiology of coccidioidomycosis in California. Demosthenes Pappagianis continued this work after Smith's death. In 1957, one year before Marshall Fiese published his masterful monograph on coccidioidomycosis, the use of the first effective agent for the therapy of coccidioidomycosis, amphotericin B, was reported. This was followed by descriptions of its appropriate clinical use by William Winn and by Hans Einstein, among others. The development of the much less toxic azole antifungal agents greatly simplified therapy in many cases, but much of the management of patients with coccidioidomycosis still relies more on art than science. The search for the “Holy Grail” - a vaccine capable of preventing this disease-continues.
Keywords: Coccidioidomycosis, history, amphotericin, Valley Fever
“More than anything else, it is important to study history.”
B.B. King, 1925–2015
Part I. Discovery, epidemiology, and clinical description
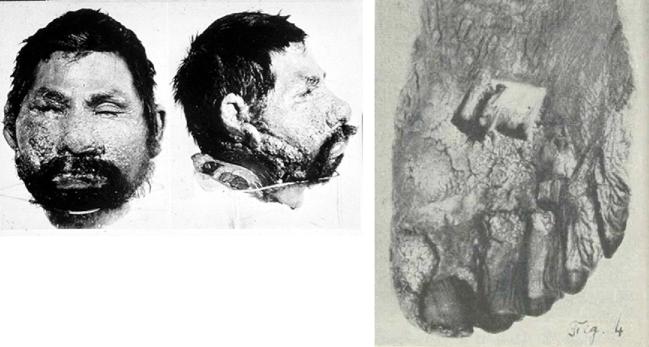
In 1948, Dr. Flavio Niño discovered a human head in a jar of formalin in the Pathology Museum of the Hospital of the University of Buenos Aires Medical School.1 The face of the decapitated head was disfigured by multiple, often confluent skin lesions (Fig. 1a),1 and Niño immediately recognized it as being that of a patient, Domingo Escurra, that had been reported by Posadas in 1892. A foot (Fig. 1b) and a hand with similar lesions, with spherules identified on microscopic examination, had been discovered the previous year by Dr. R. Sammartino in a jar in the museum of the Telémaco Susini Institute of Pathologic Anatomy and Physiology of the Faculty of Medical Sciences in Buenos Aires. Escurra's head remains on display as Exhibit No. 1 at the Institute of Parasitology in Buenos Aires.
Figure 1.
1a. Domingo Escurra. The head of Domingo Escurra was discovered in a jar of formalin by Dr. Flavio Niño in November 1948 in the Pathology Museum of the Hospital of the University of Buenos Aires Medical School. It remains on display as Exhibit No. 1 at the Institute of Parasitology.1 Permission: Creative Commons. 1b. The left foot of Domingo Escurra discovered in 1947 by Dr. R. Sammartino in the museum of the Telémaco Susini Institute of Pathologic Anatomy and Physiology of the Faculty of Medical Sciences in Buenos Aires.1 Permission: Creative Commons. This Figure is reproduced in color in the online version of Medical Mycology.
This is how Niño told the story of discovery, as translated from Spanish by our colleague, Dr. Shanthi Kappagoda: “On the 3rd of November 1948, looking at the museum pieces collected in the Cátedra [literally, “Chair,” here referring to a “professorial team”], my attention was drawn to a human head contained in a jar with no identifying information. Asking the staff of the Cátedra did not yield any further information. Careful examination of the specimen and the type of lesions it exhibited, immediately brought to mind the patient whom Alejandro Posadas painstakingly studied over a 7-year period and who was the subject of his doctoral thesis in Medicine…. Direct comparison of the head with the photographs Posadas published of his patient, converted my hunch into a near-certainty and histopathological examination of two small pieces taken from the most exuberant and striking lesions subsequently removed all doubt in my mind.”
“Last year, in a review and organization of the anatomic specimens in the museum of the Telémaco Susini Institute of Pathologic Anatomy and Physiology of the Faculty of Medical Sciences in Buenos Aires, Dr. R. Sammartino found a left foot and hand in a jar labeled ‘tuberous leprosy’ with macroscopic lesions matching those described by Posadas in his patient. Histologic study of the lesions allowed me to determine they were coccidioides granulomas and to identify the specimens as belonging— with high probability—to Posadas’ patient.”
Posadas’ patient was, of course, Domingo Escurra. In 1889, Escurra, a 33-year-old cavalryman in the pampas of the Chaco Frontier in northern Argentina, noted what he believed to be a spider bite on his cheek.2 This lesion worsened and additional ones appeared despite his application of tobacco and incisions with a penknife. Upon presentation to a military hospital, a diagnosis of lupus vulgaris was made, but, due to a lack of improvement despite local treatment with nitric acid, he was transferred to Rawson Hospital in San Juan, Argentina, where the diagnosis was changed to mycosis fungoides. Failing to respond to mercury and potassium iodide treatment, he was finally transferred to the University Hospital Clinics in Buenos Aires where he came under the care of an intern, Alejandro Posadas.
Posadas (Fig. 2),3 working under the supervision of Dr. Robert Wernicke, microscopically examined biopsies of Escurra's lesions, which by now had appeared on his extremities and trunk3,4 (Fig. 3).1 They observed multinucleated giant cells together with “psorospermiae” (i.e., protozoa) consisting of variably sized spherical sporangia, with granular contents within doubly refractile cell envelopes.4 Having accepted the diagnosis of mycosis fungoides that had been made by Dr. M. Bengolea at the Rawson Hospital, Posadas believed he had discovered its etiology. Escurra's disease was progressive and ultimately fatal—he died in 1898, 6 years after Posadas published his landmark description of the illness. Posadas, whose 1898 professional thesis,5 had dealt with the surgical treatment of tuberculosis, died of consumption in 1902 at just 31 years of age.6
Figure 2.

Alejandro Posadas. Posadas, whose father had emigrated from Spain, was born in Argentina in 1870. He attended the University of Buenos Aires Medical School and published the first description of coccidioidomycosis. He received his MD from the University of Buenos Aires Medical School two years later, in 1894, and subsequently died of consumption in 1902.3 Permission: Creative Commons.
Figure 3.
3a. Domingo Escurra as seen by Posadas. Frontal view demonstrating involvement of his face, trunk and arms.3 3b, 3c. Domingo Escurra as seen by Posadas. Extensive confluent verrucous lesions of coccidioidal granuloma involving the face.3 3d. Domingo Escurra as seen by Posadas. Large confluent verrucous lesion of his back.3 Permission: Creative Commons. This Figure is reproduced in color in the online version of Medical Mycology.
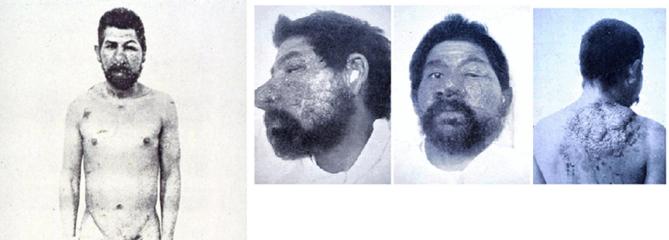
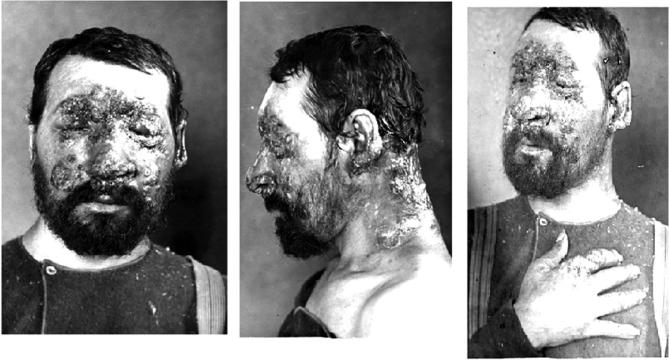
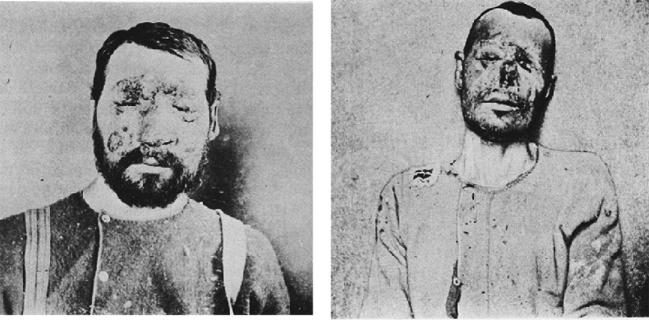
In April 1894, Emmett Rixford (Fig. 4),7 then Adjunct Professor of Surgery at Cooper Medical College (Fig. 5) (which was to become Stanford Medical School in 1912), together with W.S. Thorne, presented the case of an Azorean immigrant farm laborer, Joas Furtado Silverra, a patient at the City and County Hospital of San Francisco, to the 24th Annual Meeting of the Medical Society of California in San Jose.8,9 Rixford reported that microscopic examination of skin lesions revealed “large numbers of spherical protozoa” which he believed to be the probable cause of his disease. Rixford, collaborating with T.C. Gilchrist of Johns Hopkins School of Medicine, published their study of Silverra (Fig. 6)10 together with that of another farm laborer from the Azores, Texeira Pereira, who had been admitted to St. Mary's Hospital, also in San Francisco, in 1894.11 While Pereira (Fig. 7) had a rapid downhill course to death, Silverra, whose illness had begun 5 years before his 1893 hospitalization, had a more slowly progressive one that, nevertheless, had the same fatal outcome (Fig. 8).11
Figure 4.

Emmett Rixford, Professor of Surgery at Stanford Medical School. Rixford, with others, described the first North American cases of coccidioidomycosis.7 Rixford lived from 1865 to 1938. Permission: Creative Commons. This Figure is reproduced in color in the online version of Medical Mycology.
Figure 5.
Cooper Medical College circa 1892 at the corner of Sacramento and Webster in San Francisco. Cooper became the Stanford Medical School in 1912.12 Permission: Stanford Medical Alumni Association.
Figure 6.
Joas Furtado Silverra. The first reported case of coccidioidal granuloma in North America (and the second overall).10 6a. Joas Furtado Silverra. Facial lesions. Permission: Stanford Medical History Center. 6b. Joas Furtado Silverra. Facial and neck lesions. Permission: Stanford Medical History Center. 6c. Joas Furtado Silverra. Facial and hand lesions. Permission: Stanford Medical History Center.
Figure 7.
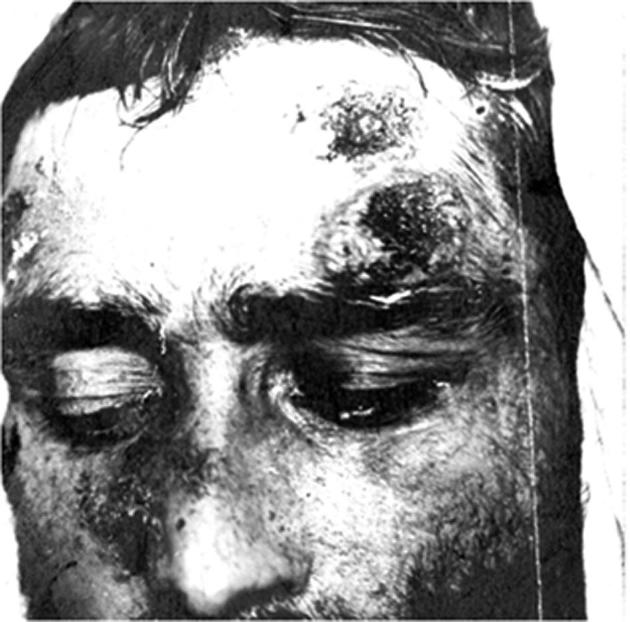
Texeira Pereira. North American patient with coccidioidal granuloma contemporaneous with Silverra.10 He was admitted to St. Mary's Hospital in San Francisco at the end of August, 1894, and died on September 21, 1894. Permission: Stanford Medical History Center.
Figure 8.
Joas Furtado Silverra. The photographs demonstrate progression of his infection (although it is possible that worsening of the skin lesions may, at least in part, have been the consequence of the therapies he received).11 8a. Joas Furtado Silverra. November 1893, 4 months after admission to the City and County Hospital of San Francisco. 8b. Joas Furtado Silverra. Later in his illness – June 26, 1894, 7 months before his death on January 31, 1895. Permission: Public domain.
Rixford and Gilchrist recognized that the organisms they were examining at appeared to be the same as those described by Posadas, and they agreed with him that they were protozoan. They placed the organism among the Sporozoa, and chose Coccidioides as the genus name because of the “coccidia-like” appearance. They named Silverra's organism Coccidioides immitis while Pereira's was called Coccidioides pyogenes, because of differences in their associated tissue inflammatory responses and clinical courses. Their strong belief that the organism was a protozoan is undoubtedly what led them to disregard the mould they repeatedly found growing on inoculated culture plates as contaminants.
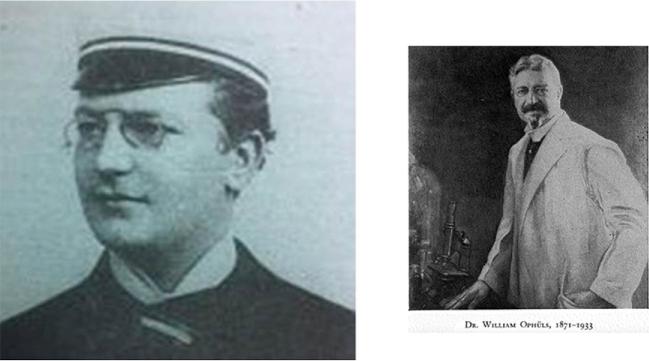
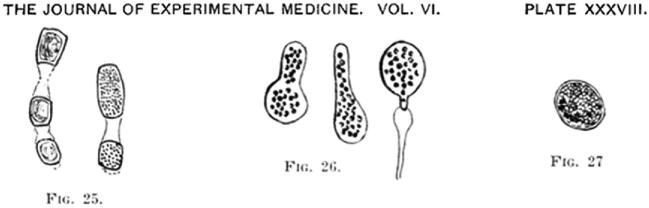
It was left to William Ophüls (Fig. 9),12 the first full-time salaried Professor of Pathology and Bacteriology at Cooper Medical College (and likely the only one with a dueling scar)13 together with Herbert Moffitt, to correctly classify the organism. Moffitt had shown Ophüls the “white mouldy growth” from pleural and splenic tissue of A.L., a 19-year old farm laborer (and, like Silverra and Pereira, an immigrant from the Azores) who died with widespread coccidioidal infection at the City and County Hospital of San Francisco on February 6, 1900, just 11 days after admission.15,16 The title of their presentation to the 30th Annual Session of the Medical Society of California in April 1900 in San Francisco was “a new pathogenic mould (formerly described as a protozoan: Coccidioides immitis, pyogenes).”15,16 In subsequent publications, Ophüls described additional work, including a description of the organism's various morphologies and life cycle, its airborne route of transmission, and the fulfillment of Koch's postulates (Fig. 10).17 He also proposed a name for the disease caused by this pathogenic mould: “coccidioidal granuloma.”
Figure 9.
William Ophüls. William Ophüls, who became the second Dean of Stanford Medical School, determined that Coccidioides was a fungus, not a protozoan, described its life cycle and, using guinea pig inoculation, fulfilled Koch's postulates. 9a. William Ophüls as a young man in Germany.14 Although of low resolution, his dueling scar appears to be visible on his cheek. 9b. William Ophüls as the 2nd Dean of the Stanford Medical School. Born in 1877, Ophüls died in 1933. Permission: Stanford Medical Alumni Association. This Figure is reproduced in color in the online version of Medical Mycology.
Figure 10.
Illustrations of the morphological forms of Coccidioides from Ophüls.17 Permission: Journal of Experimental Medicine.
Ophüls, who went on to become the second Dean of the Stanford Medical School, speculated that soil constituted an important reservoir for Coccidioides and this was confirmed in 1931 when the organism was recovered from soil under a bunkhouse in Delano, California, in which four infected farm laborers had slept.18 This, of course, was consistent with Ophüls' suggestion that infection occurred by the airborne route. The first demonstration of the ability to recover the fungus from air samples was not, however, reported until decades later.18a And if the soil is the reservoir for airborne transmission of the fungus, then animals that sniff soil should be frequently affected. And who sniffs soil more often than dogs? The first description of coccidioidomycosis in a dog (in Arizona), was not, however, published until 1940.19 Subsequent evidence indicates that the recognition of coccidioidal infections in dogs has sentinel value in defining areas of risk for human infection. Other animals also, of course, become infected. Coccidioides infection may often be found in pen-fed or confined cattle at the time of slaughter in Arizona but, in contrast to, for example, dogs and humans, it does not appear to be associated with progressive disease.19a The annals of the San Diego Zoo indicate that their first recorded animal death due to coccidioidomycosis was that of a tropical American monkey in 1936.20 Six years later, Mbongo, one of the Zoo's gorillas, died of coccidioidomycosis, 11 years after its arrival.20 It has more recently been recognized that, despite its soil reservoir, Coccidioides is surprisingly the most common cause of systemic mycotic infection among sea mammals along the California coast. These include southern sea otters and California sea lions found stranded along the state's central coast.21
Besides coccidioidal granuloma, there was another clinical illness plaguing residents of the San Joaquin Valley of California that was more common but whose etiology was unknown. This much more benign and common malady, that was called, among other things, San Joaquin Valley fever, was often accompanied by eosinophilia and, in many instances, erythema nodosum, in addition to respiratory symptoms. The presence of eosinophilia led to the hypothesis that this illness was caused by a parasitic infection. This motivated Myrnie Ada Gifford (Fig. 11),22 who had received her MD from Stanford and MPH from Johns Hopkins and in 1934 had become the Chief Assistant Health Officer of Kern County, California (in the heart of the endemic area in the San Joaquin Valley), to spend a portion of her first 17 months on the job fruitlessly trying to identify ascariasis as the cause of the illness. Her approach shifted when Coccidioides was recovered by guinea pig inoculation from the sputum of a patient with respiratory symptoms and erythema nodosum—that is, San Joaquin Valley fever.23 Gifford subsequently reported that she had presented this case, together with the fact that 3 of 15 patients with “coccidioides fungus infection” of the lungs had concomitant erythema nodosum, to Ernest Dickson in January 1936.24 Dickson (Fig. 12),25 a Stanford Professor of Public Health and Preventive Medicine, was visiting Bakersfield to obtain assistance in further investigating his observation that the occurrence of coccidioidal granuloma was often preceded by pneumonia. Gifford followed up her presentation by asking Dickson “if he thought there might be a relationship between San Joaquin Valley fever and the disease due to the Coccidioides fungus”, which was known to be the cause of coccidioidal granuloma.24 She went on in her report to say that: “He was immediately interested, as he recalled that their one laboratory case, Dr. C., had developed an unexplained erythema nodosum…”.
Figure 11.
Myrnie Ada Gifford. Gifford received her MD from Stanford in 1920 and, subsequently, an MPH from Johns Hopkins, and became Chief Assistant Health Officer of Kern County, California. She was key in the determination that San Joaquin Valley fever was caused by Coccidioides, the same fungus that caused coccidioidal granuloma. The first Coccidioidomycosis Symposium was held in her honor and a library in the Kern County Public Health Department was named after her. Gifford lived from 1892 to 1966. Permission: Bakersfield Observer, courtesy of Kern County Museum.
Figure 12.

Ernest C. Dickson. Dickson received his MD from the University of Toronto in 1907. Arriving at Stanford in 1908 as an assistant to Ophüls, he became Professor of Medicine at Stanford in 1923 and Professor of Public Health and Preventive Medicine in 1926 and was Chair of that department. He was internationally known for his work on botulism. With Gifford, Dickson provided evidence that San Joaquin Valley fever was caused by Coccidioides. He died in 1939. Permission: Kern County Health Department. http://kerncountyvalleyfever.com/history-of-valley-fever/#prettyPhoto. This Figure is reproduced in color in the online version of Medical Mycology.
“Dr. C.” was Harold Chope, a Stanford medical student working in Dickson's laboratory who had accidentally inhaled a “brownish cloud” of Coccidioides arthrospores (now arthroconidia) and became ill 9 days later. On October 8, 1929, 41 days after the laboratory accident, the San Francisco Examiner reported that this “Modern Hero” a “youthful research man, faces death in a local hospital because of a mysterious mould breathed in while making an important experiment.”25,26 Chope (Fig. 13)27 (who was ironically sent to Arizona to recuperate) did not, in fact, die until 1976 at the age of 72 years after a remarkable career in Public Health. The recognition that Chope's lengthy illness, which included pneumonia and the development of erythema nodosum with eventual spontaneous recovery, closely resembled descriptions of San Joaquin Valley fever had apparently remained fresh in Dickson's mind.
Figure 13.

Harold Chope. His self-limited illness consisting of pneumonia and erythema nodosum after accidental laboratory exposure to Coccidioides greatly contributed to the determination that San Joaquin Valley fever was caused by the fungus. Chope received his MD at Stanford in 1931 and went on to an MPH (1933) and PhD (1940), both from Harvard and became a Clinical Professor of Medicine at Stanford in 1955. He had a remarkable career in public health and in 1970 the name of the San Mateo County General Hospital was changed to the Harold C. Chope Community Hospital. The name subsequently reverted back to the original in 1989 (but was subsequently changed once again, this time to the San Mateo County Medical Center). Chope lived from 1904 to 1976. Permission: Open Access. NIH National Library of Medicine Digital Collections NLM Unique ID: 101411966 NLM Image ID: B04617. http://resource.nlm.nih.gov/101411966.
In May 1937, 16 months after Gifford had informed him of her findings, Dickson presented the case of Chope, together with an additional four similar cases, at the 66th Annual Session of the California Medical Association in Del Monte and, without acknowledging his conversation with Gifford, concluded that the cases offered “complete proof that the disease [i.e., San Joaquin Valley fever] is a primary acute manifestation of infection with coccidioides fungus.”28 Four months later, however, the follow-up publication of this presentation, while still not acknowledging the conversation with Gifford, had an addendum: “Since this paper was presented, my attention has been called to an item, under the heading “San Joaquin Fever,” by the County Health Officer of Kern County, Dr. Joe Smith of Bakersfield, in the annual report of the Kern County Health Department for the fiscal year 1935–1936…”.25 Thus, despite the clear chronological priority (as subsequently confirmed by Charles Smith), Gifford seems to have initially failed to contemporaneously receive acknowledgement for her seminal observations. Myrnie Gifford retired in 1954 after 20 years at the Kern County Public Health Department without ever having been promoted from her position of Chief Assistant Health Officer. In 1957, however, the first Coccidioidomycosis Symposium was held in her honor.29
All these observations were prologue to the subsequent remarkable accomplishments of Charles Smith, who received his MD from Stanford (where he had been a fraternity brother of Harold Chope) and his MPH from the University of Toronto,30 before returning to Stanford where Dickson had acquired funding for him. Smith (Fig. 14)29–31 was tasked with studying the epidemiology of coccidioidomycosis and, as a consequence, he became the wandering epidemiologist of Kern and Tulare counties, traveling in a Ford that was nicknamed the “Flying Chlamydospore.”31 Over 18 months, ending in May 1939, he saw >400 patients with erythema nodosum/multiforme and found that all reacted to skin testing with coccidioidin, a preparation derived from an extract of the mycelial phase of the fungus. He also went on to confirm an earlier observation by Gifford that most infections were asymptomatic as well as to establish the incubation period and demonstrate that skin test reactivity was associated with a favorable outcome. He defined endemic areas by studying coccidioidin skin test reactivity rates.
Figure 14.

Charles Smith. Smith, affectionately nicknamed Snuffy after the then popular cartoon “Barney Google and Snuffy Smith,” received his MD from Stanford in 1931 where he was Professor of Public Health and Preventive Medicine from 1948 to 1952, during which time he was also President of the California State Board of Health. He became Dean of the School of Public Health at the University of California at Berkeley in 1952. Smith defined the geographic and clinical epidemiology of coccidioidomycosis, among many other important contributions to our knowledge of the disease. Born in 1904, he died in 1967. Permission: Creative Commons. Permission: Open Access, U.S. Army Medical History Department Office of Medical History. http://history.amedd.army.mil/booksdocs/historiesofcomsn/section1.html.
Maddy subsequently defined such areas in Arizona and identified them as largely coextensive with the Lower Sonoran Life Zone ecological niche.32 Hugenholtz, working at Williams Air Force Base in Arizona, identified relationships between local weather conditions, particularly rainfall, dust, and temperature and the incidence of coccidioidomycosis.32a
Smith's further investigations established the frequency of complicated (including disseminated) infection and its association with certain ethnic groups. He developed serological means of diagnosis with the serendipitously discovered tube precipitin test which detected anti-coccidioidal immunoglobulin M (IgM) antibody and the complement fixing antibody (CFA) test that detected immunoglobulin G (IgG) antibody to antigens of the fungus. Smith also defined the time course of the rise and fall of these antibody titers and demonstrated the key clinically useful correlation between the height of the CFA titer and the extent of infection and risk of dissemination.33,34 In 1956 (also the year of the first meeting of the Coccidioidomycosis Study Group), at which time he was Dean of the School of Public Health at the University of California at Berkeley, Smith, along with Margaret Saito and Susan Simons reported the results of an astounding 39 500 serological tests.34 Smith died in 1967 and his colleague at Berkeley, Demosthenes Pappagianis (Fig. 15),33 (also a Stanford Medical School graduate but with a PhD from UC Berkeley), continued the work at the University of California at Davis Medical School, where he became Chairman of the Department of Microbiology. He was also appointed Medical Director of the Coccidioidomycosis Serology Laboratory, which has remained central to the diagnosis and management of patients (animal, as well as human) with coccidioidomycosis throughout the country, providing personalized test interpretation and other relevant information to clinicians.
Figure 15.
Demosthenes Pappagianis – back row, center. The photograph, taken at the last meeting of the US Army Commission on Acute Respiratory Diseases in 1972, includes (clockwise from Pappagianis) Ted Eickhoff, Maxwell Finland, Jay Sanford, and Harry Feldman. Pappagianis received an MD from Stanford and a PhD from the University of California, Berkeley. He worked with Smith and took over the coccidioidomycosis activities upon Smith's death in 1967 moving to the University of California at Davis, where, among other things, he became Chair of the Department of Microbiology and Medical Director of the Coccidioidomycosis Serology Group Laboratory. In the latter capacity, he has been invaluable to clinicians by his ready availability to them, providing insightful advice regarding test interpretation and sharing his vast knowledge about coccidioidomycosis. Permission: Open Access, US Army Medical History Department, Office of Medical History, http://history.amedd.army.mil/booksdocs/historiesofcomsn/section1.html.
The years since then have provided additional challenges and advances. Epidemiological events of interest included the identification of additional endemic foci quite distant from those previously defined, such as areas of northeastern Utah35 and southeastern Washington State.36 In December 1977, a massive dust storm, the “Tempest from Tehachapi,” originating in the southern San Joaquin Valley made its way northward and westward, resulting in coccidioidal infections being acquired in the San Francisco Bay area37—including by a gorilla at the San Francisco Zoo who died of coccidioidomycosis,38 echoing the fate of Mbongo of the San Diego Zoo some 35 years earlier. The dust clouds generated in the surrounding terrain by strong aftershocks and resultant landslides following the 6.7 magnitude Northridge earthquake near Los Angeles on January 17, 1994, resulted in an outbreak of coccidioidomycosis, including in Simi Valley, which had not been previously been recognized as an endemic area.39,40 The recent recognition of the high rate of coccidioidomycosis in prisoners in the California Central Valley41 created both a medical and a political disturbance—but one that should have been anticipated from experiences during and after World War II. The stationing of military personnel in endemic areas of Arizona and California and the internment of Japanese at sites along the Gila River in Arizona at that time led to frequent infections among these coccidioidal naïve populations.42 Also affected were German prisoners of war in Florence, Arizona, and in California.42
Noteworthy were the reviews of the known geographic extent of coccidioidomycosis in Mexico by Gonzalez-Ochoa and in the Americas in general by Negroni.43,44 Epidemiologists and clinicians have been confronted with an increased incidence of coccidioidomycosis.45 They have been further tested by the need to manage an increasing number of cases in immunocompromised patients,46 including those with acquired immunodeficiency syndrome as well as in hematopoietic stem cell and solid organ transplant recipients.47,48
Other events include the recent renewed commercial availability of a reformulated spherule-derived skin test preparation (no skin test reagent had been available since 1999)49 and of a coccidioidal antigen test.50 Finally, Fisher and colleagues discovered that Rixford had, in a sense, been correct when he decided a century earlier that there was a second coccidioidal species to accompany Coccidioides immitis—it just was not “Coccidioides pyogenes.” The distinction between species has nothing to do with the clinical picture or histopathological response, but rather to genetic polymorphisms. The newly designated species was named in recognition of the physician who first reported the disease – Coccidioides posadasii.51 Work utilizing molecular biological and genetic methodologies continues to expand, allowing improved understanding of the life cycle, biology, and population structure of Coccidioides, as well as the immune response to the fungus.
The knowledge that surviving infection due to Coccidioides generally provides life-long protection from clinically apparent illness due to subsequent exogenous infection makes coccidioidomycosis a potential target for vaccine development. Attempts to date, however, have proven unsuccessful, but possible new paths forward have been proposed and are under exploration.52
Part II. Treatment
When Domingo Escurra's head was discovered in 1948, the chemotherapy of coccidioidomycosis was no more advanced, although perhaps less brutal, than it was when he had first presented for medical care more than a half-century earlier. Escurra had unsuccessfully self-treated his lesions with topical applications of tobacco as well as incisions.2 The treatment of Joas Furtado Silverra included topical and intralesional methyl violet, iodine, and bromine, all without result, aside from the infliction of pain and, probably, further disfigurement.10,11 In fact, his treatment with intralesional bromine was discontinued because “injections were attended with so much suffering, notwithstanding the previous use of cocaine, that the attempt to continue them was abandoned.” On March 17, 1894, under chloroform anesthesia, “all the affected areas … were most thoroughly scraped with a sharp spoon. The raw surfaces were then vigorously scrubbed with a bristle brush and an abundance of bichlorite solution … was applied.”
In 1931, the California Department of Public Health summarized the status of the treatment of coccidioidomycosis:53
“Some … have reported apparent benefit from” X-ray therapy for superficial lesions.
Vaccines were used “with, at best, very doubtful benefit.”
Iodides, thymol, volatile oils, salvarsan and other arsenicals “have not proved of any value.”
Intramuscular colloidal copper, intravenous antimony potassium tartrate gave “more encouraging” reports.
Amputation was the only curative intervention—but obviously only applicable to disease localized to an extremity.
In 1942, Charles Smith was asked to provide recommendations for the treatment of John Fulton, the Sterling Professor and Chair of the Department of Physiology at Yale University who developed severe pulmonary and disseminated coccidioidomycosis (possibly acquired during a visit to Smith's Stanford laboratory).54 He suggested the following:
Artificial pneumothorax.
(Continuation of) sulfa administration.
Trial of coccidioidin as a therapeutic vaccine.
Thymol injections into an abdominal wall abscess.
Smith was, at a minimum, undoubtedly skeptical that following his own advice would produce favorable results for Fulton. In fact, Smith's image later appeared in a 1949 Saturday Evening Post article entitled “The Mystery of the Deadly Dust” and which contained the following caption: “Thus far, no drug or vaccine has proved effective in prevention or cure.”55
Marshall Fiese, in a 1954 presentation at the Stanford Medical Alumni Day Program, provided the following assessment:56
Nystatin is active in an animal model, but is toxic.
Cycloheximide is reported to be “of value,” but its activity is “feeble.”
Prodigiosin is reported to be “lifesaving” in disseminated infection, but “seems not to be the answer.”
Stilbamidines are associated with “isolated reports of apparent cures,” but no series were reported.
Ethyl vanillate is “perhaps the most generally useful drug at the present time … but not the final answer” but it “at least … demonstrates that disseminated coccidioidomycosis, which has heretofore mocked our best efforts, is not necessarily incurable.”
A year later, ethyl vanillate, a byproduct in the production of paper pulp, received a rousing endorsement from Dr. Robert Cohen who stated “I have used ethyl vanillate since 1951 and it has not let me down.”57 He went on to describe its preparation for therapeutic use: “for two years the Wisconsin Institute for Paper Chemistry sent me bulk quantities of ethyl vanilla, which I melted on my wife's kitchen stove and poured into thousands of gelatin capsules.”
Just a few years after his presentation to the Stanford Medical Alumni, Fiese, shaking off the final remnants of a widely shared therapeutic illusion (a common affliction of clinicians to this day), revised his earlier assessment,58 “an honest appraisal of 60 years of various forms of treatment…” compelled him to make these astute observations (among a total of 5):
“The course of acute, fulminating, disseminated coccidioidomycosis has never been altered by any form of therapy, past or present.”
“Dramatic improvement of chronic, disseminated lesions has coincided with treatment by several methods and with many drugs. It has also occurred with no treatment whatsoever.”
Fiese went on to publish a remarkably comprehensive monograph in 1958 that proved to be a tour de force, summarizing all that was known about coccidioidomycosis at the time.59 He unfortunately tragically died 2 years later when the passenger train in which he was traveling (the San Francisco Chief) collided with a fuel truck near Bakersfield, California.60
Littman and colleagues, also in 1958,61 summed up the then current state of therapeutics—using the phrase “clinical trials” very loosely: “Unsuccessful therapeutic agents which have received clinical trials include heavy metals, ethyl vanillate, gentian violet, sodium caprylate, stilbamidine, 2-hydroxystilbamidine, methyltestosterone, diethylstilbestrol, isonicotinic acid hydrazide, para-aminosalicylic acid, nitrogen mustard, potassium iodide, sulfonamides, the antibiotics [sic], penicillin, streptomycin, oxy-tetracycline, chlortetracycline, prodigiosin, protoanemonin, fradicin, thiolutin, rimocidin, actidione and nystatin, roentgen therapy, culture filtrates and extracts of C. immitis and immune human serum.”
While Fiese's therapeutic nihilism was a rational assessment of the situation at the time, things were about to change for the better. In January 1953, Streptomyces nodosum had been recovered from culture M 475 obtained from the soil of the Orinoco River Valley in Venezuela and was subsequently found to secrete 2 molecules with antifungal activity that were given the names amphotericin A and B. The latter proved more suitable for further development by Squibb Laboratories,62 which had previously developed Brown and Hazen's Nystatin. Amphotericin B was quickly demonstrated to be effective in the treatment of Coccidioides infection in a murine model,63 and its first intravenous administration to humans was reported in 1957.63,64 Prior to this, however, it had been given by the oral route with, at least in one case, some apparent degree of efficacy. Fiese had initiated the administration of 800 mg of amphotericin B by mouth every 8 hours in a patient with chronic disfiguring coccidioidal granuloma of the face on February 24, 1956, with subsequent clinical improvement that he documented with serial photographs.65 In his discussion, however, Fiese was extremely apologetic for reporting this single case, acknowledging the prior history of enthusiasm for what ultimately proved to be ineffective therapies of this disease.
Therapeutic investigations by Hans Einstein (Fig. 16) and William Winn (Fig. 17), each working separately in the San Joaquin Valley, quickly led to the determination of the basics of administration of amphotericin B and indications for its use in patients with coccidioidomycosis, which were comprehensively summarized by 1963.66 Importantly, this included the necessity of intrathecal administration of the drug when used for the treatment of coccidioidal meningitis, although the subsequent development of azole antifungals has significantly reduced this need.
Figure 16.

Hans Einstein. Einstein, a distant relative of Albert (his grandfather was a first cousin), spent most of his life as a physician in Bakersfield, California. He and another physician, William Winn, in Springville, California, were crucial in defining the clinical course of coccidioidomycosis and defining the use of amphotericin B in its treatment. His font of knowledge led to continual requests for consultative advice by physicians dealing with the disease. Born on February 3, 1923, Einstein died at age 89 on August 11, 2012. Permission: the Bakersfield Observer. This Figure is reproduced in color in the online version of Medical Mycology.
Figure 17.

William A. Winn. Winn was a personal friend of Charles Smith. He received his MD from Harvard after obtaining his undergraduate degree at Stanford. From 1935 to 1966, Winn was the Medical Director of the Springville County Hospital, which had been founded as the Tulare County and Kings County Joint Tuberculosis Sanitarium in 1918. Both Einstein and Winn realized that many patients they encountered who had received a diagnosis of tuberculosis had, instead, coccidioidomycosis. Winn delineated many of the clinical aspects of coccidioidomycosis and the appropriate use of amphotericin B, including its intrathecal administration for the treatment of coccidioidal meningitis. He was born on September 22, 1903, in Butte, Montana and died in 1967. Courtesy of William R. Winn. This Figure is reproduced in color in the online version of Medical Mycology.
The toxicities of amphotericin B unfortunately often necessitated limitations to its use, making the development of antifungal azole drugs a welcome advance. The first of the azoles, miconazole, however, required intravenous administration and was toxic, leading to its abandonment for systemic use. The approval of ketoconazole, an orally bioavailable azole, in 1981 was an important initial step forward, but it was subsequently supplanted by fluconazole and, later, itraconazole as well. Among other things, these drugs often proved effective in the treatment of coccidioidal meningitis, reducing the necessity intrathecal amphotericin B administration. The newer triazoles (voriconazole, posaconazole, isavuconazonium) provide additional alternatives, but their precise roles remain largely undetermined. In addition, the development of lipid-associated amphotericin B products has improved the systemic tolerability of this polyene antifungal, which is believed to remain the most potent agent available in the treatment of coccidioidomycosis.
Despite these advances, it can be argued that the treatment of coccidioidomycosis remains more art than science and that, despite the work of the Mycoses Study Group and others, progress has proceeded at a glacial pace. Among all the recommendations in the 2000 Infectious Diseases Society of America guideline for the treatment of coccidioidomycosis, none were rated as A-I (good strength of evidence based on at least one randomized controlled trial).67 The 2016 revision of these guidelines switched to the GRADE rating system, but the results were the same—of the 61 recommendations, 54 were judged as strong— but none were based on high-level evidence!68 This knowledge deficit can only be resolved through organizations such as the Coccidioidomycosis Center for Excellence and the Mycoses Study Group fostering collaborative efforts of clinicians in designing and implementing multicenter clinical trials—and with funding agencies to provide the necessary resources to advance therapeutics and vaccine development.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper. The authors wish to acknowledge Dr. Shanthi Kapagoda for her insightful translation.
References
- 1. Niño FL. Finding of an anatomical specimen; its importance in the history of psorospermosis or coccidioidal granuloma. Bol Univ B Aires. 1950; 26: 3–14. [in Spanish: ]. [PubMed] [Google Scholar]
- 2. Posadas A. Un nuovo caso de micosis fungoides con psorospermias. Anales del Circulo médico Argentino, Buenos Aires. 1892; 15: 585–597. [in Spanish]. [Google Scholar]
- 3. Negroni R. Historia del descubrimiento de la coccidioidomicosis. Rev Argent Dermatol. 2011; 92: n.3 [cited 2017-12-28], 0–0. Available at: http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1851-300X2011000300001&lng=en&nrm=iso> ISSN 1851-300X. [in Spanish]. [Google Scholar]
- 4. Wernicke R. Uber einen protozoen befund bei Mycosis fungoides. Zentrabl B aketeroll. 1892; 12: 859–861. [in German]. [Google Scholar]
- 5. Posadas A. Toracoplastia Temporaria y Parcial Para La Extirpación De Los Quistes Hidáticos Del Pulmón. Tesis, Universidad de Buenos Aires Facultad de Medicina; Imprenta, Litografía y Encuadernación de J. Peuser 1898. [in Spanish]. [Google Scholar]
- 6. Esteva H. Alejandro Posadas, Argentinian pioneer: thoracic surgery in the Western world in his time. Ann Thorac Surg. 2004; 78: 741–745. [DOI] [PubMed] [Google Scholar]
- 7. Eloesser L. Emmett Rixford 1865–1938. Ann Surg. 1939; 110: 786–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rixford E. A case of protozoic skin disease. Occid Med Times. 1894; 8:704–707. [Google Scholar]
- 9. Thorne WS. A case of protozoic skin disease. OccidMed Times. 1894; 8: 703–704. [Google Scholar]
- 10. Rixford E. Society Proceedings. Occid Med Times. May 1894. [Google Scholar]
- 11. Rixford E, Gilchrist TC. Two cases of protozoan (coccidioidal) infection of the skin and other organs. Johns Hopkins Hosp Rep. 1896; 1: 209–268. [Google Scholar]
- 12. Barkan H. Cooper Medical College, Founded by Levi Cooper Lane. An Historical Sketch. Stanford Medical Bulletin. 1954; 12: gd145–184. [PubMed] [Google Scholar]
- 13. Death of Dean Ophüls http://lane.stanford.edu/med-history/wilson/chap35.html.
- 14. Welsh O, Vera-Cabrera L, Rendon A, Gonzalez G, Bonifaz A. Coccidioidomycosis. Clin Dermatol. 2012; 30: 573–591. [DOI] [PubMed] [Google Scholar]
- 15. Ophüls W, Moffitt HC. A new pathogenic mould (formerly described as a protozoan: Coccidioides immitis pyogenes). Preliminary report. Transactions of the Medical Society of California 30th Annual Session, San Francisco, April1900; 30: 29–35. [Google Scholar]
- 16. Ophüls W, Moffitt HC. A new pathogenic mould (formerly described as a protozoan: Coccidioides immitis pyogenes). Preliminary report. Philadelphia Med J. 1900; 5: 1471–1472. [Google Scholar]
- 17. Ophüls W. Further observations on a pathogenic mould (formerly described as a protozoan: Coccidioides immitis pyogenes). J Exp Med. 1905; 6: 443–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stewart RA, Meyer KF. Isolation of Coccidioides immitis from soil. Proc Soc Exp Biol Med. 1932; 29: 937–938. [Google Scholar]
- 18a. Ajello L, Maddy K, Crecelius G, Hugenholtz PG, Hall LB. Recovery of Coccidioides immitis from the air. Sabouraudia. 1965; 4: 92–95. [PubMed] [Google Scholar]
- 19. Farness OJ. Coccidioidal infection in a dog. J Am Vet Med Assn. 1940; 97: 63. [Google Scholar]
- 19a. Maddy KT. Coccidioidomycosis of cattle in the Southwestern United States. J Am Vet Med Assoc. 1954; 124: 456–464. [PubMed] [Google Scholar]
- 20. San Diego Zoo Global History Timeline. http://library.sandiegozoo.org/history.htm#1940.
- 21. Huckabone SE, Gulland FM, Johnson SM et al. Coccidioidomycosis and other systemic mycoses of marine mammals stranding along the central California, USA coast: 1998–2012. J Wildl Dis. 2015; 51: 295–308. [DOI] [PubMed] [Google Scholar]
- 22. History: Don't forget these women who helped shape Kern County. Bakersfield.com http://www.bakersfield.com/bakersfield_life/history-don-t-forget-these-women-who-helped-shape-kern/article_9819d1c1-bb1f-5bab-9911-12213b36f84f.html.
- 23. Gifford MA. Annual Report of the Kern County (Calif.) Health Department for the Fiscal; Year, July 1, 1935 to June 30, 1936. [Google Scholar]
- 24. Gifford MA. Coccidioidomycosis, Kern County. Session on Fungal Infections, Sixth Pacific Science Congress of the Pacific Science Association. August 2, 1939; Reprinted from Kern County Department of Public Health, Annual Report for the Fiscal Year July 1, 1938 to June 30, 1939. [Google Scholar]
- 25. Dickson EC. “Valley Fever” of the San Joaquin Valley and fungus Coccidioides. Cal West Med. 1937; 47: 151–155. [PMC free article] [PubMed] [Google Scholar]
- 26. Modern Hero. San Francisco Examiner. December 2, 1929.
- 27. Harold D. Chope. NIH National Library of Medicine Digital Collections. NLM Unique ID: 101411966. NLM Image ID: B04617 http://resource.nlm.nih.gov/101411966. [Google Scholar]
- 28. Dickson EC. “Valley Fever” of the San Joaquin Valley and fungus Coccidioides. Read before the Pathology and Bacteriology Section of the California Medical Association at the 66th annual session, Del Monte, May 2–6, 1937.
- 29. Proceedings of the Symposium on Coccidioidomycosis. 11–13 February 1957, Phoenix AZ, Washington DC: Public Health Service; 168–170. [Google Scholar]
- 30. Huntington RW. Four great coccidioidomycologists: William Ophüls (1871–1933), Myrnie Gifford (1892–1966), and Charles Edward Smith (1904–1967) and William A. Winn (1903–1967). Sabouraudia. 1985; 23: 361–370. [PubMed] [Google Scholar]
- 31. Smith C. Reminiscences of the flying chlamydospore and its allies. In: Ajello L. (ed). Coccidioidomycosis, Tucson: Arizona University Press, 1967: xiii–xxii. [Google Scholar]
- 32. Maddy KT. The geographic distribution of coccidioides immitis and possible ecologic implications. Ariz Med. 1958; 15:178–188. [PubMed] [Google Scholar]
- 32a. Hugenholtz P. Climate and coccidioidomycosis. In: Proceedings of the Symposium on Coccidioidomycosis, Phoenix, Arizona. Publication 575. Washington, DC: U.S Public Health Services, 1957; 136–143. [Google Scholar]
- 33. Commission on Acute Respiratory Diseases. Coccidioidomycosis. http://history.amedd.army.mil/booksdocs/historiesofcomsn/section1.html.
- 34. Smith C, Saito M, Simons S. Patterns of 39,500 serological tests in coccidioidomycosis. JAMA. 1956; 160: 546–552. [DOI] [PubMed] [Google Scholar]
- 35. Petersen LR, Marshall SL, Barton-Dickson C et al. Coccidioidomycosis among workers at an archeological site, northeastern Utah. Emerg Infect Dis. 2004; 10: 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marsden-Haug N, Goldoft M, Ralston C et al. Coccidioidomycosis acquired in Washington State. Clin Infect Dis. 2013; 56: 847–850. [DOI] [PubMed] [Google Scholar]
- 37. Flynn NM, Hoeprich PD, Kawachi MM et al. An unusual outbreak of windborne coccidioidomycosis. N Engl J Med. 1979; 301: 358–361. [DOI] [PubMed] [Google Scholar]
- 38. Schneider E, Hajjeh RA, Spiegel RA et al. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA. 1997; 277: 904–908. [PubMed] [Google Scholar]
- 39. MacLean ML. The epidemiology of coccidioidomycosis: 15 California Counties, 2007–2011. http://vfce.arizona.edu/sites/vfce/files/the_epidemiology_of_coccidioidomycosis_collaborative_county_report.pdf.
- 40. Morain D. Valley Fever outbreak hits record level. Los Angeles Times. Jan 30, 1992. http://articles.latimes.com/1992-01-30/news/mn-1424_1_san-joaquin-valley-fever.
- 41. Wheeler C, Lucas KD, Mohle-Boetani JC. Rates and risk factors for Coccidioidomycosis among prison inmates, California, USA, 2011. Emerg Infect Dis. 2015; 21: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith CE, Coates JB, Hoff EC, Hoff MA. Coccidioidomycosis. Preventive Medicine in World War II: Communicable Diseases Transmitted Chiefly through Respiratory and Alimentary Tracts, vol. 4 Washington, DC: Office of the Surgeon General, Department of the Army, 1958: 285–316. [Google Scholar]
- 43. Gonzalez-Ochoa A. Coccidioidomycosis in Mexico. In Ajello L, ed. Coccidioidomycosis. Tucson: University of Arizona Press, 1967: 293–299. [Google Scholar]
- 44. Negroni R. Historical evolution of some clinical and epidemiological knowledge of coccidioidomycosis in the Americas. Rev Argent Microbiol. 2008; 40: 246–256. [Spanish: ]. [PubMed] [Google Scholar]
- 45. Cooksey GS, Nguyen A, Knutson K et al. Notes from the Field: Increase in Coccidioidomycosis - California, 2016. MMWR Morb Mortal Wkly Rep. 2017; 66: 833–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deresinski SC, Stevens DA. Coccidioidomycosis in compromised hosts. Experience at Stanford University Hospital. Medicine (Baltimore). 1975; 54: 377–395. [DOI] [PubMed] [Google Scholar]
- 47. Ampel NM. Coccidioidomycosis in persons infected with HIV type 1. Clin Infect Dis. 2005; 41: 1174–1178. [DOI] [PubMed] [Google Scholar]
- 48. Blair JE. Coccidioidomycosis in patients who have undergone transplantation. Ann N Y Acad Sci. 2007; 1111: 365–376. [DOI] [PubMed] [Google Scholar]
- 49. Wack EE, Ampel NM, Sunenshine RH, Galgiani JN. The return of delayed-type hypersensitivity skin testing for coccidioidomycosis. Clin Infect Dis. 2015; 61: 787–791. [DOI] [PubMed] [Google Scholar]
- 50. Bamberger DM, Pepito BS, Proia LA et al. Cerebrospinal fluid coccidioides antigen testing in the diagnosis and management of central nervous system coccidioidomycosis. Mycoses. 2015; 58: 598–602. [DOI] [PubMed] [Google Scholar]
- 51. Fisher MC, Koenig GL, White TJ, Taylor JW. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia. 2002; 94: 73–84. [PubMed] [Google Scholar]
- 52. Kirkland TN. The quest for a vaccine against coccidioidomycosis: a neglected disease of the Americas. J Fungi (Basel). 2016; 2: 34; 10.3390/jof2040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Coccidioidal granuloma. State of California Dept Publ Health. Special Bulletin No. 57, 1931. [Google Scholar]
- 54. Tager M. Fulton, coccidioidomycosis, and penicillin. Yale J Biol Med. 1976; 49: 391–398. [PMC free article] [PubMed] [Google Scholar]
- 55. Silverman M. The mystery of the deadly dust. Saturday Evening Post, December 17, 1949. [Google Scholar]
- 56. Fiese M. Recent experiences in the treatment of disseminated coccidioidomycosis. Stanford Med Bull. 1955; 13: 91–97. Presented Nov 19, 1954, at the Stanford Medical Alumni Day Program. [PubMed] [Google Scholar]
- 57. Cohen R. Proc Internat Symp on therapy of fungus infections, Univ Calif. June 23–25, 1955. [Google Scholar]
- 58. Fiese MJ. Therapy of coccidioidomycosis. In Arizona State Department of Public Health, Proceedings of the Symposium on Coccidioidomycosis. Phoenix, Arizona: February 11–13, 1957; Public Health Service publication; 575, 79–82. [Google Scholar]
- 59. Fiese MJ. Coccidioidomycosis. Springfield, IL: Charles C. Thomas; 1958. [Google Scholar]
- 60. 40 Hurt In fiery Bakersfield collision. Madera Tribune. Number 207. 2 March 1960.
- 61. Littman ML, Horowitz PL, Swadey JG. Coccidioidomycosis and its treatment with amphotericin B. Am J Med. 1958; 24: 568–592. [DOI] [PubMed] [Google Scholar]
- 62. Gold W, Stout HA, Pagano JF, Donovick R. Amphotericins A and B, antifungal antibiotics produced by a streptomycete. I. In vitro studies. In: Antibiotics Annual, 1955–1956, New York: Med Encyclopedia, Inc, 1956: 579–586. [PubMed] [Google Scholar]
- 63. Halde C, Newcomer VD, Wright ET, Sternberg TH. An evaluation of amphotericin B in vitro and in vivo in mice against Coccidioides immitis and Candida albicans, and preliminary observations concerning the administration of amphotericin B to man. J Invest Dermatol. 1957; 28: 217–231. [DOI] [PubMed] [Google Scholar]
- 64. Littman ML. Preliminary observations on the intravenous use of amphotericin B, an antifungal antibiotic, in the therapy of acute and chronic coccidioidal osteomyelitis. Conference on coccidioidomycosis. U S Dept. Health, Education and Welfare and Arizona State Health Department, Phoenix, Arizona, February 1–3, 1957.
- 65. Fiese MJ. Treatment of disseminated coccidioidomycosis with amphotericin B; report of a case. Calif Med. 1957; 86: 119–120. [PMC free article] [PubMed] [Google Scholar]
- 66. Winn WA. Coccidioidomycosis and amphotericin B. Med Clin North Am. 1963;47: 1131–1148. [DOI] [PubMed] [Google Scholar]
- 67. Galgiani JN, Ampel NM, Catanzaro A et al. Practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2000; 30: 658–661. [DOI] [PubMed] [Google Scholar]
- 68. Galgiani JN, Ampel NM, Blair JE et al. 2016 Infectious Diseases Society of America (IDSA) Clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016; 63: e112–46. [DOI] [PubMed] [Google Scholar]