Abstract
The archetypal imaging characteristics of meningiomas are among the most stereotypic of all central nervous system (CNS) tumors. In the era of plain film and ventriculography, imaging was only performed if a mass was suspected, and their results were more suggestive than definitive. Following more than a century of technological development, we can now rely on imaging to non-invasively diagnose meningioma with great confidence and precisely delineate the locations of these tumors relative to their surrounding structures to inform treatment planning. Asymptomatic meningiomas may be identified and their growth monitored over time; moreover, imaging routinely serves as an essential tool to survey tumor burden at various stages during the course of treatment, thereby providing guidance on their effectiveness or the need for further intervention. Modern radiological techniques are expanding the power of imaging from tumor detection and monitoring to include extraction of biologic information from advanced analysis of radiological parameters. These contemporary approaches have led to promising attempts to predict tumor grade and, in turn, contribute prognostic data. In this supplement article, we review important current and future aspects of imaging in the diagnosis and management of meningioma, including conventional and advanced imaging techniques using CT, MRI, and nuclear medicine.
Keywords: CT, imaging, meningioma, MRI, perfusion, PET, radiology
Although reports on tumors of meningeal attachment existed in the nineteenth century, the canonization of meningioma as a distinct entity occurred upon the publication of Cushing and Eisenhardt’s seminal work, “Meningiomas: Their Classification, Regional Behaviour, Life History, and Surgical End Results.”1 Strikingly though, the discussion of imaging is relatively absent in this monumental monograph, save for infrequent radiographs, given the central role that imaging plays today in the diagnosis and management of meningiomas.
Of the pivotal developments in our understanding of intracranial meningiomas—including evolution of surgical technique, a role for radiation, and a resurgent interest in their biology—perhaps none has been as central as the application of imaging to their study, beginning with the first radiological report of a meningioma in a living patient who presented with hemiparesis, hemianopia, and headaches in 1902 by radiograph.2 The radiographic features of meningioma were expanded in an analysis of 95 patients operated by Harvey Cushing, noting the cardinal features of vascularity, osteomatous changes, spicule formation, diffuse thickening, enlargement of the meningeal artery channel, and calcification.3 Walter Dandy integrated the role of mass effect exerted by tumors with their radiographic stigmata with his introduction of ventriculography and pneumoencephalography, wherein directional distortion of the ventricular system as cast by injected air aided in meningioma localization. Moreover, Dandy emphasized the importance of tumor localization prior to surgery, extrapolating from neurological symptoms, signs, and inferences derived from these early imaging modalities. This was a staple of cranial imaging for decades after its initial description, although Cushing himself was reluctant to adopt it. Collectively, early imaging modalities relied upon inference—Cushing noted that he assumed recurrence when surgical clips shifted in position on radiographs over time.
The next important development in the history of imaging of meningioma was the application of cerebral angiography to the radiographic diagnosis of meningioma—the marriage of intra-arterial dye injection to existing methods of radiography. Moniz introduced the concept of angiography in 1927 and was the first to describe the angiographic findings in intracranial meningioma with colleagues 2 years later.4 List and Hodges reported on the first large series of angiography in brain tumors, and highlighted concurrent contribution by both extracranial and intracranial circulations with an intervening intratumoral vascular blush as being pathognomonic for meningioma, when present.5 Understanding the coaptation of vascular feeders by meningioma further inspired clinicians to diminish surgical risk and blood loss through preoperative embolization of critical feeders, transforming angiography from a diagnostic tool to an interventional strategy. Angiography, in either catheter-based or non-invasive form, is routinely employed to clarify important anatomy of adjacent arterial and venous structures.
The introduction of CT by Sir Godfrey Hounsfield and MRI by Paul Lauterbur in 1973 launched the central role of imaging as we know it today in the diagnosis and management of meningiomas.6,7 These powerful modalities, coupled with the administration of intravenous contrast, elucidated the extra-axial anatomic origin of meningiomas, their patterns of growth, dural spread frequently far beyond the limits of the tumor bulk, variable involvement of adjacent bone with either lytic or hyperostotic changes, and stereotypic involvement and deflection of neurovascular structures. The unique resolution of meningioma appearance and involvement of neighboring tissue by CT and MRI spurred parallel developments in preoperative planning and surgical techniques, sharpening awareness of operative risk and allowing greater definitive resection of meningioma from multiple compartments. Additionally, important arterial and venous relationships could now be studied in greater detail with CT or MR angiography.8
The radiological diagnosis of meningioma is typically certain on CT and MRI evaluation. However, differentiating residual or recurrent tumor from postsurgical or radiation treatment changes may prove challenging on occasion. In these instances, modern imaging modalities have exploited specific attributes of meningioma biology: for example, nuclear scintigraphy or positron emission tomography (PET) may detect radiolabeled octreotide, a somatostatin agonist, and its binding to tumor-specific somatostatin receptors (eg, SSTR II).
The routine availability of CT and now MRI has provided a reliable non-invasive means to track tumor growth, allowing the derivation of the natural history of meningioma in cases where asymptomatic patients are followed with imaging over time. While in the early days of neurosurgery, imaging was used to verify and localize a tumor after patient presentation with a neurologic symptom, tumors are now frequently incidentally discovered. The decision to treat or follow the presumed meningioma with serial imaging has fueled interest in predicting tumor behavior—and even grade—from imaging features alone. This forces a critical evaluation of which patients need treatment if tumors are small and asymptomatic at the time of initial identification. This has led to scrutiny of specific features on conventional MRI as well as advanced imaging modalities like MR spectroscopy or PET in order to infer tumor behavior from imaging characteristics.
Applications of advanced computational methods like machine learning and radiomics to existing imaging data hold promise in acquiring such biologic data as meningioma grade, the most powerful predictor of tumor behavior and recurrence available to date, or to improve prognostication independently of grade.9 Below, we review important salient current and future aspects of imaging in the diagnosis and management of meningioma.
Conventional Imaging Features of Meningiomas
Meningiomas usually occur as intracranial extra-axial masses attached to the dura mater.10 While the typical appearance is of a mass with a broad-based dural attachment, meningiomas can also extend along a wide expanse of dura in a sheet-like appearance (en plaque meningioma).
Histologically, meningiomas arise from meningoepithelial cells, also known as arachnoid cap cells. As such, meningiomas most commonly occur where these cells are most numerous, such as where the arachnoid granulations are concentrated along the dural venous sinuses.10 Of all intracranial meningiomas, approximately half are located along the skull base, 40% along the calvarial convexity, 10% along the falx and parasagittal region, and a small fraction within the ventricle or across multiple locations.11,12 The majority of meningiomas are considered primarily intradural tumors.13,14 However, 1–2% of meningiomas are extradural and arise in locations other than the dura, including within the calvarium, scalp, paranasal sinuses, nasopharynx, neck, and skin with rarer locations also reported.13,14 While the locations of primary extradural meningiomas vary, two-thirds are made up of primary intraosseous meningiomas, which are postulated to arise from arachnoid cap cells that become caught between cranial sutures during birth.13,14 Meningiomas are most often solitary, with one series demonstrating multiple meningiomas on CT in 8.9% of patients.15
CT Features
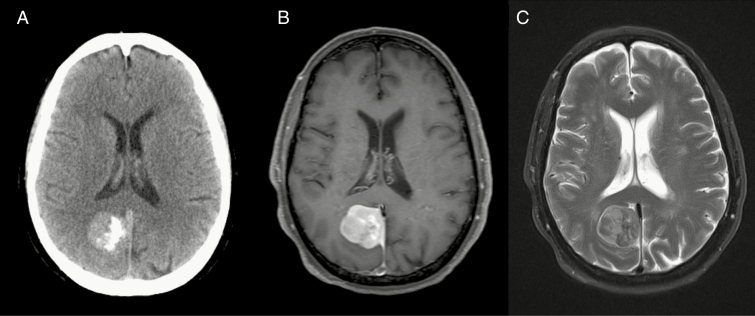
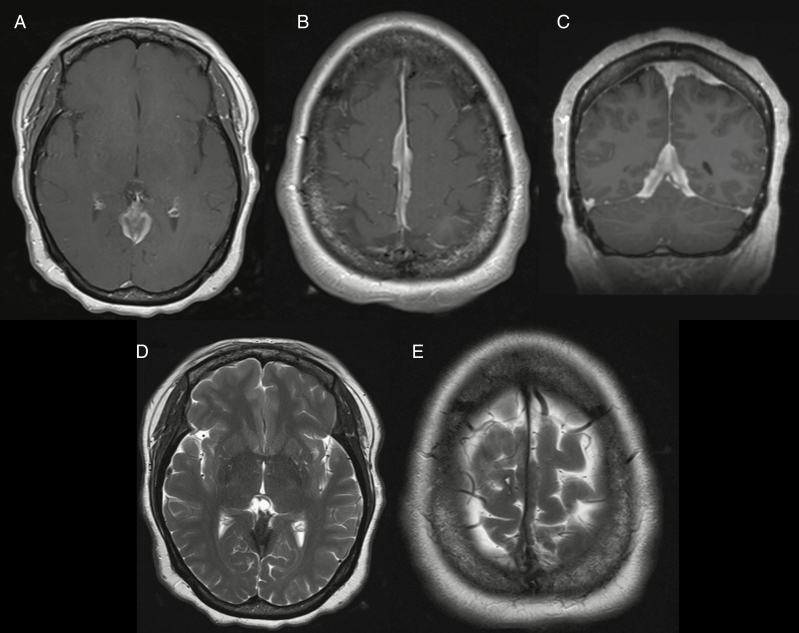
Typical diagnostic features are found on CT in 72–85% of cases, including a sharply circumscribed lobular mass with a broad-based dural attachment.10 On unenhanced CT, meningiomas typically appear as homogeneous and hyperdense extra-axial masses, which demonstrate homogeneous enhancement following contrast administration.10,16 Furthermore, meningiomas are classically associated with intratumoral calcification as befitting their slow growth rate, although specific subtypes may trigger dystrophic or metaplastic calcification as well, which lends a speckled hyperdense appearance on CT seen in 15–20% of cases (Figs. 1, 2).17 CT also best demonstrates the bony changes sometimes associated with meningiomas. These changes can include hyperostosis, osteolysis, and, in the setting of an anterior skull base meningioma, pneumosinus dilatans. Hyperostosis, which is the most commonly associated bony finding, manifests as bony thickening on CT and is seen in up to 25–49% of meningiomas, with the convexity and sphenoid wing the most common locations (Fig. 3).18 Hyperostosis may be reactive or associated with osseous tumor invasion; the differentiation may be difficult on imaging, but strong enhancement within hyperostotic bone makes tumor invasion more likely.
Fig. 1.
(A) Noncontrast CT, (B) T1-weighted gadolinium-enhanced MRI, and (C) T2-weighted MRI of a partially calcified right falcine meningioma.
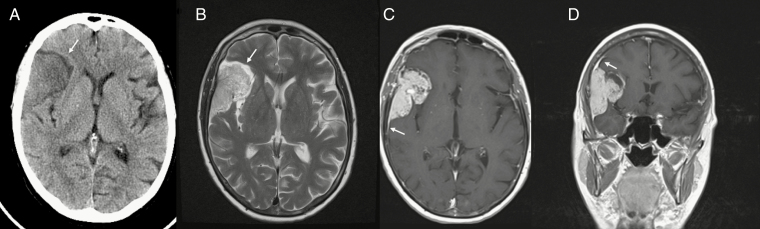
Fig. 2.
(A) Noncontrast CT shows an extra-axial mass with a low-density CSF cleft (arrow) between the mass and adjacent right frontal lobe. (B) MRI T2-weighted image shows an intermediate signal mass with a CSF cleft (arrow). Gadolinium-enhanced MRI in (C) axial and (D) coronal planes demonstrates avid enhancement within the mass and a dural tail sign (arrowhead).
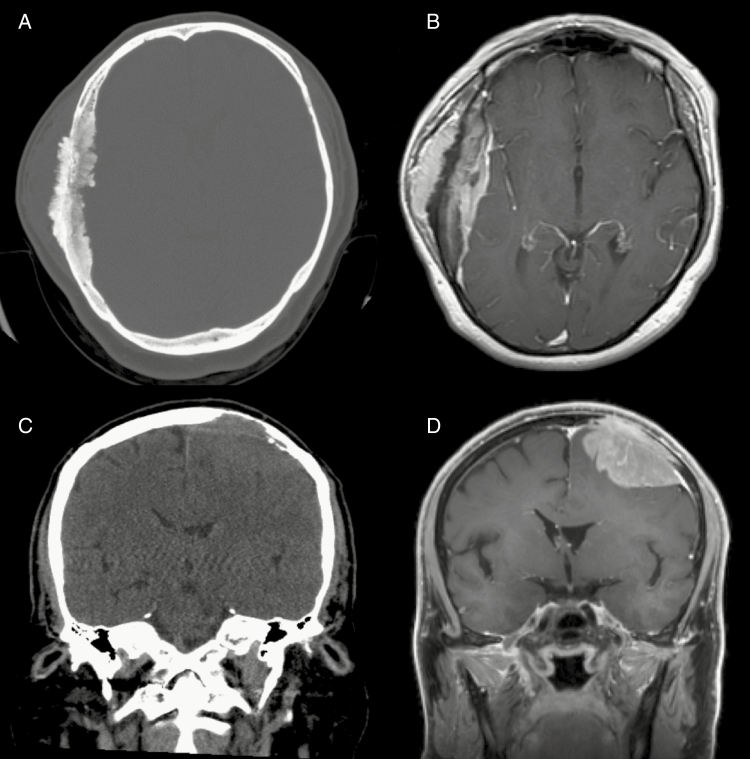
Fig. 3.
Noncontrast CT and T1-weighted gadolinium-enhanced MRI demonstrating (A, B) a grade I meningioma with profound hyperostosis and extracalvarial enhancing tumor, and (C, D) a grade III meningioma with lytic erosion of adjacent bone from tumor invasion.
MRI Features
On MRI, meningiomas are usually hypo- to isointense relative to cerebral cortex on T1-weighted sequences and iso- to hyperintense on T2-weighted sequences (Fig. 1, 2).10,19 As on CT, most meningiomas enhance avidly on MRI, and nearly all enhance at least in part, even if heavily calcified. The presence of intratumoral cysts, hemorrhage, or necrosis can produce a heterogeneous appearance and may be associated with more aggressive behavior of the tumor. A dural tail is seen on postcontrast imaging in up to 72% of meningiomas.16 While a dural tail may represent reactive dural changes, one study found that nearly two-thirds of dural tails were invaded by tumor cells.20,21 Intraoperatively, microscopic spread of tumor cells may also extend beyond the apparent dural tail on imaging. Yet despite the well-known association between dural tails and meningiomas, the sign is not specific, as other dural neoplasms can also demonstrate this finding.16 Nevertheless, this feature is useful in confirming the extra-axial location of a large meningioma that is inseparable from brain and in distinguishing meningiomas from other extra-axial tumors such as schwannomas and pituitary adenomas that typically do not exhibit dural tails.
In diffusion-weighted imaging (DWI), the apparent diffusion coefficient (ADC) values of meningiomas are variable, though ADC may be relatively low, particularly in higher grades but also in grade I meningiomas (Supplementary Fig. 1).15 On MR spectroscopy, elevated choline and alanine levels and diminished N-acetylaspartate (NAA) levels are anticipated; elevated alanine is relatively specific for meningioma but can be difficult to identify.22,23 Perfusion imaging will generally reveal high relative cerebral blood flow (rCBF) and relative cerebral blood volume (rCBV) (Supplementary Fig. 1), although substantial gadolinium leakage with the dynamic susceptibility contrast (DSC) technique confounds rCBV quantitation. Less than 50% return to baseline of signal intensity after gadolinium bolus injection with DSC is described with meningiomas,24,25 though this percent signal recovery is technique dependent (eg, dependent upon gadolinium preload use, some pulse sequence parameters, and software leakage correction). Arterial spin labeling (ASL) perfusion has also shown increased rCBF in meningiomas, particularly the angiomatous histological subtype,26,27 while dynamic contrast enhanced (DCE) MR permeability imaging parameters did not reliably predict meningioma microvascularization parameters in another study.28
As meningiomas grow larger, they demonstrate inward displacement of the underlying brain parenchyma.10 MRI helps delineate the extra-axial nature of the tumor, often revealing a CSF cleft between the mass and the brain which appears as a T2-weighted crescent. Such clefts can be absent, however, particularly when higher-grade meningiomas invade the brain. Although most meningiomas demonstrate typical imaging characteristics, approximately 15% of benign meningiomas are multiple or demonstrate features such as tumor necrosis, cystic change, hemorrhage, and fatty infiltration.29
Edema in adjacent brain is present with slightly over half of meningiomas, and this phenomenon does not correlate well with tumor size.30,31 While atypical and malignant meningiomas may cause edema by invading the brain, World Health Organization (WHO) grade I meningiomas not infrequently have peritumoral brain edema without brain invasion. There are various proposed etiologies to account for this edema, including compressive ischemia with compromise of the blood–brain barrier, vascular shunting due to parasitization of pial microvessels, mechanical venous obstruction, elevated hydrostatic pressure within the tumor, as well as secretory-excretory phenomena within the tumor cells.17,32,33 As such, peritumoral brain edema does not reliably distinguish between benign and atypical or malignant meningiomas.
Vasculature
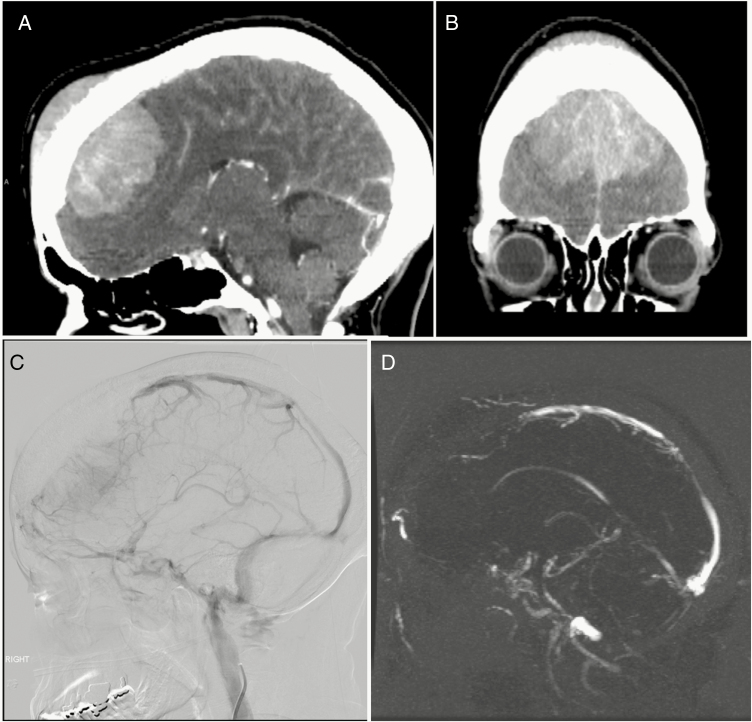
Given their vascular nature, flow voids or enhancing vessels are commonly seen within and around the periphery of meningiomas on MRI and contrast enhanced CT. Nearly three-quarters of meningiomas receive their primary vascular supply from dural vessels, although they may also parasitize the pial supply from the carotid or vertebrobasilar circulation.17 The blood supply to meningiomas characteristically includes a prominent central vascular pedicle from which smaller vessels radiate in a “spoke wheel”–like pattern and the surface is often supplied by a peripheral plexus of pial vessels.17 On conventional angiography, meningiomas usually demonstrate hypervascularity and a prominent tumor blush with delayed washout.10,16 Meningiomas may displace, encase and narrow adjacent vessels, or sometimes invade or occlude dural venous sinuses (Fig. 4).
Fig. 4.
(A, B) WHO grade I meningioma with diffuse infiltration of bone and occlusion of (C) anterior superior sagittal sinus on angiography and (D) MRV with venous rerouting.
Differential Diagnosis
Meningiomas account for over a third of all primary CNS tumors and over half of nonmalignant CNS tumors, and predominate among extra-axial lesions.34 Despite this prevalence, broad differential diagnoses of meningioma exist, including dural-based metastasis (eg, from lung, breast, or prostate primaries), lymphoma and leukemia (ie, granulocytic sarcoma), solitary fibrous tumor, and hemangiopericytoma (now considered within the solitary fibrous tumor spectrum). Enhancing dural masses may be seen with sarcoidosis and tuberculosis but are often multiple (Fig. 5). Idiopathic hypertrophic pachymeningitis, extranodal sinus histiocytosis,35 and immunoglobulin G4–related disease can cause a diffuse thickening of the dura which could be occasionally confused with en plaque or multiple meningiomas.16 Imaging features which distinguish meningioma mimics from meningioma itself can include homogeneous T2 hypo- or hyperintensity in the tumor, adjacent osseous destruction, leptomeningeal or pial extension, and absence of a dural tail.36
Fig. 5.
Dural sarcoidosis mimicking en plaque meningioma. (A, B, C) Gadolinium-enhanced T1-weighted images and (D, E) T2-weighted image show nodular dural thickening and enhancement along the falx and tentorium.
Molecular Imaging for Meningioma
PET is an imaging modality capable of providing biochemical and physiologic data about a tumor.37 The most widely used radiopharmaceutical in PET imaging is 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG), which is a glucose analog actively transported into metabolically active cells.37 FDG-PET has been used in patients with primary brain tumors for tumor grading, prognosis, and differentiating recurrent tumor from radiation necrosis.38 However, the high physiological FDG uptake in the cerebral cortex as well as the accumulation of FDG in inflammatory processes hamper the diagnostic accuracy of FDG-PET in brain tumors.39 Therefore, different and more specific radioligands have been proposed for brain tumor diagnostics.
Meningiomas are known to have increased somatostatin receptor II (SSTR II) expression in almost all cases.40,41 Radiolabeled SSTR ligands are increasingly used in PET imaging. The high specific binding to the SSTR in meningioma, as well as the very low uptake in adjacent structures like bone or brain tissue, the latter due to the inability of these tracers to cross the blood–brain barrier, result in a very high tumor-to-background contrast. This is of special interest in those cases with low CT or MR contrast due to osseous infiltration or in skull base meningiomas, where the exact delineation is often very challenging.42,43
Recent studies revealed a higher sensitivity of PET with SSTR ligands labeled with gallium-68 (Ga-68) compared with contrast enhanced MRI, which detected only 171 out of 190 (92%) of the meningiomas that were revealed by PET/CT.44 Thus, SSTR-PET is useful for differential diagnosis—for instance, in discriminating optic sheath meningioma.45 Initial data also demonstrated that SSTR-PET enables a more exact tumor delineation, especially in those tumors with limited MR contrast because of their localizations, such as at the skull base, falcine area, orbit, as well as sagittal and cavernous sinus or due to transosseous growth (Supplementary Fig. 2).46 For the detection of osseous involvement, a higher sensitivity of SSTR-PET compared with MRI (98.5% vs 53.7%) but only a slightly decreased specificity (86.7% vs 93.3%) were confirmed in a recent study. Interestingly, intraosseous meningioma involvement as assessed with SSTR-PET was significantly larger than in contrast enhanced MRI alone (P < 0.001) and comparable for the extraosseous part of transosseous meningiomas (P = 0.636) and for extraosseous meningiomas (P = 0.132).47 In addition, SSTR-PET was shown to differentiate between viable tumor and scar tissue by using semi-quantitative PET data analysis, since the semi-quantitative uptake value (SUV) was shown to correlate with SSTR II expression in immunostaining.48 While further validation is needed to confirm the utility of SSTR II imaging, an evidence-based recommendation for the use of molecular imaging in meningioma has been proposed recently.46
Natural History of Meningiomas
Meningiomas are generally considered slowly growing lesions, often with an indolent clinical course depending on the location.49 Meningiomas are often clinically detected when already of large size or with increasing age.34 However, with increasing availability of cranial imaging, incidentally discovered meningiomas are becoming more frequent. While it is common practice to follow most asymptomatic patients by MR imaging, understanding the natural history and growth dynamics are essential for clinical decision making, especially when histological analysis is not established.
The benign behavior of WHO grade I meningioma was recently demonstrated by using retrospective radiocarbon birth dating in 12 cases, which revealed a mean tumor age of 22.1 ± 6.5 years.50 In contrast, 2 WHO grade II meningiomas assessed by this method developed only 1.5 ± 0.1 years prior to surgical resection. Several studies have indicated differing growth rates and doubling times between WHO grade I and WHO grade II meningiomas.51–55 In a retrospective evaluation of 50 meningiomas, there were significant different presurgical annual volumetric growth rates between WHO grade I (1.34 cm3, range 0.07–3.26 cm3) and WHO grade II meningiomas (6.40 cm3, 3.61–13.29 cm3).55 In this patient cohort, a volumetric growth rate of 3.05 cm3/year was suggested as a threshold for a higher-grade meningioma.55 Similar growth rates were found for incompletely resected WHO grades I and II meningiomas during follow-up.52 The growth rates and dynamics among the group of benign meningiomas display a huge variability.52,56 Long-term analyses revealed changing growth dynamics in WHO grade I meningioma with periods of exponential, linear, or no growth, whereas most atypical meningiomas display an exponential growth pattern.53,54,57 Although some evidence suggests a relationship between histological grading and tumor growth rate, substantial validation is limited.56
The reported natural history in cohorts of incidental and untreated meningiomas varies significantly and may follow complex growth patterns.58–60 Volumetric growth rates of less than 1 cm3/year in 66% of incidental meningioma have been reported with a range of 0.03–2.62 cm3/year and a mean tumor doubling time of 21.6 years ranging from 1.27 to 143.5 years.61 Many incidental meningiomas grow extremely slowly or do not grow at all, but more aggressive growth patterns can occur and may display growth rates of up to 4.0962 or 10.3 cm3/year.58 During a minimum follow-up period of 10 years in 65 patients with asymptomatic and untreated meningiomas, 35.4% of patients exhibited progression defined by >2 mm increase of any diameter. Based on life-table statistics, the authors calculated a 75% 15-year growth rate, indicating that the majority of patients may eventually progress.63
Many patient-related factors and radiological characteristics have been investigated to identify predictors of aggressive or clinically relevant tumor progression in incidental and untreated meningioma, with inconsistent results. Younger age (≤60 years),61,62 male sex,55 radiological tumor characteristics such as hyperintensity on T2-weighted MRI,55,57,59,61,62 absence of tumor calcification,52,55,57,58,61–63 or presence of peritumoral edema55,62 may be associated with a risk for relevant meningioma progression. A greater degree of tumor proliferation as measured by MIB-1 index was reported in meningiomas presenting with peritumoral edema, an ambiguous brain–tumor border on T2-weighted MRI, and irregular tumor shape.64 The impact of meningioma location on the natural history is not well understood. Tumor location has been evaluated as a predictor for growth rate; in a volumetric MRI analysis of 113 incidental meningiomas, non–skull base meningiomas had a higher tendency for progression with a significantly higher growth rate than skull base meningiomas.65 In contrast, 2 single center studies58,62 and a meta-analysis on the published natural history of meningioma59 were not able to establish a link between location and tumor growth pattern.
As meningiomas may display different growth dynamics over time, the initial tumor volume may have a significant impact on the risk of clinically relevant progression and outcome. Untreated meningiomas below the size of 25 mm in maximum diameter tend to show a benign behavior in a 5-year follow up period.59,62 Nevertheless, even for small tumors an increase in tumor size >10% is significantly associated with the development of clinical symptoms, underscoring the importance of follow-up imaging especially in the first years.59 A weighted scoring system for estimating the risk of rapid growth (defined as growth rate >2 cm3/y) of untreated meningiomas, the Asan Intracranial Meningioma Scoring System (AIMSS), has been proposed.57 The AIMSS is based on the categorization in 3 tumor sizes with cutoffs at <2.5 cm and ≥4 cm maximum diameter. With inclusion of the known risk factors such as absence of calcification, presence of peritumoral edema, and tumor signal on T2-weighted MRI, the resulting score allows the attribution to a low-, intermediate-, or high-risk group for the probability of rapid tumor growth.57,66
Although a number of studies have been conducted to analyze the natural history of meningiomas, the results are limited due to retrospective single center study design with different varieties of methods in assessing the growth dynamics. Volumetric analysis during follow-up imaging is more accurate in determining changes of tumor volume than measurement of maximum tumor diameter.60,62 Newly diagnosed and radiologically presumed meningiomas are best followed by MRI if there is no indication for surgical resection. If no other differential diagnosis is suspected, an initial follow-up after 6 months and then annually if the patient remains stable has been proposed49 but may be individually adjusted according to known risk factors. Based on reported growth patterns of nontreated meningiomas and the European Association of Neuro-Oncology guidelines49 for follow-up of resected WHO grade I meningiomas, MR imaging every 2 years after a follow-up period of 5 years may be reasonable. Long-term data on the natural history63 implicates that patients should be subjected to routine surveillance imaging.
Radiographic Distinction of Meningioma Grade
The clinical course of patients with meningiomas is strongly dictated by their histopathologic grade. The majority of meningiomas, particularly WHO grade I meningiomas, are effectively managed by complete surgical resection, with radiotherapy used in select cases to augment local control, especially in patients with multiple recurrences and medical comorbidities, or in those cases where surgery alone is insufficient.49 However, tumor recurrence remains a problem, particularly for meningiomas with subtotal resection, with universal recurrence for grade III tumors and recurrence rates of 20–75% for grade II meningiomas at 10 years follow-up, even after aggressive multimodality treatment.
Given the prognostic and therapeutic implications of tumor grade, specifically between benign tumors and their atypical/malignant counterparts, preoperative knowledge of tumor grade may influence patient management, including decisions of whether to observe, operate, or administer adjuvant therapies, as well as facilitate patient counseling at an earlier stage of clinical care. Furthermore, in the contemporary era of routine and easily accessible imaging, meningiomas are increasingly diagnosed as incidental findings, making this differentiation all that more relevant.
In general, meningiomas can be fairly confidently diagnosed by MRI and CT alone, typically presenting as sharply circumscribed masses with a broad-based dural attachment, which demonstrate homogeneous enhancement on postcontrast imaging.10,49 However, moving from simple diagnosis toward prediction of tumor behavior such as through the use of non-invasive imaging biomarkers would further enhance clinical decision making.9
Morphologic Features
Based on imaging morphology alone, a number of features have been shown to be associated with more aggressive or higher-grade behavior, including an indistinct tumor–brain interface, irregular tumor shape and margins, heterogeneous enhancement, large tumor size, absence of calcification, and the presence of peritumoral edema.64,67–69
The amalgamation of specific imaging features has also been combined with clinical characteristics, such as older age, into models capable of predicting meningiomas with advanced histopathologic grades.70 In addition, when serial imaging data are available, the volumetric growth rate of meningiomas has also been shown to correlate with tumor grade.51,71,72
Despite the correlation of certain morphologic features with advanced tumor grade, the fact remains that no feature is specific for atypical/malignant lesions, as similar findings can also be seen in low-grade tumors.73 As such, the need remains for additional imaging biomarkers able to complement these morphologic features in assessing tumor grade.
Diffusion Weighted/Diffusion Tensor Imaging
DWI is an MR technique sensitive to the motion of water molecules and which, along with ADC measurements, provides important tissue microstructural information.74 In biological tissue, water diffusion is highly dependent on the ratio of extracellular to intracellular space, with water diffusivity being greater in the extracellular space than in the intracellular space.75 As such, increased cellularity and a subsequent decrease in the fraction of extracellular space cause restriction of water diffusion.75
Histologically, atypical and malignant meningiomas have more compact architecture with more tightly packed and smaller cells with increased mitotic activity, prominent nuclei, and high nucleus-to-cytoplasm ratio, all of which are factors expected to decrease water diffusivity and thus presumably lead to lower ADC values.74,76,77
A number of previous studies have evaluated the ability of ADC values to differentiate benign from atypical/malignant meningiomas, with variable conclusions.74–85 One study analyzing 177 meningiomas (135 benign, 37 atypical, and 5 malignant) found that while the mean ADC values and ADC ratios of benign meningiomas were higher than those of atypical and malignant tumors, the difference was not statistically significant.77 Similar findings were observed by several others who reported no statistically significant difference in respective ADC values based on meningioma grade.79–81,84 Other studies, however, have found significant differences in ADC values between benign and atypical/malignant meningiomas.74–76,78,83,85,86 The largest of those analyzed 138 meningiomas (106 benign, 19 atypical, and 13 malignant) and found the mean ADC values and mean normalized ADC (NADC) ratios in atypical/malignant meningiomas to be significantly lower than in benign meningiomas.85 A number of additional studies have made similar findings, all showing atypical/malignant meningioma subtypes to have lower ADC values.74–76,78,83,86
The variability of these findings can result from differences in sample size, histologic criteria, and imaging methodology. Studies have also assessed ADC cutoff values in this grading assessment.74,76 An ADC cutoff value of 0.70 × 10−3 mm2/s provided a sensitivity for diagnosing atypical/aggressive meningiomas of 29%, specificity of 94%, positive predictive value of 67%, and negative predictive value of 75%.74 Using receiver operating characteristic curves, others found that the optimal ADC and NADC thresholds for differentiating atypical/malignant meningiomas were 0.80 × 10−3 mm2/s and 0.99, respectively.76 Using these thresholds, ADC correctly predicted 96% of atypical/ malignant and 83% of benign meningiomas, and NADC correctly predicted 96% of atypical/malignant and all of the benign meningiomas.76
In contrast to isotropic DWI, which eliminates directional or anisotropic diffusion, diffusion tensor imaging (DTI) analyzes the 3D shape of the diffusion, providing information about the magnitude and directionality of water diffusion.83,87 DTI allows for the calculation of eigenvalues (λ) and quantification of tensor shapes, as well as the generation of a fractional anisotropy (FA) map.87Grade I meningiomas may have significantly lower FA, greater λ2 and λ3 values, and a greater proportion of spherical tensors compared with atypical meningiomas, indicating more disorganized microscopic water motion in grade I versus grade II meningiomas.83 The authors of this particular study speculated that the histologic attributes of meningiomas accounted for these differences, with the whorls and fascicles found in grade I meningiomas serving as physical barriers to the linear movement of water molecules, with the sheet-like growth of atypical meningiomas possibly facilitating the directional movement of water within the tumor.83
However, a later study using histogram analysis of DTI metrics to analyze differences between meningioma grades and subtypes found conflicting results.88 In this study, WHO grades II and III meningiomas had a significantly reduced planar anisotropy coefficient along with histogram skewness measurements indicating a distribution shift toward a higher spherical anisotropy coefficient in comparison to their typical counterparts (grade I).88 An alternative explanation is that the loss of normal internal architecture seen in atypical meningiomas, such as the absence of whorls and fascicles in atypical meningiomas, results in less directionality in atypical meningiomas compared with benign meningiomas.88
Perfusion Imaging
Perfusion imaging is a method for assessing the flow of blood occurring at the tissue level and is used frequently in neuro-oncologic imaging, particularly in the evaluation of gliomas.89,90 Prior studies assessing meningiomas with MR perfusion have successfully demonstrated a correlation between cerebral blood volume (CBV) and histologic measures of tumor vascularity, including microvessel density and area.91,92 A significant correlation between CBV and expression of vascular endothelial growth factor has been observed, suggesting that perfusion imaging may help to identify those meningiomas that may be refractory to conventional treatment and respond best to anti-angiogenic therapies.93 In addition to correlation with vascularity, maximum rCBV has been shown to positively correlate with the Ki-67 proliferative index in meningiomas.79
While correlation between perfusion values and vascularity has been demonstrated, correlation between perfusion values and meningioma grade has had mixed results. Several studies found no statistically significant difference in blood volume measures between typical/benign and atypical/malignant meningiomas.94–96 Others, however, reported significant differences in rCBV, although with conflicting results. In a study of 24 low-grade and 24 high-grade meningiomas, normalized maximum CBV ratios were higher in high-grade meningiomas compared with low-grade meningiomas.93 In contrast, others have reported that the rCBV of benign meningiomas may be significantly higher than that of malignant meningiomas.92 The authors in the latter study hypothesized that malignant meningiomas might be in a state of relative ischemia and hypoxia because of their rapid growth, leading to a reduction in the number of tumor microvessels and a subsequent decrease in rCBV.92 Evaluation of mean rCBV values in the peritumoral edema revealed that the maximal rCBV in the peritumoral edema was higher in malignant versus benign meningiomas.96 This observation suggests that the increase in rCBV in the peritumoral edema of malignant meningiomas might be attributed to tumor invasion and angiogenesis in the adjacent brain tissue.96
Unlike intra-axial tumors, meningiomas typically derive blood supply from extracranial arteries which do not contain a blood–brain barrier and are therefore permeable to contrast material. Therefore, meningiomas are generally associated with increased vascular permeability, and the impact of gadolinium contrast leakage on perfusion measurements can be substantial using the DSC technique. This effect may have led to the mixed results of tumor grade prediction using the DSC method reported in the literature. DCE perfusion technique allows evaluation of vascular permeability directly. The Ktrans values measured by DCE were higher in atypical meningiomas compared with benign meningiomas, and the difference may reflect increased capillary leakiness due to micronecrosis in the high-grade tumors.95 Arterial-spine-labeling MR perfusion, a technique that can provide regional flow information without the confounding permeability factor and does not require gadolinium contrast, also has been shown to differentiate WHO grade I from WHO grades II and III intracranial meningiomas.27
Machine Learning and Radiomics
Radiomics, a field of quantitative imaging focused on converting large sets of digital medical images into minable high-dimensional data, is of increasing interest in neuro-oncology. These large image datasets, which include a variety of imaging features, can offer information on imaging phenotype that can be used in conjunction with clinical information and correlated with clinical outcomes.97 In practice, 2 types of imaging features can be extracted—“semantic” and “agnostic.”97 Semantic features are those that are visually assessed by the radiologist, such as bone invasion or necrosis, and as such, are subject to interobserver variability.9,97 In contrast, agnostic features assess lesion heterogeneity through quantitative descriptors, such as skewness and texture.97
Radiomic analysis of the preoperative T1-weighted postcontrast MRI features across 175 meningiomas revealed both quantitative and qualitative features that significantly associated with meningioma grade, with high-grade tumors exhibiting more necrosis or hemorrhage, intratumoral heterogeneity, nonspherical shape, and larger volumes.9 Intriguingly, quantitative radiomic features demonstrated significant association with number of atypical features among grade I meningiomas, suggesting that further development of computational methods may afford detailed insights into meningioma biology beyond current capabilities.9 An additional study hypothesized that tumor heterogeneity and irregular shape may aid in meningioma grading given their association with higher tumor grade, and observed several texture and shape features that contributed to the prediction of meningioma grade.98 Furthermore, combination of quantitative and qualitative analyses may provide synergistic capability to predict meningioma grade and behavior, opening venues for future exploration.9
Positron Emission Tomography
Although PET does not play a major role in the primary diagnosis of meningiomas, PET imaging may be helpful for treatment stratification and precise delineation of meningioma, as well as in differentiating between viable tumor and scar tissue. Studies assessing the ability of FDG PET to differentiate meningioma grade have shown mixed results. The ratio of tumor to gray matter in atypical/malignant meningiomas has been shown to be higher than that in low-grade tumors and correlated with proliferative potential of the tumor, as well as being a significant predictor for tumor recurrence, although the sensitivity for detecting high-grade meningioma was only 43%.38 Additional studies have also shown the ability of FDG-PET to differentiate benign and atypical/malignant meningiomas,99,100 as well as to be able to differentiate recurrent or growing meningiomas from static meningiomas.101 However, opposite results have been reported to show a lack of correlation between FDG uptake and WHO grading, MIB-1 labeling index, or tumor doubling time.102–104
Another retrospective analysis demonstrated that high SSTR expression assessed by SUV measurements with PET correlated with higher tumor growth rate in WHO grades I and II meningiomas, thus providing additional information beyond morphology that might be useful for patient treatment stratification.105
Summary of preoperative meningioma grade prediction
To date, histologic grades of meningiomas have been correlated to qualitative and quantitative features of many conventional and advanced imaging techniques, although most of these imaging markers, apart from those with obvious signs of brain invasion, are not used in routine clinical care, since they do not provide sufficiently high accuracy for determination of tumor grade. For example, incidentally discovered meningiomas still require follow-up imaging to determine their proliferative characteristics, which would then influence the decision for the need of treatment and if so, the timing and type of treatment. Recent advances in radiomic technique combined with machine learning algorithms provide a pathway for improving the accuracy in tumor grade prediction, and the accuracy of radiomic tumor grading models will also be enhanced by advancement of novel imaging techniques.
Beyond Histopathologic Grade
While tumor grade is important in providing population-based risk stratification, histopathologic grade does not always reliably predict which patients will progress or recur after treatment.106 The fact remains that sometimes high-grade tumors behave less aggressively and low-grade tumors more aggressively.106,107 In addition, histopathologic grading is often based on examination of only a limited sample of tissue, which can be a potential issue in more heterogeneous lesions, whereas imaging can more easily evaluate the entirety of the lesion.
Given this potential limitation of histopathologic grading, identification of imaging biomarkers able to provide prognostic information independently of histologic tumor grade is also of clinical importance, as it may offer complementary information allowing for more individualized prognostic information and therapeutic recommendations. One recent study assessed the ability of preoperative imaging and clinical characteristics to stratify patients based on risk for disease progression or recurrence following initial treatment.106 That study found that the combination of extent of resection and ADC values outperformed WHO histopathologic grade for predicting which patients will suffer progression/recurrence after initial treatment. Similarly, preoperative radiological classification was able to supplement WHO histopathologic grading to accurately predict the aggressive behavior of convexity meningiomas in another study.108 These may eventually lead to incorporation of imaging into the preoperative grading of meningiomas.
Summary
Preoperative MR and PET imaging are increasingly appreciated to provide information regarding differential diagnosis, extent of tumor, and histologic grade of meningioma. Quantitative analytical methods are further augmenting the interpretation of imaging data and helping to correlate imaging features with clinical outcome. Continued investigation is needed to further validate and refine these imaging biomarkers as well as to develop strategies of how to integrate these imaging methods into clinical practice. However, it is becoming increasingly evident that imaging will play a key role in further individualizing care for patients with meningiomas, both in managing patient expectations and in guiding therapy.
Imaging Considerations for Meningioma Treatment Planning
Surgical Planning Considerations
The operative strategy for meningiomas is influenced by the tumor location, its relationship with adjacent structures, the vascular supply to the tumor and the surgical corridor, the anticipated tumor consistency, and the interface between tumor and brain—all of which can be appreciated with increasing clarity with imaging. The most common preoperative study for meningioma treatment planning is a contrast enhanced MRI, which aptly delineates the size, location, heterogeneity, and vascularity of the tumor, as well as important features that influence the planning of the craniotomy and resection, such as peritumoral edema and extent of dural involvement. Conventional MRI, as well as advanced sequences such as MR elastography (MRe), may also suggest the consistency and the nature of the tumor–brain interface by the extent of tumor nodularity at its boundary or a CSF cleft between the tumor and the surrounding parenchyma, the presence or absence of which may alter the surgical strategy. Bony involvement by meningioma may manifest as either erosion or hyperostosis and is best appreciated on CT. Visualization of affected bone not only suggests the point of origin of the meningioma but is also important to recognize to ensure total removal of potentially invasive tumor cells for long-term disease control, as removal of bulk tumor, involved dura, and affected bone remains one of the most powerful influences on preventing recurrence in meningioma.109 SSTR-PET can add valuable information to plan the extent of resection especially. Specific considerations and imaging modalities are detailed below.
Vascularity
Keen awareness of vasculature is critical to treatment planning for a presumed meningioma. First, the extent and source of vascular supply to meningioma dictates the surgical approach and preoperative consideration for embolization. Early devascularization is a critical step in surgery for meningioma and promotes the ease of resection while minimizing blood loss. Meningiomas derive their major blood supply from branches of the external carotid artery or meningeal branches of the internal carotid and vertebrobasilar arteries, which may be clearly visualized on MRI, CT angiography (CTA), or catheter-based angiography. In general, convexity, parasagittal, and sphenoid wing meningiomas are primarily supplied by middle meningeal artery branches; olfactory groove meningiomas are supplied by branches of the ethmoidal arteries along with dural branches of the internal carotid artery. Lateral posterior fossa meningiomas are generally supplied by branches of the occipital and ascending pharyngeal artery, whereas posteromedial posterior fossa meningiomas are typically fed by meningeal branches of the vertebral artery and the posterior meningeal artery. By nature of their central anatomical location, clival and tentorial meningiomas may receive their blood supply from various feeders such as the tentorial artery, cavernous internal carotid artery, and middle meningeal artery.
For hypervascular meningiomas, branches that are difficult to reach during the exposure may be important to recognize and consider for preoperative embolization. On occasion, abundant intratumoral vascularity in an extra-axial intracranial tumor may suggest a diagnosis of hemangiopericytoma, rather than meningioma, which mandates a surgical technique that avoids precipitating significant intraoperative blood loss and possible preoperative embolization. Additionally, dynamic CT angiogram/venogram (dCTA/V) offers visualization of arterial and venous flow in a time- and phase-resolved fashion, similar to catheter angiography, and provides a unique vantage point in preoperative planning of skull base or paravenous meningiomas.8
Second, delineation of traversing vessels which pass through or along the surface of the tumor to supply normal brain rather than the tumor itself is important to avoid intra- and postoperative ischemia. This may be appreciated on T2-weighted and postcontrast T1-weighted MRI sequences or CTA, which allow for visualization of the tumor mass in relation to the vessels. Prominent curvilinear signal void or enhancement within or surrounding the tumor mass may suggest high tumor vascularity, and the intra- or peritumoral vascular anatomy including presence of thrombosis can be delineated using an imaging modality with temporal resolution, such as dCTA, MR angiography, or catheter angiography. Distinction between a high-flow state versus intraluminal thrombosis is important to distinguish before venturing into tumor debulking (Figure 4). Preservation of en passage vessels is also critical to avoid unintentional ischemia in the surrounding brain.
Third, clear appreciation of partial or complete venous sinus occlusion, as well as the course of venous rerouting along the periphery of sinus-invasive meningiomas, influences the operative strategy and preparation for sinus reconstruction or avoidance with use of a staged surgery and radiosurgery approach.110,111 While catheter-based angiography affords the highest specificity and sensitivity in determining sinus patency, its cost and risk, albeit low, decrease its utility compared with less invasive modalities. CT venogram (CTV) and MR venogram (MRV) are frequently performed in this context, with varying sensitivity and specificity. In comparison, dCTA/V provides 3D flow-dependent resolution of venous sinuses and alternative venous channels, allowing for the advantage of catheter-based angiography in a non-invasive test.
Tracts and nerves
As extrinsic tumors which arise from outside the brain parenchyma, meningiomas classically displace rather than disrupt or infiltrate into adjacent white matter tracts. For meningiomas abutting the corticospinal tract, reduction in diffusion tensor tractography fiber number and density, but not deviation, correlated with postoperative temporary paresis in one study.112 While such imaging analysis suggests a means for risk stratification in meningioma surgery, it does not obviate the contribution of surgical influences on postoperative function, including technique, presence, and treatment of the arachnoid plane around the meningioma, and extent of traction during tumor resection. Tractography has also been applied to resolve the course of cranial nerves from their nucleus of origin to foramen of exit, as well as the optic chiasm and pathway, in relation to skull base tumors; however, this is subject to distortion by large tumors and the computational algorithms applied to resolve the tracts. Among conventional MRI sequences, cranial nerves within cisternal spaces are best appreciated on high-resolution CSF-sensitive sequences such as constructive interference in steady state (CISS), with their identification critical in planning safe resection of skull base meningiomas.
Tumor consistency
The texture of meningiomas, especially those at the cranial base or which surround neurovascular structures, influences the complexity, time, and risk during surgery for meningioma; it is among the most critical of surgical variables and can be very difficult to ascertain preoperatively from imaging alone. Soft lesions are more amenable to suction aspiration than are tough, fibrous lesions; should firm lesions surround vessels and/or nerves, it may be far more challenging to dissect these normal structures.
On conventional MRI, meningioma hyperintensity on T2-weighted images and hypointensity on T1-weighted images serve as a surrogate for softer texture, while T2-weighted hypointensity suggests firmer tumor, although the predictive value can be inconsistent.113,114 Additionally, the association of DWI with meningioma texture has been variable, with high FA values, hyperintensity on FA maps, and isointensity on mean diffusivity maps suggestive of firmer texture in some studies.114,115
Complementary to anatomic imaging, MRe is a developing technology which shows promise in determining tumor firmness and its relationship with adjacent structures.116 Specifically, MRe captures the tissue response after delivering a source of motion and then mathematically calculates a viscoelastic model that estimates firmness. Differing stiffness on either side of a tissue boundary generates a “slip” interface, or a measurement of the freedom with which adjacent tissue planes can slide past one another and create an estimate of marginal invasiveness. In clinical series of meningioma which underwent surgical resection, prediction of tumor firmness by MRe correlated with intraoperative observations in two-third of cases, with greater error for vascular and small tumors.117 Shear line and octahedral shear strain (OSS) values derived from MRe sequences concurred with intraoperative annotation of tumor adhesion with the brain in 72% of cases, while normalized OSS values offered over 90% concordance with surgical observations.118 Note that MRe is suboptimal in the evaluation of small meningiomas given its limited spatial resolution.
Imaging Considerations for Radiation Therapy
MRI and CT play a tantamount role in demarcation of meningiomas for radiation planning. While tumor bulk and dural involvement are well appreciated on gadolinium-enhanced T1 MRI, fat-saturation sequences are particularly useful for delineation of meningioma boundaries at the skull base and abutting venous sinuses. Thin-slice 3D views, especially the coronal plane, are important for fine separation of optic apparatus, brainstem, and other sensitive structures during treatment plan contouring. Tractography and high-resolution CSF-sensitive sequences such as CISS can also be critical in helping identify cranial nerves during planning.
Molecular Imaging and Targeted Therapy Considerations
Regarding meningioma delineation, several different tissues are to be respected as background (eg, brain, bone, blood; in the case of pretreated lesions, fibrotic tissue). Due to usually high levels of glucose in healthy brain parenchyma causing a poor tumor-to-background contrast, the tracer 18F-FDG is not suitable for precise tumor delineation.119 In contrast, because of its high specificity, SSTR-PET can add valuable information to plan the extent of resection. Especially in tumors with transosseous growth and complex location and in pretreated lesions, sensitivity and specificity are higher compared with MRI (Supplementary Figure 2). Thus, the surgeon can tailor the resection more accordingly. Integration of PET imaging into neuronavigation systems allows for retrieval of this information even without additional intraoperative imaging.120 Similarly, precision of targeting radiation can be improved especially in pretreated or transosseous lesions.121–123
Finally, the first centers are currently investigating the therapeutic approach with DOTA-conjugated SSTR ligands in “out-treated” meningioma patients under progression after several lines of therapy.124–127 Because the same SSTR ligand can either be radiolabeled with the positron emitter Ga-68 for PET imaging or with the ß-emitters lutetium-177 and yttrium-90 for peptide receptor radionuclide therapy (PRRT), semi-quantitative SSTR-PET measurement prior to PRRT is considered to be crucial for an adequate patient selection, since higher SSTR expression as measured by PET was shown to be associated with higher radiation dose in the tumor.128 Even more, tracer uptake and response correlated in patients with multiple lesions.129
Posttreatment Imaging Considerations
Postsurgical Artifacts to Consider on Conventional MRI
When achievable, total surgical resection (Simpson grade 1) is associated with lower recurrence and longer overall survival (OS) for benign, atypical, and malignant meningiomas.130–132 Besides providing prognostic value, extent of resection also provides predictive value for early adjuvant radiation treatment of atypical meningioma, since there is no benefit of survival or recurrence for patients with gross total surgical resection (Simpson grades 1–3) of those tumors.133 Evaluation of residual meningioma is most commonly done on contrast enhanced MRI performed within 24–72 hours after surgery. After 72 hours, contrast enhancement from granulation tissues at the resection site often begins to develop, making it difficult to distinguish from residual tumor.134 More recently, presence of non–tumor related reactive enhancement can be detected between 48 and 72 hours on 3T MRI,135,136 although reactive changes tend to appear linear, while residual tumor enhancement is often nodular.136 Novel radiotracer imaging such as 68Ga-DOTATATE PET appears to be more specific for residual meningioma and can be considered when MRI is equivocal.137,138
During the immediate postoperative period, significant susceptibility artifact is common due to the presence of air and blood product at the resection site obscuring sites of enhancing tumor. To reduce susceptibility artifact related to surgery or at locations near the skull base, 3D spin echo MR technique is preferred over 3D gradient echo techniques.139 It is advantageous to apply a fat-suppression technique to the acquisition pulse sequence to distinguish enhancing tissues from fat graft material. Fat suppression also allows delineation of tumor from marrow or extracranial fat if meningiomas extend to the calvarium, skull base, or extracranial structures.
Posttreatment Follow-Up Guidelines
Even though MRI is routinely performed for following meningiomas after surgery, radiation, or systemic therapy, consensus for the frequency of imaging follow-up is lacking. Moreover, the degree of progression or growth of residual tumor that is clinically relevant to initiate a second therapeutic intervention is not known. The National Comprehensive Cancer Network provides a guideline for monitoring WHO grades I and II meningiomas at 3, 6, and 12 months after initial surgery or radiation, followed by every 6–12 months for 5 years, and then every 1–3 years as clinically indicated (NCCN version 1.2018). However, meningiomas tend to exhibit delayed recurrence on the order of 10 to 20 years,109 and require long-term vigilance in a young patient. For WHO grade III meningiomas, and for meningiomas of any grade that are treated for recurrence or with chemotherapy, more frequent imaging may be necessary and may depend on symptoms and expected risk of recurrence. One challenge in defining optimal follow-up imaging frequency for meningiomas is the variability in their growth trajectories. In one series, atypical meningiomas grew exponentially, while benign meningiomas exhibited exponential, linear, or no growth.140 For benign meningiomas, a sigmoid growth curve has been used to model the alteration of growth pattern over time for meningiomas that eventually slow or stop growing.141 For this group of patients, less frequent imaging may be appropriate once the growth rate appears to plateau. Slowing of growth rate has been associated with the appearance of intratumoral calcification.140 For meningiomas that grow exponentially, the period of accelerated growth is associated with increase in the Ki-67 proliferative index.142 It has been shown that tumor ADC values on DWI are associated with Ki-67 index,143 and meningiomas with low ADC are also at significantly increased risk of recurrence.106,144 This marker may potentially be used to monitor tumor proliferation over time. As a general rule, surveillance imaging schemes after surgery vary based on the perceived risk of recurrence, with regrowth firmly predicated on inherent biological tumor characteristics (eg, grade, MIB-1) and the completeness of surgical resection.131,145 Given that distinct DNA methylation signatures have been shown to identify distinct risk groups, it is possible that the frequency of surveillance imaging may ultimately be governed by the molecular alterations of a given tumor (see the Biomolecular Landscape paper elsewhere in this supplement).
Response Criteria for Clinical Trials of Meningiomas
Currently, no standard imaging criteria exist for determining response or progression in clinical trials of meningioma. Variations of 1D (Response Evaluation Criteria In Solid Tumors)146 and 2D (Macdonald) criteria147 have been used in previous clinical trials, although these imaging criteria have been developed for clinical trials of systemic tumors and high-grade gliomas. Unlike in these cancers, OS is often very long for meningioma, and even progression-free survival requires long-term follow-up. To capture more tumors that exhibit small changes, the Response Assessment in Neuro-Oncology (RANO) Meningioma working group has proposed a modification of 2D criteria to include a 25% reduction in dimensional product of tumor area as “minor response” (RANO Meningioma paper under review), which will need to be validated in future trials. In addition to 1D and 2D measurements, volumetric approach has been evaluated as response criteria for meningiomas (meningioma paper under review). Due to the often irregular contour of meningiomas that conform to the cranial vault or skull base, a volumetric approach is expected to be more accurate in depicting tumor burden than 1D or 2D cross-sectional measurements. Making 1D and 2D measurements can be quite variable in irregularly shaped tumors, and they may inadequately represent the size of irregular tumors. Since it is technically much more challenging to obtain tumor volume, this approach may not be easily implemented in clinical trials at the present and require more data in validating its advantage over cross-sectional methods.
Treatment Effect versus True Progression or Response
While radiation necrosis can be observed following radiation therapy, and stereotactic radiosurgery in particular, for meningiomas, it is typically feasible to distinguish treatment effect from tumor on MRI, as the former will appear as intra-axial enhancement rather than as dural thickening or nodular enhancement observed with residual or recurrent meningiomas. In a small subset of atypical or malignant meningiomas with brain invasion, it can be challenging to discern tumor from treatment necrosis at the brain–tumor interface and requires serial MRI to determine growth of the enhancing regions.
The anti-angiogenic agent bevacizumab has been evaluated for treatment of meningiomas.148–150 Due to its effect on vascular permeability, bevacizumab can reduce the intensity of contrast enhancement within a tumor, a phenomenon commonly observed among high-grade gliomas receiving this treatment. Since meningiomas are primarily measured by the size of contrast enhancement, it is possible that perceived reductions in tumor burden could result from contrast enhancement suppression by treatment rather than actual tumor shrinkage.
Perspectives on the Future of Imaging in the Management of Meningiomas
A multitude of imaging features based on CT, MRI, and nuclear medicine techniques show promising values in the clinical management of meningiomas, including diagnosis, grading, treatment planning, prognostication, and treatment monitoring. These imaging markers are derived from novel methods of acquisition, postprocessing, and image feature extraction. Dual-energy CT has also been applied to differentiate meningiomas from pituitary adenomas of the sella/tuberculum.151 High magnetic field scanners at 7T provide more detailed delineation of peritumoral vascularity and brain–tumor interface that may facilitate surgical planning.152 Amide proton transfer imaging is a novel MRI method based on endogenous mobile proteins and peptides in tissue and has been applied to differentiate atypical meningiomas from benign meningiomas.153 In order for widespread clinical implementations of these imaging techniques, their accuracy and reproducibility need to be validated within well-defined patient populations, tumor subtypes, and treatment modalities.
While many imaging markers evaluated to date show correlations with biological characteristics of meningioma, patient outcome, or both, there is a significant degree of heterogeneity among the reported accuracies when they are used as diagnostic or prognostic makers. For example, reports on the use of low preoperative ADC to predict higher histological grade and greater risk of recurrence differed in their diagnostic performance and the threshold values for ADC. These differences can be due to variations in imaging acquisition and postprocessing method as well as patient selection. Other potential confounding factors include the effect of susceptibility from calcifications within tumors, which can alter ADC measurements.154 Since calcification may be suggestive of meningiomas with more indolent clinical course, low ADC values resulting from calcifications may result in misclassification of these tumors to higher grade and higher risk of recurrence.
PET imaging might gain further importance—however, more prospective multi-institutional studies for validation are in need. Therefore, technical guidelines for imaging acquisition and readout are necessary, which are currently being developed by the RANO-PET task force. Introduction of new, specific ligands/tracers might open new avenues for metabolic imaging in meningiomas.
Strategies that combine multiple imaging features to generate diagnostic and predictive markers are therefore more likely to provide a more comprehensive assessment of tumor biology. Recent radiomic approaches in combination with machine learning algorithms have been increasingly employed to generate and refine imaging markers.9 One major advantage of this approach is its ability to incorporate clinical, anatomical, genomic features into a combined model. Due to inter-institutional variations of imaging acquisition equipment, imaging protocol, and postprocessing method, this approach requires a large sample size for model training to achieve high accuracy, and the resultant models also need to be validated for generalizability using independent datasets. A public database for meningioma including imaging, clinical, and genomic data is urgently needed.
Conclusion
The central role of imaging in the contemporary treatment and management of meningioma is in its descriptive strength; its ability to inform location, growth over time, effect on adjacent tissues, and adjacency to critical structures, for instance, is vital. The predictive power of imaging, however, is in its infancy. With advances in our understanding of the fundamental biology of meningioma, targeted and immunologic approaches are being developed and evaluated for treatment in recurrent disease to complement current standard approaches of surgery and radiation therapy. In this era, imaging will be relied upon for the non-invasive determination of factors such as histologic grade and genetic constitution and, ultimately, for the prediction of tumor behavior, whose influence on management strategies cannot be underestimated.
Recommendations
With respect to advances in imaging and diagnosis, the International Consortium on Meningiomas recommends:
• Use of routine imaging modalities for diagnosis and surveillance of meningiomas
• Use of standardized definition for tumor progression or recurrence (which will be established through the RANO working group) across all centers for both clinical and research use
• In cases where the certainty of recurrence or tumor progression is equivocal, advancing imaging modalities such as PET may be considered.
Funding
The collaborative effort of the International Consortium on Meningiomas (ICOM) is supported by The Brain Tumour Charity Quest for Cures: Collaborative Team Award and the Canadian Institute of Health Research.
Conflict of interest statement. J.C.T. was on the speaker’s bureau of and received honoraria by BrainLab company.
Authorship statement. The generation of all manuscripts has been supported by the membership of the consortium, which at the time of supplement generation includes: Kenneth Aldape, Karolyn Au, Jill Barnhartz-Sloan, Wenya Linda Bi, Priscilla K. Brastianos, Nicholas Butowski, Carlos Carlotti, Michael D. Cusimano, Francesco DiMeco, Katharine Drummond, Ian F. Dunn, Evanthia Galanis, Caterina Giannini, Roland Goldbrunner, Brent Griffith, Rintaro Hashizume, C. Oliver Hanemann, Christel Herold-Mende, Craig Horbinski, Raymond Y. Huang, David James, Michael D. Jenkinson, Christine Jungk, Timothy J. Kaufman, Boris Krischek, Daniel Lachance, Christian Lafougère, Ian Lee, Jeff C. Liu, Yasin Mamatjan, Alireza Mansouri, Christian Mawrin, Michael McDermott, David Munoz, Farshad Nassiri, Houtan Noushmehr, Ho-Keung Ng, Arie Perry, Farhad Pirouzmand, Laila M Poisson, Bianca Pollo, David Raleigh, Felix Sahm, Andrea Saladino, Thomas Santarius, Christian Schichor, David Schultz, Nils O. Schmidt, Warren Selman, Andrew Sloan, Julian Spears, James Snyder, Suganth Suppiah, Ghazaleh Tabatabai, Marcos Tatagiba, Daniela Tirapelli, Joerg C. Tonn, Derek Tsang, Michael A. Vogelbaum, Andreas von Deimling, Patrick Y. Wen, Tobias Walbert, Manfred Westphal, Adriana M. Workewych, Gelareh Zadeh.
Sponsorship statement. This supplement was supported by an unrestricted grant from the MacFeeters Hamilton Neuro-Oncology Program at the Princess Margaret Cancer Center and by the Dr. Mary Hunter Brain Tumor Research Funds from the Toronto General & Western Hospital Foundation.
Supplementary Material
Contributor Information
International Consortium on Meningiomas:
Kenneth Aldape, Karolyn Au, Jill Barnhartz-Sloan, Wenya Linda Bi, Priscilla K Brastianos, Nicholas Butowski, Carlos Carlotti, Michael D Cusimano, Francesco DiMeco, Katharine Drummond, Ian F Dunn, Evanthia Galanis, Caterina Giannini, Roland Goldbrunner, Brent Griffith, Rintaro Hashizume, C Oliver Hanemann, Christel Herold-Mende, Craig Horbinski, Raymond Y Huang, David James, Michael D Jenkinson, Christine Jungk, Timothy J Kaufman, Boris Krischek, Daniel Lachance, Christian Lafougère, Ian Lee, Jeff C Liu, Yasin Mamatjan, Alireza Mansouri, Christian Mawrin, Michael McDermott, David Munoz, Farshad Nassiri, Houtan Noushmehr, Ho-Keung Ng, Arie Perry, Farhad Pirouzmand, Laila M Poisson, Bianca Pollo, David Raleigh, Felix Sahm, Andrea Saladino, Thomas Santarius, Christian Schichor, David Schultz, Nils O Schmidt, Warren Selman, Andrew Sloan, Julian Spears, James Snyder, Suganth Suppiah, Ghazaleh Tabatabai, Marcos Tatagiba, Daniela Tirapelli, Joerg C Tonn, Derek Tsang, Michael A Vogelbaum, Andreas von Deimling, Patrick Y Wen, Tobias Walbert, Manfred Westphal, Adriana M Workewych, and Gelareh Zadeh
References
- 1. Cushing H. Meningiomas. Springfield, IL: Thomas; 1938. [Google Scholar]
- 2. Mills CK, Pfahler GE. Tumor of the brain localized clinically and by the roentgen rays. PMJ. 1902;9:268–273. [Google Scholar]
- 3. Sosman MC, Putnam TJ. Roentgenological aspects of brain tumors: meningiomas. Am J Roentgenol. 1925;13:1–12. [Google Scholar]
- 4. Moniz EP, Pinto A, Lima A. Le diagnostic differentiel entre les meningiomes et les autres tumeurs cerebrales par l’epreuve de l’encephalographie arterielle. Revista de Neurologia. 1929;1:1126–1135. [Google Scholar]
- 5. List CF, Hodges FJ. Differential diagnosis of intracranial neoplasms by cerebral angiography. Radiology. 1947;48(5):493–508. [DOI] [PubMed] [Google Scholar]
- 6. Hounsfield GN. Computerized transverse axial scanning (tomography). 1. Description of system. Br J Radiol. 1973;46(552):1016–1022. [DOI] [PubMed] [Google Scholar]
- 7. Lauterbur P. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 8. Bi WL, Brown PA, Abolfotoh M, Al-Mefty O, Mukundan S Jr, Dunn IF. Utility of dynamic computed tomography angiography in the preoperative evaluation of skull base tumors. J Neurosurg. 2015;123(1):1–8. [DOI] [PubMed] [Google Scholar]
- 9. Coroller TP, Bi WL, Huynh E, et al. . Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS One. 2017;12(11): e0187908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buetow MP, Buetow PC, Smirniotopoulos JG. Typical, atypical, and misleading features in meningioma. Radiographics. 1991;11(6):1087–1106. [DOI] [PubMed] [Google Scholar]
- 11. Magill ST, Young JS, Chae R, Aghi MK, Theodosopoulos PV, McDermott MW. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg Focus. 2018;44(4):E4. [DOI] [PubMed] [Google Scholar]
- 12. Wang DJ, Xie Q, Gong Y, et al. . Histopathological classification and location of consecutively operated meningiomas at a single institution in China from 2001 to 2010. Chin Med J. 2013;126(3):488–493. [PubMed] [Google Scholar]
- 13. Lang FF, Macdonald OK, Fuller GN, DeMonte F. Primary extradural meningiomas: a report on nine cases and review of the literature from the era of computerized tomography scanning. J Neurosurg. 2000;93(6):940–950. [DOI] [PubMed] [Google Scholar]
- 14. Chen TC. Primary intraosseous meningioma. Neurosurg Clin N Am. 2016;27(2):189–193. [DOI] [PubMed] [Google Scholar]
- 15. Lusins JO, Nakagawa H. Multiple meningiomas evaluated by computed tomography. Neurosurgery. 1981;9(2):137–141. [DOI] [PubMed] [Google Scholar]
- 16. O’Leary S, Adams WM, Parrish RW, Mukonoweshuro W. Atypical imaging appearances of intracranial meningiomas. Clin Radiol. 2007;62(1):10–17. [DOI] [PubMed] [Google Scholar]
- 17. Sheporaitis LA, Osborn AG, Smirniotopoulos JG, Clunie DA, Howieson J, D’Agostino AN. Intracranial meningioma. AJNR Am J Neuroradiol. 1992;13(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 18. Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107(5):905–912. [DOI] [PubMed] [Google Scholar]
- 19. Tamrazi B, Shiroishi MS, Liu CS. Advanced imaging of intracranial meningiomas. Neurosurg Clin N Am. 2016;27(2):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aoki S, Sasaki Y, Machida T, Tanioka H. Contrast-enhanced MR images in patients with meningioma: importance of enhancement of the dura adjacent to the tumor. AJNR Am J Neuroradiol. 1990;11(5):935–938. [PMC free article] [PubMed] [Google Scholar]
- 21. Wen M, Jung S, Moon KS, et al. . Immunohistochemical profile of the dural tail in intracranial meningiomas. Acta Neurochir (Wien). 2014;156(12):2263–2273. [DOI] [PubMed] [Google Scholar]
- 22. Demir MK, Iplikcioglu AC, Dincer A, Arslan M, Sav A. Single voxel proton MR spectroscopy findings of typical and atypical intracranial meningiomas. Eur J Radiol. 2006;60(1):48–55. [DOI] [PubMed] [Google Scholar]
- 23. Kousi E, Tsougos I, Fountas K, et al. . Distinct peak at 3.8 ppm observed by 3T MR spectroscopy in meningiomas, while nearly absent in high-grade gliomas and cerebral metastases. Mol Med Rep. 2012;5(4):1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223(1):11–29. [DOI] [PubMed] [Google Scholar]
- 25. Hakyemez B, Yildirim N, Erdoğan C, Kocaeli H, Korfali E, Parlak M. Meningiomas with conventional MRI findings resembling intraaxial tumors: can perfusion-weighted MRI be helpful in differentiation?Neuroradiology. 2006;48(10):695–702. [DOI] [PubMed] [Google Scholar]
- 26. Koizumi S, Sakai N, Kawaji H, et al. . Pseudo-continuous arterial spin labeling reflects vascular density and differentiates angiomatous meningiomas from non-angiomatous meningiomas. J Neurooncol. 2015;121(3):549–556. [DOI] [PubMed] [Google Scholar]
- 27. Qiao XJ, Kim HG, Wang DJJ, et al. . Application of arterial spin labeling perfusion MRI to differentiate benign from malignant intracranial meningiomas. Eur J Radiol. 2017;97:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keil VC, Pintea B, Gielen GH, et al. . Meningioma assessment: kinetic parameters in dynamic contrast-enhanced MRI appear independent from microvascular anatomy and VEGF expression. J Neuroradiol. 2018;45(4):242–248. [DOI] [PubMed] [Google Scholar]
- 29. Russell EJ, George AE, Kricheff II, Budzilovich G. Atypical computed tomography features of intracranial meningioma: radiological-pathological correlation in a series of 131 consecutive cases. Radiology. 1980;135(3):673–682. [DOI] [PubMed] [Google Scholar]
- 30. Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999;85(4):936–944. [DOI] [PubMed] [Google Scholar]
- 31. Lee KJ, Joo WI, Rha HK, et al. . Peritumoral brain edema in meningiomas: correlations between magnetic resonance imaging, angiography, and pathology. Surg Neuro. 2008;69(4):350–355; discussion 355. [DOI] [PubMed] [Google Scholar]
- 32. Go KG, Wilmink JT, Molenaar WM. Peritumoral brain edema associated with meningiomas. Neurosurgery. 1988;23(2):175–179. [DOI] [PubMed] [Google Scholar]
- 33. Tamiya T, Ono Y, Matsumoto K, Ohmoto T. Peritumoral brain edema in intracranial meningiomas: effects of radiological and histological factors. Neurosurgery. 2001;49(5):1046–1051; discussion 1051–1052. [DOI] [PubMed] [Google Scholar]
- 34. Ostrom QT, Gittleman H, Fulop J, et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015; 17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prayson RA, Rowe JJ. Dural-based Rosai-Dorfman disease: differential diagnostic considerations. J Clin Neurosci. 2014;21(11):1872–1873. [DOI] [PubMed] [Google Scholar]
- 36. Starr CJ, Cha S. Meningioma mimics: five key imaging features to differentiate them from meningiomas. Clin Radiol. 2017;72(9):722–728. [DOI] [PubMed] [Google Scholar]
- 37. Wong WL, Campbell H, Saunders M. Positron emission tomography (PET)–evaluation of ‘indeterminate pulmonary lesions’. Clin Oncol (R Coll Radiol). 2002;14(2):123–128. [DOI] [PubMed] [Google Scholar]
- 38. Lee JW, Kang KW, Park SH, et al. . 18F-FDG PET in the assessment of tumor grade and prediction of tumor recurrence in intracranial meningioma. Eur J Nucl Med Mol Imaging. 2009;36(10):1574–1582. [DOI] [PubMed] [Google Scholar]
- 39. la Fougère C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13(8):806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barresi V, Alafaci C, Salpietro F, et al. . Sstr2A immunohistochemical expression in human meningiomas: is there a correlation with the histological grade, proliferation or microvessel density. Oncol Rep. 2008;20(3):485–492. [PubMed] [Google Scholar]
- 41. Dutour A, Kumar U, Panetta R, et al. . Expression of somatostatin receptor subtypes in human brain tumors. Int J Cancer. 1998;76(5):620–627. [DOI] [PubMed] [Google Scholar]
- 42. Grzbiela H, Tarnawski R, D’Amico A, Stąpór-Fudzińska M. The uUse of 68Ga-DOTA-(Tyr3)-octreotate PET/CT for improved target definition in radiotherapy treatment planning of meningiomas—a case report. Curr Radiopharm. 2015;8(1):45–48. [DOI] [PubMed] [Google Scholar]
- 43. Zollner BD, Vettermann F, Ilhan H, et al. . [Ga-68]DOTATATE-PET-based target volume definition in meningiomas WHO grade I. Strahlenther Onkol. 2017;193:S152–S152. [Google Scholar]
- 44. Afshar-Oromieh A, Giesel FL, Linhart HG, et al. . Detection of cranial meningiomas: comparison of ⁶⁸Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging. 2012;39(9):1409–1415. [DOI] [PubMed] [Google Scholar]
- 45. Klingenstein A, Haug AR, Miller C, Hintschich C. Ga-68-DOTA-TATE PET/CT for discrimination of tumors of the optic pathway. Orbit. 2015;34(1):16–22. [DOI] [PubMed] [Google Scholar]
- 46. Galldiks N, Albert NL, Sommerauer M, et al. . PET imaging in patients with meningioma—report of the RANO/PET Group. Neuro Oncol. 2017;19(12):1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kunz WG, Jungblut LM, Kazmierczak PM, et al. . Improved detection of transosseous meningiomas using 68Ga-DOTATATE PET/CT compared with contrast-enhanced MRI. J Nucl Med. 2017;58(10):1580–1587. [DOI] [PubMed] [Google Scholar]
- 48. Rachinger W, Thon N, Haug A, Schuller U, Schichor C, Tonn JC. Increased [Ga-68]-DOTATOC uptake in pet imaging discriminates meningioma and tumorfree tissue. Neuro-oncology. 2013;15:203-203. [Google Scholar]
- 49. Goldbrunner R, Minniti G, Preusser M, et al. . EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 50. Huttner HB, Bergmann O, Salehpour M, et al. . Meningioma growth dynamics assessed by radiocarbon retrospective birth dating. EBioMedicine. 2018;27:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jääskeläinen J, Haltia M, Laasonen E, Wahlström T, Valtonen S. The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg Neurol. 1985;24(2):165–172. [DOI] [PubMed] [Google Scholar]
- 52. Nakamura M, Roser F, Michel J, Jacobs C, Samii M. Volumetric analysis of the growth rate of incompletely resected intracranial meningiomas. Zentralbl Neurochir. 2005;66(1):17–23. [DOI] [PubMed] [Google Scholar]
- 53. Nakasu S, Fukami T, Nakajima M, Watanabe K, Ichikawa M, Matsuda M. Growth pattern changes of meningiomas: long-term analysis. Neurosurgery. 2005;56(5):946–955; discussion 946. [PubMed] [Google Scholar]
- 54. Nakasu S, Nakasu Y, Fukami T, Jito J, Nozaki K. Growth curve analysis of asymptomatic and symptomatic meningiomas. J Neurooncol. 2011;102(2):303–310. [DOI] [PubMed] [Google Scholar]
- 55. Soon WC, Fountain DM, Koczyk K, et al. . Correlation of volumetric growth and histological grade in 50 meningiomas. Acta Neurochirurgica. 2017;159(11):1–9. [DOI] [PubMed] [Google Scholar]
- 56. Fountain DM, Soon WC, Matys T, Guilfoyle MR, Kirollos R, Santarius T. Volumetric growth rates of meningioma and its correlation with histological diagnosis and clinical outcome: a systematic review. Acta Neurochirurgica. 2017;159(3):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee EJ, Kim JH, Park ES, et al. . A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J Neurosurg. 2017;127(5):971–980. [DOI] [PubMed] [Google Scholar]
- 58. Hashiba T, Hashimoto N, Izumoto S, et al. . Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. J Neurosurg. 2009;110(4):675–684. [DOI] [PubMed] [Google Scholar]
- 59. Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, Parsa AT. Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg. 2010;113(5):1036–1042. [DOI] [PubMed] [Google Scholar]
- 60. Chang V, Narang J, Schultz L, et al. . Computer-aided volumetric analysis as a sensitive tool for the management of incidental meningiomas. Acta Neurochir (Wien). 2012;154(4):589–597; discussion 597. [DOI] [PubMed] [Google Scholar]
- 61. Nakamura M, Roser F, Michel J, Jacobs C, Samii M. The natural history of incidental meningiomas. Neurosurgery. 2003;53(1):62–70; discussion 70. [DOI] [PubMed] [Google Scholar]
- 62. Oya S, Kim SH, Sade B, Lee JH. The natural history of intracranial meningiomas. J Neurosurg. 2011;114(5):1250–1256. [DOI] [PubMed] [Google Scholar]
- 63. Jadid KD, Feychting M, Höijer J, Hylin S, Kihlström L, Mathiesen T. Long-term follow-up of incidentally discovered meningiomas. Acta Neurochir (Wien). 2015;157(2):225–30; discussion 230. [DOI] [PubMed] [Google Scholar]
- 64. Hashiba T, Hashimoto N, Maruno M, et al. . Scoring radiologic characteristics to predict proliferative potential in meningiomas. Brain Tumor Pathol. 2006;23(1):49–54. [DOI] [PubMed] [Google Scholar]
- 65. Hashimoto N, Rabo CS, Okita Y, et al. . Slower growth of skull base meningiomas compared with non-skull base meningiomas based on volumetric and biological studies. J Neurosurg. 2012;116(3):574–580. [DOI] [PubMed] [Google Scholar]
- 66. Lee EJ, Park JH, Park ES, Kim JH. “Wait-and-see” strategies for newly diagnosed intracranial meningiomas based on the risk of future observation failure. World Neurosurg. 2017;107:604–611. [DOI] [PubMed] [Google Scholar]
- 67. Nakasu S, Nakajima M, Matsumura K, Nakasu Y, Handa J. Meningioma: proliferating potential and clinicoradiological features. Neurosurgery. 1995;37(6):1049–1055. [DOI] [PubMed] [Google Scholar]
- 68. Kasuya H, Kubo O, Tanaka M, Amano K, Kato K, Hori T. Clinical and radiological features related to the growth potential of meningioma. Neurosurg Rev. 2006;29(4):293–296; discussion 296–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kawahara Y, Nakada M, Hayashi Y, et al. . Prediction of high-grade meningioma by preoperative MRI assessment. J Neurooncol. 2012;108(1):147–152. [DOI] [PubMed] [Google Scholar]
- 70. Lin BJ, Chou KN, Kao HW, et al. . Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg. 2014;121(5):1201–1208. [DOI] [PubMed] [Google Scholar]
- 71. Fountain DM, Soon WC, Matys T, Guilfoyle MR, Kirollos R, Santarius T. Volumetric growth rates of meningioma and its correlation with histological diagnosis and clinical outcome: a systematic review. Acta Neurochir (Wien). 2017;159(3):435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Soon WC, Fountain DM, Koczyk K, et al. . Correlation of volumetric growth and histological grade in 50 meningiomas. Acta Neurochir (Wien). 2017;159(11):2169–2177. [DOI] [PubMed] [Google Scholar]
- 73. Mahmood A, Caccamo DV, Tomecek FJ, Malik GM. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. 1993;33(6):955–963. [DOI] [PubMed] [Google Scholar]
- 74. Tang Y, Dundamadappa SK, Thangasamy S, et al. . Correlation of apparent diffusion coefficient with Ki-67 proliferation index in grading meningioma. AJR Am J Roentgenol. 2014;202(6):1303–1308. [DOI] [PubMed] [Google Scholar]
- 75. Hakyemez B, Yildirim N, Gokalp G, Erdogan C, Parlak M. The contribution of diffusion-weighted MR imaging to distinguishing typical from atypical meningiomas. Neuroradiology. 2006;48(8):513–520. [DOI] [PubMed] [Google Scholar]
- 76. Nagar VA, Ye JR, Ng WH, et al. . Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol. 2008;29(6):1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sanverdi SE, Ozgen B, Oguz KK, et al. . Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas?Eur J Radiol. 2012;81(9):2389–2395. [DOI] [PubMed] [Google Scholar]
- 78. Filippi CG, Edgar MA, Uluğ AM, Prowda JC, Heier LA, Zimmerman RD. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR Am J Neuroradiol. 2001;22(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- 79. Ginat DT, Mangla R, Yeaney G, Wang HZ. Correlation of diffusion and perfusion MRI with Ki-67 in high-grade meningiomas. AJR Am J Roentgenol. 2010;195(6):1391–1395. [DOI] [PubMed] [Google Scholar]
- 80. Pavlisa G, Rados M, Pazanin L, Padovan RS, Ozretic D, Pavlisa G. Characteristics of typical and atypical meningiomas on ADC maps with respect to schwannomas. Clin Imaging. 2008;32(1):22–27. [DOI] [PubMed] [Google Scholar]
- 81. Santelli L, Ramondo G, Della Puppa A, et al. . Diffusion-weighted imaging does not predict histological grading in meningiomas. Acta Neurochir (Wien). 2010;152(8):1315–1319; discussion 1319. [DOI] [PubMed] [Google Scholar]
- 82. Surov A, Gottschling S, Mawrin C, et al. . Diffusion-weighted imaging in meningioma: prediction of tumor grade and association with histopathological parameters. Transl Oncol. 2015;8(6):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Toh CH, Castillo M, Wong AM, et al. . Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29(9):1630–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yamasaki F, Kurisu K, Satoh K, et al. . Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235(3):985–991. [DOI] [PubMed] [Google Scholar]
- 85. Yin B, Liu L, Zhang BY, Li YX, Li Y, Geng DY. Correlating apparent diffusion coefficients with histopathologic findings on meningiomas. Eur J Radiol. 2012;81(12):4050–4056. [DOI] [PubMed] [Google Scholar]
- 86. Watanabe Y, Yamasaki F, Kajiwara Y, et al. . Preoperative histological grading of meningiomas using apparent diffusion coefficient at 3T MRI. Eur J Radiol. 2013;82(4):658–663. [DOI] [PubMed] [Google Scholar]
- 87. Huisman TA. Diffusion-weighted and diffusion tensor imaging of the brain, made easy. Cancer Imaging. 2010;10 Spec no A:S163–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang S, Kim S, Zhang Y, et al. . Determination of grade and subtype of meningiomas by using histogram analysis of diffusion-tensor imaging metrics. Radiology. 2012;262(2):584–592. [DOI] [PubMed] [Google Scholar]
- 89. Konstas AA, Goldmakher GV, Lee TY, Lev MH. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: technical implementations. AJNR Am J Neuroradiol. 2009;30(5):885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Griffith B, Jain R. Perfusion imaging in neuro-oncology: basic techniques and clinical applications. Magn Reson Imaging Clin N Am. 2016;24(4):765–779. [DOI] [PubMed] [Google Scholar]
- 91. Kimura H, Takeuchi H, Koshimoto Y, et al. . Perfusion imaging of meningioma by using continuous arterial spin-labeling: comparison with dynamic susceptibility-weighted contrast-enhanced MR images and histopathologic features. AJNR Am J Neuroradiol. 2006;27(1):85–93. [PMC free article] [PubMed] [Google Scholar]
- 92. Shi R, Jiang T, Si L, Li M. Correlations of magnetic resonance, perfusion-weighed imaging parameters and microvessel density in meningioma. J BUON. 2016;21(3):709–713. [PubMed] [Google Scholar]
- 93. Ginat DT, Mangla R, Yeaney G, Schaefer PW, Wang H. Correlation between dynamic contrast-enhanced perfusion MRI relative cerebral blood volume and vascular endothelial growth factor expression in meningiomas. Acad Radiol. 2012;19(8):986–990. [DOI] [PubMed] [Google Scholar]
- 94. Zhu F, Zhou Y, Wang C, Gao J, Qi J. Perfusion MRI evaluation of correlating perfusion constants with histologic findings in meningiomas. Paper presented at: Annual Meeting of the International Society for Magnetic Resonance in Medicine2002; Berkeley, CA. [Google Scholar]
- 95. Yang S, Law M, Zagzag D, et al. . Dynamic contrast-enhanced perfusion MR imaging measurements of endothelial permeability: differentiation between atypical and typical meningiomas. AJNR Am J Neuroradiol. 2003;24(8):1554–1559. [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang H, Rödiger LA, Shen T, Miao J, Oudkerk M. Perfusion MR imaging for differentiation of benign and malignant meningiomas. Neuroradiology. 2008;50(6):525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yan PF, Yan L, Hu TT, et al. . The potential value of preoperative mri texture and shape analysis in grading meningiomas: a preliminary investigation. Transl Oncol. 2017;10(4):570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cremerius U, Bares R, Weis J, et al. . Fasting improves discrimination of grade 1 and atypical or malignant meningioma in FDG-PET. J Nucl Med. 1997;38(1):26–30. [PubMed] [Google Scholar]
- 100. Okuchi S, Okada T, Yamamoto A, et al. . Grading meningioma: a comparative study of thallium-SPECT and FDG-PET. Medicine (Baltimore). 2015;94(6):e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Di Chiro G, Hatazawa J, Katz DA, Rizzoli HV, De Michele DJ. Glucose utilization by intracranial meningiomas as an index of tumor aggressivity and probability of recurrence: a PET study. Radiology. 1987;164(2):521–526. [DOI] [PubMed] [Google Scholar]
- 102. Arita H, Kinoshita M, Okita Y, et al. . Clinical characteristics of meningiomas assessed by 11C-methionine and 18F-fluorodeoxyglucose positron-emission tomography. J Neurooncol. 2012;107(2):379–386. [DOI] [PubMed] [Google Scholar]
- 103. Park YS, Jeon BC, Oh HS, Lee SM, Chun BK, Chang HK. FDG PET/CT assessment of the biological behavior of meningiomas. J Korean Neurosurg Soc. 2006;40:428–433. [Google Scholar]
- 104. Iuchi T, Iwadate Y, Namba H, et al. . Glucose and methionine uptake and proliferative activity in meningiomas. Neurol Res. 1999;21(7):640–644. [DOI] [PubMed] [Google Scholar]
- 105. Sommerauer M, Burkhardt JK, Frontzek K, et al. . 68Gallium-DOTATATE PET in meningioma: a reliable predictor of tumor growth rate?Neuro Oncol. 2016;18(7):1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hwang WL, Marciscano AE, Niemierko A, et al. . Imaging and extent of surgical resection predict risk of meningioma recurrence better than WHO histopathological grade. Neuro Oncol. 2016;18(6):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21(12):1455–1465. [DOI] [PubMed] [Google Scholar]
- 108. Liu Y, Chotai S, Chen M, Jin S, Qi ST, Pan J. Preoperative radiologic classification of convexity meningioma to predict the survival and aggressive meningioma behavior. PLoS One. 2015;10(3):e0118908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. SIMPSON D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sughrue ME, Rutkowski MJ, Shangari G, Parsa AT, Berger MS, McDermott MW. Results with judicious modern neurosurgical management of parasagittal and falcine meningiomas. Clinical article. J Neurosurg. 2011;114(3):731–737. [DOI] [PubMed] [Google Scholar]
- 111. Raza SM, Gallia GL, Brem H, Weingart JD, Long DM, Olivi A. Perioperative and long-term outcomes from the management of parasagittal meningiomas invading the superior sagittal sinus. Neurosurgery. 2010;67(4):885–893; discussion 893. [DOI] [PubMed] [Google Scholar]
- 112. Ng WH, Cheong DL, Khu KJ, Venkatesh G, Ng YK, Lim CC. Diffusion tensor tractography: corticospinal tract fiber reduction is associated with temporary hemiparesis in benign extracerebral lesions. Neurosurgery. 2008;63(3):452–458; discussion 458–459. [DOI] [PubMed] [Google Scholar]
- 113. Hoover JM, Morris JM, Meyer FB. Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency. Surg Neurol Int. 2011;2:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shiroishi MS, Cen SY, Tamrazi B, et al. . Predicting meningioma consistency on preoperative neuroimaging studies. Neurosurg Clin N Am. 2016;27(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Romani R, Tang WJ, Mao Y, et al. . Diffusion tensor magnetic resonance imaging for predicting the consistency of intracranial meningiomas. Acta Neurochir (Wien). 2014;156(10):1837–1845. [DOI] [PubMed] [Google Scholar]
- 116. Murphy MC, Huston J 3rd, Glaser KJ, et al. . Preoperative assessment of meningioma stiffness using magnetic resonance elastography. J Neurosurg. 2013;118(3):643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hughes JD, Fattahi N, Van Gompel J, et al. . Higher-resolution magnetic resonance elastography in meningiomas to determine intratumoral consistency. Neurosurgery. 2015;77(4):653–658; discussion 658–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yin Z, Hughes JD, Glaser KJ, et al. . Slip interface imaging based on MR-elastography preoperatively predicts meningioma-brain adhesion. J Magn Reson Imaging. 2017;46(4):1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Galldiks N, Albert NL, Sommerauer M, et al. . PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro Oncol. 2017;19(12):1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Terpolilli NA, Rachinger W, Kunz M, et al. . Orbit-associated tumors: navigation and control of resection using intraoperative computed tomography. J Neurosurg. 2016;124(5):1319–1327. [DOI] [PubMed] [Google Scholar]
- 121. Gehler B, Paulsen F, Oksüz MO, et al. . [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. 2009;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Graf R, Nyuyki F, Steffen IG, et al. . Contribution of 68Ga-DOTATOC PET/CT to target volume delineation of skull base meningiomas treated with stereotactic radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):68–73. [DOI] [PubMed] [Google Scholar]
- 123. Stade F, Dittmar JO, Jäkel O, et al. . Influence of 68Ga-DOTATOC on sparing of normal tissue for radiation therapy of skull base meningioma: differential impact of photon and proton radiotherapy. Radiat Oncol. 2018;13(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kwekkeboom DJ, de Herder WW, Kam BL, et al. . Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26(13):2124–2130. [DOI] [PubMed] [Google Scholar]
- 125. Bartolomei M, Bodei L, De Cicco C, et al. . Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur J Nucl Med Mol Imaging. 2009;36(9):1407–1416. [DOI] [PubMed] [Google Scholar]
- 126. Imhof A, Brunner P, Marincek N, et al. . Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29(17):2416–2423. [DOI] [PubMed] [Google Scholar]
- 127. Marincek N, Radojewski P, Dumont RA, et al. . Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: long-term results of a phase II clinical trial. J Nucl Med. 2015;56(2):171–176. [DOI] [PubMed] [Google Scholar]
- 128. Hänscheid H, Sweeney RA, Flentje M, et al. . PET SUV correlates with radionuclide uptake in peptide receptor therapy in meningioma. Eur J Nucl Med Mol Imaging. 2012;39(8):1284–1288. [DOI] [PubMed] [Google Scholar]
- 129. Seystahl K, Stoecklein V, Schüller U, et al. . Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. 2016;18(11):1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wang YC, Chuang CC, Wei KC, et al. . Long term surgical outcome and prognostic factors of atypical and malignant meningiomas. Sci Rep. 2016;6:35743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. de Almeida AN, Pereira BJA, Pires Aguiar PH, et al. . Clinical outcome, tumor recurrence, and causes of death: a long-term follow-up of surgically treated meningiomas. World Neurosurg. 2017;102:139–143. [DOI] [PubMed] [Google Scholar]
- 132. Nanda A, Bir SC, Maiti TK, Konar SK, Missios S, Guthikonda B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization grade I meningioma. J Neurosurg. 2017;126(1):201–211. [DOI] [PubMed] [Google Scholar]
- 133. Jenkinson MD, Waqar M, Farah JO, et al. . Early adjuvant radiotherapy in the treatment of atypical meningioma. J Clin Neurosci. 2016;28:87–92. [DOI] [PubMed] [Google Scholar]
- 134. Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34(1):45–60; discussion 60-41. [DOI] [PubMed] [Google Scholar]
- 135. Lescher S, Schniewindt S, Jurcoane A, Senft C, Hattingen E. Time window for postoperative reactive enhancement after resection of brain tumors: less than 72 hours. Neurosurg Focus. 2014;37(6):E3. [DOI] [PubMed] [Google Scholar]
- 136. Bette S, Gempt J, Huber T, et al. . Patterns and time dependence of unspecific enhancement in postoperative magnetic resonance imaging after glioblastoma resection. World Neurosurg. 2016;90:440–447. [DOI] [PubMed] [Google Scholar]
- 137. Slotty PJ, Behrendt FF, Langen KJ, Cornelius JF. (68)Ga-DOTATATE-positron emission tomography imaging in spinal meningioma. J Craniovertebr Junction Spine. 2014;5(1):44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rachinger W, Stoecklein VM, Terpolilli NA, et al. . Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015;56(3):347–353. [DOI] [PubMed] [Google Scholar]
- 139. Jeevanandham B, Kalyanpur T, Gupta P, Cherian M. Comparison of post-contrast 3D-T1-MPRAGE, 3D-T1-SPACE and 3D-T2-FLAIR MR images in evaluation of meningeal abnormalities at 3-T MRI. Br J Radiol. 2017;90(1074):20160834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nakasu S, Fukami T, Nakajima M, Watanabe K, Ichikawa M, Matsuda M. Growth pattern changes of meningiomas: long-term analysis. Neurosurgery. 2005;56(5):946–955; discussion 946. [PubMed] [Google Scholar]
- 141. Nakasu S, Nakasu Y, Fukami T, Jito J, Nozaki K. Growth curve analysis of asymptomatic and symptomatic meningiomas. J Neurooncol. 2011;102(2):303–310. [DOI] [PubMed] [Google Scholar]
- 142. Faraji AH, Tonetti DA, Flickinger JC, Engh JA. Alteration of the Ki-67 proliferative index following surgical resection with or without radiation therapy of intracranial meningiomas. Cureus. 2017;9(11):e1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Surov A, Meyer HJ, Wienke A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: a meta-analysis. Part 1: ADCmean. Oncotarget. 2017;8(43):75434–75444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ko CC, Lim SW, Chen TY, Chen JH, Li CF, Shiue YL. Prediction of progression in skull base meningiomas: additional benefits of apparent diffusion coefficient value. J Neurooncol. 2018;138(1):63–71. [DOI] [PubMed] [Google Scholar]
- 145. Mansouri A, Klironomos G, Taslimi S, et al. . Surgically resected skull base meningiomas demonstrate a divergent postoperative recurrence pattern compared with non-skull base meningiomas. J Neurosurg. 2016;125(2):431–440. [DOI] [PubMed] [Google Scholar]
- 146. Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 147. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 148. Lou E, Sumrall AL, Turner S, et al. . Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. J Neurooncol. 2012;109(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Furtner J, Schöpf V, Seystahl K, et al. . Kinetics of tumor size and peritumoral brain edema before, during, and after systemic therapy in recurrent WHO grade II or III meningioma. Neuro Oncol. 2016;18(3):401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Shih KC, Chowdhary S, Rosenblatt P, et al. . A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neurooncol. 2016;129(2):281–288. [DOI] [PubMed] [Google Scholar]
- 151. Wu LM, Li YL, Yin YH, et al. . Usefulness of dual-energy computed tomography imaging in the differential diagnosis of sellar meningiomas and pituitary adenomas: preliminary report. PLoS One. 2014;9(3):e90658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Song SW, Son YD, Cho ZH, Paek SH. Experience with 7.0 T MRI in patients with supratentorial meningiomas. J Korean Neurosurg Soc. 2016;59(4):405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Joo B, Han K, Choi YS, et al. . Amide proton transfer imaging for differentiation of benign and atypical meningiomas. Eur Radiol. 2018;28(1):331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Schwyzer L, Berberat J, Remonda L, Roelcke U. Susceptibility changes in meningiomas influence the apparent diffusion coefficient in diffusion-weighted MRI. J Neuroradiol. 2015;42(6):332–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.