Abstract
Hypertension, or elevated blood pressure (BP), has been extensively researched over decades and clearly demonstrated to be caused due to a combination of host genetic and environmental factors. Although much research remains to be conducted to pin-point the precise genetic elements on the host genome that control BP, new lines of evidence are emerging to indicate that, besides the host genome, the genomes of all indigenous commensal micro-organisms, collectively referred to as the microbial metagenome or microbiome, are important, but largely understudied, determinants of BP. Unlike the rigid host genome, the microbiome or the “second genome” can be altered by diet or microbiotal transplantation in the host. This possibility is attractive from the perspective of exploiting the microbiotal composition for clinical management of inherited hypertension. Thus, focusing on the limited current literature supporting a role for the microbiome in BP regulation, this review highlights the need to further explore the role of the co-existence of host and the microbiota as an organized biological unit called the “holobiont” in the context of BP regulation.
Evolution of complex life forms, such as humans, is often thought of as occurring due to the contributions of “nature” and “nurture,” interpreted in biology as genes and environment. In the post-genomic era, armed with advanced technologies to query our genomes, we are learning more and more about the dividing line between nature and nurture as a fallacy. This is because we are now able to appreciate that there is a “plasticity” from both ends. On one hand, there is the plasticity of the genome to accommodate changes in response to the environment in the form of epigenetic changes, and, on the other hand, there is the plasticity of environmental factors to include genomes other than that of the host, i.e., the microbiomes. Microbes have colonized and co-evolved with plants and animals to the extent that they are perhaps constantly remodeling inside their hosts to suit their survival, regardless of the fact that we, their hosts, consider them our environmental factors. Another way of looking at this symbiotic relationship between the host and the microbes living within the host is to look at this relationship as an ecosystem. Coined by Lynn Margulis in 1991, the term “holobiont” describes how macro-species, such as mammals, live in symbiosis with other micro-species, whereby all individuals that participate in a particular symbiosis are “bionts,” and the entire organism that comprises these bionts is a holobiont (70).
In the human body, there are ~10:1 numbers of microbial to human cells, and ~130 times the numbers of microbial genes (4). Although these numbers are staggering, it is only in recent years that attention is beginning to be given to explore ways by which microbes contribute to governing host physiology and the transition to pathophysiology. The reason for this surge in recent interest can be attested to rapid technological advancements in low-cost sequencing, whereby sequence variability in the 16S RNA genes of microbes, collectively called “microbiota,” are exploited to determine their identities (18, 85). To attest to this point, note that a survey of articles published in PubMed using the search term “microbiota” shows a surge in publications since the turn of the 21st century, which marks the beginning of the genome sequencing era (FIGURE 1). Furthermore, of these articles, there are very few that focus on microbiota and hypertension (FIGURE 1). (Note: Less than half of the number of the articles retrieved for microbiota and hypertension are original articles. The remainder are review articles on the topic of microbiota and hypertension.) The term microbiota in the literature largely refers to bacteria but should encompass all microscopic organisms, including viruses, bacteriophages, fungi, and protozoans. The genomes of microbiota are collectively referred to as the microbiome, and similar newer terms are emerging to describe the collective genomes of viruses, fungi, and protozoa living in the holobiont as virome, mycobiome, and protozoan genome, respectively. To date, there are no reports of viromes, mycobiomes, or protozoan genomes described for their associations with blood pressure (BP) regulation. For the purpose of this review, the term microbiota will be used for describing bacteria investigated for their links to the regulation of BP.
FIGURE 1.
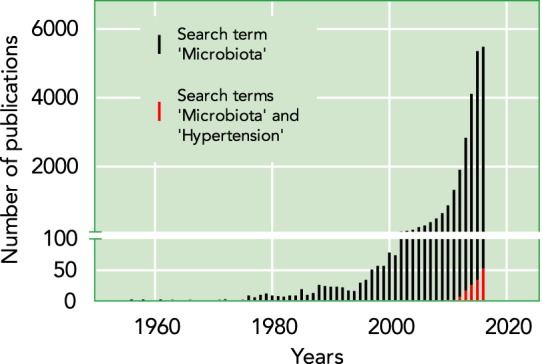
Relative number of publications on microbiota vs. publications on microbiota in the field of hypertension
Data were collected using the search feature from PubMed on 1/4/2017 using the search terms “microbiota” or “microbiota AND hypertension.” Each bar represents the total number of research articles plus review articles published per year.
The distal gut is the major site for microbiota colonization in our bodies and has therefore received the most attention for research. Commensal gut microbiota perform a variety of functions that are important to the host. Perhaps one of the most obvious functions of gut microbiota is to help the host to digest food and generate energy (47). For example, the microbiota in the colon ferment plant-derived complex carbohydrates (dietary fiber), as well as other carbohydrates that resist host digestive enzymes (83), and generate short-chain fatty acids (SCFAs), which provide additional calories to the host (67, 102). These SCFAs are then transported throughout the body, where they exert epigenetic and physiological effects (87, 88). Microbiota are also important for the metabolism of host lipids (45). Additionally, gut bacteria are an important source for biotin, vitamins K (47) and B12 (62), and essential amino acids (60). Furthermore, the gut microbiota are also important for strengthening the barrier function of the intestinal epithelium by inducing the expression of small proline-rich protein 2A, which is important for desmosome maintenance (66). Symbiotic microbiota also act as a first line of defense against invading microbes. The microbiota compete with invading microbes for the same environment and resources, thus inhibiting the growth of invading enteropathogens by a process known as colonization resistance/competitive exclusion (50). There is also evidence that gut microbiota influence the host immune system 1) by affecting the expression of immunoglobulins (IgA) in Peyer’s patches (25, 68) and 2) by influencing differentiation of regulatory T cells through SCFAs (109). Newer functions of gut microbiota are being discovered due to the observations that they contribute importantly to the pathophysiology of a variety of disorders such as obesity, colitis, inflammatory diseases, metabolic syndrome, liver disease, and kidney disease (3, 7, 8, 11–17, 19, 20, 29, 31, 33–37, 46, 55, 57, 59, 64, 65, 69, 72, 75–77, 80, 86, 94, 96, 97, 100, 103, 107, 108, 112–114, 116, 119, 120, 122, 125, 128, 131, 133).
Gut Microbiota, the Host Genome and Hypertension
A study reported using toll-like receptor 5 (Tlr5) knockout mice was among the first to describe the relationship between the host genome, gut microbiota, and BP regulation. Tlr5 is a receptor for bacterial flagellin, and deletion of Tlr5 in mice led to alterations in their gut microbiotal composition (119), resulting in the development of spontaneous colitis and metabolic syndrome. An interesting point to note is that the widely held notion that the relative abundance ratio of Firmicutes to Bacteroidetes (F/B ratio) is indicative of microbiotal dysbiosis, or microbial imbalance, was not the case with Tlr5−/− mice (119). The gut bacterial communities of Tlr5−/− and wild-type (WT) mice had similar relative abundances of Firmicutes and Bacteroidetes. Instead, consistent differences were noted in a total of 116 bacterial phylotypes from various phyla, which were either enriched or reduced in Tlr5−/− mice relative to WT mice. Importantly, the Tlr5−/− mice presented with elevated BP as one of the features in addition to other hallmark features of metabolic syndrome. To determine whether the changes in the gut microbiota were a cause or a consequence of the metabolic syndrome in Tlr5−/− mice, cecal microbiota from Tlr5−/− mice were transplanted into WT germ-free mice. The transplanted microbiota from Tlr5−/− mice conferred many phenotypes to the WT germ-free hosts, including hyperphagia, obesity, hyperglycemia, insulin resistance, colomegaly, and elevated colonic proinflammatory cytokines, but, unfortunately, BP was not examined in this study (119). In terms of defining the underlying molecular mechanism, the authors of this primary finding speculated that the absence of Tlr5 produced alterations in the gut microbiota that induce low-grade inflammatory signaling, which in turn could cross-desensitize metabolic (insulin) receptor signaling, leading to hyperphagia and associated metabolic syndrome (119). Taken together, although these studies suggest that the changes in the gut microbiota observed in the Tlr5−/− mice are likely to be a contributing factor in the development of metabolic syndrome in the mice, it remains to be formally tested to see whether this is indeed the case with BP.
Since this study was reported, other groups have obtained evidence for additional, but functionally different, receptors on the host genome that also interact with gut microbiota to regulate BP. These are the G-protein-coupled receptors and olfactory receptors. The commonality for these two classes of receptors is that several family members of these two groups of proteins are receptors for SCFAs. The most common SCFAs synthesized by microbiota are butyrate, propionate, and acetate. The idea of SCFAs having physiological effects has been around for a while. In 1991, Kristev et al. (56) found that, when butyrate and other SCFAs were added to smooth muscle, there was increased prostaglandin synthesis, smooth muscle contraction, and hypertension. They concluded that SCFAs can adversely regulate BP and contribute to hypertension. Precisely how SCFAs influenced BP mechanistically was unknown at that time.
The notion that SCFAs bind to specific receptors to regulate BP is relatively nascent. One of the first receptors implicated in SCFA control of BP is an olfactory receptor found in the kidney, Olfr78 (87–89). Olfr78 was localized to the major branches of the renal artery and the juxtaglomerular afferent arteriole, an important site for renin secretion, as well as some smooth muscle cells in the heart, diaphragm, skeletal muscle, and skin (88). Olfr78 localized to the juxtaglomerular apparatus has been implicated in renin release (89). It was found that this receptor can use SCFAs as a ligand, particularly propionate (88). Propionate was found to induce a hypotensive response acutely, theoretically through its binding to Olfr78. However, when Olfr78 was knocked out, the hypotensive response was still present and even increased. Therefore, Pluznick et al. concluded that the Olfr78 receptor acts to increase BP through release of renin and that there must be other receptors that propionate binds to to lower BP (87). Two of these receptors were subsequently identified as G-protein-coupled receptors Gpr41 and Gpr43, and have been localized to small resistance vessels (88). When propionate binds to these receptors, they induce vasodilation of the vessels, thus lowering BP (87). Gpr41 knockout mice had elevated systolic BP that was not salt-sensitive (79). However, high levels of propionate were also found to activate Olfr78-induced renin release, thereby raising BP (87).
Hydrogen sulfide is another gut microbiotal metabolite (105) that plays a role in the regulation of BP. It has been found that hydrogen sulfide released in the colon helps lower BP (111), mostly through vasodilation. The mechanism behind this vasodilation is still not clear. In addition to hydrogen sulfide, gut bacteria also release indole as a metabolite (23), which may have cardiovascular and renal effects (6, 132).
Gut Microbiotal Studies Using Genetic Models of Hypertension
Beyond the above-mentioned single-gene effects, evidence for the host-microbiotal cross talk emanated from studies reported with two of the widely used genetic models of hypertension, the Dahl salt-sensitive (S) rat and the spontaneously hypertensive rat (SHR). The genetic origins of these two hypertensive strains are distinct and are thoroughly described in the review by Rapp (92). The S rats originated from Sprague-Dawley rats, whereas the SHR originated from Wistar rats. The normotensive counterparts of S rats and SHR are the Dahl salt-resistant (R) rats and the Wistar Kyoto (WKY) rats, respectively. Mell et al. reported that fecal microbiota of hypertensive S rats and normotensive R rats had different microbiotal compositions (71). In particular, the S rats had higher levels of the phylum Bacteroidetes (71). Yang et al. studied SHR and WKY rats, and reported that there was a difference in their microbiotal composition (129). They found an increased Firmicutes-to-Bacteroidetes (F/B) ratio in the SHR, which has previously been implicated in metabolic disease (30, 53, 123), as well as decreased diversity of the microbiota (129). SHR were also found to have less acetate and butyrate-fermenting bacteria, such as Coprococcus and Pseudobutyrivibrio. It was also found that there were more lactate-producing bacteria in SHR, such as Streptococcus and Turicibacter (129). In another study, it was found that feeding high-fructose and -salt diets to Wistar rats resulted in higher body weights, insulin resistance, and BP, and resulted in elevated levels of acetate (130). Since acetate is one of the SCFAs produced by gut microbiota, it was concluded that the high fructose and salt caused a disturbance in the gut microbiota (130). Perry et al. found that increased acetate can activate the parasympathetic nervous system and lead to obesity through hyperphagia, increased ghrelin, and increased insulin secretion (84). These findings suggest that the gut microbiota might be a target for the treatment of obesity (84).
Different approaches were used to further examine the effects of resident microbiota of S rats and SHR on hypertension. In the S rat, Mell et al. performed a cecal transplant by oral gavage and found that the S rats that received cecal contents from R rats had higher BP compared with the BP of S rats that received cecal content from S rats (71). Increased circulating acetate and heptanoate were associated with the observed increase in BP of S rats given cecal content from R rats (71). A similar cecal transplant study was reported by Durgan et al. by oral gavage of cecal content from either normotensive or hypertensive obstructive sleep apnea rats to normotensive rats (24). They found that rats that received the cecal contents of the hypertensive obstructive sleep apnea rats demonstrated an increase in BP, whereas those receiving cecal contents from normotensive rats did not. They concluded that hypertension is transferable by gut microbiota (24). In another study, Adnan et al. performed a gut microbiota transplant between spontaneously hypertensive stroke-prone (SHRSP) rats and WKY rats. They found that the WKY rats, which received gut microbiota from SHRSP rats, had an increased systolic BP. Although these results are compelling, the BP data were collected by tail-cuff method only (1), an indirect method of recording BP, as opposed to telemetry, which can result in a lower sensitivity. In the SHR, however, cecal transplantation studies are not reported. Instead, treatment using the antibiotic minocycline is reported to be sufficient to eliminate microbiotal dysbiosis and lower BP (129). This, however, was not the case with S rats, wherein treatment with an antibiotic per se did not alter BP (71). There are potentially two reasons for this observed dichotomy. First, S rats are not reported to have dysbiosis as measured by the typical F/B ratio. Second, the antibiotic used in the study with S rats was vancomycin, which, unlike minocycline, does not cross the blood-brain barrier. In any case, the evidence from both strains is definitive to point to alterations in gut microbiotal composition that are linked to hypertension. Further studies are required to clarify whether the genomes of S rats and SHR are permissive to specifically different microbiota to reside in them and thereby influence the extent of their BP.
Another interesting aspect pertaining to S rats and SHR is that these two models, despite being hypertensive, are divergent in many aspects. For example, the BP of the S rat is highly sensitive to dietary salt, whereas the BP of the SHR is not. Second, the S rat is susceptible to renal disease, which the SHR is not. Major gaps in knowledge exist on how dietary salt impacts microbiotal composition and the extent to which microbiota influence hypertension independent of influencing renal disease. It is also worth noting that differences in phenotypes such as insulin-resistance and abnormalities in carbohydrate and lipid metabolism and others co-exist along with differences in BP between the hypertensive rats (S and SHR) and their normotensive controls, the R and WKY rats, respectively (91, 93, 95, 104). Therefore, any observations of changes in microbiota between these strains cannot be interpreted as directly related to BP alone. Knowledge gained from these host-microbiotal associations are intriguing to ask the even more important question of whether there are alleles that were fixed during the selection process that cause a differential microbiotal inhabitation in the guts of selectively bred hypertensive rats compared with their relative normotensive “control” strains. In other words, is there a genetic basis for the host genome-microbiotal associations to be passed on as an inherited feature from one generation to the next? Although the differences observed in microbiotal populations between inbred strains is appreciative, the experimental design of comparing inbred strains is insufficient to detect and pinpoint a genetic basis for the observed host-microbiotal associations. This is because these strains differ by millions of genomic variants throughout their genomes, inheritance of only some, but not all, of which could be related to host-microbiotal interactions.
Differences in microbiota could not have influenced the genome of the host because the genome of the host is inherited from its parents even before it developed its gut, where microbiota reside. The use of the unidirectional nature of this genetic argument depends completely on being able to demonstrate Mendelian segregation of discrete genotypes and associated microbiota in segregating populations for BP and associating the microbiotal populations with BP differences. Therefore, it follows by logic that genetic linkage studies to track Mendelian inheritance must be employed to define and understand the inherited nature of these observations of host-microbiotal correlations in inbred genetic models of hypertension. This, for now, is a completely unexplored area of research. If quantitative trait loci (QTLs) that causally influence gut microbiotal compositions are detected, our goal to pin-point host genomic factors causally influencing microbiotal compositions can be furthered by constructing and comparing congenic strains or genetically modified strains with appropriate control strains that are identical in genomic background throughout the genome except for the QTL region.
Questions on the mechanisms by which gut microbiota impact hypertension are drawing focused attention to the view that the gut along with its microbiota serve as a central node interacting with multiple other organs, whereby the connections are referred to as “axes” interacting with the node. Dominating current thinking about such axes for hypertension and microbiota is the “gut-brain-bone marrow axis” proposed by Santisteban et al., who suggest that the dysfunction of the gut-brain-bone marrow axis could be associated with hypertension (101). Data in support of this proposed axis as a hypothesis is largely drawn from reports on phenotypes other than hypertension, whereby studies testing this “triad” hypothesis for hypertension per se are anticipated. Similarly, driven by the influences of the gut microbiota on specific renal G-protein-coupled and Olfactory receptors, another emerging school of thought is the “gut-renal axis.” Evidence in support of the gut-renal axis can be found in the literature pertaining to independent reports of gut microbiota impacting renal disease (5, 39, 40, 48, 49, 51, 58, 61, 63, 71, 73, 80, 99, 110, 117, 118, 126, 127). Another axis that remains to be explored is the gut-liver axis. Given that hypertension is a hallmark of metabolic syndrome, there is an intriguing article in the literature that prompts research in the gut-liver axis for hypertension (106). Tlr5−/− mice, which present with elevated BP, also display elevated hepatic neutral lipids (cholesterol esters and triglycerides) enriched with oleate and increased liver stearoyl CoA desaturase (SCD1) expression, both of which were dependent on the gut microbiotal composition (106). Deletion of hepatic SCD1 not only prevented elevated hepatic neutral lipids but also resulted in amelioration of metabolic syndrome in Tlr5−/− mice, thus demonstrating a key role of the gut microbiota-liver axis in the pathogenesis of metabolic diseases. Because Tlr5−/− mice have elevated BP compared with the WT control, it is plausible that the gut-liver axis is also important for BP regulation (119).
Beyond Gut Microbiota, Other Organ-Specific Microbiota Links to Hypertension
Although the idea of experimentally approaching the link between hypertension and gut microbiota is emerging, it may be limiting because the microbiota in our bodies are not limited to the gut. Oral and dermal microbiota are pertinent to consider as modulators of salt-sensitive hypertension not only because of their exposed surfaces to bacteria but also because the oral cavity is the primary site in contact with diet (including salt) and the skin is an excretory organ for salt via sweat. To date, there are no studies reported on dermal microbiota and their role in hypertension. However, although there are no direct studies on the oral microbiome, there are reports suggesting a link. For example, a direct relationship is reported between the levels of subgingival periodontal bacteria and the prevalence of hypertension. An increase in the number of oral microbiota has also been reported in hypertensive patients taking antihypertensive medications (22, 81). Additionally, an increase in certain oral microbiotal species such as Anaeroglobus has also been identified in symptomatic atherosclerosis (26). Besides, the oral cavity has a central role in regulating nitric oxide (NO) production from dietary nitrates and nitrites, which are mainly present in green leafy vegetables (41). Reduction from nitrate to nitrite by oral commensal bacteria is an obligatory step for further NO generation. This central role of the oral microbiota in regulating a vasodilator such as NO presents an intriguing possibility to study the role of oral microbiota in hypertension. In fact, interruption of this pathway through the use of antibacterial mouthwash was paralleled by a small elevation of systolic BP (10, 41). Clearly, further studies are warranted for both the oral and dermal microbiota.
The Host as a Holobiont
Experimental considerations of such organ-specific microbiotal relationships with the host (gut, oral, or dermal) to control BP may still be limiting to understand the full spectrum of the host-microbiotal symbiosis. This is because such experiments are pairwise evaluations of limited defined relationships of the microbiota with the whole host. The co-existence of the host and all the microorganisms living within it is defined by evolution, ecology, and the sum effect of the genomes of the microbiota and the host. The concept that the host has evolutionarily never been autonomous but is an organized biological unit called the holobiont (70) composed of millions of individual microorganisms further expands the limitation of pairwise comparisons of microbes in any particular organ. This allows for thinking in broader terms for host-microbiotal interactions to shape physiology. Thus our current research strategy of exploring gut microbiota in hypertension or other similar organ-specific microbiota in focused axes such as the gut-brain, gut-liver, or gut-kidney, etc. may still lack the ability to capture the full extent to which microbiota may influence hypertension. For example, Vikram et al. found that the gut microbiome remotely controls vascular microRNA-204 to regulate endothelial vasorelaxation (121). The questions of whether other microorganisms besides bacteria, such as viruses, fungi, and protozoa that also exist as part of the holobiont, influence BP regulation remain currently unknown (FIGURE 2).
FIGURE 2.
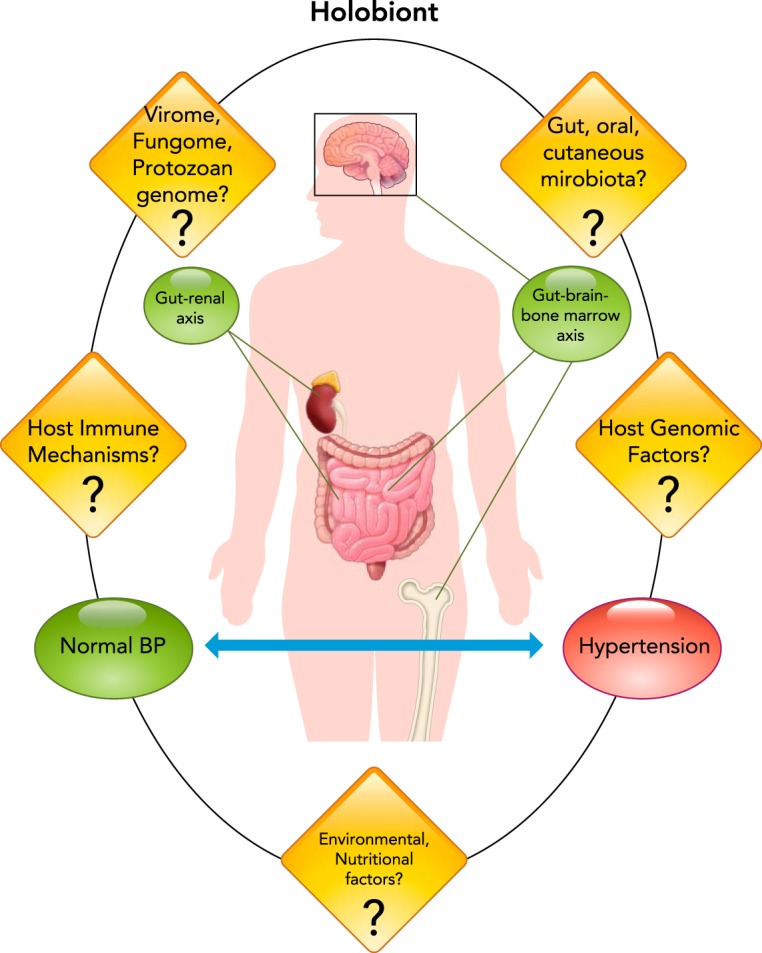
Current status and future prospects for research on microbiota as a factor contributing to blood pressure homeostasis
The current literature in the field, which is limited to the contributions of the gut-renal and gut-brain-bone marrow axes are shown in green. Significant knowledge gaps in the field are represented by the various question marks, linked to the contemplation of the total factors from the host and microbiota, represented by the holobiont.
Despite these limitations, studies are emerging that capture host genomic associations with the microbiome that are not limited to organ-specific experimental designs. For example, recent genome-wide association studies are reporting, in an unbiased manner, the precise points on the host genome that are associated with the abundant presence of certain microbiota (9, 115, 124). It is interesting to note that, in these human studies, many loci showing significant association with microbiome traits were found in close proximity to loci having effects on complex disease risks (9, 115, 124). Understanding host-microbiome interactions in this context may further illuminate historical and evolutionary events affecting the emergence and distribution of disease predisposition in different populations. This field of research remains vastly unexplored, but interesting clues are emerging for this to be promising at least in BP regulation. For example, the inheritance of BP is known to be linked to genes in the steroid biosynthetic pathway (32), and, interestingly, the study by Wang et al. found that associations with several individual microbiotal taxa and expression QTL analyses converged on genomic loci involved in sterol biosynthesis, implying that genetic variation in sterol biosynthesis may shape the microbiotal composition (124).
The Translational Value of Understanding the Host-Microbial Relationships Within a Holobiont
If normal physiology is attributed to symbiotic relationships between the host and the microbes in a holobiont, disruptions of such symbiotic relationships are to be viewed as contributing to pathophysiology. Disruptions are possible either through mutations on the host genome or through alterations in the microbial genomes. Because host mutations are not easily remedied, the microbes causing the disruptions of symbiotic relationships with the host are relatively easier targets for alleviating pathologies. A time-tested way to do this is to use antibiotic or probiotic therapy. Honor (42) draws attention to this topic as being as old as 30 years, wherein experimental increase of BP in Sprague-Dawley rats through the action of corticosterone or ACTH was preventable by the administration of vancomycin or neomycin. Honor et al. also note that oral neomycin decreased the development of high BP in the SHR (43). Clinically, hypertension in patients with a rare disorder of synthesis of cortisol due to a genetic defect of steroid cytochrome 17-hydroxylase is attributed to an excess of deoxycorticosterone, which causes renal sodium retention and therefore hypertension. The urinary steroid metabolites of these patients were analyzed and found to have a high proportion of 21-deoxycorticosterone steroids, and the microorganism Eubacterium lentum is reported to be likely responsible for catalyzing 21-dehydroxylation of corticosteroids (27, 28). However, formal antibiotic administration studies in this case are not reported. These steroids may inhibit dehydrogenase activity to decrease protection of the kidney mineralocorticoid receptors, leading to the renal sodium retention (74). Previously, Honor et al. found 11-oxygenated 21-deoxysteroids in the urine of patients with 17α-hydroxylase deficiency syndrome and concluded that it was caused by microbial 21-dehydroxylation (44). In recent years, evidence is accumulating to suggest an overall variation in gut microbiota from hypertensive and normotensive subjects. Kim et al. analyzed fecal samples from hypertensive and normotensive patients and found that there was less diversity and abundance of microbiota in hypertensive patients (54). Yang et al. also report such a variation between hypertensive and normotensive subjects, albeit using a small cohort (129). Trials are currently underway to further test the possibility of the antibiotic minocycline as an agent to lower BP of resistant hypertensives (129).
Probiotics and Hypertension
Several studies report beneficial effects of the use of probiotics on hypertension. Hata et al. found that when sour milk with Lactobacillus helveticus and Saccharomyces cerevisiae were given to hypertensive patients, both the systolic and diastolic BPs were lower (38). In another study, Agerholm-Larsen et al. found that Enterococcus faecium and Streptococcus thermophiles, when added to yogurt administered to participants over an 8-wk period, decreased their systolic BP (2). Kawase et al. reported that Lactobacillus casei and S. thermophilus in fermented milk in healthy males caused a decrease in systolic BP, also over an 8-wk period (52). Naruszewicz et al. discovered that, in heavy smokers, Lactobacillus plantarum added to rose-hip drink significantly reduced systolic BP. They concluded that probiotics could be used as a preventative measure against cardiovascular disease in those who are at risk (78).
Overall, the older and more recent data in humans and rat models provide a fundamental rationale to further explore possibilities for treating hypertension by altering microbiota with antibiotic, probiotic, or other dietary factors, as well as through fecal transplantations. Fecal transplantations are already being used to successfully treat relapsing Clostridium difficile infections (21, 82, 90, 98). Whether or not fecal transplantation would be successful in treating hypertension needs to be studied further.
Conclusions
The role of microbiota in hypertension is a relatively new field of study. To date, there are limited publications, most of which are focused on the gut microbiota. Nevertheless, the evidence is strong for further expanding our questions on the identities of the microbiota and the mechanisms by which they operate to exert BP regulation. Although there is great potential for using microbiota in treatments for hypertension and other diseases, there are some obvious limitations that are thwarting full exploration of this possibility. The work that is currently reported focuses on correlative observations, whereby key questions on cause-effect relationships remain to be tested with appropriate experimental designs. There is a perceived “rush” to answer questions on mechanistic aspects even before fundamental studies are conducted to move the field from mere correlative studies to cause-effect relationships. A concerted, systems biology approach exploring microbiome-metagenomics-metabolomics is lacking to delineate how the microbiome could causally impact hypertension.
Although technology may no longer be a limiting factor for querying microbiota or their metabolites, there are other perceivable technical difficulties to study the gut microbiota such as the inability to culture all the gut bacteria in the laboratory, whereby the bacteria cultured and studied in the laboratory may not be entirely representative of what is present in the gut. This technical challenge is, however, not limited to the field of hypertension research. Overall, a broader emerging perspective of the host as being a part of an ecosystem called the holobiont is needed to expand our current understanding of bi-directional relationships between the macro- (host) and micro-species to impact BP and contemplate appropriate clinical management strategies for hypertension.
Summary
Gut microbiota have been implicated in a number of diseases, including obesity, colitis, inflammatory diseases, metabolic syndrome, liver disease, and kidney disease. Although hypertension is a common disorder in America, with one-third of the population being affected, it has been studied less than the other diseases with regard to the impact of gut microbiota. There is definitive evidence in the literature pointing to a link between gut microbiota and blood pressure. This review surveys the existing literature linking microbiota to hypertension and explores the idea that host-microbiotal interactions are an important contributor to the etiology of hypertension. The review identifies gaps in knowledge, including the important lack of our current understanding of whether alterations in gut microbiota observed to be associated with elevated blood pressure cause hypertension or result as a consequence of hypertension. In any case, this field of research is nascent and exciting due to the overarching possibility that manipulating microbiota is clinically feasible and could be contemplated as a treatment for hypertensive subjects.
Footnotes
This work was supported by National Heart Lung and Blood Institute Grant HL-020176 to B. Joe.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: S.G. prepared figures; S.G., S.C., and B.M. drafted manuscript; S.G., S.C., B.M., M.V.-K., and B.J. edited and revised manuscript; S.G., S.C., B.M., M.V.-K., and B.J. approved final version of manuscript; M.V.-K. and B.J. conceived and designed research.
References
- 1.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics : 96–104, 2017. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agerholm-Larsen L, Raben A, Haulrik N, Hansen AS, Manders M, Astrup A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur J Clin Nutr : 288–297, 2000. doi: 10.1038/sj.ejcn.1600937. [DOI] [PubMed] [Google Scholar]
- 3.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology : 1006–1016, 2012. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annalisa N, Alessio T, Claudette TD, Erald V, Antonino L, Nicola DD. Gut microbioma population: an indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediators Inflamm : 901308, 2014. doi: 10.1155/2014/901308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ay N, Kaya S, Anil M, Alp V, Sevuk U, Danis R. Management of a resistant hypotension developing after reperfusion of a living-donor kidney transplant. Exp Clin Transplant. In press. doi: 10.6002/ect.2015.0179. [DOI] [PubMed] [Google Scholar]
- 6.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uremic Toxin Work Group (EUTox) . Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol : 1551–1558, 2009. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchi J, Goret J, Mégraud F. Clostridium difficile Infection: a model for disruption of the gut microbiota equilibrium. Dig Dis : 217–220, 2016. doi: 10.1159/000443355. [DOI] [PubMed] [Google Scholar]
- 8.Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc : 227–234, 2015. doi: 10.1017/S0029665114001700. [DOI] [PubMed] [Google Scholar]
- 9.Bonder MJ, Kurilshikov A, Tigchelaar EF, Mujagic Z, Imhann F, Vila AV, Deelen P, Vatanen T, Schirmer M, Smeekens SP, Zhernakova DV, Jankipersadsing SA, Jaeger M, Oosting M, Cenit MC, Masclee AAM, Swertz MA, Li Y, Kumar V, Joosten L, Harmsen H, Weersma RK, Franke L, Hofker MH, Xavier RJ, Jonkers D, Netea MG, Wijmenga C, Fu J, Zhernakova A. The effect of host genetics on the gut microbiome. Nat Genet : 1407–1412, 2016. doi: 10.1038/ng.3663. [DOI] [PubMed] [Google Scholar]
- 10.Bondonno CP, Liu AH, Croft KD, Considine MJ, Puddey IB, Woodman RJ, Hodgson JM. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am J Hypertens : 572–575, 2015. doi: 10.1093/ajh/hpu192. [DOI] [PubMed] [Google Scholar]
- 11.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med : 42, 2016. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cammarota G, Ianiro G, Cianci R, Bibbò S, Gasbarrini A, Currò D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy. Pharmacol Ther : 191–212, 2015. doi: 10.1016/j.pharmthera.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Cani PD. Gut microbiota and obesity: lessons from the microbiome. Brief Funct Genomics : 381–387, 2013. doi: 10.1093/bfgp/elt014. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho FA, Aitken JD, Vijay-Kumar M, Gewirtz AT. Toll-like receptor-gut microbiota interactions: perturb at your own risk! Annu Rev Physiol : 177–198, 2012. doi: 10.1146/annurev-physiol-020911-153330. [DOI] [PubMed] [Google Scholar]
- 15.Chassaing B, Aitken JD, Gewirtz AT, Vijay-Kumar M. Gut microbiota drives metabolic disease in immunologically altered mice. Adv Immunol : 93–112, 2012. doi: 10.1016/B978-0-12-394300-2.00003-X. [DOI] [PubMed] [Google Scholar]
- 16.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature : 92–96, 2015. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chassaing B, Kumar M, Baker MT, Singh V, Vijay-Kumar M. Mammalian gut immunity. Biomed J : 246–258, 2014. doi: 10.4103/2319-4170.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhary N, Sharma AK, Agarwal P, Gupta A, Sharma VK. 16S classifier: a tool for fast and accurate taxonomic classification of 16S rRNA hypervariable regions in metagenomic datasets. PLoS One : e0116106, 2015. doi: 10.1371/journal.pone.0116106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chehoud C, Albenberg LG, Judge C, Hoffmann C, Grunberg S, Bittinger K, Baldassano RN, Lewis JD, Bushman FD, Wu GD. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis : 1948–1956, 2015. doi: 10.1097/MIB.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung H, Kasper DL. Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Curr Opin Immunol : 455–460, 2010. doi: 10.1016/j.coi.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Costello SP, Tucker EC, La Brooy J, Schoeman MN, Andrews JM. Establishing a fecal microbiota transplant service for the treatment of clostridium difficile infection. Clin Infect Dis : 908–914, 2016. doi: 10.1093/cid/civ994. [DOI] [PubMed] [Google Scholar]
- 22.Desvarieux M, Demmer RT, Jacobs DR Jr, Rundek T, Boden-Albala B, Sacco RL, Papapanou PN. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens : 1413–1421, 2010. doi: 10.1097/HJH.0b013e328338cd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donia MS, Fischbach MA. Small molecules from the human microbiota. Science : 1254766, 2015. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM Jr. Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension : 469–474, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durkin HG, Bazin H, Waksman BH. Origin and fate of IgE-bearing lymphocytes. I. Peyer’s patches as differentiation site of cells. Simultaneously bearing IgA and IgE. J Exp Med : 640–648, 1981. doi: 10.1084/jem.154.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fåk F, Tremaroli V, Bergström G, Bäckhed F. Oral microbiota in patients with atherosclerosis. Atherosclerosis : 573–578, 2015. doi: 10.1016/j.atherosclerosis.2015.10.097. [DOI] [PubMed] [Google Scholar]
- 27.Feighner SD, Bokkenheuser VD, Winter J, Hylemon PB. Characterization of a C21 neutral steroid hormone transforming enzyme, 21-dehydroxylase, in crude cell extracts of Eubacterium lentum. Biochim Biophys Acta : 154–163, 1979. doi: 10.1016/0005-2760(79)90094-8. [DOI] [PubMed] [Google Scholar]
- 28.Feighner SD, Hylemon PB. Characterization of a corticosteroid 21-dehydroxylase from the intestinal anaerobic bacterium, Eubacterium lentum. J Lipid Res : 585–593, 1980. [PubMed] [Google Scholar]
- 29.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol : 16079–16094, 2014. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finucane MM, Sharpton TJ, Laurent TJ, Pollard KS. A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS One : e84689, 2014. doi: 10.1371/journal.pone.0084689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flowers SA, Ellingrod VL. The microbiome in mental health: potential contribution of gut microbiota in disease and pharmacotherapy management. Pharmacotherapy : 910–916, 2015. doi: 10.1002/phar.1640. [DOI] [PubMed] [Google Scholar]
- 32.Garrett MR, Rapp JP. Defining the blood pressure QTL on chromosome 7 in Dahl rats by a 177-kb congenic segment containing Cyp11b1. Mamm Genome : 268–273, 2003. doi: 10.1007/s00335-002-2245-9. [DOI] [PubMed] [Google Scholar]
- 33.Gérard P. Gut microbiota and obesity. Cell Mol Life Sci : 147–162, 2016. doi: 10.1007/s00018-015-2061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gkolfakis P, Dimitriadis G, Triantafyllou K. Gut microbiota and non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int : 572–581, 2015. doi: 10.1016/S1499-3872(15)60026-1. [DOI] [PubMed] [Google Scholar]
- 35.Goto Y, Kurashima Y, Kiyono H. The gut microbiota and inflammatory bowel disease. Curr Opin Rheumatol : 388–396, 2015. doi: 10.1097/BOR.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 36.Greenhill C. Obesity: Gut microbiota, host genetics and diet interact to affect the risk of developing obesity and the metabolic syndrome. Nat Rev Endocrinol : 630, 2015. doi: 10.1038/nrendo.2015.152. [DOI] [PubMed] [Google Scholar]
- 37.Guo M, Ding S, Zhao C, Gu X, He X, Huang K, Luo Y, Liang Z, Tian H, Xu W. Red Ginseng and Semen Coicis can improve the structure of gut microbiota and relieve the symptoms of ulcerative colitis. J Ethnopharmacol : 7–13, 2015. doi: 10.1016/j.jep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 38.Hata Y, Yamamoto M, Ohni M, Nakajima K, Nakamura Y, Takano T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am J Clin Nutr : 767–771, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol : 1339–1343, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Hatch M, Vaziri ND. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin Sci (Lond) : 511–516, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Hezel MP, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis : 7–16, 2015. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- 42.Honour JW. Historical perspective: gut dysbiosis and hypertension. Physiol Genomics : 443–446, 2015. doi: 10.1152/physiolgenomics.00063.2015. [DOI] [PubMed] [Google Scholar]
- 43.Honour JW, Borriello SP, Ganten U, Honour P. Antibiotics attenuate experimental hypertension in rats. J Endocrinol : 347–350, 1985. doi: 10.1677/joe.0.1050347. [DOI] [PubMed] [Google Scholar]
- 44.Honour JW, Tourniaire J, Biglieri EG, Shackleton CH. Urinary steroid excretion in 17 alpha-hydroxylase deficiency. J Steroid Biochem : 495–505, 1978. doi: 10.1016/0022-4731(78)90115-2. [DOI] [PubMed] [Google Scholar]
- 45.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science : 881–884, 2001. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 46.Imhann F, Vich Vila A, Bonder MJ, Fu J, Gevers D, Visschedijk MC, Spekhorst LM, Alberts R, Franke L, van Dullemen HM, Ter Steege RW, Huttenhower C, Dijkstra G, Xavier RJ, Festen EA, Wijmenga C, Zhernakova A, Weersma RK. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2016. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol : 8787–8803, 2015. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joe B, Letwin NE, Garrett MR, Dhindaw S, Frank B, Sultana R, Verratti K, Rapp JP, Lee NH. Transcriptional profiling with a blood pressure QTL interval-specific oligonucleotide array. Physiol Genomics : 318–326, 2005. doi: 10.1152/physiolgenomics.00164.2004. [DOI] [PubMed] [Google Scholar]
- 49.Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens : 403–409, 2015. doi: 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol : 685–690, 2013. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang JY. The gastrointestinal tract in uremia. Dig Dis Sci : 257–268, 1993. doi: 10.1007/BF01307542. [DOI] [PubMed] [Google Scholar]
- 52.Kawase M, Hashimoto H, Hosoda M, Morita H, Hosono A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci : 255–263, 2000. doi: 10.3168/jds.S0022-0302(00)74872-7. [DOI] [PubMed] [Google Scholar]
- 53.Kim H, Kim DH, Seo KH, Chon JW, Nah SY, Bartley GE, Arvik T, Lipson R, Yokoyama W. Modulation of the intestinal microbiota is associated with lower plasma cholesterol and weight gain in hamsters fed chardonnay grape seed flour. J Agric Food Chem : 1460–1467, 2015. doi: 10.1021/jf5026373. [DOI] [PubMed] [Google Scholar]
- 54.Kim S, Rodriguez V, Santisteban M, Yang T, Qi Y, Raizada M, Pepine C. 6B.07: Hypertensive patients exhibit gut microbial dysbiosis and an increase in TH17 cells. J Hypertens , Suppl 1: e77–e78, 2015. doi: 10.1097/01.hjh.0000467562.03337.a5. [DOI] [Google Scholar]
- 55.Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med : 14, 2011. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kristev A, Mitkov D, Lukanov J. Influence of short-chain fatty acids on vascular tone. Int J Exp Pathol : 475–480, 1991. [PMC free article] [PubMed] [Google Scholar]
- 57.Kuczynski J, Costello EK, Nemergut DR, Zaneveld J, Lauber CL, Knights D, Koren O, Fierer N, Kelley ST, Ley RE, Gordon JI, Knight R. Direct sequencing of the human microbiome readily reveals community differences. Genome Biol : 210, 2010. doi: 10.1186/gb-2010-11-5-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumarasamy S, Waghulde H, Gopalakrishnan K, Mell B, Morgan E, Joe B. Mutation within the hinge region of the transcription factor Nr2f2 attenuates salt-sensitive hypertension. Nat Commun : 6252, 2015. doi: 10.1038/ncomms7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lankelma JM, Nieuwdorp M, de Vos WM, Wiersinga WJ. The gut microbiota in internal medicine: implications for health and disease. Neth J Med : 61–68, 2015. [PubMed] [Google Scholar]
- 60.Larsen T, Ventura M, Maraldo K, Triadó-Margarit X, Casamayor EO, Wang YV, Andersen N, O’Brien DM. The dominant detritus-feeding invertebrate in Arctic peat soils derives its essential amino acids from gut symbionts. J Anim Ecol : 1275–1285, 2016. doi: 10.1111/1365-2656.12563. [DOI] [PubMed] [Google Scholar]
- 61.Lau WL, Liu SM, Pahlevan S, Yuan J, Khazaeli M, Ni Z, Chan JY, Vaziri ND. Role of Nrf2 dysfunction in uremia-associated intestinal inflammation and epithelial barrier disruption. Dig Dis Sci : 1215–1222, 2015. doi: 10.1007/s10620-014-3428-4. [DOI] [PubMed] [Google Scholar]
- 62.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol : 160–168, 2013. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Lee JR, Muthukumar T, Dadhania D, Toussaint NC, Ling L, Pamer E, Suthanthiran M. Gut microbial community structure and complications after kidney transplantation: a pilot study. Transplantation : 697–705, 2014. doi: 10.1097/TP.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI. Evolution of mammals and their gut microbes. Science : 1647–1651, 2008. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopetuso LR, Petito V, Zambrano D, Orlando D, Dal Lago A, Serrichhio L, Papa A, Gasbarrini A, Scaldaferri F. Gut microbiota: a key modulator of intestinal healing in inflammatory bowel disease. Dig Dis : 202–209, 2016. doi: 10.1159/000444460. [DOI] [PubMed] [Google Scholar]
- 66.Lutgendorff F, Akkermans LM, Söderholm JD. The role of microbiota and probiotics in stress-induced gastro-intestinal damage. Curr Mol Med : 282–298, 2008. doi: 10.2174/156652408784533779. [DOI] [PubMed] [Google Scholar]
- 67.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc : 67–72, 2003. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 68.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science : 1662–1665, 2004. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 69.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut : 330–339, 2016. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Margulis L, Fester R. Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis. Cambridge, MA: The MIT Press, 1991. [PubMed] [Google Scholar]
- 71.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics : 187–197, 2015. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miele L, Giorgio V, Alberelli MA, De Candia E, Gasbarrini A, Grieco A. Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr Cardiol Rep : 120, 2015. doi: 10.1007/s11886-015-0671-z. [DOI] [PubMed] [Google Scholar]
- 73.Miyazaki T, Ise M, Hirata M, Endo K, Ito Y, Seo H, Niwa T. Indoxyl sulfate stimulates renal synthesis of transforming growth factor-beta 1 and progression of renal failure. Kidney Int Suppl : S211–S214, 1997. [PubMed] [Google Scholar]
- 74.Morris DJ, Latif SA, Brem AS. An alternative explanation of hypertension associated with 17α-hydroxylase deficiency syndrome. Steroids : 44–48, 2014. doi: 10.1016/j.steroids.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Munyaka PM, Eissa N, Bernstein CN, Khafipour E, Ghia JE. Antepartum antibiotic treatment increases offspring susceptibility to experimental colitis: a role of the gut microbiota. PLoS One : e0142536, 2015. doi: 10.1371/journal.pone.0142536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res : 127–138, 2016. doi: 10.5217/ir.2016.14.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagpal R, Kumar M, Yadav AK, Hemalatha R, Yadav H, Marotta F, Yamashiro Y. Gut microbiota in health and disease: an overview focused on metabolic inflammation. Benef Microbes : 181–194, 2016. doi: 10.3920/bm2015.0062. [DOI] [PubMed] [Google Scholar]
- 78.Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr : 1249–1255, 2002. [DOI] [PubMed] [Google Scholar]
- 79.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G-protein coupled receptor 41. Physiol Genomics : 826–834, 2016. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noel S, Martina-Lingua MN, Bandapalle S, Pluznick J, Hamad AR, Peterson DA, Rabb H. Intestinal microbiota-kidney cross talk in acute kidney injury and chronic kidney disease. Nephron Clin Pract : 139–143, 2014. doi: 10.1159/000363209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nonzee V, Manopatanakul S, Khovidhunkit SO. Xerostomia, hyposalivation and oral microbiota in patients using antihypertensive medications. J Med Assoc Thai : 96–104, 2012. [PubMed] [Google Scholar]
- 82.Orenstein R, Griesbach CL, DiBaise JK. Moving fecal microbiota transplantation into the mainstream. Nutr Clin Pract : 589–598, 2013. doi: 10.1177/0884533613497516. [DOI] [PubMed] [Google Scholar]
- 83.Ouwehand A, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur J Nutr , Suppl 1: I32–I37, 2002. doi: 10.1007/s00394-002-1105-4. [DOI] [PubMed] [Google Scholar]
- 84.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature : 213–217, 2016. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe : 417–427, 2008. doi: 10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pietroiusti A, Magrini A, Campagnolo L. New frontiers in nanotoxicology: Gut microbiota/microbiome-mediated effects of engineered nanomaterials. Toxicol Appl Pharmacol : 90–95, 2016. doi: 10.1016/j.taap.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 87.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes : 202–207, 2014. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan L-X, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA : 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pluznick JL, Zou D-J, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA : 2059–2064, 2009. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ponte A, Pinho R, Mota M, Silva J, Vieira N, Oliveira R, Pinto-Pais T, Fernandes C, Ribeiro I, Rodrigues J, Lopes P, Teixeira T, Carvalho J. Initial experience with fecal microbiota transplantation in Clostridium difficile infection - transplant protocol and preliminary results. Rev Esp Enferm Dig : 402–407, 2015. [DOI] [PubMed] [Google Scholar]
- 91.Pravenec M, Kurtz TW. Genetics of Cd36 and the hypertension metabolic syndrome. Semin Nephrol : 148–153, 2002. doi: 10.1053/snep.2002.2002.30218. [DOI] [PubMed] [Google Scholar]
- 92.Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol Rev : 135–172, 2000. [DOI] [PubMed] [Google Scholar]
- 93.Rapp JP, McPartland RP, Sustarsic DL. Inheritance of blood pressure and pituitary colloid protein in Dahl rats. J Hered : 169–174, 1979. doi: 10.1093/oxfordjournals.jhered.a109228. [DOI] [PubMed] [Google Scholar]
- 94.Ray A, Dittel BN. Interrelatedness between dysbiosis in the gut microbiota due to immunodeficiency and disease penetrance of colitis. Immunology : 359–368, 2015. doi: 10.1111/imm.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reaven GM, Twersky J, Chang H. Abnormalities of carbohydrate and lipid metabolism in Dahl rats. Hypertension : 630–635, 1991. [DOI] [PubMed] [Google Scholar]
- 96.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science : 1241214, 2013. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rogers CJ, Prabhu KS, Vijay-Kumar M. The microbiome and obesity-an established risk for certain types of cancer. Cancer J : 176–180, 2014. doi: 10.1097/PPO.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 98.Rohlke F, Stollman N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv Gastroenterol : 403–420, 2012. doi: 10.1177/1756283X12453637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rossi M, Campbell KL, Johnson DW, Stanton T, Vesey DA, Coombes JS, Weston KS, Hawley CM, McWhinney BC, Ungerer JP, Isbel N. Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3-4 chronic kidney disease. Arch Med Res : 309–317, 2014. doi: 10.1016/j.arcmed.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 100.Sanmiguel C, Gupta A, Mayer EA. Gut microbiome and obesity: a plausible explanation for obesity. Curr Obes Rep : 250–261, 2015. doi: 10.1007/s13679-015-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-gut-bone marrow axis: implications for hypertension and related therapeutics. Circ Res : 1327–1336, 2016. doi: 10.1161/CIRCRESAHA.116.307709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology : 577–594, 2008. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 103.Schulberg J, De Cruz P. Characterisation and therapeutic manipulation of the gut microbiome in inflammatory bowel disease. Intern Med J : 266–273, 2016. doi: 10.1111/imj.13003. [DOI] [PubMed] [Google Scholar]
- 104.Sechi LA, Griffin CA, Zingaro L, Catena C, De Carli S, Schambelan M, Bartoli E. Glucose metabolism and insulin receptor binding and mRNA levels in tissues of Dahl hypertensive rats. Am J Hypertens : 1223–1230, 1997. doi: 10.1016/S0895-7061(97)00220-3. [DOI] [PubMed] [Google Scholar]
- 105.Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med : 195–200, 2013. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh V, Chassaing B, Zhang L, San Yeoh B, Xiao X, Kumar M, Baker MT, Cai J, Walker R, Borkowski K, Harvatine KJ, Singh N, Shearer GC, Ntambi JM, Joe B, Patterson AD, Gewirtz AT, Vijay-Kumar M. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab : 983–996, 2015. doi: 10.1016/j.cmet.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh V, Kumar M, San Yeoh B, Xiao X, Saha P, Kennett MJ, Vijay-Kumar M. Inhibition of interleukin-10 signaling induces microbiota-dependent chronic colitis in apolipoprotein E deficient mice. Inflamm Bowel Dis : 841–852, 2016. doi: 10.1097/MIB.0000000000000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh V, Yeoh BS, Xiao X, Kumar M, Bachman M, Borregaard N, Joe B, Vijay-Kumar M. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat Commun : 7113, 2015. doi: 10.1038/ncomms8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science : 569–573, 2013. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res : 448–455, 2015. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tomasova L, Dobrowolski L, Jurkowska H, Wrobel M, Huc T, Ondrias K, Ostaszewski R, Ufnal M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide : 50–58, 2016. doi: 10.1016/j.niox.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 112.Toyonaga T, Nakase H, Ueno S, Matsuura M, Yoshino T, Honzawa Y, Itou A, Namba K, Minami N, Yamada S, Koshikawa Y, Uede T, Chiba T, Okazaki K. Osteopontin deficiency accelerates spontaneous colitis in mice with disrupted gut microbiota and macrophage phagocytic activity. PLoS One : e0135552, 2015. doi: 10.1371/journal.pone.0135552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol : 4153–4158, 2009. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature : 480–484, 2009. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turpin W, Espin-Garcia O, Xu W, Silverberg MS, Kevans D, Smith MI, Guttman DS, Griffiths A, Panaccione R, Otley A, Xu L, Shestopaloff K, Moreno-Hagelsieb G, Paterson AD, Croitoru K; GEM Project Research Consortium . Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet : 1413–1417, 2016. doi: 10.1038/ng.3693. [DOI] [PubMed] [Google Scholar]
- 116.Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, Deng L, Bry L, Gordon JI, Kahn CR. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab : 516–530, 2015. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vaziri ND, Freel RW, Hatch M. Effect of chronic experimental renal insufficiency on urate metabolism. J Am Soc Nephrol : 1313–1317, 1995. [DOI] [PubMed] [Google Scholar]
- 118.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int : 308–315, 2013. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 119.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science : 228–231, 2010. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vijay-Kumar M, Gewirtz AT. Is predisposition to NAFLD and obesity communicable? Cell Metab : 419–420, 2012. doi: 10.1016/j.cmet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 121.Vikram A, Kim YR, Kumar S, Li Q, Kassan M, Jacobs JS, Irani K. Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1. Nat Commun : 12565, 2016. doi: 10.1038/ncomms12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Villanueva-Millán MJ, Pérez-Matute P, Oteo JA. Gut microbiota: a key player in health and disease. A review focused on obesity. J Physiol Biochem : 509–525, 2015. doi: 10.1007/s13105-015-0390-3. [DOI] [PubMed] [Google Scholar]
- 123.Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett : 4223–4233, 2014. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, Degenhardt F, Heinsen FA, Rühlemann MC, Szymczak S, Holm K, Esko T, Sun J, Pricop-Jeckstadt M, Al-Dury S, Bohov P, Bethune J, Sommer F, Ellinghaus D, Berge RK, Hübenthal M, Koch M, Schwarz K, Rimbach G, Hübbe P, Pan WH, Sheibani-Tezerji R, Häsler R, Rosenstiel P, D’Amato M, Cloppenborg-Schmidt K, Künzel S, Laudes M, Marschall HU, Lieb W, Nöthlings U, Karlsen TH, Baines JF, Franke A. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet : 1396–1406, 2016. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, Prescott SL, MacKay C, Salminen S, Wong G, Sinn J, Stokholm J, Bisgaard H, Pawankar R, Noakes P, Kesper D, Tulic M; in-FLAME Microbiome Interest Group . The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol : 3–13, 2015. doi: 10.1016/j.jaci.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 126.Wing MR, Patel SS, Ramezani A, Raj DS. Gut microbiome in chronic kidney disease. Exp Physiol : 471–477, 2016. doi: 10.1113/EP085283. [DOI] [PubMed] [Google Scholar]
- 127.Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol : 230–237, 2014. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao S, Zhao L. Gut microbiota-based translational biomarkers to prevent metabolic syndrome via nutritional modulation. FEMS Microbiol Ecol : 303–314, 2014. doi: 10.1111/1574-6941.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension : 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang Y, Zheng L, Wang L, Wang S, Wang Y, Han Z. Effects of high fructose and salt feeding on systematic metabonome probed via (1) H NMR spectroscopy. Magn Reson Chem : 295–303, 2015. doi: 10.1002/mrc.4198. [DOI] [PubMed] [Google Scholar]
- 131.Yeoh BS, Aguilera Olvera R, Singh V, Xiao X, Kennett MJ, Joe B, Lambert JD, Vijay-Kumar M. Epigallocatechin-3-gallate inhibition of myeloperoxidase and its counter-regulation by dietary iron and lipocalin 2 in murine model of gut inflammation. Am J Pathol : 912–926, 2016. doi: 10.1016/j.ajpath.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshikawa D, Ishii H, Suzuki S, Takeshita K, Kumagai S, Hayashi M, Niwa T, Izawa H, Murohara T. Plasma indoxyl sulfate and estimated glomerular filtration rate. Circ J : 2477–2482, 2014. doi: 10.1253/circj.CJ-14-0401. [DOI] [PubMed] [Google Scholar]
- 133.Zhang C, Yin A, Li H, Wang R, Wu G, Shen J, Zhang M, Wang L, Hou Y, Ouyang H, Zhang Y, Zheng Y, Wang J, Lv X, Wang Y, Zhang F, Zeng B, Li W, Yan F, Zhao Y, Pang X, Zhang X, Fu H, Chen F, Zhao N, Hamaker BR, Bridgewater LC, Weinkove D, Clement K, Dore J, Holmes E, Xiao H, Zhao G, Yang S, Bork P, Nicholson JK, Wei H, Tang H, Zhang X, Zhao L. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine : 968–984, 2015. doi: 10.1016/j.ebiom.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


