Abstract
The identification of the low-density lipoprotein receptor (LDLR) provided a foundation for subsequent studies in lipoprotein metabolism, receptor-mediated endocytosis, and many other fundamental biological functions. The importance of the LDLR led to numerous studies that identified homologous molecules and ultimately resulted in the description of the LDL-receptor superfamily, a group of proteins that contain domains also found in the LDLR. Subsequent studies have revealed that members of the LDLR-related protein family play roles in regulating many aspects of signal transduction. This review is focused on the roles of selected members of this protein family in skeletal development and disease. We present background on the identification of this subgroup of receptors, discuss the phenotypes associated with alterations in their function in human patients and mouse models, and describe the current efforts to therapeutically target these proteins to treat human skeletal disease.
I. INTRODUCTION
A. The Low-Density Lipoprotein Receptor Superfamily
The body of work that led to the identification and characterization of the low-density lipoprotein receptor (LDLR) is one of the most influential sets of studies ever performed. Its impact on basic and translational research has been immense, perhaps best reflected by the fact that Michael Brown and Joseph Goldstein received the Nobel Prize for this work in 1985. This provided the foundation for our understanding of how cells regulate intercellular cholesterol levels, significantly increased our understanding of the mechanisms underlying endocytosis, and helped provide the rationale for the use of statins in treating cardiovascular disease. For those interested in a more detailed history of this work, we recommend the article by Goldstein and Brown (51).
Given the importance of the LDLR, there has been significant interest in understanding the function of its various domains and identifying other molecules that share homologous structures. Initial comparisons of LDLR sequences from different species revealed several conserved domains. One such example is the class A repeat (LDL-A) (12, 32, 100, 142). Each of the seven or eight LDL-A repeats in the LDLR is composed of ~40 amino acids, among which are three pairs of cysteines that form intra-repeat disulfide bonds. In addition, each LDL-A domain contains a series of acidic residues required for the coordination of a calcium ion.
A second example is the LDLR class B (LDL-B) repeat. Each LDL-B repeat contains the four-amino-acid sequence of Tyr-Trp-Thr-Asp (YWTD). Domains containing LDL-B repeats typically are composed of two epidermal growth factor (EGF) repeats, followed by six LDL-B repeats, followed by another EGF repeat (76, 77). The coordinated folding of the six LDL-B repeats creates a structure referred to as the β-propeller (76). The crystal structures of β-propeller motifs have been solved (24, 77) and have been described as being reminiscent of an amphitheater or funnel.
An Asn-Pro-X-Tyr motif (NPXY; with X representing any amino acid) is found in several LDL receptor family members. This motif was first recognized as a sequence conserved in the LDLRs of species ranging from frogs to humans (25). This motif is also found in other membrane receptors, such as EGF receptor (EGFR), insulin-like growth factor receptors (IGFRs), and integrins (177). Most NPXY sequences are found in the cytoplasmic portion of LDLR-related proteins, within 50 amino acids of the transmembrane domain. Generally, phosphorylation on the Tyr of NPXY provides a docking site for proteins containing a PTB (phosphorylated tyrosine binding) domain (167). The NPXY motif in the LDLR also facilitates coated-pit-mediated endocytosis (25).
Given the crucial importance of the LDL in lipid metabolism, several laboratories set up screens and identified molecules with structural similarities to the LDLR, particularly those that contained LDL-A and LDL-B repeats. This led to the identification of several related molecules that are now classified as members of the LDLR superfamily or of the LDLR-related proteins (LRPs) (11, 57, 61, 130). While many human proteins contain either LDL-A or -B repeats, a smaller number contain at least one domain of each repeat.
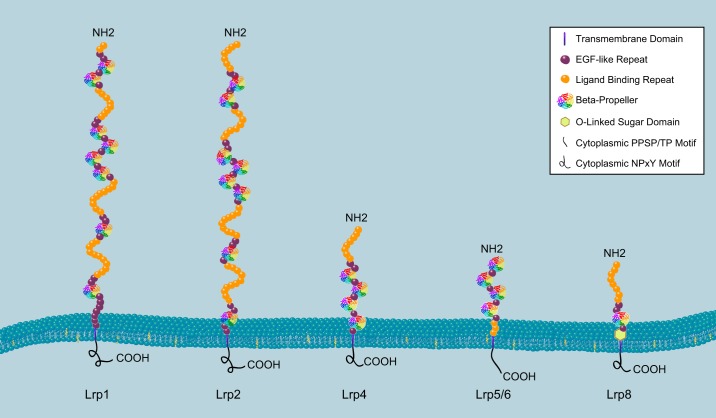
While the historical foundation of studies on these LDLR family members was their ability to bind lipoproteins, more recent work has identified their other important functions. For example, these receptors play crucial roles in neuronal development, and their dysregulation has been strongly associated with the development of Alzheimer’s disease and cardiovascular diseases (106, 129). Furthermore, over the past 15 yr, the demonstration that several members of this family either directly or indirectly regulate Wnt signaling (80) and other developmental signaling pathways has stimulated additional interest in understanding the molecular mechanisms by which they signal. In this review, we primarily focus on the LDLR superfamily members, in which alterations in function have been shown to effect skeletal development and/or bone homeostasis, specifically, LDLR, LRP1, LRP2, LRP4, LRP5, LRP6, and LRP8 (APOER2) (FIGURE 1). We will first briefly discuss the processes underlying normal skeletal development and its dysregulation in disease.
FIGURE 1.
Schematic diagram of selected low-density lipoprotein receptor family members. The domain configurations and relative sizes of LRP1, LRP2, LRP4, LRP5/6, and LRP8 are shown. Each commonly shared domain is indicated within the structure by symbols shown in the box at the top right. The COOH terminus of the protein is on the cytoplasmic side of the membrane, and the NH2 terminus is extracellular.
B. Skeletal Development and Repair
The skeletal system originates from cells of the mesenchymal lineage, which aggregate and condense to form a pattern for the future skeletal elements during embryogenesis [details on patterning and development of nascent bone can be found in two excellent reviews by Berendsen and Olsen (14) and Franz-Odendaal (41)]. The majority of skeletal elements develop through endochondral ossification, in which the mesenchymal cells at the core of the condensations start differentiating into chondrocytes and secrete cartilage matrix components such as type II collagen and aggrecan (122). These chondrocytes initially proliferate rapidly and unidirectionally to enlarge the skeletal elements. Subsequently, the chondrocytes from the inner region become hypertrophic and express type X collagen, a chondrocyte terminal differentiation marker. The polarized chondrocyte maturation establishes a finely zoned structure of the growth plate, i.e., the resting zone containing naive chondrocytes, the proliferating zone containing the columnar proliferative chondrocytes, the hypertrophic zone of terminally differentiated chondrocytes, and a thin prehypertrophic zone between the proliferating and hypertrophic zones (122).
In addition to secreting type X collagen, hypertrophic chondrocytes release mineralization vesicles (171) and express vascular endothelial growth factor (VEGF; which induces angiogenesis) and matrix metalloproteinases (MMPs; which degrade the cartilaginous matrix for blood vessel extension into the primary ossification center) (124). Then, the hematopoietic-cell-derived osteoclasts remove cartilaginous matrix to create space for the bone marrow. At the same time, mesenchymal progenitors within the perichondrium, a fibrotic tissue surrounding the cartilage, initiate osteoblast differentiation and migrate to bone marrow cavities, where they replace cartilage with bone. In a similar way, secondary ossification occurs in the resting zone (136). The bony secondary ossification center separates the original growth plate into two parts: the growth plate residing in between the two ossification centers and the articular cartilage that covers the ends of bone and provides a smooth surface for joint movement.
A less widespread process called intramembranous ossification is how a few craniofacial bones and a portion of the clavicle form; this process does not depend on the intermediate cartilage template formation step (41). Within the mesenchymal condensation, some cells aggregate to form clusters and directly differentiate into osteoblasts and osteocytes. New bone is progressively built up around the outer skirts of these ossified clusters. In later development stages, vascularization, osteoclast formation, and bone marrow establishment occur. The mesenchymal tissues surrounding the bone become periosteum, providing progenitors to support the growth and repair of these bones.
The two types of ossification also contribute to bone fracture healing (reviewed in Ref. 37). Inflammatory responses to fracture recruit skeletal progenitor cells from the periosteum and marrow (121). If the bone vasculature is severely damaged, the hypoxic environment first induces chondrogenesis of skeletal progenitors and forms new bone through endochondral ossification. In contrast, if the vasculature is partly preserved, the recruited skeletal progenitors directly differentiate into osteoblasts to repair the bone through intramembranous ossification. During the ossification process, inflammatory responses need to be restrained to allow chondrogenesis and ossification.
C. Bone Homeostasis and Osteoporosis
Bone is a dynamic tissue that undergoes continuous remodeling, a process coupling osteoblast-dependent bone formation and osteoclast-dependent bone resorption (reviewed in Ref. 165). Bone remodeling is required to replace microdamaged old bone with new bone, thus maintaining bone strength. It also helps bone to adapt to changes in loading and to maintain systemic homeostasis of calcium and phosphate.
Osteoporosis is a disease characterized by low bone mass (bone quantity), deteriorated bone microarchitecture (bone quality), and increased risk of fracture (outcome) (reviewed in Ref. 15). In persons with osteoporosis, the hip and the vertebrae are the most susceptible fracture sites. Generally, osteoporosis is caused either by excessive bone resorption due to overactive osteoclast activity or by insufficient bone formation due to defective osteoblast activity/function, or a combination of both. Annually in the United States, osteoporosis-related fractures cost some $18 billion. Risk factors of osteoporosis include obesity, nutrition deficits (vitamin D, minerals, etc.), gender and age (hormone levels), life style (alcohol consumption, exercise, etc.), and genetic predisposition. Genetic linkage and genome-wide association studies (GWAS) studies have been successful in uncovering candidate genes responsible for low bone mass and osteoporosis. Many of those genes and their corresponding pathways have been used as drug targets for osteoporosis treatments (for example, see Ref. 154).
D. Cartilage Homeostasis and Osteoarthritis
In articular joints, adjacent bone elements are held together with ligaments, tendons, and synovia. This structure facilitates motion by effectively transducing muscle force to bone and by providing smooth cartilage surfaces between bones. Osteoarthritis (OA) is the most common and disabling disease of the joints. Traditionally, OA has been considered as resulting from the wear and tear of the articular cartilage, but an evolving concept suggests that OA is a degenerative disorder progressively impairing almost every component of joints, including articular cartilage, subchondral bone, synovia, and ligaments (40). Eventually, this results in the “failure of the joint as an organ.” The risk factors for OA include joint injury, age, sex, weight, genetic predisposition, and abnormal joint alignment or shape, among others (48, 50). In healthy joints, the articular chondrocytes are quiescent, with minimal matrix remodeling. However, in OA joints, some chondrocytes undergo proliferation, differentiation, apoptosis, or senescence. These “activated” chondrocyte clusters express mineralizable matrix proteins (type 10 collagen, type 1 collagen, etc.), proteases (such as ADAMTS-5, MMP13, and MMP3), and inflammatory cytokines and effectors (interleukins, CCLs, cyclooxygenase-2, inducible nitric oxide synthase, etc.). These cells also show activity in enhanced stress response pathways, such as NFκB and mitogen-activated protein kinase (MAPK) signals (49, 118).
II. SKELETAL PHENOTYPES ASSOCIATED WITH DYSREGULATION OF LDLR FAMILY MEMBERS
A. LDLR
The LDLR was the first identified member of the LRP family (169). As a transport protein that facilitates cholesterol entry into cells by receptor-mediated endocytosis, the LDLR plays a crucial role in regulating the amount of plasma cholesterol in animals (20, 51). Mutations that inactivate the LDLR are an underlying cause of familial hypercholesterolemia, an autosomal recessive condition causing premature cardiovascular diseases (65).
The LDLR is expressed in vascular smooth muscle cells (VSMCs), which behave similarly to osteochondroprogenitors within atherosclerotic plaques, contributing to the development of calcified cartilage or bonelike structures within these lesions (137). Ldlr–/– mice fed a high-fat diet develop arterial intima calcification; this process does not occur in VSMCs lacking RUNX2, the master transcription factor of osteogenesis (115). Moreover, cultured Ldlr–/– VSMCs show enhanced osteogenesis upon lipid treatment, suggesting that LDLR-mediated lipid intake lessens osteogenesis (45). However, compared with wild-type (WT) controls, Ldlr–/– mice on a normal diet showed a lower bone mass, which became more severe when these mice were fed a high-fat diet. Decreased Runx2 expression and higher TRAP expression were detected in the mutant mouse bone. In addition, Ldlr–/– bone marrow stromal cells (BMSCs) had increased adipogenesis and decreased osteogenesis associated with elevated PPARγ expression (26). The context-dependent LDLR functions in regulating osteogenesis and calcification in VSMCs versus BMSCs remain unclear.
The LDLR is constitutively expressed in osteoclast precursors in a RANKL-independent manner and plays a regulatory role in osteoclastogenesis. Ldlr–/– mice show increased bone mass and decreased bone resorption but no changes in bone formation. Ldlr-deficient osteoclast precursors have impaired osteoclast differentiation and aberrant cell-cell fusion, consistent with the smaller size of and fewer nuclei in the mutant osteoclasts. While RANKL/RANK downstream signaling through ERK, AKT, and NFATc1 (a master transcription factor of osteoclastogenesis) is unchanged, the levels of osteoclast-fusion related genes encoding v-ATPase V (0) subunit d2 (ATP6V0d2) and dendritic cell-specific transmembrane protein (DC-STAMP) are reduced in the Ldlr–/– pre-osteoclasts. These findings provide a connection between osteoclast formation and lipid metabolism (147). However, these data differ from the study described above showing that Ldlr−/− mice fed with either a normal or a high-fat diet have decreased osteogenesis but enhanced bone resorption (26). The mice fed with normal diets were similar for both studies in that they used 8-wk-old male mice on a C57BL/6J background, so it is unclear what accounts for the differences in the bone mass observed.
B. LRP1
LRP1 was first cloned from a human liver cDNA library (59). It has an extraordinarily large extracellular domain (ECD) and a small intracellular domain (ICD) of 100 amino acids (FIGURES 1 and 2). The ECD contains four clusters of cysteine-rich, complement-like repeats that are pivotal for interacting with LRP1's ligands. The ICD has two YXXL motifs, a dileucine motif responsible for endocytosis, and two NPXY motifs (59). A study using neurons as a model showed that the membrane-proximal NPXY4473 motif, although not crucial for endocytosis, facilitates recycling of LRP1 in the early endosome, thus maintaining the abundance of LRP1 on the cell surface (179). Moreover, phosphorylation of the NPXY motifs within the ICD provides binding sites for a set of signaling proteins having PTB domains. For example, phosphorylation of Tyr4507 within LRP1 by platelet-derived growth factor (PDGF) treatment or v-Src overexpression enhances Shc-LRP1 interactions (8); this site also binds to DAB1, JIP1, and PDGFR, among others. Tyrosine phosphorylation on NPXY4473 depends on the phosphorylation of NPXY4507, which recruits SRC to the ICD. Phosphorylation on both NPXY4473 and NPXY4507 are required for Shc1 recruitment to the ICD. Mutation of NPXY4473 in mice leads to embryonic death and liver degeneration, while mutation of NPXY4507 causes no distinctive phenotype (156). This suggests that NPXY4473 is pivotal for mediating LRP1 downstream signaling.
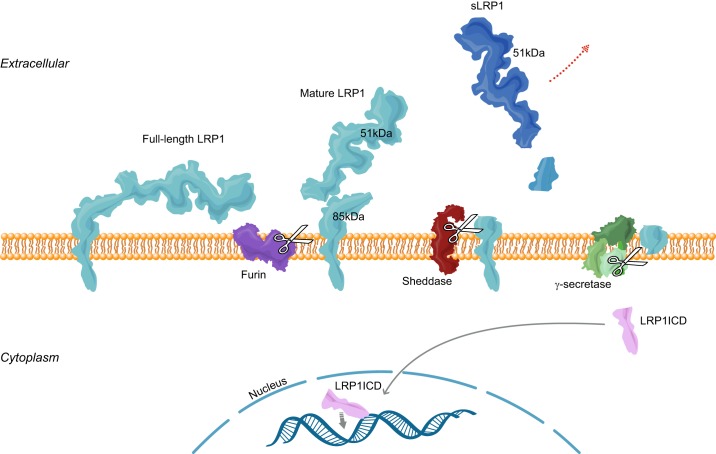
FIGURE 2.
LRP1 proteolytic processing. Full-length LRP1 is cleaved by furin into 515 and 85 kDa subunits, which associate with each other noncovalently as “mature LRP1.” The 85-kDa subunit can be processed by sheddases to free the larger subunit (soluble LRP1, or sLRP1; dark blue) from the membrane. The remaining transmembrane subunit may be further cleaved by γ-secretase to release the LRP1 intracellular domain (LRP1ICD; pink), which can translocate to the nucleus to regulate gene transcription.
LRP1 undergoes a cascade of proteolytic modifications in a context-dependent manner (FIGURE 2). Most typically it is cleaved in the ECD by furin convertase into an α unit (515 kDa) and a β unit (85 kDa), both of which remain in a complex noncovalently (59, 60). This “matured” LRP1 may be further cleaved by cell surface proteases such as MMPs or BACE1 to produce the soluble LRP1 (sLRP1) (161, 182, 204), and/or processed intracellularly by γ-secretase to release the 10-kDa ICD from the membrane (128, 205).
LRP1 transports a broad spectrum of ligands into the cell, such as apoprotein E (APOE)-enriched lipoproteins (APOE function in skeletal tissue will be discussed in a later section), proteases and protease inhibitors, and amyloid-β; thus it has been referred to as a scavenger receptor (58, 103, 114). LRP1-mediated endocytosis is commonly involved in regulating the availability of signaling components on cell surface. For example, LRP1 suppresses the hypertrophy of vascular smooth vessels by inhibiting the PDGF receptor and transforming growth factor (TGF)-β pathways (16, 17), and it cooperates with glypicans to inhibit hedgehog signaling by promoting sonic hedgehog (SHH) endocytosis (22). In addition, LRP1 regulates adipocyte differentiation by enhancing Wnt signaling (175).
1. LRP1 in chronic medical conditions
Alterations in both the scavenger and signaling functions of LRP1 contribute to the development and progression of several chronic diseases. LRP1 is abundantly expressed in the VSMCs of the cerebrovasculature and acts as a potent amyloid-β (Aβ) clearance receptor. Conditional deletion of Lrp1 in the VSMCs of APP/PS1 mice, an amyloid model, accelerated Aβ accumulation in the brain and exacerbated Aβ deposition without affecting its production. Because the impaired clearance of Aβ is a major pathogenic event in Alzheimer's disease (AD), LRP1 is likely to play a preventive role in AD (82).
LRP1 is also a key regulator of atherosclerosis development. Mice with VSMC-specific Lrp1 knockout have elevated PDGFR (PDGF receptor) signaling, resulting in VSMC overproliferation, aneurysm formation, and a marked susceptibility to cholesterol-induced atherosclerosis (16). Subsequent studies indicated that LRP1 suppresses TGF-β signaling to inhibit atherosclerosis (17). Macrophage-specific LRP1 function has also been found to play a role in atherosclerotic pathogenesis. Transplanting the bone marrow of macrophage-specific Lrp1-knockout mice into lethally irradiated Ldlr−/− recipient mice resulted in a 40% increase in atherosclerosis, accompanied by enhanced proximal aorta macrophage cellularity and the elevated expression of monocyte chemoattractant protein type 1 (MCP1), tumor necrosis factor alpha (TNF-α), and MMP9 (148). Moreover, follow-up studies show that macrophage-derived LRP1 is required for anti-atherogenic effects mediated by inhibition of TNF-α (203).
Recent evidence has indicated that LRP1 is a multifaceted inflammatory suppressor. In macrophages, LRP1 decreases the level of TNF receptor 1 (TNFR1) on the cell surface, blocks nuclear factor κB (NFκB) signaling, and reduces the expression of iNos, Ccl2, and Il6 (44). Moreover, macrophage-specific Lrp1 KO mice show exacerbated responses to lipopolysaccharide (LPS), with dramatically enhanced Il6 and Ccl2 expression (125). Lrp1 knockdown enhances TNF-α-induced inflammation, apoptosis, and MMP13 expression in chondrocytes by promoting the NFκB pathway (193). Interestingly, inflammation stimulates the proteolysis of LRP1, and the cleaved LRP1 derivatives (including secreted LRP1 or sLRP1) are potent inflammation suppressors. LPS can promote sLRP1 production from LRP1 shedding, and the plasma sLRP1 level is increased in inflammatory diseases such as rheumatoid arthritis and lupus (53). sLRP1 was also found to bind and suppress CD11b on macrophages, thus inhibiting inflammation (150). Peripheral nerve injury enhances LRP1 shedding from Schwan cells and augments the level of sLRP1, which suppresses TNF-α-induced MAPK signaling and alleviates neuropathic pain (43). Yet, sLRP1 has also been reported to interact with TNFR1 to activate p38 and JNK signaling in macrophages (53). This discrepancy is still unresolved. Moreover, the intracellular domain of LRP1 (LRP1ICD) is released from the membrane by γ-secretase when macrophages are stimulated by LPS, and it then interacts with and stimulates the export of interferon regulatory transcription factor (IRF3) from the nucleus, blocking the production of IL-6 and TNF-α and restraining the amplification inflammation-induced signaling (205).
2. LRP1 function in skeletal tissues
LRP1 SNPs have been associated with decreases in bone mineral density, bone mineral content, and femoral area (166). This provided a tantalizing clue that LRP1 may play a role in bone accrual or remodeling. LRP1 is highly expressed in osteoblastic cell lines (54, 139). It promotes the endocytosis of lactoferrin, a pleotropic factor abundant in milk and other secreted fluids that has antibacterial and pro-proliferation activity. Lactoferrin-treated osteoblastic cells show activated Erk1/2 (p42/44) and enhanced cell proliferation and survival. This lactoferrin-induced mitogenesis can be abolished by LRP1 antibody or the LRP1 inhibitor RAP (LRP receptor–associated protein, which is an ER-resident chaperone facilitating the folding of LRP1 and other LDL receptor family members) (54). However, the pro-survival and pro-differentiation functions of lactoferrin in osteoblasts are LRP1 independent (55, 198). Hence, the lactoferrin-dependent LRP1 function likely contributes to osteoblast proliferation. Moreover, LRP1 cooperates with an unidentified 170-kDa protein to facilitate MMP13 internalization and degradation in UMR 106–01 cells, a rat osteoblastic osteosarcoma cell line. This suggests that LRP1 may regulate bone extracellular matrix remodeling by modulating MMP13 availability (7).
LRP1 also regulates bone accrual by promoting vitamin K intake (139). The lipophilic vitamin K serves as a cofactor of γ-carboxylase, which converts three glutamic acid residues of osteocalcin into γ-carboxyglutamic acid. These modifications enhance the interaction of osteocalcin with the mineral hydroxyapatite, thus augmenting the strength and integrity of the bone matrix (69). Vitamin K1 in the diet is carried by chylomicrons and their remnants. The osteoblast-expressed LRP1 robustly endocytoses remnants and co-localizes with them. Moreover, dietary vitamin K intake can be competed for or blocked by lactoferrin, supporting the idea that LRP1 is a receptor for chylomicron remnants in osteoblasts and that it plays a crucial role in vitamin K intake (139).
Osteoblast-derived LRP1 has been reported to play a regulatory role in osteoclastogenesis. LRP1 promotes lactoferrin translocation to the cytoplasm and its interaction with TRAF6, thus blocking TNF-α and RANKL production by osteoblasts and inhibiting the osteoclastogenesis of OB-OC co-cultures (73). More recently, LRP1 was found to facilitate galectin-8 internalization, which lessens galectin-8-induced RANKL expression and the osteoclastogenesis in OB-OC co-cultures (181).
LRP1 is expressed at a high level in articular chondrocytes, and it protects the cartilage matrix in joints by suppressing cartilage-degrading enzymes. MMP13 (collagenase 3) is such an enzyme; it degrades fibrillar native collagens and aggrecan, damaging joint surfaces, and it is highly expressed in arthritic joints (134, 151, 162). The articular chondrocytes and synoviocytes in osteoarthritis have decreased amounts of LRP1, leading to an impaired ability to bind, internalize, and degrade MMP13 (183). Another enzyme, ADAMTS-5, specifically degrades aggrecan (an abundant extracellular matrix of articular cartilage) and promotes osteoarthritis progression. Blocking LRP1 by using siRNA or RAP treatment dramatically impairs ADAMTS5 endocytosis and causes ADAMTS5 over-activation (96). In addition, LRP1 mediates ADAMTS4 endocytosis and degradation in articular cartilage via a similar mechanism, albeit at a slower rate (192).
LRP1 has been implicated in regulating chondrocyte differentiation. LRP1 expression is high in resting and proliferating chondrocytes but low in hypertrophic chondrocytes (88, 89). LRP1 facilitates CCN2 (CTGF) transcytosis in the growth plate. Exogenously added recombinant CCN2 is colocalized with endogenously expressed LRP1 in clathrin-coated vesicles and endosomes, while LRP1 knock-down can block CCN2 transcytosis and retention in chondrocytes. Moreover, RAP is highly expressed in the resting zone of growth plate, while CCN2 expression is limited to the prehypertrophic zone. Therefore, LRP1 is proposed to transport CCN2 in forming a CCN2 gradient from the prehypertrophic zone to the hypertrophic zone.
Recently, LRP1-SNRNP25, a recurrent fusion gene within the chromosome12q locus, was identified in osteosarcoma patients by transcriptome analyses. This gene fusion event is specific to osteosarcoma and has not been identified in other types of sarcomas. In addition, the ectopically overexpressed LRP1-SNRNP25 fusion gene promotes SAOS-2 osteosarcoma cell migration and invasion (194). Further validation and more detailed mechanistic studies of LRP1-SNRNP25 in osteosarcoma pathogenesis will be important.
C. LRP2
LRP2 (also called megalin or gp330) is a 650-kDa transmembrane protein that has the highest homology to LRP1 (FIGURE 1). It has four clusters of cysteine-rich, complement-like repeats in its ECD and two NPXY motifs in its ICD.
LRP2 mutations are the underlying cause of two inherited human clinical conditions, Donnai-Barrow syndrome (DBS) and facio-oculo-acoustico-renal (FOAR) syndrome, which are characterized by developmental defects of the forebrain and facial structure (84). Studies on Lrp2-deficient mice have provided mechanistic insights into these phenotypes. Most Lrp2–/– mice die perinatally of respiratory insufficiency caused by bloated alveoli and thickened alveolar walls. Lrp2–/– mice also show distinctive holoprosencephalic phenotypes (fusion of the forebrain hemispheres and loss of the olfactory bulb) and defects in facial structures (shortened nasal bone and flattened forehead) that develop from the forebrain-derived neural crest and contiguous mesoderm. Before the vasculature system is established in embryonic development, the nutrition of the rapidly dividing neuroepihelium relies on uptake from the surrounding fluids. LRP2 is highly expressed in the apical surface of neuroectoderm and later within the neuroepithelium, and it mediates uptake of cholesterol-rich lipoproteins from the amniotic fluid. Therefore, the holoprosencephalic phenotypes of Lrp2–/– mice may be partly due to cellular starvation for cholesterol and fat-soluble vitamins (188).
LRP2 also regulates craniofacial development through sonic hedgehog (SHH) signaling, the loss of which is the most common cause of holoprosencephaly (13, 157). During neurulation, forebrain midline structures are specified by signals originating from the prechordal plate (PrCP), which is a mesodermal tissue underlying the rostral diencephalon ventral midline (RDVM). PrCP-derived SHH acts on the overlying RDVM to establish ventral forebrain identity. Consequently, RDVM-produced SHH induces ventral cell populations and antagonizes dorsal signals governed by bone morphogenetic protein (BMP) signaling. In its target areas, LRP2 can act as an auxiliary sonic hedgehog (SHH) receptor that internalizes the SHH/patched-1 complex, thus relieving patched-1-dependent suppression of smoothened. Moreover, these internalized SHH molecules can then be recycled back to the extracellular space to continue activating smoothened signaling. Consistent with this model, Lrp2-deficient mice have an impaired response to SHH (29). LRP2 can also downregulate BMP signaling by serving as a BMP4 clearance receptor, which could contribute to LRP2-mediated forebrain and facial development (168).
About 2% of Lrp2–/– mice survive through to adult age, and although the skeletal patterning is largely normal, they have severe growth retardation and low bone density with overactive osteoclast activity. The urine of these mice shows elevated alkaline phosphatase and hydroxyproline, suggesting a defect in bone mineralization and a high bone turnover rate. These skeletal phenotypes are primarily due to dysregulated vitamin D3 production in the kidney. Vitamin D binding protein (DBP) forms a complex with the vitamin D3 precursor 25-OH-D3 and is filtered out of the glomeruli. Then the DBP-25-OH-D3 complex is resorbed in the kidney proximal tubules, where the 25-OH-D3 can be further converted into the active vitamin, 1,25-(OH)2-vitamin D3. LRP2 is highly expressed in the renal proximal tubule and serves as a DBP-25-OH-D3 receptor to avoid excessive excretion of 25-OH-D3. In Lrp2–/– mice, large amounts of DBP and vitamin D3 appeared in the urine, and the serum level of vitamin D3 was dramatically decreased. This explains the rickets-like skeletal phenotype of Lrp2–/– mice (143).
Human osteoblasts express LRP2 and 1α-hydroxylase, a key enzyme for 1,25-(OH)2-vitamin D3 synthesis. This suggests that vitamin D3 may be an auto/paracrine factor within bone (178). However, the extent to which osteoblast-specific LRP2 expression contributes to bone homeostasis has yet to be clearly defined.
D. LRP4
Lrp4 was first identified based on a strategy to identify large proteins that contain EGF-like motifs; it was originally named MEGF7 (multiple epidermal growth factor-like motifs 7) (163). The following year, it was independently identified in a screen for mRNAs that are enriched in the postsynaptic fraction within the neuromuscular junction and was termed short synaptic LRP (ssLRP) (163, 176). The LRP4 protein is a 212-kDa protein that contains eight LDL type A repeats followed by four β-propeller motifs; the cytoplasmic domain contains one NPXY motif (FIGURE 1). Subsequent work has demonstrated a key role for LRP4 in mediating the functions of the agrin/MUSK (muscle-specific kinase) signaling complex. This function of LRP4 is the underlying cause of the perinatal death of mice that are globally deficient for Lrp4; they retain their in utero posture after birth, failing to either move or breath (184). As this review will focus on the role of LRP4 within the skeleton, we will not further discuss this aspect of LRP4 signaling, but excellent reviews are available (155, 163).
A role for LRP4 within the skeletal system is clear from the phenotypes associated with human patient Cenani-Lenz syndrome. In this syndrome, loss-of-function mutations in LRP4 cause a variety of developmental defects linked to skeletal malformation, including syndactyly and tooth abnormality (85, 94, 112, 116). Some patients also display renal abnormalities. In addition, two independent point mutations in the extracellular domain of LRP4 have been associated with bone overgrowth (110).
Animal models carrying mutations in Lrp4 further emphasize its role in skeletal patterning and homeostasis. For example, bovines carrying a specific mutation in Lrp4 develop the autosomal recessively inherited disorder referred to as mulefoot disease, in which the usually cloven bovine hoof is fused (79). Mice carrying germline loss-of-function mutations in Lrp4 display phenotypes similar to those of their human counterparts, differing in severity depending on the specific Lrp4 mutation (79, 86, 144, 145).
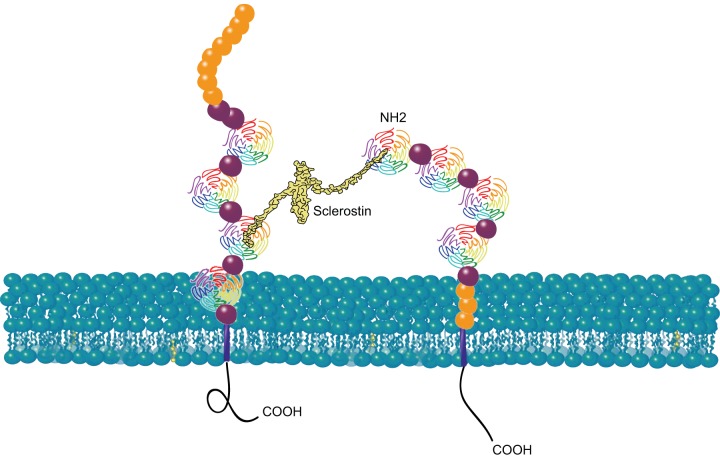
The observation that mutations in human LRP4 are associated with high-bone-mass phenotypes was reminiscent of the phenotypes associated with loss-of-function mutations in the sclerostin gene. The protein product of this gene is most highly expressed in osteocytes and acts as an inhibitor of Wnt/β-catenin signaling (see a more detailed discussion in sect. IID). Subsequent work showed that LRP4 serves as a receptor for SOST, allowing it to be presented to LRP5 and/or LRP6 to inhibit Wnt signaling (23, 28, 91, 110) (FIGURE 3). In an elegant set of experiments, Chang et al. (23) developed antibodies that specifically interfere with the interaction between LRP4 and SOST without affecting the LRP4/agrin interaction, and they showed that they could specifically increase bone mass without affecting the function of neuromuscular junctions. A role for agrin signaling via LRP4 in cartilage formation has recently been demonstrated (38), emphasizing the importance of specifically targeting the LRP4/SOST interaction.
FIGURE 3.
LRP4 facilitates presentation of sclerostin to LRP5/6. LRP4 and LRP5/6 are shown with the same domain symbols as in FIGURE 1. Sclerostin is shown in yellow. The COOH termini of LRP4 and LRP5/6 are on the cytoplasmic side of the plasma membrane; the NH2 termini are extracellular.
E. LRP5
Lrp5 was first identified by three groups in 1998. Kim et al. (95) identified the gene based on screening a rabbit cDNA library with a probe derived from the human LRP8 gene (FIGURE 1). Their initial characterization showed that the protein product of LRP5 interacted specifically with apolipoprotein E. Hey et al. (62) isolated LRP5 during an examination of a genomic region that had been linked with a genetic susceptibility to type 1 diabetes and noted its homology to members of the LDL receptor family. Finally, Dong and colleagues identified the LRP5 gene and originally designated it as LR3 (and also later as LRP7) (24a, 35a). The LRP5 protein contains four β-propeller motifs followed by three LDL type A repeats in the extracellular domain. A single-pass transmembrane domain is then followed by several serine-rich motifs that are subject to posttranslational phosphoregulation.
Shortly after the cloning of LRP5, work from three laboratories simultaneously showed that LRP5 and the highly related LRP6 protein function as key components of the Wnt signal transduction pathway (149, 173, 186). Wnt proteins are a family of 19 cysteine-rich glycoproteins that initiate signals by binding to a protein complex containing a member of the Frizzled family of seven-transmembrane receptors and either LRP5 or LRP6 (80, 127). This leads to downregulation of glycogen synthase kinase-3 (GSK-3) activity. In the absence of Wnt, GSK-3 phosphorylates β-catenin, marking it for ubiquitin-dependent proteolysis (1). Inactivation of GSK-3 increases β-catenin in the cytosol and nucleus, allowing β-catenin to interact with TCFs and LEF (135), and these complexes modulate the transcriptional activity of target promoters (135). Several proteins downregulate Wnt signaling by binding to and preventing Wnts from interacting with LRP5 and LRP6; these include Dickkopf-1 (DKK1) and sclerostin (SOST). Sclerostin has been of particular interest in the context of skeletal biology, because patients homozygous for loss-of-function mutations—either directly in the SOST gene or in a regulatory element necessary for normal SOST expression—develop phenotypes similar to those in patients carrying high-bone-mass-associated mutations in LRP5.
Wnt-induced inactivation of GSK-3 activity also regulates other signaling pathways independent of β-catenin, many of which are just now being identified (70, 71, 98, 172). Wnts also signal through GSK3-independent mechanisms that have been collectively referred to as “noncanonical” signaling (180). LRP5 and LRP6 were originally thought to not be required for noncanonical signaling, although some studies have suggested a role (4).
Shortly after the cloning of LRP5, several groups linked its mutation to dramatic changes in human bone mass. The first such report, from Warman and colleagues, demonstrated that loss-of-function mutations in LRP5 were the underlying cause of the rare autosomal recessive syndrome, osteoporosis pseudoglioma (OPPG) (52a). OPPG is characterized by extremely low bone mass at young ages and a high propensity for bone fractures. OPPG patients also suffer from vision problems related to several factors that affect the ability of the retina to develop normal vascularization (107).
Soon after, two reports linked a point mutation in LRP5 to individuals who had extremely high bone mass (18, 117). These reports, along with subsequent work, led to the demonstration that this point mutation (a Gly to Val change, G171V) produced a LRP5 protein that could no longer interact with proteins that normally negatively regulate its activity (2). These proteins included the chaperone protein MESD (199) and the negative regulators DKK1 and SOST. Numerous genetic association studies have since provided strong evidence that many other mutations in LRP5 produce more-subtle changes in human bone mass and have established that LRP5-dependent pathways are key modulators of bone homeostasis.
The evaluation of the mechanisms underlying the control of bone homeostasis by LRP5 was greatly aided by the use of genetically engineered mouse models. Several laboratories, starting with the laboratory of Gerard Karsenty, reported that mice homozygous for a global deletion of the Lrp5 gene were viable and fertile and had phenotypes similar to those of their human counterparts, including decreased bone mass and inappropriate retinal vascularization (66, 75, 87). Furthermore, mice that carry a transgene that directs expression of the LRP5 allele coding for G171V specifically within the osteoblast lineage developed high bone mass, modeling that is seen in their human counterparts (3, 6).
The use of the Cre-lox system in mice to delete or activate Lrp5 within specific cell types has provided a great deal of information on the mechanisms by which Lrp5 controls bone mass. The first such report involved the G171V allele in the intestine using the Villin-Cre driver and in osteoblasts using Col1a1-Cre (191). The data were consistent with a model in which Lrp5 functions within the enterrochromaffin cells of the intestine to control the expression and secretion of serotonin. The changing amount of serotonin in the blood then affects serotonin receptors on the surface of osteoblasts to regulate osteoblast activity and function. Subsequent reports indicated that modulation of serotonin levels by drugs in low-bone-mass models could normalize bone mass (72, 190). However, other reports are consistent with LRP5 functioning within the osteoblast, and not within the intestine, to control bone mass (30, 31, 78, 153, 201). Another report failed to demonstrate correlation between serotonin levels and either the LRP5 genotype or bone mass (108). In addition, a model in which LRP5 functions within the osteoblast is consistent with a significant amount of research showing that LRP5 is necessary to increase bone mass through exposure to mechanical loading (83, 141, 158, 200). The reasons for the reported differences in the role of serotonin in Lrp5-dependent regulation of bone mass remain unclear (30, 99).
Several studies have evaluated the role of LRP5 in osteoarthritis. A SNP within the LRP5 locus is significantly associated with osteoarthritis susceptibility (196). A recent analysis of genome-wide distribution of hydroxymethylated cytosine (5hmC) in osteoarthritic cartilage revealed that LRP5 has more differentially hydroxymethylated regions (DhMRs), suggesting that increased expression of LRP5 is a potential risk factor of OA (174). Consistent with these reports, LRP5 expression is increased in human and mouse osteoarthritic cartilage, while Lrp5–/– mice exhibit resistance to cartilage destruction caused by surgical destabilization of the medial meniscus or aging (164). However, another study showed that Lrp5–/– mice have significantly more cartilage degradation and OA phenotypes following such surgery, likely due to low bone mass density caused by Lrp5 loss (120).
The role of LRP5 in fracture healing has been examined. Animal studies show that Lrp5–/– mice have decreased osseous tissue in the healing sites and a reduced rigidity score of fracture callus (113). This is in line with the current understanding that canonic Wnt signaling promotes fracture healing (27, 152).
F. LRP6
LRP6 was first identified by screening a human liver cDNA with a probe derived from LRP5 (21). The initial analysis of the coding sequence of LRP6 revealed a 71% identity with LRP5 at the amino acid level (FIGURE 1). As described above, less than 2 yr after their discovery, the two proteins were linked to key roles as co-receptors for the Wnt family. While both proteins clearly serve as Wnt receptors in various cell contexts, initial reports suggested that LRP6 may be more active than LRP5 in stimulating signaling activity (67, 173). The molecular mechanisms that underlie such differences are still being evaluated (123), but they may be related to intrinsic differences in the ability to interact with specific Wnt molecules (67, 123).
Mice homozygous for a loss-of-function mutations in Lrp6 die late in embryonic development or shortly after birth and have multiple developmental defects (149). Mice carrying heterozygous inactivating mutations in the gene have low bone mass (66). Heterozygosity for a spontaneous mutation in the mouse Lrp6 gene, termed ringelschwanz, also produces lowered bone mass (101, 105).
Consistent with the high degree of homology between Lrp5 and Lrp6, embryos homozygous for null alleles of both genes die shortly after gastrulation, displaying phenotypes similar to those of mice lacking other components of the Wnt signaling pathway (92). Mice carrying other combinations of compound global mutations in these two genes display synergistic phenotypes. This is certainly true in skeletal development, where the most severely affected mice that survive to adulthood, i.e., those heterozygous for a null mutation in Lrp6 and homozygous for a null mutation in Lrp5, have limb patterning defects and very low bone mass (66).
The deletion of Lrp6 within the osteoblast lineage is associated with low bone mass. Consistent with the synergistic interactions seen between global deletions of Lrp5 and Lrp6, combined deletion of both genes in several tissues demonstrates aspects of redundancy in numerous tissues (195, 201). During early stages of osteochondral differentiation, either Lrp5 or Lrp6 is required for normal commitment to the osteoblast lineage (78), consistent with phenotypes seen after deletion of β-catenin at the same stages. The deletion of either Lrp5 or Lrp6 during this time has no discernable effect. During later stages of osteoblast differentiation, the genes act in a partially redundant fashion: deletion of either gene results in a reduction in bone mass, while simultaneous deletion causes a severe loss of bone mass and a phenotype similar to that seen when β-catenin is deleted (47, 68) or when the ability to secrete Wnts from the lineage is disrupted (202).
Lrp6 haploinsufficiency in mice causes decreased β-catenin levels in cartilage and enhances surgery-induced OA progression accompanied by decreased bone mass (81). To date, no association between genetic variation within the human LRP6 locus and OA has been reported (93).
G. LRP8 and VLDLR
LRP8, also called APOER2, was first identified in the brain. Its extracellular domain has a cluster of seven LDL-A repeats followed by LDL-B repeats and a β-propeller motif; its ICD contains an NPXY motif that interacts with PTD domain-containing proteins (FIGURE 1) (160). LRP8 regulates neuronal differentiation and migration by enhancing endocytosis of reelin, which is a large, secreted extracellular matrix glycoprotein that promotes DAB1-mediated recruitment of PI3K and GSK3B to the LRP8 ICD (5). Among all LRP family members, LRP8 has highest homology (50% in protein sequence) with the very-low-density lipoprotein receptor (VLDLR). VLDLR has eight LDL-A repeats in the extracellular domain and an ICD with NPXY motif, which is also involved in reelin signaling (63).
The components of the reelin signaling pathway exhibit a dynamic expression pattern in developing limbs and digits. LRP8 and VLDLR, as reelin receptors, are expressed in cartilage and tendons, respectively, and showed temporal expression differences in micromass cultures. LRP8 gene silencing downregulates Sox9 and other chondrogenic markers in micromass cultures, while VLDLR gene silencing upregulates scleraxis, an important transcription factor for tenogenesis. This study suggests that reelin signaling plays diverse roles in skeletogenesis by promoting chondrogenesis through LRP8 while inhibiting tenogenic differentiation through VLDLR (34).
LRP8 has also been shown to be a novel activator of Wnt/β-catenin signaling during osteoblast differentiation. The LRP8 ICD is required for LRP8's interaction with AXIN1 and AXIN2 upon Wnt3a stimulation. Knocking down LRP8 inhibits WNT3a-induced β-catenin stabilization and Axin2 expression, and it also impairs osteoblast progenitor differentiation and mineralization (197). Moreover, LRP8 inhibits osteoclastogenesis by internalizing F-spondin, a secreted glycoprotein located on the root surface of teeth (146). Future work is warranted to further define the mechanism(s) of LRP8 signaling within the skeletal lineage.
H. APOE
APOE, a 34-kDa glycoprotein highly expressed in liver, macrophages, and neurons, is a common ligand for many LRPs (46). As its role in the skeleton may partly reflect the synergistic actions of these LRPs, it is appropriate to include it in this review.
APOE plays a key role in lipid metabolism by facilitating lipid clearance from the circulation. ApoE-deficient mice have high plasma cholesterol levels and spontaneously develop atherosclerotic lesions (74). The exact role of APOE in human bone mass regulation remains controversial. APOE SNPs have been reported to be associated with either high or low BMD, or to be uncorrelated with BMD (140).
Genetically engineered mouse models have been used to assess the effects of APOE on the skeleton. Human express three major APOE isoforms: APOE ε2, ε3, and ε4. A study in which these human isoforms were knocked into the mouse ApoE locus found that APOE ε2 allele caused decreased bone mass and bone formation as well as a lower OPG/RANKL ratio, suggesting that APOE ε2 may be a risk factor for human osteoporosis (35).
Female ApoE–/– mice were reported to show high bone mass with increased bone formation, but bone resorption appears unchanged. The authors proposed that the high bone mass in these knockout mice was due to a decreased uptake by osteoblasts of the triglyceride-rich lipoproteins that carry vitamin K, leading to an elevated level of undercarboxylated osteocalcin in the serum (159).
Another study showed that ApoE-deficient mice on a normal diet gain less fat mass but higher vertebrate bone mass than the WT littermates. However, the knockout mice on a high-fat diet had decreased bone mass and a lower bone formation rate, while the WT controls showed no changes (9).
In another study, ApoE–/– mice on a normal diet had a normal bone phenotype, but when fed a high-fat diet, they had decreased cortical bone volume and reduced bone formation rates in both trabecular and cortical bone. Osteoblast apoptosis in the long bone was higher on a normal diet in the ApoE–/– mice than in WT controls. Moreover, osteoblast apoptosis was even more pronounced in ApoE–/– mice fed a high-fat diet. p53 mRNA expression was significantly increased in the ApoE–/– adherent bone marrow stromal cells, with a strong positive correlation with the concentration of LDL in culture medium. The authors concluded that ApoE loss augments the bone loss resulting from a high-fat diet by stimulating p53-mediated apoptosis of osteoblastic cells (64).
A recent study showed that ApoE–/– mice fed a high-fat diet had higher levels of oxidized lipids and lower femur and vertebrae bone mass. These mice also had fewer osteoblast progenitors and more monocyte/macrophages in the bone marrow; produced more IL-1β, IL-6, and TNF-α; and showed lower Wnt signaling and lower expression of Wnts. These data suggest that ApoE loss induced the accumulation of oxidized lipids, which decreased bone mass by increasing anti-osteoblastogenic inflammatory cytokines and decreasing pro-osteoblastogenic Wnts (119).
APOE has been reported as a negative regulator of osteoclastogenesis. During osteoclastic differentiation, ApoE expression is decreased. Overexpression of ApoE can block the RANKL-induced expression of c-Fos and Nfatc1, as well as the cell-cell fusion-related genes for osteoclastogenesis, including Dc-Stamp and Atp6v0d2. In addition, ApoE knock-down promotes osteoclast differentiation by enhancing NFκB signaling, but it does not affect ERK, JNK, and p38 MAPK (97).
Despite the discrepancy, it seems more data support that in the female mice on normal diet, loss of APOE promotes bone mass. However, a high-fat diet generally causes low bone mass in the knockout mice. The differences in gender, diet, and bone compartments need to be carefully taken into consideration when interpretation these data.
III. THERAPEUTIC CONSIDERATIONS
Most therapeutic development for skeletal diseases related to these proteins has focused on either the β-propeller regions or proteins that interact with those regions. This has primarily been in the context of LRP5 and LRP6. As discussed above, there are several effectors which bind to LRP5 and LRP6 and prevent their activation by Wnts. One example is the Dkk family, of which three members (DKK1, DKK2, and DKK4) bind to LRP5 and LRP6 (138) Antibodies that bind to DKK1 and interfere with its ability to bind LRP5 and LRP6 have been developed and tested for use in treating osteoporosis (19, 33, 42, 56, 90, 102, 111, 126). However, they lag behind the development of anti-sclerostin approaches, perhaps due to the relatively specific expression of sclerostin in osteocytes (104, 109, 187, 189).
Antibodies that interfere with the ability of sclerostin to bind and inhibit Wnt-induced activation of LRP5 and LRP6 have been developed. Two pharmaceutical companies have agents in clinical trials to evaluate their efficacy against osteoporosis. Amgen has developed a partially humanized antibody named romosozumab which has shown some efficacy in increasing bone mass in phase II clinical trials (131). Eli Lilly has developed a fully humanized antibody referred to as blosozumab that is also in early-stage clinical trials (170). While there is some initial promise associated with these agents, important questions have emerged from the early trial data. For example, it appears that increases in bone mass are seen only during the first few months of treatment. Gaining insight into why increases in bone mass are not more persistent may allow improvements in the therapy. More recently, reports that sclerostin inhibition can promote the progression of rheumatoid arthritis symptoms in mouse models have added a cautionary note (185).
Several reports have focused on the development of antibodies that bind directly to the extracellular β-propeller motifs of LRP6 (39, 52). These efforts have not yet yielded agents that have been entered into human clinical trials. However, they have given insights into the complexity of Wnt signaling, with some antibodies showing inhibition or activation of signaling depending on the specific Wnt molecule being examined.
The elegant work on antibodies that specifically interfere with the ability of LRP4 to interact with sclerostin and thus regulate osteoblastogenesis is another potential therapeutic approach (23). The utility of targeting LRP1, LRP2, or LRP8 for the treatment of skeletal diseases is unexplored at this point.
IV. FUTURE DIRECTIONS
Despite sharing several conserved structure domains, these LDL receptor-related family members have diverse functions. In some cases, differences in signaling mechanisms are apparent. For example, evidence suggests that LRP1 and LRP2 can undergo a cascade of proteolytic processing to release intercellular domains (ICD) that carry out signaling processes, in a manner reminiscent of the Notch ICD (36). It will be of interest to see if the ICDs of other LRP family members function in a similar manner. For example, there have been reports that the ICDs of LRP5 and LRP6 can participate in signaling (10, 132, 133).
Future studies will undoubtedly uncover additional roles for LRP proteins in skeletal development. Additional insights into how apolipoprotein interactions may facilitate signaling within the skeletal compartment will add to our understanding of these key molecules and will better guide the effective targeting of these receptors and their downstream signaling pathways to treat skeletal diseases.
GRANTS
B. O. Williams is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR053293.
DISCLOSURES
B. O. Williams has received an honorarium and travel support to present seminars at Amgen, Novartis, Surrozen, and Vertex Pharmaceuticals. B. O. Williams also is a member of the Board of Scientific Advisors for Surrozen.
ACKNOWLEDGMENTS
We thank David Nadziejka for editorial assistance and Nicole Ethen for preparation of the figures. We also thank Van Andel Research Institute for support.
Address for reprint requests and other correspondence: T. Yang and/or B. O. Williams, Program in Skeletal Disease and Tumor Microenvironment, Center for Cancer and Cell Biology, Van Andel Research Institute, 333 Bostwick Ave. NE, Grand Rapids, MI 49503 (e-mail: tao.yang@vai.org or bart.williams@vai.org).
REFERENCES
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J : 3797–3804, 1997. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol : 4946–4955, 2005. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, Babij P, Allen KM, Yaworsky PJ, Bex F, Recker RR. Bone biomechanical properties in LRP5 mutant mice. Bone : 162–169, 2004. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Andersson ER, Bryjova L, Biris K, Yamaguchi TP, Arenas E, Bryja V. Genetic interaction between Lrp6 and Wnt5a during mouse development. Dev Dyn : 237–245, 2010. doi: 10.1002/dvdy.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D’Arcangelo G, Clark GD. Interaction of reelin signaling and Lis1 in brain development. Nat Genet : 270–276, 2003. doi: 10.1038/ng1257. [DOI] [PubMed] [Google Scholar]
- 6.Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res : 960–974, 2003. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 7.Barmina OY, Walling HW, Fiacco GJ, Freije JM, López-Otín C, Jeffrey JJ, Partridge NC. Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization. J Biol Chem : 30087–30093, 1999. doi: 10.1074/jbc.274.42.30087. [DOI] [PubMed] [Google Scholar]
- 8.Barnes H, Larsen B, Tyers M, van der Geer P. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (Lrp1) associates with the adaptor protein SHC in SRC-transformed cells. J Biol Chem : 19119–19125, 2001. doi: 10.1074/jbc.M011437200. [DOI] [PubMed] [Google Scholar]
- 9.Bartelt A, Beil FT, Schinke T, Roeser K, Ruether W, Heeren J, Niemeier A. Apolipoprotein E-dependent inverse regulation of vertebral bone and adipose tissue mass in C57Bl/6 mice: modulation by diet-induced obesity. Bone : 736–745, 2010. doi: 10.1016/j.bone.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Beagle B, Mi K, Johnson GV. Phosphorylation of PPP(S/T)P motif of the free LRP6 intracellular domain is not required to activate the Wnt/beta-catenin pathway and attenuate GSK3beta activity. J Cell Biochem : 886–895, 2009. doi: 10.1002/jcb.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beffert U, Stolt PC, Herz J. Functions of lipoprotein receptors in neurons. J Lipid Res : 403–409, 2004. doi: 10.1194/jlr.R300017-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Beglova N, Blacklow SC. The LDL receptor: how acid pulls the trigger. Trends Biochem Sci : 309–317, 2005. doi: 10.1016/j.tibs.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HHQ, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet : 353–356, 1996. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- 14.Berendsen AD, Olsen BR. Bone development. Bone : 14–18, 2015. doi: 10.1016/j.bone.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med : 254–262, 2016. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 16.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science : 329–332, 2003. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 17.Boucher P, Li WP, Matz RL, Takayama Y, Auwerx J, Anderson RG, Herz J. LRP1 functions as an atheroprotective integrator of TGFbeta and PDFG signals in the vascular wall: implications for Marfan syndrome. PLoS One : e448, 2007. doi: 10.1371/journal.pone.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med : 1513–1521, 2002. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 19.Brommage R. Genetic approaches to identifying novel osteoporosis drug targets. J Cell Biochem : 2139–2145, 2015. doi: 10.1002/jcb.25179. [DOI] [PubMed] [Google Scholar]
- 20.Brown MS, Goldstein JL. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci USA : 3330–3337, 1979. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown SD, Twells RC, Hey PJ, Cox RD, Levy ER, Soderman AR, Metzker ML, Caskey CT, Todd JA, Hess JF. Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem Biophys Res Commun : 879–888, 1998. doi: 10.1006/bbrc.1998.9061. [DOI] [PubMed] [Google Scholar]
- 22.Capurro MI, Shi W, Filmus J. LRP1 mediates Hedgehog-induced endocytosis of the GPC3-Hedgehog complex. J Cell Sci : 3380–3389, 2012. doi: 10.1242/jcs.098889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang MK, Kramer I, Huber T, Kinzel B, Guth-Gundel S, Leupin O, Kneissel M. Disruption of Lrp4 function by genetic deletion or pharmacological blockade increases bone mass and serum sclerostin levels. Proc Natl Acad Sci USA : E5187–E5195, 2014. doi: 10.1073/pnas.1413828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CK, Chan NL, Wang AH. The many blades of the β-propeller proteins: conserved but versatile. Trends Biochem Sci : 553–561, 2011. doi: 10.1016/j.tibs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 24a.Chen D, Lathrop W, Dong Y. Molecular cloning of mouse Lrp7(Lr3) cDNA and chromosomal mapping of orthologous genes in mouse and human. Genomics : 313–321, 1999. doi: 10.1006/geno.1998.5688. [DOI] [PubMed] [Google Scholar]
- 25.Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem : 3116–3123, 1990. [PubMed] [Google Scholar]
- 26.Chen X, Wang C, Zhang K, Xie Y, Ji X, Huang H, Yu X. Reduced femoral bone mass in both diet-induced and genetic hyperlipidemia mice. Bone : 104–112, 2016. doi: 10.1016/j.bone.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med : e249, 2007. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One : e7930, 2009. doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christ A, Christa A, Kur E, Lioubinski O, Bachmann S, Willnow TE, Hammes A. LRP2 is an auxiliary SHH receptor required to condition the forebrain ventral midline for inductive signals. Dev Cell : 268–278, 2012. doi: 10.1016/j.devcel.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Cui Y, Niziolek PJ, MacDonald BT, Alenina N, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Powell DR, He X, Bader M, Williams BO, Warman ML, Robling AG. Reply to Lrp5 regulation of bone mass and gut serotonin synthesis. Nat Med : 1229–1230, 2014. doi: 10.1038/nm.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG. Lrp5 functions in bone to regulate bone mass. Nat Med : 684–691, 2011. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daly NL, Scanlon MJ, Djordjevic JT, Kroon PA, Smith R. Three-dimensional structure of a cysteine-rich repeat from the low-density lipoprotein receptor. Proc Natl Acad Sci USA : 6334–6338, 1995. doi: 10.1073/pnas.92.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G. Dickkopf-1 is a master regulator of joint remodeling. Nat Med : 156–163, 2007. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 34.Diaz-Mendoza MJ, Lorda-Diez CI, Montero JA, Garcia-Porrero JA, Hurle JM. Reelin/DAB-1 signaling in the embryonic limb regulates the chondrogenic differentiation of digit mesodermal progenitors. J Cell Physiol : 1397–1404, 2014. doi: 10.1002/jcp.24576. [DOI] [PubMed] [Google Scholar]
- 35.Dieckmann M, Beil FT, Mueller B, Bartelt A, Marshall RP, Koehne T, Amling M, Ruether W, Cooper JA, Humphries SE, Herz J, Niemeier A. Human apolipoprotein E isoforms differentially affect bone mass and turnover in vivo. J Bone Miner Res : 236–245, 2013. doi: 10.1002/jbmr.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Dong Y, Lathrop W, Weaver D, Qiu Q, Cini J, Bertolini D, Chen D. Molecular cloning and characterization of LR3, a novel LDL receptor family protein with mitogenic activity. Biochem Biophys Res Commun : 784–790, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Ehebauer M, Hayward P, Martinez-Arias A. Notch signaling pathway. Sci STKE : cm7, 2006. doi: 10.1126/stke.3642006cm7. [DOI] [PubMed] [Google Scholar]
- 37.Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol : 45–54, 2015. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eldridge S, Nalesso G, Ismail H, Vicente-Greco K, Kabouridis P, Ramachandran M, Niemeier A, Herz J, Pitzalis C, Perretti M, Dell’Accio F. Agrin mediates chondrocyte homeostasis and requires both LRP4 and α-dystroglycan to enhance cartilage formation in vitro and in vivo. Ann Rheum Dis : 1228–1235, 2016. doi: 10.1136/annrheumdis-2015-207316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD, Schuller AG, Yuan J, Ospina B, Green J, Yu Q, Walsh R, Li S, Schmitz R, Heine H, Bilic S, Ostrom L, Mosher R, Hartlepp KF, Zhu Z, Fawell S, Yao YM, Stover D, Finan PM, Porter JA, Sellers WR, Klagge IM, Cong F. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci USA : 15473–15478, 2010. doi: 10.1073/pnas.1007428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med : 841–848, 2006. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 41.Franz-Odendaal TA. Induction and patterning of intramembranous bone. Front Biosci (Landmark Ed) : 2734–2746, 2011. doi: 10.2741/3882. [DOI] [PubMed] [Google Scholar]
- 42.Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood : 371–379, 2009. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaultier A, Arandjelovic S, Li X, Janes J, Dragojlovic N, Zhou GP, Dolkas J, Myers RR, Gonias SL, Campana WM. A shed form of LDL receptor-related protein-1 regulates peripheral nerve injury and neuropathic pain in rodents. J Clin Invest : 161–172, 2008. doi: 10.1172/JCI32371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaultier A, Arandjelovic S, Niessen S, Overton CD, Linton MF, Fazio S, Campana WM, Cravatt BF III, Gonias SL. Regulation of tumor necrosis factor receptor-1 and the IKK-NF-kappaB pathway by LDL receptor-related protein explains the anti-inflammatory activity of this receptor. Blood : 5316–5325, 2008. doi: 10.1182/blood-2007-12-127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geng Y, Hsu JJ, Lu J, Ting TC, Miyazaki M, Demer LL, Tintut Y. Role of cellular cholesterol metabolism in vascular cell calcification. J Biol Chem : 33701–33706, 2011. doi: 10.1074/jbc.M111.269639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Getz GS, Reardon CA. Apoprotein E as a lipid transport and signaling protein in the blood, liver, and artery wall. J Lipid Res , Suppl: S156–S161, 2009. doi: 10.1194/jlr.R800058-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glass DA II, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell : 751–764, 2005. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis : 269–285, 2012. doi: 10.1177/1759720X12448454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldring MB, Berenbaum F. Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol : 51–63, 2015. doi: 10.1016/j.coph.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol : 471–478, 2011. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol : 431–438, 2009. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong Y, Bourhis E, Chiu C, Stawicki S, DeAlmeida VI, Liu BY, Phamluong K, Cao TC, Carano RA, Ernst JA, Solloway M, Rubinfeld B, Hannoush RN, Wu Y, Polakis P, Costa M. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS One : e12682, 2010. doi: 10.1371/journal.pone.0012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52a.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Jüppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML, Osteoporosis-Pseudoglioma Syndrome Collaborative Group . LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell : 513–523, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Gorovoy M, Gaultier A, Campana WM, Firestein GS, Gonias SL. Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J Leukoc Biol : 769–778, 2010. doi: 10.1189/jlb.0410220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grey A, Banovic T, Zhu Q, Watson M, Callon K, Palmano K, Ross J, Naot D, Reid IR, Cornish J. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol : 2268–2278, 2004. doi: 10.1210/me.2003-0456. [DOI] [PubMed] [Google Scholar]
- 55.Grey A, Zhu Q, Watson M, Callon K, Cornish J. Lactoferrin potently inhibits osteoblast apoptosis, via an LRP1-independent pathway. Mol Cell Endocrinol : 96–102, 2006. doi: 10.1016/j.mce.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD Jr, Evans HR, Snowden JA, Stover DR, Vanderkerken K, Croucher PI. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res : 425–436, 2009. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 57.Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem : 405–434, 2002. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- 58.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell : 411–421, 1992. doi: 10.1016/0092-8674(92)90511-A. [DOI] [PubMed] [Google Scholar]
- 59.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J : 4119–4127, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herz J, Kowal RC, Goldstein JL, Brown MS. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J : 1769–1776, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herz J, Marschang P. Coaxing the LDL receptor family into the fold. Cell : 289–292, 2003. doi: 10.1016/S0092-8674(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 62.Hey PJ, Twells RC, Phillips MS, Yusuke Nakagawa, Brown SD, Kawaguchi Y, Cox R, Guochun Xie, Dugan V, Hammond H, Metzker ML, Todd JA, Hess JF. Cloning of a novel member of the low-density lipoprotein receptor family. Gene : 103–111, 1998. doi: 10.1016/S0378-1119(98)00311-4. [DOI] [PubMed] [Google Scholar]
- 63.Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron : 481–489, 1999. doi: 10.1016/S0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 64.Hirasawa H, Tanaka S, Sakai A, Tsutsui M, Shimokawa H, Miyata H, Moriwaki S, Niida S, Ito M, Nakamura T. ApoE gene deficiency enhances the reduction of bone formation induced by a high-fat diet through the stimulation of p53-mediated apoptosis in osteoblastic cells. J Bone Miner Res : 1020–1030, 2007. doi: 10.1359/jbmr.070330. [DOI] [PubMed] [Google Scholar]
- 65.Hobbs HH, Brown MS, Russell DW, Davignon J, Goldstein JL. Deletion in the gene for the low-density-lipoprotein receptor in a majority of French Canadians with familial hypercholesterolemia. N Engl J Med : 734–737, 1987. doi: 10.1056/NEJM198709173171204. [DOI] [PubMed] [Google Scholar]
- 66.Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res : 2033–2040, 2004. doi: 10.1359/jbmr.040907. [DOI] [PubMed] [Google Scholar]
- 67.Holmen SL, Salic A, Zylstra CR, Kirschner MW, Williams BO. A novel set of Wnt-Frizzled fusion proteins identifies receptor components that activate beta -catenin-dependent signaling. J Biol Chem : 34727–34735, 2002. doi: 10.1074/jbc.M204989200. [DOI] [PubMed] [Google Scholar]
- 68.Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem : 21162–21168, 2005. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 69.Houben RJ, Rijkers DT, Stanley TB, Acher F, Azerad R, Käkönen SM, Vermeer C, Soute BA. Characteristics and composition of the vitamin K-dependent gamma-glutamyl carboxylase-binding domain on osteocalcin. Biochem J : 323–328, 2002. doi: 10.1042/bj3640323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang YL, Anvarian Z, Döderlein G, Acebron SP, Niehrs C. Maternal Wnt/STOP signaling promotes cell division during early Xenopus embryogenesis. Proc Natl Acad Sci USA : 5732–5737, 2015. doi: 10.1073/pnas.1423533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell : 955–968, 2006. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 72.Inose H, Zhou B, Yadav VK, Guo XE, Karsenty G, Ducy P. Efficacy of serotonin inhibition in mouse models of bone loss. J Bone Miner Res : 2002–2011, 2011. doi: 10.1002/jbmr.439. [DOI] [PubMed] [Google Scholar]
- 73.Inubushi T, Kawazoe A, Miyauchi M, Kudo Y, Ao M, Ishikado A, Makino T, Takata T. Molecular mechanisms of the inhibitory effects of bovine lactoferrin on lipopolysaccharide-mediated osteoclastogenesis. J Biol Chem : 23527–23536, 2012. doi: 10.1074/jbc.M111.324673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: tests of the hypothesis in “knockout” mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc Natl Acad Sci USA : 4431–4435, 1994. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iwaniec UT, Wronski TJ, Liu J, Rivera MF, Arzaga RR, Hansen G, Brommage R. PTH stimulates bone formation in mice deficient in Lrp5. J Bone Miner Res : 394–402, 2007. doi: 10.1359/jbmr.061118. [DOI] [PubMed] [Google Scholar]
- 76.Jeon H, Blacklow SC. An intramolecular spin of the LDL receptor beta propeller. Structure : 133–136, 2003. doi: 10.1016/S0969-2126(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 77.Jeon H, Meng W, Takagi J, Eck MJ, Springer TA, Blacklow SC. Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol : 499–504, 2001. doi: 10.1038/88556. [DOI] [PubMed] [Google Scholar]
- 78.Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol : 222–229, 2011. doi: 10.1016/j.ydbio.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson EB, Steffen DJ, Lynch KW, Herz J. Defective splicing of Megf7/Lrp4, a regulator of distal limb development, in autosomal recessive mulefoot disease. Genomics : 600–609, 2006. doi: 10.1016/j.ygeno.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Joiner DM, Ke J, Zhong Z, Xu HE, Williams BO. LRP5 and LRP6 in development and disease. Trends Endocrinol Metab : 31–39, 2013. doi: 10.1016/j.tem.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joiner DM, Less KD, Van Wieren EM, Hess D, Williams BO. Heterozygosity for an inactivating mutation in low-density lipoprotein-related receptor 6 (Lrp6) increases osteoarthritis severity in mice after ligament and meniscus injury. Osteoarthritis Cartilage : 1576–1585, 2013. doi: 10.1016/j.joca.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 82.Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-β. J Neurosci : 16458–16465, 2012. doi: 10.1523/JNEUROSCI.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang KS, Robling AG. New insights into Wnt-Lrp5/6-β-catenin signaling in mechanotransduction. Front Endocrinol (Lausanne) : 246, 2015. doi: 10.3389/fendo.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, Chassaing N, Lacombe D, Devriendt K, Teebi A, Loscertales M, Robson C, Liu T, MacLaughlin DT, Noonan KM, Russell MK, Walsh CA, Donahoe PK, Pober BR. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet : 957–959, 2007. doi: 10.1038/ng2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kariminejad A, Stollfuß B, Li Y, Bögershausen N, Boss K, Hennekam RC, Wollnik B. Severe Cenani-Lenz syndrome caused by loss of LRP4 function. Am J Med Genet A : 1475–1479, 2013. doi: 10.1002/ajmg.a.35920. [DOI] [PubMed] [Google Scholar]
- 86.Karner CM, Dietrich MF, Johnson EB, Kappesser N, Tennert C, Percin F, Wollnik B, Carroll TJ, Herz J. Lrp4 regulates initiation of ureteric budding and is crucial for kidney formation--a mouse model for Cenani-Lenz syndrome. PLoS One : e10418, 2010. doi: 10.1371/journal.pone.0010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA II, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol : 303–314, 2002. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawata K, Eguchi T, Kubota S, Kawaki H, Oka M, Minagi S, Takigawa M. Possible role of LRP1, a CCN2 receptor, in chondrocytes. Biochem Biophys Res Commun : 552–559, 2006. doi: 10.1016/j.bbrc.2006.04.109. [DOI] [PubMed] [Google Scholar]
- 89.Kawata K, Kubota S, Eguchi T, Aoyama E, Moritani NH, Kondo S, Nishida T, Takigawa M. Role of LRP1 in transport of CCN2 protein in chondrocytes. J Cell Sci : 2965–2972, 2012. doi: 10.1242/jcs.101956. [DOI] [PubMed] [Google Scholar]
- 90.Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev : 747–783, 2012. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 91.Kedlaya R, Veera S, Horan DJ, Moss RE, Ayturk UM, Jacobsen CM, Bowen ME, Paszty C, Warman ML, Robling AG. Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome. Sci Transl Med : 211ra158, 2013. doi: 10.1126/scitranslmed.3006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development : 2803–2815, 2004. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 93.Kerkhof JM, Uitterlinden AG, Valdes AM, Hart DJ, Rivadeneira F, Jhamai M, Hofman A, Pols HA, Bierma-Zeinstra SM, Spector TD, van Meurs JB. Radiographic osteoarthritis at three joint sites and FRZB, LRP5, and LRP6 polymorphisms in two population-based cohorts. Osteoarthritis Cartilage : 1141–1149, 2008. doi: 10.1016/j.joca.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Khan TN, Klar J, Ali Z, Khan F, Baig SM, Dahl N. Cenani-Lenz syndrome restricted to limb and kidney anomalies associated with a novel LRP4 missense mutation. Eur J Med Genet : 371–374, 2013. doi: 10.1016/j.ejmg.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Kim DH, Inagaki Y, Suzuki T, Ioka RX, Yoshioka SZ, Magoori K, Kang MJ, Cho Y, Nakano AZ, Liu Q, Fujino T, Suzuki H, Sasano H, Yamamoto TT. A new low density lipoprotein receptor related protein, LRP5, is expressed in hepatocytes and adrenal cortex, and recognizes apolipoprotein E. J Biochem : 1072–1076, 1998. doi: 10.1093/oxfordjournals.jbchem.a022223. [DOI] [PubMed] [Google Scholar]
- 96.Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, Lee G, Rhee J, Ryu JH, Chun CH, Chun JS. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell : 730–743, 2014. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Kim WS, Kim HJ, Lee ZH, Lee Y, Kim HH. Apolipoprotein E inhibits osteoclast differentiation via regulation of c-Fos, NFATc1 and NF-κB. Exp Cell Res : 436–446, 2013. doi: 10.1016/j.yexcr.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell : 1225–1236, 2015. doi: 10.1016/j.cell.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 99.Kode A, Obri A, Paone R, Kousteni S, Ducy P, Karsenty G. Lrp5 regulation of bone mass and serotonin synthesis in the gut. Nat Med : 1228–1229, 2014. doi: 10.1038/nm.3698. [DOI] [PubMed] [Google Scholar]
- 100.Koduri V, Blacklow SC. Folding determinants of LDL receptor type A modules. Biochemistry : 12801–12807, 2001. doi: 10.1021/bi011344k. [DOI] [PubMed] [Google Scholar]
- 101.Kokubu C, Heinzmann U, Kokubu T, Sakai N, Kubota T, Kawai M, Wahl MB, Galceran J, Grosschedl R, Ozono K, Imai K. Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development : 5469–5480, 2004. doi: 10.1242/dev.01405. [DOI] [PubMed] [Google Scholar]
- 102.Komatsu DE, Mary MN, Schroeder RJ, Robling AG, Turner CH, Warden SJ. Modulation of Wnt signaling influences fracture repair. J Orthop Res : 928–936, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc Natl Acad Sci USA : 5810–5814, 1989. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kramer I, Keller H, Leupin O, Kneissel M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab : 237–244, 2010. doi: 10.1016/j.tem.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 105.Kubota T, Michigami T, Sakaguchi N, Kokubu C, Suzuki A, Namba N, Sakai N, Nakajima S, Imai K, Ozono K. Lrp6 hypomorphic mutation affects bone mass through bone resorption in mice and impairs interaction with Mesd. J Bone Miner Res : 1661–1671, 2008. doi: 10.1359/jbmr.080512. [DOI] [PubMed] [Google Scholar]
- 106.Lämsä R, Helisalmi S, Herukka SK, Tapiola T, Pirttilä T, Vepsäläinen S, Hiltunen M, Soininen H. Genetic study evaluating LDLR polymorphisms and Alzheimer’s disease. Neurobiol Aging : 848–855, 2008. doi: 10.1016/j.neurobiolaging.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 107.Lee DH, Wenkert D, Whyte MP, Trese MT, Cruz OA. Congenital blindness and osteoporosis-pseudoglioma syndrome. J AAPOS : 75–77, 2003. doi: 10.1016/S1091-8531(03)00051-X. [DOI] [PubMed] [Google Scholar]
- 108.Lee GS, Simpson C, Sun BH, Yao C, Foer D, Sullivan B, Matthes S, Alenina N, Belsky J, Bader M, Insogna KL. Measurement of plasma, serum, and platelet serotonin in individuals with high bone mass and mutations in LRP5. J Bone Miner Res : 976–981, 2014. doi: 10.1002/jbmr.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res : 1957–1967, 2007. doi: 10.1359/jbmr.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leupin O, Piters E, Halleux C, Hu S, Kramer I, Morvan F, Bouwmeester T, Schirle M, Bueno-Lozano M, Fuentes FJ, Itin PH, Boudin E, de Freitas F, Jennes K, Brannetti B, Charara N, Ebersbach H, Geisse S, Lu CX, Bauer A, Van Hul W, Kneissel M. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem : 19489–19500, 2011. doi: 10.1074/jbc.M110.190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lewiecki EM. Monoclonal antibodies for the treatment of osteoporosis. Expert Opin Biol Ther : 183–196, 2013. doi: 10.1517/14712598.2012.740006. [DOI] [PubMed] [Google Scholar]
- 112.Li Y, Pawlik B, Elcioglu N, Aglan M, Kayserili H, Yigit G, Percin F, Goodman F, Nürnberg G, Cenani A, Urquhart J, Chung BD, Ismail S, Amr K, Aslanger AD, Becker C, Netzer C, Scambler P, Eyaid W, Hamamy H, Clayton-Smith J, Hennekam R, Nürnberg P, Herz J, Temtamy SA, Wollnik B. LRP4 mutations alter Wnt/beta-catenin signaling and cause limb and kidney malformations in Cenani-Lenz syndrome. Am J Hum Genet : 696–706, 2010. doi: 10.1016/j.ajhg.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liedert A, Röntgen V, Schinke T, Benisch P, Ebert R, Jakob F, Klein-Hitpass L, Lennerz JK, Amling M, Ignatius A. Osteoblast-specific Krm2 overexpression and Lrp5 deficiency have different effects on fracture healing in mice. PLoS One : e103250, 2014. doi: 10.1371/journal.pone.0103250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost : 1884–1893, 2005. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 115.Lin ME, Chen TM, Wallingford MC, Nguyen NB, Yamada S, Sawangmake C, Zhang J, Speer MY, Giachelli CM. Runx2 deletion in smooth muscle cells inhibits vascular osteochondrogenesis and calcification but not atherosclerotic lesion formation. Cardiovasc Res : 606–616, 2016. doi: 10.1093/cvr/cvw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lindy AS, Bupp CP, McGee SJ, Steed E, Stevenson RE, Basehore MJ, Friez MJ. Truncating mutations in LRP4 lead to a prenatal lethal form of Cenani-Lenz syndrome. Am J Med Genet A : 2391–2397, 2014. doi: 10.1002/ajmg.a.36647. [DOI] [PubMed] [Google Scholar]
- 117.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet : 11–19, 2002. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol : 35–44, 2015. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y, Almeida M, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL. Skeletal inflammation and attenuation of Wnt signaling, Wnt ligand expression, and bone formation in atherosclerotic ApoE-null mice. Am J Physiol Endocrinol Metab : E762–E773, 2016. doi: 10.1152/ajpendo.00501.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lodewyckx L, Luyten FP, Lories RJ. Genetic deletion of low-density lipoprotein receptor-related protein 5 increases cartilage degradation in instability-induced osteoarthritis. Rheumatology (Oxford) : 1973–1978, 2012. doi: 10.1093/rheumatology/kes178. [DOI] [PubMed] [Google Scholar]
- 121.Loi F, Córdova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone : 119–130, 2016. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol : a008334, 2013. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.MacDonald BT, Semenov MV, Huang H, He X. Dissecting molecular differences between Wnt coreceptors LRP5 and LRP6. PLoS One : e23537, 2011. doi: 10.1371/journal.pone.0023537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maes C. Role and regulation of vascularization processes in endochondral bones. Calcif Tissue Int : 307–323, 2013. doi: 10.1007/s00223-012-9689-z. [DOI] [PubMed] [Google Scholar]
- 125.Mantuano E, Brifault C, Lam MS, Azmoon P, Gilder AS, Gonias SL. LDL receptor-related protein-1 regulates NFκB and microRNA-155 in macrophages to control the inflammatory response. Proc Natl Acad Sci USA : 1369–1374, 2016. doi: 10.1073/pnas.1515480113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mason JJ, Williams BO. SOST and DKK: Antagonists of LRP family signaling as targets for treating bone disease. J Osteoporos : 460120, 2010. doi: 10.4061/2010/460120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maupin KA, Droscha CJ, Williams BO. A comprehensive overview of skeletal phenotypes associated with alterations in Wnt/β-catenin signaling in humans and mice. Bone Res : 27–71, 2013. doi: 10.4248/BR201301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J Biol Chem : 37386–37392, 2003. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- 129.May P, Herz J. LDL receptor-related proteins in neurodevelopment. Traffic : 291–301, 2003. doi: 10.1034/j.1600-0854.2003.00086_4_5.x. [DOI] [PubMed] [Google Scholar]
- 130.May P, Herz J, Bock HH. Molecular mechanisms of lipoprotein receptor signalling. Cell Mol Life Sci : 2325–2338, 2005. doi: 10.1007/s00018-005-5231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med : 412–420, 2014. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 132.Mi K, Johnson GV. Regulated proteolytic processing of LRP6 results in release of its intracellular domain. J Neurochem : 517–529, 2007. doi: 10.1111/j.1471-4159.2007.04447.x. [DOI] [PubMed] [Google Scholar]
- 133.Mi K, Johnson GV. Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J Cell Biochem : 328–338, 2005. doi: 10.1002/jcb.20400. [DOI] [PubMed] [Google Scholar]
- 134.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest : 761–768, 1996. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet : 691–701, 2004. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 136.Morini S, Continenza MA, Ricciardi G, Gaudio E, Pannarale L. Development of the microcirculation of the secondary ossification center in rat humeral head. Anat Rec A Discov Mol Cell Evol Biol : 419–427, 2004. doi: 10.1002/ar.a.20016. [DOI] [PubMed] [Google Scholar]
- 137.Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM, Speer MY. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res : 545–554, 2012. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene : 7469–7481, 2006. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 139.Niemeier A, Kassem M, Toedter K, Wendt D, Ruether W, Beisiegel U, Heeren J. Expression of LRP1 by human osteoblasts: a mechanism for the delivery of lipoproteins and vitamin K1 to bone. J Bone Miner Res : 283–293, 2005. doi: 10.1359/JBMR.041102. [DOI] [PubMed] [Google Scholar]
- 140.Niemeier A, Schinke T, Heeren J, Amling M. The role of apolipoprotein E in bone metabolism. Bone : 518–524, 2012. doi: 10.1016/j.bone.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 141.Niziolek PJ, Warman ML, Robling AG. Mechanotransduction in bone tissue: the A214V and G171V mutations in Lrp5 enhance load-induced osteogenesis in a surface-selective manner. Bone : 459–465, 2012. doi: 10.1016/j.bone.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.North CL, Blacklow SC. Solution structure of the sixth LDL-A module of the LDL receptor. Biochemistry : 2564–2571, 2000. doi: 10.1021/bi992087a. [DOI] [PubMed] [Google Scholar]
- 143.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell : 507–515, 1999. doi: 10.1016/S0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 144.Ohazama A, Johnson EB, Ota MS, Choi HY, Porntaveetus T, Oommen S, Itoh N, Eto K, Gritli-Linde A, Herz J, Sharpe PT. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One : e4092, 2008. doi: 10.1371/journal.pone.0004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ohazama A, Porntaveetus T, Ota MS, Herz J, Sharpe PT. Lrp4: a novel modulator of extracellular signaling in craniofacial organogenesis. Am J Med Genet A : 2974–2983, 2010. doi: 10.1002/ajmg.a.33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oka H, Kitagawa M, Takata T. F-spondin inhibits differentiation of clastic precursors via lipoprotein receptor-related protein 8 (LRP8). J Periodontol : 465–472, 2015. doi: 10.1902/jop.2014.140419. [DOI] [PubMed] [Google Scholar]
- 147.Okayasu M, Nakayachi M, Hayashida C, Ito J, Kaneda T, Masuhara M, Suda N, Sato T, Hakeda Y. Low-density lipoprotein receptor deficiency causes impaired osteoclastogenesis and increased bone mass in mice because of defect in osteoclastic cell-cell fusion. J Biol Chem : 19229–19241, 2012. doi: 10.1074/jbc.M111.323600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res : 670–677, 2007. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 149.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature : 535–538, 2000. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 150.Ranganathan S, Cao C, Catania J, Migliorini M, Zhang L, Strickland DK. Molecular basis for the interaction of low density lipoprotein receptor-related protein 1 (LRP1) with integrin alphaMbeta2: identification of binding sites within alphaMbeta2 for LRP1. J Biol Chem : 30535–30541, 2011. doi: 10.1074/jbc.M111.265413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest : 2011–2019, 1996. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Regard JB, Zhong Z, Williams BO, Yang Y. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb Perspect Biol : a007997, 2012. doi: 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Riddle RC, Diegel CR, Leslie JM, Van Koevering KK, Faugere MC, Clemens TL, Williams BO. Lrp5 and Lrp6 exert overlapping functions in osteoblasts during postnatal bone acquisition. PLoS One : e63323, 2013. doi: 10.1371/journal.pone.0063323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rivadeneira F, Styrkársdottir U, Estrada K, Halldórsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG; Genetic Factors for Osteoporosis (GEFOS) Consortium . Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet : 1199–1206, 2009. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rodríguez Cruz PM, Palace J, Beeson D. Inherited disorders of the neuromuscular junction: an update. J Neurol : 2234–2243, 2014. doi: 10.1007/s00415-014-7520-7. [DOI] [PubMed] [Google Scholar]
- 156.Roebroek AJ, Reekmans S, Lauwers A, Feyaerts N, Smeijers L, Hartmann D. Mutant Lrp1 knock-in mice generated by recombinase-mediated cassette exchange reveal differential importance of the NPXY motifs in the intracellular domain of LRP1 for normal fetal development. Mol Cell Biol : 605–616, 2006. doi: 10.1128/MCB.26.2.605-616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet : 357–360, 1996. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- 158.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem : 23698–23711, 2006. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 159.Schilling AF, Schinke T, Münch C, Gebauer M, Niemeier A, Priemel M, Streichert T, Rueger JM, Amling M. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res : 274–282, 2005. doi: 10.1359/JBMR.041101. [DOI] [PubMed] [Google Scholar]
- 160.Schneider WJ, Nimpf J. LDL receptor relatives at the crossroad of endocytosis and signaling. Cell Mol Life Sci : 892–903, 2003. doi: 10.1007/s00018-003-2183-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Selvais C, D’Auria L, Tyteca D, Perrot G, Lemoine P, Troeberg L, Dedieu S, Noël A, Nagase H, Henriet P, Courtoy PJ, Marbaix E, Emonard H. Cell cholesterol modulates metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 (LRP-1) and clearance function. FASEB J : 2770–2781, 2011. doi: 10.1096/fj.10-169508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol : 602–608, 1998. doi: 10.1016/S0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 163.Shen C, Xiong WC, Mei L. LRP4 in neuromuscular junction and bone development and diseases. Bone : 101–108, 2015. doi: 10.1016/j.bone.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 164.Shin Y, Huh YH, Kim K, Kim S, Park KH, Koh JT, Chun JS, Ryu JH. Low-density lipoprotein receptor-related protein 5 governs Wnt-mediated osteoarthritic cartilage destruction. Arthritis Res Ther : R37, 2014. doi: 10.1186/ar4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Siddiqui JA, Partridge NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology (Bethesda) : 233–245, 2016. doi: 10.1152/physiol.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sims AM, Shephard N, Carter K, Doan T, Dowling A, Duncan EL, Eisman J, Jones G, Nicholson G, Prince R, Seeman E, Thomas G, Wass JA, Brown MA. Genetic analyses in a sample of individuals with high or low BMD shows association with multiple Wnt pathway genes. J Bone Miner Res : 499–506, 2008. doi: 10.1359/jbmr.071113. [DOI] [PubMed] [Google Scholar]
- 167.Songyang Z, Margolis B, Chaudhuri M, Shoelson SE, Cantley LC. The phosphotyrosine interaction domain of SHC recognizes tyrosine-phosphorylated NPXY motif. J Biol Chem : 14863–14866, 1995. doi: 10.1074/jbc.270.25.14863. [DOI] [PubMed] [Google Scholar]
- 168.Spoelgen R, Hammes A, Anzenberger U, Zechner D, Andersen OM, Jerchow B, Willnow TE. LRP2/megalin is required for patterning of the ventral telencephalon. Development : 405–414, 2005. doi: 10.1242/dev.01580. [DOI] [PubMed] [Google Scholar]
- 169.Südhof TC, Goldstein JL, Brown MS, Russell DW. The LDL receptor gene: a mosaic of exons shared with different proteins. Science : 815–822, 1985. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sugiyama T, Torio T, Miyajima T, Kim YT, Oda H. Romosozumab and blosozumab: alternative drugs of mechanical strain-related stimulus toward a cure for osteoporosis. Front Endocrinol (Lausanne) : 54, 2015. doi: 10.3389/fendo.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun MM, Beier F. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth Defects Res C Embryo Today : 74–82, 2014. doi: 10.1002/bdrc.21062. [DOI] [PubMed] [Google Scholar]
- 172.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell : 1136–1148, 2010. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature : 530–535, 2000. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 174.Taylor SE, Li YH, Wong WH, Bhutani N. Genome-wide mapping of DNA hydroxymethylation in osteoarthritic chondrocytes. Arthritis Rheumatol : 2129–2140, 2015. doi: 10.1002/art.39179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Terrand J, Bruban V, Zhou L, Gong W, El Asmar Z, May P, Zurhove K, Haffner P, Philippe C, Woldt E, Matz RL, Gracia C, Metzger D, Auwerx J, Herz J, Boucher P. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem : 381–388, 2009. doi: 10.1074/jbc.M806538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tian QB, Nakayama K, Okano A, Suzuki T. Identification of mRNAs localizing in the postsynaptic region. Brain Res Mol Brain Res : 147–157, 1999. doi: 10.1016/S0169-328X(99)00214-4. [DOI] [PubMed] [Google Scholar]
- 177.Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol : 1–20, 2005. doi: 10.1016/j.jmb.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 178.Van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J : 2417–2419, 2006. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 179.Van Kerkhof P, Lee J, McCormick L, Tetrault E, Lu W, Schoenfish M, Oorschot V, Strous GJ, Klumperman J, Bu G. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J : 2851–2861, 2005. doi: 10.1038/sj.emboj.7600756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell : 367–377, 2003. doi: 10.1016/S1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 181.Vinik Y, Shatz-Azoulay H, Vivanti A, Hever N, Levy Y, Karmona R, Brumfeld V, Baraghithy S, Attar-Lamdar M, Boura-Halfon S, Bab I, Zick Y. The mammalian lectin galectin-8 induces RANKL expression, osteoclastogenesis, and bone mass reduction in mice. eLife : e05914, 2015. doi: 10.7554/eLife.05914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Von Einem B, Schwanzar D, Rehn F, Beyer AS, Weber P, Wagner M, Schneckenburger H, von Arnim CA. The role of low-density receptor-related protein 1 (LRP1) as a competitive substrate of the amyloid precursor protein (APP) for BACE1. Exp Neurol : 85–93, 2010. doi: 10.1016/j.expneurol.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 183.Walling HW, Raggatt LJ, Irvine DW, Barmina OY, Toledano JE, Goldring MB, Hruska KA, Adkisson HD, Burdge RE, Gatt CJ Jr, Harwood DA, Partridge NC. Impairment of the collagenase-3 endocytotic receptor system in cells from patients with osteoarthritis. Osteoarthritis Cartilage : 854–863, 2003. doi: 10.1016/S1063-4584(03)00170-5. [DOI] [PubMed] [Google Scholar]
- 184.Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development : 4993–5000, 2006. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- 185.Wehmeyer C, Frank S, Beckmann D, Böttcher M, Cromme C, König U, Fennen M, Held A, Paruzel P, Hartmann C, Stratis A, Korb-Pap A, Kamradt T, Kramer I, van den Berg W, Kneissel M, Pap T, Dankbar B. Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction. Sci Transl Med : 330ra35, 2016. doi: 10.1126/scitranslmed.aac4351. [DOI] [PubMed] [Google Scholar]
- 186.Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. Arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature : 527–530, 2000. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 187.Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res : 171–178, 2009. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA : 8460–8464, 1996. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J : 6267–6276, 2003. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, Guo XE, Mann JJ, Balapure AK, Gershon MD, Medhamurthy R, Vidal M, Karsenty G, Ducy P. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med : 308–312, 2010. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell : 825–837, 2008. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamamoto K, Owen K, Parker AE, Scilabra SD, Dudhia J, Strickland DK, Troeberg L, Nagase H. Low density lipoprotein receptor-related protein 1 (LRP1)-mediated endocytic clearance of a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4): functional differences of non-catalytic domains of ADAMTS-4 and ADAMTS-5 in LRP1 binding. J Biol Chem : 6462–6474, 2014. doi: 10.1074/jbc.M113.545376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang E, Zheng H, Peng H, Ding Y. Lentivirus-induced knockdown of LRP1 induces osteoarthritic-like effects and increases susceptibility to apoptosis in chondrocytes via the nuclear factor-κB pathway. Exp Ther Med : 97–105, 2015. doi: 10.3892/etm.2015.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang J, Annala M, Ji P, Wang G, Zheng H, Codgell D, Du X, Fang Z, Sun B, Nykter M, Chen K, Zhang W. Recurrent LRP1-SNRNP25 and KCNMB4-CCND3 fusion genes promote tumor cell motility in human osteosarcoma. J Hematol Oncol : 76, 2014. doi: 10.1186/s13045-014-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP. β-Catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology : 964–976, 2014. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yerges-Armstrong LM, Yau MS, Liu Y, Krishnan S, Renner JB, Eaton CB, Kwoh CK, Nevitt MC, Duggan DJ, Mitchell BD, Jordan JM, Hochberg MC, Jackson RD. Association analysis of BMD-associated SNPs with knee osteoarthritis. J Bone Miner Res : 1373–1379, 2014. doi: 10.1002/jbmr.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang J, Zhang X, Zhang L, Zhou F, van Dinther M, Ten Dijke P. LRP8 mediates Wnt/β-catenin signaling and controls osteoblast differentiation. J Bone Miner Res : 2065–2074, 2012. doi: 10.1002/jbmr.1661. [DOI] [PubMed] [Google Scholar]
- 198.Zhang W, Guo H, Jing H, Li Y, Wang X, Zhang H, Jiang L, Ren F. Lactoferrin stimulates osteoblast differentiation through PKA and p38 pathways independent of lactoferrin’s receptor LRP1. J Bone Miner Res : 1232–1243, 2014. doi: 10.1002/jbmr.2116. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Y, Wang Y, Li X, Zhang J, Mao J, Li Z, Zheng J, Li L, Harris S, Wu D. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol : 4677–4684, 2004. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhao L, Shim JW, Dodge TR, Robling AG, Yokota H. Inactivation of Lrp5 in osteocytes reduces young’s modulus and responsiveness to the mechanical loading. Bone : 35–43, 2013. doi: 10.1016/j.bone.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhong Z, Baker JJ, Zylstra-Diegel CR, Williams BO. Lrp5 and Lrp6 play compensatory roles in mouse intestinal development. J Cell Biochem : 31–38, 2012. doi: 10.1002/jcb.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, Rao S, Yao W, Guan M, Helms JA, Lane NE, Lang RA, Williams BO. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci USA : E2197–E2204, 2012. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhu L, Giunzioni I, Tavori H, Covarrubias R, Ding L, Zhang Y, Ormseth M, Major AS, Stafford JM, Linton MF, Fazio S. Loss of macrophage low-density lipoprotein receptor-related protein 1 confers resistance to the antiatherogenic effects of tumor necrosis factor-α inhibition. Arterioscler Thromb Vasc Biol : 1483–1495, 2016. doi: 10.1161/ATVBAHA.116.307736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem : 1077–1089, 2010. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci Signal : ra15, 2008. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]