To the Editors:
People who inject drugs (PWID) are disproportionately affected by both HIV and Hepatitis C virus (HCV). While HIV incidence rates have since decreased to relatively low numbers among PWID in the United States, 1–4 the current opioid epidemic poses a new threat for HIV outbreaks among PWID.5 HCV prevalence and incidence still remains high among PWID in the United States with increases in HCV incidence linked to the opioid epidemic.4,6,7,8 Syringe service programs (SSPs) distribute clean syringes to prevent the spread of blood-borne diseases including HIV and HCV, and may provide important health services for PWID who are not otherwise engaged in health care.9 For example, in one study conducted among PWID in California, 44% reported a primary care visit in the past 6 months whereas 78% had two or more SSP visits in the past 30 days.10 The majority of SSPs in the United States reported offering on-site HIV testing (88%) and HCV testing (76%) in 2013.11 We describe current HIV and HCV on-site testing and treatment services provided at SSPs in the United States and identify opportunities to improve the provision of services for PWID.
Data are from the Mount Sinai Beth Israel and North American Syringe Exchange Network (NASEN) survey administered annually by mail or email to SSPs from a list developed with NASEN. The survey collects program level information about harm reduction and health services provided to clients in 2015, as reported by the program director. Additional questions about on-site HIV and HCV testing and treatment services, as well as about organizational leadership12 and funding were piloted and added to the survey. A total of 205 SSPs that were operating in 2015 were invited to complete the survey in September 2016, and 134 programs responded with a response rate of 65% as of June 2017. Analysis was limited to SSPs that responded to the HIV and HCV on-site testing questions, resulting in a sample size of 127 SSPs.
The distributions of key program characteristics for SSPs offering both HIV and HCV testing (n=100) were similar to those offering only HIV (n=11) or only HCV (n=2) on-site testing; therefore, we combined these groups into one category of on-site HIV and/or HCV (hereafter on-site HIV/HCV) testing for analysis. Program characteristics of SSPs with on-site HIV/HCV testing (n = 113) are compared with characteristics of the remaining SSPs without on-site HIV/HCV testing (n = 14). The percentage of SSP clients tested was constructed using the reported number of HIV/HCV tests conducted on-site and number of SSP clients, which may overestimate testing uptake if individuals receive multiple tests. SSP size was categorized by the number of syringes distributed (small=1-9,999; medium=10,000-55,000; large=55,001-99,999; very large ≥100,000 syringes). We completed the analysis using Pearson’s Chi-squared test, Fisher’s test, or t-tests. Chi-squared tests were used to compare HIV and HCV testing and treatment responses for categorical variables with two response levels.
Using program data or estimation, SSP directors reported that on average, the majority of clients were white (68%), male (64%), and 30-50 years old (48%). SSPs were predominantly large in terms of number of syringes distributed (54%), part of a larger organization (77%), located in urban areas (69%), and received public funding (64%) (Table, Supplemental Digital Content). On-site HIV and HCV testing was very common at SSPs, with 79% providing both HIV and HCV on-site testing services, 9% only offering only on-site HIV testing, 1% only offering only on-site HCV testing, and 11% offering neither on-site HIV nor HCV testing. On average, 17% (SD=22) of SSP clients were tested for HIV and 15% (SD=21) were tested for HCV on site during 2015.
SSPs with on-site HIV/HCV testing were significantly more likely to have a larger budget (mean $184,733 vs. $30,882), receive public funding (67% vs 36%), be part of a larger organization (81% vs. 43%), and be located in the Northeast and Midwest (56% vs. 14%) than SSPs without on-site testing services (p-value<0.05). While not statistically significant, SSPs without on-site HIV/HCV testing services were more frequently located in rural locations (36% vs. 19%) and were small to medium in size. Overall, 11% (14/127) provided on-site buprenorphine and/or methadone for opioid use disorders (OUDs). Leaders at SSPs with on-site HIV/HCV testing were significantly more likely to spend on average more hours in a month communicating with funding or monitoring organizations, making public presentations and appearances in the community, identifying possible new payment or funding sources, and recruiting more staff.
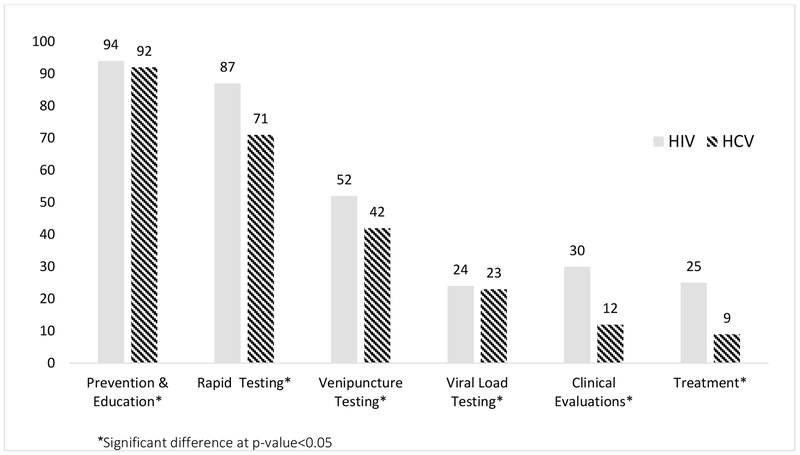
Most SSPs offered on-site HIV and HCV prevention and education (94% and 92%, respectively) and many provided HIV and HCV rapid testing services (87% and 71%, respectively). For clinical services such as venipuncture and viral load testing, clinical evaluations, and treatment, slightly less than half of programs offered those services on-site, and more frequently provided each those services for HIV than for HCV (p<0.05) (Figure). The approach for HIV and HCV testing practices were similar with about half of the programs reporting that testing is available to everyone and clients can ask for testing. The majority of programs encourage re-testing for both HIV and HCV, although re-testing is encouraged more strongly and frequently (every 3 months) for HIV than HCV (p<0.05).
Figure: Percent of syringe service programs providing on-site HIV or HCV education, testing and treatment services (N=127).
Statistical significance determined using Chi-square or Fisher’s test at p-value < 0.05. Human immunodeficiency virus is abbreviated to HIV and hepatitis c virus is abbreviated to HCV.
SSPs were significantly more likely to provide on-site medical care, refer clients to agencies/programs with a formal arrangement, make medical appointments for clients at other agencies/programs, and accompany clients to medical appointments for HIV than for HCV positive clients (p<0.05). Clients testing HCV positive at SSPs were more likely to be referred to treatment by receiving a list of contact information for agencies/programs than clients testing HIV positive (p<0.05). For clients testing positive who were referred to care, 63% of SSPs tracked whether clients testing HIV positive engaged in care compared to only 42% of SSPs that did the same for clients testing HCV positive (p<0.05).
Across the testing and treatment services spectrum (e.g., venipuncture testing, purchasing rapid test kits, performing rapid testing, and programs for positive clients), SSPs were significantly more likely to have grants or contract funding for HIV-related services than HCV services (p<0.05). Very few programs received reimbursement from billing health insurers (<3%). HCV-related testing and care services were more likely to not have grant or contract funding sources than HIV-related services. Less than half of SSPs received donated rapid test kits, with the majority of those which did receiving donated HIV and HCV test kits from the local health department. However, SSPs received more donated HCV test kits from testing or pharmaceutical companies than HIV tests kits (33% and 16%, p<0.05).
We found that even though a large proportion of SSPs offer on-site HIV/HCV testing, uptake was low with an average of 15–17% of clients testing on-site. Further research is needed to study barriers to uptake including financial, organizational capacity, and client-related barriers to testing. For example, determining whether SSPs offering on-site testing by outside organizations test less frequently than SSPs that use their own staff to provide these services would be an area of future research. Addressing these barriers requires improved funding for testing and treatment services in SSPs, investing more into improving staff capacity at SSPs, and moving towards the integration of care into SSPs, including possible expansion of on-site buprenorphine/methadone, to expand health service access for PWID.
We also found that the minority of SSPs that did not provide on-site HIV and HCV testing services were smaller and more organizationally isolated. Providing on-site HIV and HCV screening services at under-resourced, smaller SSPs is critical to meeting the needs of PWID living in rural areas. Rural PWID report a significantly longer times between tests for blood-borne diseases compared to PWID living in metropolitan areas and therefore are at high need for screening services.13 This may be attributed to generally less health care access in rural areas,14 less access to SSPs due to distance or limited hours,13 and greater fear of stigma in health care settings.15 SSPs may serve to fill this gap by providing more culturally sensitive, stigma-free health services and connecting PWID to appropriate care.
SSPs conducting on-site testing had fewer on-site services available for HCV compared to HIV, despite reporting clients testing positive for HCV more frequently than for HIV. The disparity between HIV and HCV on-site service provision at SSPs may not only be a result of differences in funding levels. Establishing referrals to culturally competent care may be a barrier to successful linkage to HCV care and treatment.15, 16 More effective linkage strategies or on-site phlebotomy-trained staff for clinical services could be more broadly implemented to improve HCV screening and treatment expansion.17,18
Our findings provide insights that further characterize our understanding of the provision of HIV and HCV services in SSPs. With deaths from HCV exceeding deaths from HIV, expansion of HCV testing and treatment with new, highly effective, direct-acting antiviral medications at SSPs provide an opportunity to cure HCV and dramatically reduce HCV prevalence and death among clients.19 Primary prevention through syringe exchange, and secondary prevention through on-site screening and linkage to care and treatment, are essential to realizing these objectives for HCV and “getting to zero” new infections for HIV.
Supplementary Material
Acknowledgements:
We would like to acknowledge the North American Syringe Exchange Network for their important contributions towards study recruitment and all of the syringe service programs for their time and participation in this study. This research was supported by the National Institute of Drug Abuse (R01DA027379, P30DA040500, and P30DA011041).
Footnotes
Meetings at which parts of data were presented:
Conference: The Association for Medical Education and Research in Substance Abuse (AMERSA), 41st Annual Conference
Location: Washington, DC
Dates: November 2-4, 2017
Conflicts of Interest:
The authors have no conflicts of interest to disclose.
References:
- 1.Wejnert C, Hess KL, Hall HI, et al. Vital Signs: Trends in HIV Diagnoses, Risk Behaviors, and Prevention Among Persons Who Inject Drugs - United States. MMWR Morb Mortal Wkly Rep. 2016;65(47):1336–1342. [DOI] [PubMed] [Google Scholar]
- 2.Broz D, Wejnert C, Pham HT, et al. HIV infection and risk, prevention, and testing behaviors among injecting drug users -- National HIV Behavioral Surveillance System, 20 U.S. cities, 2009. MMWR Surveill Summ. 2014;63(6):1–51. [PubMed] [Google Scholar]
- 3.Des Jarlais DC, Arasteh K, McKnight C, et al. Consistent Estimates of Very Low HIV Incidence Among People Who Inject Drugs: New York City, 2005-2014. Am J Public Health. 2016;106(3):503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neaigus A, Reilly KH, Jenness SM, et al. Trends in HIV and HCV Risk Behaviors and Prevalent Infection Among People Who Inject Drugs in New York City, 2005-2012. J Acquir Immune Defic Syndr. 2017;75 Suppl 3:S325–s332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conrad C, Bradley HM, Broz D, et al. Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone--Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443–444. [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan AE, Des Jarlais DC, Arasteh K, McKnight C, Nash D, Perlman DC. Incidence and prevalence of hepatitis c virus infection among persons who inject drugs in New York City: 2006-2013. Drug Alcohol Depend. 2015;152:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang TJ, Ward JW. Hepatitis C in Injection-Drug Users — A Hidden Danger of the Opioid Epidemic. New England Journal of Medicine. 2018;378(13):1169–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zibbell JE, Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahern J, Stuber J, Galea S. Stigma, discrimination and the health of illicit drug users. Drug Alcohol Depend. 2007;88(2-3):188–196. [DOI] [PubMed] [Google Scholar]
- 10.Heinzerling KG, Kral AH, Flynn NM, et al. Unmet need for recommended preventive health services among clients of California syringe exchange programs: implications for quality improvement. Drug Alcohol Depend. 2006;81(2):167–178. [DOI] [PubMed] [Google Scholar]
- 11.Des Jarlais DC, Nugent A, Solberg A, et al. Syringe Service Programs for Persons Who Inject Drugs in Urban, Suburban, and Rural Areas - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(48):1337–1341. [DOI] [PubMed] [Google Scholar]
- 12.D’Aunno T, Vaughn TE, McElroy P. An institutional analysis of HIV prevention efforts by the nation’s outpatient drug abuse treatment units. J Health Soc Behav . 1999;40(2):175–192. [PubMed] [Google Scholar]
- 13.Day C, Conroy E, Lowe J, Page J, Dolan K. Patterns of drug use and associated harms among rural injecting drug users: comparisons with metropolitan injecting drug users. Aust J Rural Health. 2006;14(3):120–125. [DOI] [PubMed] [Google Scholar]
- 14.Benitez JA, Seiber EE. US Health Care Reform and Rural America: Results From the ACA’s Medicaid Expansions. J Rural Health. 2017. [DOI] [PubMed] [Google Scholar]
- 15.McKnight C, Shumway M, Masson CL, et al. Perceived discrimination among racial and ethnic minority drug users and the association with health care utilization. J Ethn Subst Abuse. 2017;16(4):404–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stopka TJ, Hutcheson M, Donahue A. Access to healthcare insurance and healthcare services among syringe exchange program clients in Massachusetts: qualitative findings from health navigators with the iDU (“I do”) Care Collaborative. Harm Reduct J. 2017;14(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burr CK, Storm DS, Hoyt MJ, et al. Integrating health and prevention services in syringe access programs: a strategy to address unmet needs in a high-risk population. Public Health Rep. 2014;129 Suppl 1:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam MM, Topp L, Conigrave KM, et al. Linkage into specialist hepatitis C treatment services of injecting drug users attending a needle syringe program-based primary healthcare centre. J Subst Abuse. 2012;43(4):440–445. [DOI] [PubMed] [Google Scholar]
- 19.Ly KN, Hughes EM, Jiles RB, et al. Rising Mortality Associated With Hepatitis C Virus in the United States, 2003-2013. Clin Infect Dis. 2016;62(10):1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.