Abstract
Background:
Both circulating tumor cell (CTC) and total circulating cell-free DNA (ccfDNA) predict cancer patient prognosis. However, no study has explored the prognostic value of the combined use of CTC and ccfDNA. We aimed to investigate individual and joint effects of CTC and ccfDNA on clinical outcomes of metastatic breast cancer (MBC) patients.
Methods:
We collected 227 blood samples from 117 MBC patients. CTCs were enumerated using the CellSearch System. ccfDNAs were quantified by quantitative real-time PCR and Qubit fluorometer. The individual and joint effects of CTC and ccfDNA levels on patient progression-free survival (PFS) and overall survival (OS) were analyzed using Cox proportional hazards models.
Results:
Compared to patients with < 5 CTCs, patients with ≥ 5 CTCs had a 2.58-fold increased risk of progression and 3.63-fold increased risk of death. High level of ccfDNA was associated with a 2.05-fold increased risk of progression and 3.56-fold increased risk of death. These associations remained significant after adjusting for other important clinical covariates and CTC/ccfDNA levels. CTC and ccfDNA levels had a joint effect on patient outcomes. Compared to patients with low levels of both CTC and ccfDNA, those with high levels of both markers exhibited a > 17-fold increased death risk (P < 0.001). Moreover, longitudinal analysis of 132 samples from 22 patients suggested that the inconsistency between CTC level and outcome in some patients could possibly be explained by ccfDNA level.
Conclusions:
CTC and total ccfDNA levels were individually and jointly associated with PFS and OS in MBC patients.
Keywords: circulating tumor cell (CTC), circulating cell-free DNA (ccfDNA), metastatic breast cancer (MBC), prognosis
1. Introduction
More than 40,000 U.S. deaths each year are attributable to breast cancer, predominantly metastatic breast cancer (MBC) [1, 2]. Precisely treating patients with MBC and prolonging survival is a significant challenge. Currently, MBC treatment decisions are based mainly on pathological examination of tumor tissues or biopsies, but these are not always obtainable and can yield inaccurate findings due to intratumoral heterogeneity [3, 4]. Moreover, treatment is often inadequate because MBC progresses quickly and dynamically to evade the attacks of systemic therapies. Novel non-invasive approaches that prospectively predict patient survival and monitor real-time treatment responses are needed in order to better manage and treat MBC.
Liquid biopsy is increasingly used for disease monitoring and therapy selection in advanced stage cancer, especially for patients whose tumor tissues or biopsies are difficult to procure [3–5]. Two major types of liquid biopsy are circulating tumor cell (CTC) and circulating tumor DNA (ctDNA). CTCs are rare malignant cells that are shed from primary or metastatic tumors, circulate in the peripheral blood, and play a critical functional role in tumor metastasis [6]. ctDNAs are also shed from primary and metastatic tumors, but do not seem to be as functionally important as CTCs [3, 7, 8]. However, compared to CTCs, which are mostly present in advanced stage cancer patients, ctDNAs can be identified in the majority of early stage cancer patients and are arguably the best marker for disease monitoring and therapy selection [9–12]. Moreover, both CTC and ctDNA levels have been associated with patient survival and treatment responses [13–19].
ctDNA comprises a small portion of total circulating cell-free DNA (ccfDNA), which is composed of both ctDNAs and DNAs that are derived from normal cells [3, 7, 20]. In recent years, ctDNAs have been used to interrogate genomic mutations in order to match patients to targeted therapies attacking those mutations [21, 22]. However, a major caveat with the use of ctDNAs is their detection accuracy, since in the majority of cancer patients, even those with metastatic diseases, the percentage of ctDNA is extremely low among total ccfDNAs [7, 21]. Determining whether the detected low-frequency mutations are bona fide mutations or false positives due to amplification and/or sequencing errors is difficult with traditional sequencing and analytical approaches [5, 23]; the recent application of various molecular barcode-based strategies alleviates this caveat but cannot completely resolve it. For example, ctDNA analyses of the same samples by two reputable service providers yielded considerably different results [24]. Moreover, due to the low quantity of ccfDNA in blood and the low percentage of ctDNA in ccfDNA, ctDNA analysis usually requires extremely high-depth sequencing, which significantly increases the cost of analysis. Thus, further evaluation is needed to assess the clinical validity and utility of most ctDNA analyses for guiding cancer treatment, according to a recent joint review by the American Society of Clinical Oncology and College of American Pathologists [25, 26].
The implication of total ccfDNA in cancer prognosis has not been as extensively studied as CTC and ctDNA. It has been reported that cancer patients have a significantly larger load of ccfDNAs than non-cancer patients [3, 27–29], although these ccfDNAs contain varying percentages of ctDNAs depending on different cancer types and patients [12, 20, 28]. Total ccfDNAs cannot be used to detect specific mutations to guide the use of targeted therapies, unlike ctDNAs; however, in a few recent studies, ccfDNA has exhibited certain prognostic performance [18, 19, 29–33]. Nonetheless, despite the fact that both CTC and ccfDNA can predict patient survival, no study has been conducted to evaluate the combined prognostic value of these two markers. Herein, we simultaneously measured CTC and total ccfDNA levels using 227 blood samples in 117 MBC patients. Based on these data, we conducted, to our best knowledge, the first analysis of the individual and joint effects of CTCs and ccfDNA on disease progression and survival in MBC patients.
2. Methods
2.1. Study population
In a hospital-based cohort started in November 2013, female MBC patients were identified and continuously recruited from breast cancer patients who visited the breast cancer clinic of the Sidney Kimmel Cancer Center at Thomas Jefferson University Hospital. MBC diagnosis was based on tissue histology, complemented by radiological evaluations. Demographic-, tumor presentation-, and treatment-related information were obtained by reviewing medical charts and consulting treating physicians. Blood samples were collected from each patient at baseline before initiation of a new therapy. Longitudinal samples at baseline and each follow-up visit were collected from a subset of patients. This study was approved by the Institutional Review Board of Thomas Jefferson University. Each patient signed a written informed consent at enrollment.
2.2. Enumeration of CTCs
Approximately 8–10 mL of whole blood was collected into a CellSave preservative tube containing a cellular fixative (Janssen Diagnostics, Raritan, NJ, USA). Blood samples were maintained at room temperature and processed within 96 h of blood draw. CTC enumeration was conducted using CELLSEARCH® CTC kits on the Food and Drug Administration-approved CellSearch System, including the CELLTRACKS® AUTOPREP® System and the CELLTRACKS ANALYZER II® System (Janssen Diagnostics) [13]. In brief, 7.5 mL of whole blood was subjected to enrichment of epithelial cells with antibody-coated ferrous particles targeting the epithelial cell adhesion molecule antigen (EpCAM+). CTC identification was achieved following the positive staining for both phycoerythrin-conjugated cytokeratins (cytokeratins 8+, 18+, or 19+) and double-stranded DNA (DAPI+) and the negative staining for CD45 (CD45-).
2.3. Measurement of total ccfDNAs
Each 8–10 mL of whole blood was collected in a vacutainer tube with K2 EDTA (Becton Dickinson, Franklin Lakes, NJ, USA) and stored at room temperature. Blood samples were processed within 3 h of collection. Plasma was separated by centrifugation at 1,700 g for 10 min at room temperature, transferred to a microcentrifuge tube and centrifuged at 20,000 g for 10 min at 4 °C to remove cell debris. Plasma was then stored at −80 °C. Before using the plasma for circulating nucleic acid extraction, tubes were thawed at room temperature and centrifuged at 16,000 g for 5 min at 4 °C to remove cryoprecipitates. ccfDNA was isolated, purified, and concentrated from 1.5 mL plasma using QIAamp Circulating Nucleic Acid Kit (Qiagen). Concentration of purified plasma DNA was determined by two methods: fluorescence-based Qubit® dsDNA HS quantitation Assay kit using a Qubit® 2.0 Fluorometer (Invitrogen) and quantitative real-time PCR (qRT-PCR) assay for ALU DNA repeats on chromosome 1, as previously described [34–37] with modifications. For the qRT-PCR assay, a dilution series of genomic DNA was used to create a standard curve. Two sets of ALU primers used in qRT-PCR were: forward 5’-CCTGAGGTCAGGAGTTCGAG-3’ and reverse 5’-CCCGAGTAGCTGGGATTACA-3’ for a 115-bp amplicon of the ALU repeat sequence (ALU115) which amplifies both shorter (apoptotic) and longer (non-apoptotic) DNA fragments [34, 35], and forward 5’-CCTGAGGTCAGGAGTTCGAG-3’ and reverse 5’-GCCCCGGCTAATTTTTGTAT-3’ for a 81-bp amplicon (ALU81), which showed high sensitivity and linearity even though the DNA was severely fragmented [37]. Briefly, 6 μl of reaction mixture for qRT-PCR included 2 μl of DNA template, 0.5 μl of each primer, and 3 μl of Maxima SYBR Green (Thermo Fisher Scientific, Waltham, MA). Real-time PCR amplification was carried out on a ViiA 7 Real-Time PCR System (Life Technologies, Carlsbad, CA) with pre-cycling heat activation of DNA polymerase at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 sec and 60 °C for 1 min, annealing at 64 °C for 30 sec, and extension at 72 °C for 1 min. All assays were conducted in duplicates with adequate positive and negative controls on each plate. Quantitative values from either 115-bp or 81-bp amplification product represent the total level of ccfDNA in ng/ml [35, 37], and they were highly consistent in our study (Pearson’s correlation coefficient [r] = 0.97, P < 0.001, Supplementary Fig. 1A). The amount of total ccfDNA in plasma was calculated based on qRT-PCR results of ALU115 and ALU81 using the formula: . A high correlation was observed between total ccfDNA level calculated by the above method and Qubit quantitation (Pearson’s r = 0.94, P < 0.001, Supplementary Fig. 1B).
2.4. Statistical analysis
The endpoints analyzed in this study were progression-free survival (PFS) and overall survival (OS). PFS was defined as the elapsed time between the date of baseline blood draw and either the date of clinical progression, death, or the last follow-up. OS was defined as the time from baseline blood collection to death or the last follow-up. Patients without an endpoint event at the last follow-up were censored. We also evaluated treatment responses on the basis of longitudinally collected samples. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were assessed by imaging-based methods following the Response Evaluation Criteria in Solid Tumors (RECIST) guideline [38]. For CTC-related analyses, a widely used cut-off of 5 CTCs per 7.5 mL whole blood was applied to stratify patients into high- and low-risk groups [13]; for ccfDNA-related analyses, the best cut-off point of ccfDNA amount, calculated based on ALU115 and ALU81 levels, was determined using Receiver Operating Characteristic (ROC) curve analysis. Comparisons of demographic and clinical variables were conducted using a χ2 test for categorical variables and an unpaired Student’s t test for continuous variables. The correlation between CTC and ccfDNA levels was evaluated by the Spearman’s rank-order correlation method. Survival curves were estimated using the Kaplan-Meier method and compared using a log-rank test. Associations of CTC or ccfDNA levels with PFS or OS were evaluated using hazard ratios (HRs) with 95% confidence intervals (CIs) by a univariate and a multivariate Cox proportional hazards model adjusting for age, ethnicity, body mass index (BMI), tumor grade, menopause status, breast cancer subtypes, previous therapies, treatments after baseline blood draw, and ccfDNA/CTC, where appropriate. The proportional hazards assumption was validated using the test based on Schoenfeld residuals. Possible interaction effect between CTC and ccfDNA on survivals was tested by including a product interaction term into the Cox proportional hazards model. Changes in CTC and ccfDNA levels over time with treatment responses and clinical outcomes were analyzed and plotted using data of longitudinally collected samples. CTC or ccfDNA levels before and after the initiations of new therapies were compared using a paired t test. SAS (Version 9.4, SAS Institute) and STATA (Version 11.0, STATA Corp.) software packages were used for the analyses in this study. All P values were 2-sided, with a P < 0.05 considered the threshold for statistical significance.
3. Results
3.1. Patient characteristics
The characteristics of 117 MBC patients are presented in Table 1. The mean age at baseline was 54.5 years. The majority of patients was Caucasians (86.3%) and had poorly differentiated tumors (66.7%). Of the 117 patients, 69 (59.0%), 15 (12.8%), and 33 (28.2%) patients had Luminal, HER2-enriched, and triple negative (TNBC) subtypes of breast cancer. Twenty-seven (23.1%) and 64 (54.7%) patients did not have previous chemotherapy or hormone therapies. There were 76.9%, 41.9%, and 70.1% of patients who received chemotherapy, hormonal therapy, and targeted therapy, respectively, after baseline blood draw. During a median follow-up of 42.1 weeks, 81 (69.2%) patients progressed and 23 (19.7%) died (Table 2).
Table 1.
Characteristics of metastatic breast cancer patients (N=117)
| Variables | N(<%) |
|---|---|
| Age (years), mean t SD | 54.5 ± 11.5 |
| Ethnicity | |
| Caucasian | 101 (86.3) |
| Black | 12 (10.3) |
| Other | 4(3.4) |
| Smoking status | |
| No | 76 (65.0) |
| Yes | 41 (35.0) |
| Drinking status | |
| No | 61 (52.1) |
| Yes | 53 (45.3) |
| Unknown | 3(2.6) |
| BMI (kg/m2) | |
| <25 | 43 (36.8) |
| 25- | 32 (27.4) |
| 30- | 42 (35.9) |
| Family history of breast cancer | |
| No | 47 (40.2) |
| Yes | 67 (57.3) |
| Unknown | 3(2.6) |
| Menopause status | |
| Pre | 18 (15.4) |
| Post | 99(84.6) |
| Tumor grades | |
| Moderately | 22 (18.8) |
| Poorly | 78 (66.7) |
| Unknown | 17 (14.5) |
| Breast cancer subtypes | |
| Luminal | 69 (59.0) |
| ER2-enriched | 15 (12.8) |
| TNBC | 33 (28.2) |
| Baseline cancer antigen 15.3 | |
| Normal | 19 (16.2) |
| Elevated | 28 (23.9) |
| Unknown | 70 (59.8) |
| Number of previous chemotherapy lines | |
| 0 | 27 (23.1) |
| 1 | 29 (24.8) |
| ≥2 | 61 (52.1) |
| Number of previous hormone therapy lines | |
| 0 | 64(54.7) |
| 1 | 19 (16.2) |
| ≥2 | 34 (29.1) |
| Chemotherapy | |
| No | 27 (23.1) |
| Yes | 90 (76.9) |
| Hormonal therapy | |
| No | 68 (58.1) |
| Yes | 49 (41.9) |
| Target therapy | |
| No | 35 (29.9) |
| Yes | 82 (70.1) |
Abbreviations: SD, standard deviation; BMI, body mass index; HER2, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer.
Table 2.
Associations between baseline CTC or/and ccfDNA level with patient PFS and OS
| Variables | Total | Events, N (%) | HR (95% Cl) | P | HR (95% Cl)* | P* | HR (95% Cl)# | P# |
|---|---|---|---|---|---|---|---|---|
|
Associated with PFS
CTC | ||||||||
| < 5 cells/7.5 ml | 85 | 51 (60.0) | 1.00 | 1.00 | 1.00 | |||
| ≥ 5 cells/7.5 ml | 32 | 30(93.8) | 2.58 (1.63–4.07) | <0.001 | 2.93(1.78–4.81) | < 0.001 | 2.34(1.33–4.12) | 0.003 |
| ccfDNA | 117 | 81(69.2) | 1.61 (1.25–2.09)§ | <0.001§ | 1.68 (1.28–2.21)§ | <0.001§ | 1.35 (0.99–1.85)§ | <0.055§ |
| Low | 73 | 46(63.0) | 1.00 | 1.00 | 1.00 | |||
| High | 44 | 35(79.5) | 2.05(1.31–3.19) | 0.002 | 2.37(1.47–3.81) | < 0.001 | 1.64 (0.98–2.76) | 0.062 |
|
Associated with OS
CTC | ||||||||
| < 5 cells/7.5 ml | 85 | 10(11.8) | 1.00 | 1.00 | 1.00 | |||
| ≥ 5 cells/7.5 ml | 32 | 13 (40.6) | 3.63 (1.58–8.34) | 0.002 | 6.76(2.55–17.92) | < 0.001 | 6.67(2.42–18.39) | <0.001 |
| ccfDNA | 117 | 23(19.7) | 1.73 (1.11–2.69)§ | 0.016§ | 1.75 (1.08–2.84)§ | 0.023§ | 1.72 (1.02–2.89)§ | 0.042§ |
| Low | 73 | 8(11.0) | 1.00 | 1.00 | 1.00 | |||
| High | 44 | 15(34.1) | 3.56(1.51–8.40) | 0.004 | 2.97(1.18–7.49) | 0.021 | 2.73 (1.05–7.06) | 0.039 |
Abbreviations: CTC, circulating tumor cell; ccfDNA, circulating cell-free DNA; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Adjusted for age, ethnicity, body mass index, tumor grade, menopause status, breast cancer subtypes, previous therapies, and treatments after baseline blood draw.
Adjusted for age, ethnicity, body mass index, tumor grade, menopause status, breast cancer subtypes, previous therapies, treatments after baseline blood draw, and ccfDNA/CTC.
ccfDNA level (log transformation) was analyzed as a continuous variable.
3.2. Associations of CTC and ccfDNA levels at baseline with patient outcomes
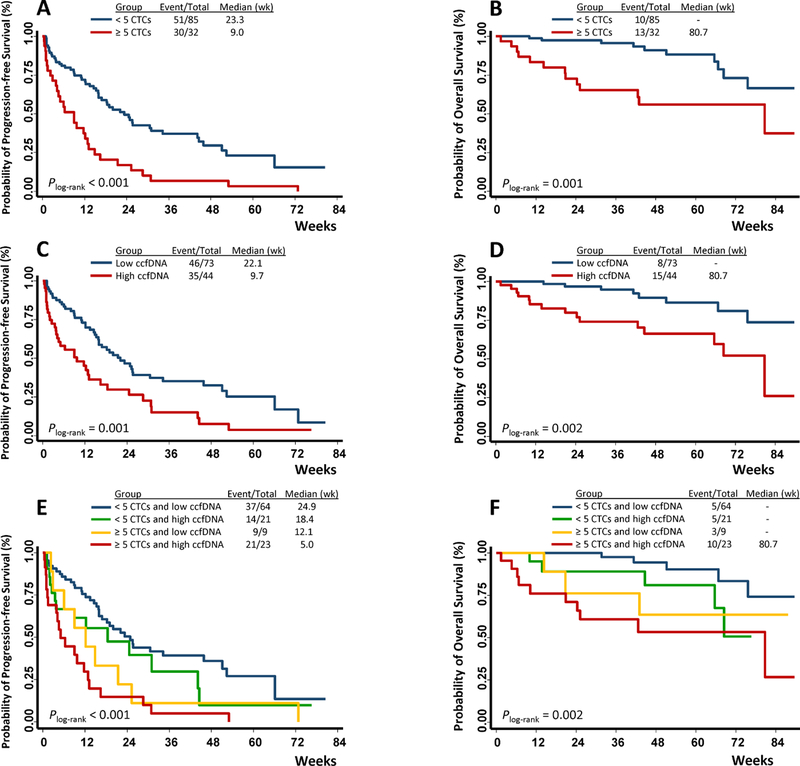
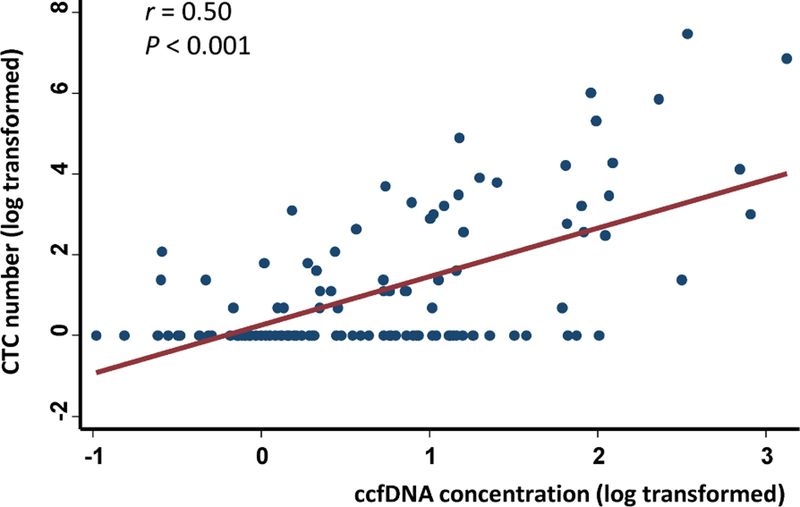
Among the 117 patients, 32 (27.4%) had elevated CTCs (≥ 5 CTCs) at baseline. This percentage was slightly lower than previous reports [14], possibly due to the larger number of TNBC patients in our cohort and to the fact that some patients in our cohort had undergone previous therapies that might have reduced the number of CTCs [19, 39]. As expected, patients with elevated CTCs exhibited significantly unfavorable PFS (Plog-rank < 0.001, Fig. 1A) and OS (Plog-rank = 0.001, Fig. 1B). Compared to the patients with < 5 CTCs, those with ≥ 5 CTCs had 2.58-fold (95% CI 1.63–4.07) increased risk for disease progression and 3.63-fold (95% CI 1.58–8.34) increased risk for death (Table 2). The associations of CTCs with patient outcomes remained significant after adjusting for age, ethnicity, BMI, tumor grade, menopause status, breast cancer subtypes, previous therapies, and treatments after baseline blood draw (HR 2.93, 95% CI 1.78–4.81 for PFS; HR 6.76, 95% CI 2.55–17.92 for OS, Table 2). The survival differences between patients with high- and low-level ccfDNAs were also statistically significant (Plog-rank = 0.001 for PFS and Plog-rank = 0.002 for OS, Fig. 1C and 1D). Compared to patients with low levels of ccfDNA, those with high levels of ccfDNA had a much higher risk for progression and death in both univariate (HR 2.05, 95% CI 1.31–3.19 for PFS; HR 3.56, 95% CI 1.51–8.40 for OS) and multivariate analyses (HR 2.37, 95% CI 1.47–3.81 for PFS; HR 2.97, 95% CI 1.18–7.49 for OS, Table 2). Similar results were obtained when total ccfDNA level was analyzed as a continuous variable (Table 2). CTC and ccfDNA exhibited a significant correlation (Spearman’s r = 0.50, P < 0.001, Fig. 2). Therefore, we also adjusted ccfDNA when analyzing CTC, and we adjusted CTC when analyzing ccfDNA. These analyses yielded similar results with moderately reduced HRs, indicating that CTC or ccfDNA affected patient outcomes in a way that depended partly on one another but mostly did not overlap (Table 2).
Fig. 1.
CTC and ccfDNA levels individually and jointly associated with clinical outcomes of MBC patients. Survival differences between patients with ≥ 5 and < 5 CTCs/7.5 mL of whole blood, or between patients with high- and low-levels of ccfDNA were compared at baseline. CTC level was associated with PFS (A) and OS (B); ccfDNA level associated with PFS (C) and OS (D); joint effect of CTC and ccfDNA levels on PFS (E) and OS (F).
Fig. 2.
Correlation between CTC and ccfDNA levels at baseline. The data were log-transformed. If the patients had 0 CTC, log (0+1) was plotted alternatively in Y-axis. The Spearman correlation coefficient between CTC count and ccfDNA level was 0.50.
3.3. Joint effects of baseline CTC and ccfDNA levels on patient outcomes
We next evaluated whether there was a joint effect between CTC and ccfDNA level on patient outcomes. To this end, we separated all patients into four risk groups based on their baseline CTC and ccfDNA levels, including: 1) 64 patients with < 5 CTCs and low ccfDNA (low risk); 2) 21 patients with < 5 CTCs and high ccfDNA (low-intermediate risk); 3) 9 patients with ≥ 5 CTCs and low ccfDNA (high-intermediate risk); and 4) 23 patients with ≥ 5 CTCs and high ccfDNA (high risk). The survival differences among these four groups were statistically significant (Plog-rank < 0.001 for PFS analysis, Plog-rank = 0.002 for OS analysis, Fig. 1E and 1F). As shown in Table 3, compared to patients with low risk, the trend of disease progression increased (Ptrend < 0.001) for patients with low-intermediate risk (adjusted HR, 1.56), high-intermediate risk (adjusted HR, 2.21), and high risk (adjusted HR, 3.90); the trend of death elevated as well (Ptrend < 0.001) for patients with low-intermediate risk (adjusted HR, 1.19), high-intermediate risk (adjusted HR, 2.46), and high risk (adjusted HR, 17.43). The P value for multiplicative interaction between CTC and ccfDNA for OS analysis was 0.156.
Table 3.
Joint effects of baseline CTC and ccfDNA level on patient PFS and OS
| Variables | Total | Events, N (%) | HR (95% Cl) | p | HR (95% Cl)* | p* |
|---|---|---|---|---|---|---|
| Associated with PFS | ||||||
| CTC < 5 cells/7.5 ml and low ccfDNA | 64 | 37(57.8) | 1.00 | 1.00 | ||
| CTC < 5 cells/7.5 ml and high ccfDNA | 21 | 14(66.7) | 1.54(0.83–2.85) | 0.174 | 1.56(0.77–3.14) | 0.215 |
| CTC ≥ 5 cells/7.5 ml and low ccfDNA | 9 | 9(100.0) | 2.07 (0.99–4.34) | 0.053 | 2.21 (1.03–4.77) | 0.043 |
| CTC ≥ 5 cells/7.5 ml and high ccfDNA | 3 | 21(91.3) | 3.42(1.99–5.89) | <0.001 | 3.90(2.17–7.04) | < 0.001 |
| Ptrend < 0.001 | Ptrend < 0.001 | |||||
| Associated with OS | ||||||
| CTC < 5 cells/7.5 ml and low ccfDNA | 64 | 5(7.8) | 1.00 | 1.00 | ||
| CTC < 5 cells/7.5 ml and high ccfDNA | 21 | 5(23.8) | 3.08 (0.89–10.66) | 0.076 | 1.19(0.31–4.61) | 0.801 |
| CTC ≥ 5 cells/7.5 ml and low ccfDNA | 9 | 3(33.3) | 3.34 (0.79–14.03) | 0.100 | 2.46 (0.49–12.46) | 0.277 |
| CTC ≥ 5 cells/7.5 ml and high ccfDNA | 23 | 10 (43.5) | 6.77 (2.30–19.93) | <0.001 | 17.43 (4.76–63.78) | < 0.001 |
| Ptrend < 0.001 | Ptrend < 0.001 |
Abbreviations: CTC, circulating tumor cell; ccfDNA, circulating cell-free DNA; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Adjusted for age, ethnicity, body mass index, tumor grade, menopause status, breast cancer subtypes, previous therapies, and treatments after baseline blood draw.
3.4. Longitudinal changes of CTC and ccfDNA levels and patient outcomes
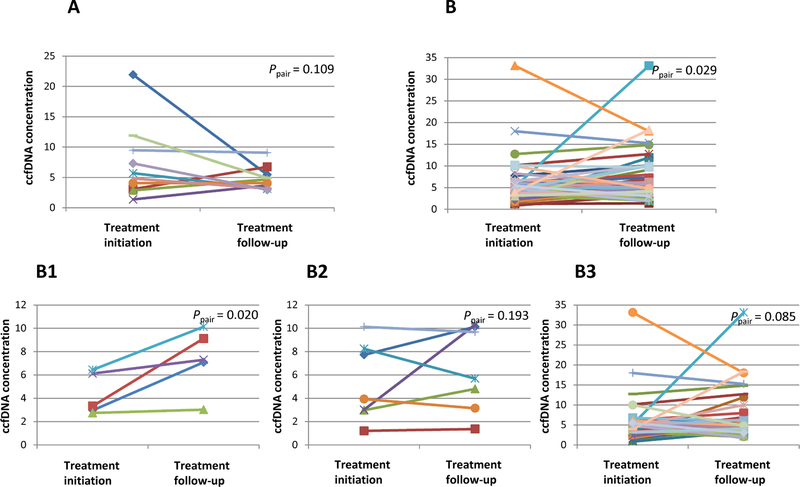
Using 132 longitudinally collected blood samples from a subset of 22 patients, we further analyzed whether changes in CTC and ccfDNA over time were correlated with treatment responses and survival. First, the correlations between CTC/ccfDNA with treatment responses were analyzed. We separated patients into two groups: responders who showed partial or complete response (PR/CR) or non-responders who had stable or progressive disease (SD/PD) [40], and then we compared the changes in CTC and ccfDNA levels between these two groups. We found that both CTC and ccfDNA levels at first follow-up were lower in responders but higher in non-responders when compared to their baseline levels (Supplementary Fig. 2A-D). The CTC and ccfDNA levels collected at each visit served the baseline for the next visit, on which treatment responses were re-defined using the most recent imaging and treatement initiation was determined. Statistically significant differences were then observed between these two time points (Supplementary Fig. 2E-H). In most cases, CTC changes were consistent with treatment responses (i.e., decreased CTCs in responders, increased CTCs in non-responders). However, in some patients, inconsistency was observed between CTC change and treatment response. For these patients, we explored whether ccfDNA changes could explain the inconsistencies. We considered two types of inconsistent situations, including: (1) responders who had ≥ 5 CTCs or stable CTCs at follow-up visit after treatment initiation (Fig. 3A); and (2) non-responders who had < 5 CTCs or decreased CTCs at follow-up visit after treatment initiation (Fig. 3B). The latter situation also included i) CTCs that decreased but were still ≥ 5, ii) CTCs that decreased from ≥ 5 to < 5, and iii) CTCs that were continuously < 5 (Fig. 3B1–3B3). As shown in Fig. 3, we observed decreased ccfDNA level in responders (Fig. 3A) and elevated ccfDNA levels in non-responders (Fig. 3B1–3B3). Therefore, the desynchrony between CTC changes and treatment responses may be largely explained by changes in ccfDNA level.
Fig. 3.
ccfDNA changes when treatment responses could not be expained by CTC change alone. Responders are defined as patients with partial or complete response; non-responders are patients with stable or progressive disease. ccfDNA level at follow-up visit after treatment initiation were compared with ccfDNA level before treatment initiation in (A) responders who had ≥ 5 CTCs or stable CTCs at follow-up visit after treatment initiation; (B) non-responders who had decreased CTCs or continuously < 5 CTCs at follow-up visits after treatment initiation, including (B1) CTCs that decreased but were still ≥ 5, (B2) CTCs that decreased from ≥ 5 to < 5, and (B3) CTCs that were continuously < 5.
Next, we plotted the dynamic changes of CTC and ccfDNA levels of individual patients in relationship to treatment response and survival. Supplementary Fig. 3A showed that in a 56-year-old patient with HER2 breast cancer, a significantly increased ccfDNA level (from 6.16 to 47.32) after the initiation of a new combined therapy of Capecitabine, Trastuzumab, and Zometa could possibly explain the observed progressive disease, although CTC number was continuously < 5. Similarly, in another patient with Luminal breast cancer whose CTC count decreased significantly (from 116 to 0) and then stayed below 5 after FEC therapy was initiated, a continuous increase in the ccfDNA level may account for the disease progression observed in this patient (Supplementary Fig. 3B). Hence, the longitudinal data from individual patients further indicated the complementary role of ccfDNA to CTC-based cancer prognostication analyses.
4. Discussion
Both CTC and total ccfDNA have been associated with the clinical outcomes of cancer patients [14, 18]. However, no prior study explored the prognostic values of the combined use of CTC and ccfDNA. In the current study, we found that CTC and ccfDNA were associated with outcomes of MBC patients individually and jointly. We also provided novel evidence that ccfDNA could provide additional prognostic value compared to CTC enumeration alone.
It is important to discern the differences between ccfDNA and ctDNA. The analysis of ctDNA is more amenable to the use of personalized treatment, especially targeted therapies, because it precisely measures the levels of mutant alleles derived from tumors. However, to date, only a few recurrently mutated genes have been identified in breast cancer, specifically TP53, PIK3CA, ESR1, and HER2, and not all of them are druggable [19, 41, 42]. In comparison, although the level of total ccfDNA is not completely tumor specific, they might reflect tumor-related pathological processes [3, 43], a notion that is supported by previous reports showing that ccfDNA levels are positively correlated with tumor severity [33, 44, 45]. Moreover, ccfDNA analysis circumvents the use of complex and expensive molecular barcode-based assays that are needed for ctDNA analysis, and thus can be more easily conducted in clinical settings.
In the current study, we isolated ccfDNAs from plasma samples and standardized pre-analytical variables, including time before isolation and isolation method that might affect ccfDNA levels [46, 47]. Total ccfDNA levels were quantified using two methods, direct quantification by Qubit, and indirect quantification by qRT-PCR of the ALU DNA repeats. The integrity of ALU sequence in ccfDNAs has been shown to be sensitive for the assessment of treatment response and disease progression in breast cancer patients [34, 36]. The results of these two methods are highly consistent (Supplementary Fig. 1), which further substantiates the quality of the ccfDNAs in our samples, and endorses the use of ccfDNA-related analyses in cancer prognostication. Furthermore, since Qubit is a highly reliable and inexpensive assay, it has the potential to supplement the more specialized and expensive assays for CTC and ctDNA analyses in predicting patient prognosis.
The prognostic value of CTC enumeration has been well documented in MBC patients [14]. A systematic review and meta-analysis also suggested an association between higher ccfDNA and worse survival in solid tumors [18]. In a recent study, Shaw et al. [19] reported that CTC count and ccfDNA level were significantly correlated and were both independent indicators for overall survival in MBC patients. However, as yet, no study has been reported to evaluate the prognostic value of the combined use of CTC and ccfDNA. In the present study, we found that either CTC or ccfDNA level was associated with clinical outcomes in MBC patients, independent of other common clinical covariates. Moreover, although a correlation was observed between CTC count and ccfDNA level (Fig. 2), the associations of CTC or ccfDNA with clinical outcomes remained significant after adjusting each other (Table 2), indicating their prognostic values are largely non-overlapping. Nonetheless, it is clear that CTC exhibited a higher prognostic value than ccfDNA, as evidenced by the higher and more significant HRs (PFS: HR = 2.34, P = 0.003 for CTC vs. HR = 1.64, P = 0.062 for ccfDNA; OS: HR = 6.67, P < 0.001 for CTC vs. HR = 2.73, P = 0.039 for ccfDNA, Table 2). Similar results were reported in Shaw’s study (OS: HR = 2.8, P = 0.005 for CTC vs. HR = 2.2, P = 0.03 for ccfDNA) [19]. Moreover, we observed an apparent joint effect between CTC and ccfDNA levels on patient outcomes. The joint effect was much more prominent on OS than PFS (Table 3), which seems to be reasonable because both CTC and ccfDNA provide more values that are prognostic of eventual survival but not predictive of response to specific treatments [31, 48–52]. Nonetheless, the multiplicative interaction between CTC and ccfDNA on OS was borderline significant, which could be due to the smaller number of deceased patients in each risk group and insufficient power in interaction analysis, and thus needs to be further confirmed.
CTC enumeration is the only FDA-approved liquid biopsy assay predicting MBC survival. Although the cut-off of ≥ 5 CTCs has been practically used for many years, discrepancies between CTC number and outcomes for individual patients were frequently observed. Therefore, additional markers are needed to more precisely stratify patients. In this study, we found that ccfDNA provided important prognostic information by further stratifying the survival of patients with different risks determined by CTC alone (Table 3). Moreover, longitudinal analysis indicated that ccfDNAs might at least partially explain the discrepancies between CTC changes and patient outcomes (Fig. 3 and Supplementary Fig. 3). These data further suggested the important prognostic value of ccfDNA independent of CTC. If validated, ccfDNA assessment holds the potential to complement the FDA-approved CTC enumeration to significantly increase the accuracy in predicting the prognosis of MBC patients in an efficient and inexpensive manner.
The major strengths of our study include the simultaneous measurement of matched CTC and ccfDNA, the potential of using Qubit assay to accurately and inexpensively measure total ccfDNA levels, the convincing data showing and the complementary role of ccfDNA in cancer prognostication over the FDA-approved CTC alone. One weakness of the study is the heterogeneity of clinical situations and the lack of relevant data on some important patient characteristics. Another weakness is the lack of data on baseline tumor markers, such as carcinoembryonic antigen, CA15.3, and CA27.29. Based on a subgroup analysis of CA15.3 obtained from 47 patients in our study, CTC and ccfDNA exhibited a much stronger association than CA15.3 (data not shown). Nonetheless, these results from the subgroup analysis should be validated in future studies with more complete data on tumor markers. The inability to detect the exact genomic mutations in ctDNA that may be used to guide therapy selection is another main limitation. A recent phase III clinical trial showed that switching therapies based on CTC enumeration alone did not prolong the survival of MBC patients, highlighting the potential importance of genomic dissection of CTCs [53]. Shaw et al. [19] recently reported that, in MBC patients with high CTC counts, individual CTCs had heterogeneous mutations and ccfDNA isolated from the same blood sample provided an accurate reflection of mutations seen in individual CTCs. This seminal study further demonstrated the significance of monitoring tumor genomic alterations in both CTC and ccfDNA. Other limitations of our study include the relatively small sample size, short follow-up time, longitudinal analyses based on data from limited number of patients, and lack of independent validations. Thus, prospectively designed, independent multi-site cohorts with large populations and longitudinally collected samples are warranted to confirm our findings.
In short, our study showed that CTC and ccfDNA levels are associated with clinical outcomes of MBC patients individually and jointly. ccfDNA could provide complementary prognostic information to CTC in an inexpensive and effective way. Future analyses combining CTC, ccfDNA, and ctDNA, with in-depth genomic characterizations, will be important for large-scale clinical applications of liquid biopsy in precision medicine.
Supplementary Material
Highlights:
CTC or ccfDNA level was associated with survivals of MBC patients, and their prognostic values were largely non-overlapping.
There was a joint effect of CTC and ccfDNA level on patient survival, especially overall survival.
The inconsistency between CTC level and outcome in some patients could possibly be explained by ccfDNA level.
Our study provided novel evidence that ccfDNA could add additional prognostic information to CTC-based prognostication, suggesting the application potential of combined CTC and ccfDNA liquid biopsy in the management of MBC patients.
Acknowledgments
Role of the funding source
This work was supported by National Cancer Institute Grant (CA207468), Thomas Jefferson University Cancer Center Support Grant (5P30CA056036–17) for the CTC Core Facility, Pennsylvania Department of Health Grant (SAP# 4100062221), and Jamie Lieberman Memorial Endowment Trust. The funding agencies were not involved in the design, conduct, analysis or interpretation of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its ?nal citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- [1].DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67(6):439–448. [DOI] [PubMed] [Google Scholar]
- [2].Schott AF, Barlow WE, Van Poznak CH, Hayes DF, Moinpour CM, Lew DL, et al. Phase II studies of two different schedules of dasatinib in bone metastasis predominant metastatic breast cancer: SWOG S0622. Breast Cancer Res Treat 2016;159(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14(9):531–548. [DOI] [PubMed] [Google Scholar]
- [4].Chen L, Bode AM, Dong Z. Circulating Tumor Cells: Moving Biological Insights into Detection. Theranostics 2017;7(10):2606–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ulz P, Heitzer E, Geigl JB, Speicher MR. Patient monitoring through liquid biopsies using circulating tumor DNA. Int J Cancer 2017;141(5):887–896. [DOI] [PubMed] [Google Scholar]
- [6].Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10(20):6897–904. [DOI] [PubMed] [Google Scholar]
- [7].Calabuig-Farinas S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer-which one will win? Transl Lung Cancer Res 2016;5(5):466–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tan CR, Zhou L, El-Deiry WS. Circulating Tumor Cells Versus Circulating Tumor DNA in Colorectal Cancer: Pros and Cons. Curr Colorectal Cancer Rep 2016;12(3):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang M, Topaloglu U, Petty WJ, Pagni M, Foley KL, Grant SC, et al. Circulating mutational portrait of cancer: manifestation of aggressive clonal events in both early and late stages. J Hematol Oncol 2017;10(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao H, Chen KZ, Hui BG, Zhang K, Yang F, Wang J. Role of circulating tumor DNA in the management of early-stage lung cancer. Thorac Cancer 2018. [DOI] [PMC free article] [PubMed]
- [11].Phallen J, Sausen M, Adleff V, Leal A, Hruban C, White J, et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med 2017;9(403). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6(224):224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N England J Med 2004;351(8):781–91. [DOI] [PubMed] [Google Scholar]
- [14].Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15(4):406–14. [DOI] [PubMed] [Google Scholar]
- [15].Heller G, McCormack R, Kheoh T, Molina A, Smith MR, Dreicer R, et al. Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison With Prostate-Specific Antigen Across Five Randomized Phase III Clinical Trials. J Clin Oncol 2018;36(6):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368(13):1199–209. [DOI] [PubMed] [Google Scholar]
- [17].O’Leary B, Hrebien S, Morden JP, Beaney M, Fribbens C, Huang X, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun 2018;9(1):896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ocana A, Diez-Gonzalez L, Garcia-Olmo DC, Templeton AJ, Vera-Badillo F, Jose Escribano M, et al. Circulating DNA and Survival in Solid Tumors. Cancer Epidemiol Biomarkers Prev 2016;25(2):399–406. [DOI] [PubMed] [Google Scholar]
- [19].Shaw JA, Guttery DS, Hills A, Fernandez-Garcia D, Page K, Rosales BM, et al. Mutation Analysis of Cell-Free DNA and Single Circulating Tumor Cells in Metastatic Breast Cancer Patients with High Circulating Tumor Cell Counts. Clin Cancer Res 2017;23(1):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001;61(4):1659–65. [PubMed] [Google Scholar]
- [21].Diaz LA, Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 2014;32(6):579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Friedrich MJ. Going With the Flow: The Promise and Challenge of Liquid Biopsies. JAMA 2017;318(12):1095–1097. [DOI] [PubMed] [Google Scholar]
- [23].Wang JF, Pu X, Zhang X, Chen K, Xi Y, Wang J, et al. Variants with a low allele frequency detected in genomic DNA affect the accuracy of mutation detection in cell-free DNA by next-generation sequencing. Cancer 2018;124(5):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kuderer NM, Burton KA, Blau S, Rose AL, Parker S, Lyman GH, et al. Comparison of 2 Commercially Available Next-Generation Sequencing Platforms in Oncology. JAMA Oncol 2017;3(7):996–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol 2018:JCO2017768671. [DOI] [PubMed]
- [26].Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. Arch Pathol Lab Med 2018. [DOI] [PubMed]
- [27].Sozzi G, Conte D, Leon M, Ciricione R, Roz L, Ratcliffe C, et al. Quantification of free circulating DNA as a diagnostic marker in lung cancer. J Clin Oncol 2003;21(21):3902–8. [DOI] [PubMed] [Google Scholar]
- [28].Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov 2014;4(6):650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Valpione S, Gremel G, Mundra P, Middlehurst P, Galvani E, Girotti MR, et al. Plasma total cell-free DNA (cfDNA) is a surrogate biomarker for tumour burden and a prognostic biomarker for survival in metastatic melanoma patients. Eur J Cancer 2018;88:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mehra N, Dolling D, Sumanasuriya S, Christova R, Pope L, Carreira S, et al. Plasma Cell-free DNA Concentration and Outcomes from Taxane Therapy in Metastatic Castration-resistant Prostate Cancer from Two Phase III Trials (FIRSTANA and PROSELICA). Eur Urol 2018. [DOI] [PMC free article] [PubMed]
- [31].Hyun MH, Sung JS, Kang EJ, Choi YJ, Park KH, Shin SW, et al. Quantification of circulating cell-free DNA to predict patient survival in non-small-cell lung cancer. Oncotarget 2017;8(55):94417–94430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hsieh CC, Hsu HS, Chang SC, Chen YJ. Circulating Cell-Free DNA Levels Could Predict Oncological Outcomes of Patients Undergoing Esophagectomy for Esophageal Squamous Cell Carcinoma. Int J Mol Sci 2016;17(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bedin C, Enzo MV, Del Bianco P, Pucciarelli S, Nitti D, Agostini M. Diagnostic and prognostic role of cell-free DNA testing for colorectal cancer patients. Int J Cancer 2017;140(8):1888–1898. [DOI] [PubMed] [Google Scholar]
- [34].Umetani N, Giuliano AE, Hiramatsu SH, Amersi F, Nakagawa T, Martino S, et al. Prediction of breast tumor progression by integrity of free circulating DNA in serum. J Clin Oncol 2006;24(26):4270–6. [DOI] [PubMed] [Google Scholar]
- [35].Deligezer U, Eralp Y, Akisik EZ, Akisik EE, Saip P, Topuz E, et al. Effect of adjuvant chemotherapy on integrity of free serum DNA in patients with breast cancer. Ann N Y Acad Sci 2008;1137:175–9. [DOI] [PubMed] [Google Scholar]
- [36].Lehner J, Stotzer OJ, Fersching DM, Nagel D, Holdenrieder S. Plasma DNA integrity indicates response to neoadjuvant chemotherapy in patients with locally confined breast cancer. Int J Clin Pharmacol Ther 2013;51(1):59–62. [DOI] [PubMed] [Google Scholar]
- [37].Park JL, Kim HJ, Choi BY, Lee HC, Jang HR, Song KS, et al. Quantitative analysis of cell-free DNA in the plasma of gastric cancer patients. Oncol Lett 2012;3(4):921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- [39].Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst 2009;101(1):61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Punnoose EA, Atwal S, Liu W, Raja R, Fine BM, Hughes BG, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012;18(8):2391–401. [DOI] [PubMed] [Google Scholar]
- [41].Ng CK, Schultheis AM, Bidard FC, Weigelt B, Reis-Filho JS. Breast cancer genomics from microarrays to massively parallel sequencing: paradigms and new insights. J Natl Cancer Inst 2015;107(5). [DOI] [PubMed] [Google Scholar]
- [42].Lehmann BD, Pietenpol JA. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 2015;24 Suppl 2:S36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11(6):426–37. [DOI] [PubMed] [Google Scholar]
- [44].Kumari S, Tewari S, Husain N, Agarwal A, Pandey A, Singhal A, et al. Quantification of Circulating Free DNA as a Diagnostic Marker in Gall Bladder Cancer. Pathol Oncol Res 2017;23(1):91–97. [DOI] [PubMed] [Google Scholar]
- [45].Hashad D, Sorour A, Ghazal A, Talaat I. Free circulating tumor DNA as a diagnostic marker for breast cancer. J Clin Lab Anal 2012;26(6):467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bronkhorst AJ, Aucamp J, Pretorius PJ. Cell-free DNA: Preanalytical variables. Clin Chim Acta 2015;450:243–53. [DOI] [PubMed] [Google Scholar]
- [47].El Messaoudi S, Rolet F, Mouliere F, Thierry AR. Circulating cell free DNA: Preanalytical considerations. Clin Chim Acta 2013;424:222–30. [DOI] [PubMed] [Google Scholar]
- [48].Massard C, Borget I, Farace F, Aspeslagh S, Le Deley MC, Le Tourneau C, et al. RECIST response and variation of circulating tumour cells in phase 1 trials: A prospective multicentric study. Eur J Cancer 2017;83:185–193. [DOI] [PubMed] [Google Scholar]
- [49].Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 2006;12(21):6403–9. [DOI] [PubMed] [Google Scholar]
- [50].Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26(19):3213–21. [DOI] [PubMed] [Google Scholar]
- [51].Lee YJ, Yoon KA, Han JY, Kim HT, Yun T, Lee GK, et al. Circulating cell-free DNA in plasma of never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. Clin Cancer Res 2011;17(15):5179–87. [DOI] [PubMed] [Google Scholar]
- [52].Tissot C, Toffart AC, Villar S, Souquet PJ, Merle P, Moro-Sibilot D, et al. Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur Respir J 2015;46(6):1773–80. [DOI] [PubMed] [Google Scholar]
- [53].Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 2014;32(31):3483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.