ABSTRACT
Necrotic enteritis toxin B (NetB)-producing Clostridium perfringens (CP) type A is the etiological agent of necrotic enteritis (NE) – an economically significant disease in broiler chickens. Understanding the immune response to CP infection in broiler chickens is becoming important to develop effective vaccines against NE. An experiment was conducted to determine the expression levels of selected cytokine genes in the intestine and cecal tonsil of CP-challenged broiler chickens. In a floor-pen housing, broiler chickens were randomly assigned to the following treatment groups: 1) bacitracin methylene disalicylate (BMD)-free control diet with no CP challenge (CX), 2) BMD-supplemented diet with no CP challenge (CM), 3) BMD-free control diet with CP challenge (PCX), or 4) BMD-supplemented diet with CP challenge (PCM). The establishment of CP infection was confirmed, with the treatment groups exposed to CP having a 1.5 to 2-fold higher CP levels (P < 0.05) compared to the non-exposed groups. On day 1 and 7 post-challenge, jejunal segments and cecal tonsils were collected from experimental chickens for quantitative real-time RT-PCR analysis to determine the expression levels of interleukin (IL)-1β, interferon-γ (IFN-γ), IL-2, IL-13, IL-17, IL-10, and transforming growth factor (TGF)-β genes. Levels of antibodies to CP recombinant proteins were also determined in the plasma of experimental chickens. Results indicated that on day 7 post-challenge, IL-1β (proinflammatory cytokine), IL-13 (Th2 cytokine), and IL-17 (Th17 cytokine) were upregulated (P < 0.05) in CP-challenged PCX and PCM treatments, compared to the unchallenged (control) CX and CM treatments. A reverse trend was observed for TGF-β (anti-inflammatory cytokine), while no change was observed in IFN-γ (Th1 cytokine). Levels of plasma antibodies (IgY) to CP recombinant proteins were higher in CP-challenged treatments (PCX and PCM; P < 0.05), compared to their corresponding controls (CX and CM). It was concluded that CP infection induced inflammatory response in the intestine of broiler chickens, and the mechanisms of inflammation are probably mediated via Th2 and Th17 cells.
Keywords: Clostridium perfringens, broiler chicken, intestine, cytokines, inflammation
INTRODUCTION
Necrotic enteritis toxin B (NetB)-producing Clostridium perfringens (CP) type A is the major etiologic agent for necrotic enteritis (NE) in broiler chickens (Cooper et al., 2013; Zhou et al., 2017). CP is a Gram-positive, spore-forming, anaerobic bacterium that is widely distributed in nature, and commonly found in soil, dust, animal production environments such as litter and contaminated feed, and in the gastrointestinal tract of animals and humans as a member of the normal (native) microbiota (Timbermont et al., 2009; Miller et al., 2010). The intestine of healthy birds typically contains approximately 104 CFU of CP/g of digesta (McDevitt et al., 2006). However, predisposing factors such as pre-existing damage to the intestinal epithelium by coccidia (Eimeria spp.), infectious bursal disease virus, high dietary levels of certain cereals and fish meal, disturbance to the normal intestinal flora, overcrowding, or a variety of management and climatic conditions (McDevitt et al., 2006; Wu et al., 2014) may favor proliferation of CP to reach a critical concentration of about 107 to 109 CFU/g of digesta (Williams, 2005). At this concentration, CP produces toxins that induce mucosal damage in the small intestine and ceca of chickens and turkeys, and results in clinical NE (Van Immerseel et al., 2004, 2009).
Host–pathogen interaction in NE is complex and involves different components of the host immune system (Oh and Lillehoj, 2016). In the chicken intestine, pathogenesis of CP begins with the bacteria penetrating the mucus barrier on the epithelial surface, followed by binding and attachment to epithelial cells. The mechanism by which bacterial pathogens (such as CP) bind to intestinal epithelial cells begins with the recognition and binding of pathogen-associated molecular patterns (PAMPs) by highly conserved pathogen recognition receptors, of which Toll-like receptors (TLRs) are the best characterized (Oh and Lillehoj, 2016). It has been established that chickens have at least 10 TLRs comprising of cell surface-located TLR1a, TLR1b, TLR2a, TLR2b, TLR4, TLR5, TLR15, and intracellular endosome-located TLR3, TLR7, and TLR21 (Chen et al., 2013). In particular, intestinal TLR2 is known to recognize peptidoglycans in Gram-positive bacteria such as CP, and its gene was upregulated in CP-challenged broiler chickens (Du et al., 2016). Interactions between bacterial PAMPs and TLRs trigger a cascade of signaling events that result in the activation of dendritic cells and cellular responses, such as the differentiation of naive T helper (Th) cells into mature effector Th1, Th2, Th17, and Treg cells (St. Paul et al., 2013; Oh and Lillehoj, 2016). These signaling events culminate in the secretion of various cytokines, such as proinflammatory interleukin (IL)-1 by naïve Th cells, interferon-γ (IFN- γ) by Th1 cells, IL-13 by Th2 cells, IL-17 by Th17 cells, and transforming growth factor (TGF)-β and IL-10 by Treg cells (Cosmi et al., 2014; Narsale et al., 2018). Cytokines are effector molecules that communicate information between cells of the immune system, and the composition of cytokines in the microenvironment of an infection site determines the nature of the immune response affected.
Although some studies have studied the response of various cytokines to CP infection or NE disease in broiler chickens (Yitbarek et al., 2012; Alizadeh et al., 2016; Du et al., 2016), robust data are needed especially since the induction of CP infection and NE disease is achieved through various challenge methods (Prescott et al., 2016; Van Waeyenberghe et al., 2016). It has been estimated that annually, NE costs the world poultry industry about $6 billion (US), thereby making it an economically important disease (Wade and Keyburn, 2015). Better understanding of the intestinal immune response to CP infection in commercial broiler chickens is important to enhance the design of more effective vaccines against NE. Our results clearly show that various cytokines and their associated immune function(s) are affected by CP infection. Plasma antibody levels to CP were also affected.
MATERIALS AND METHODS
All the procedures used in this study were approved by the Auburn University Institutional Animal Care and Use Committee.
Experimental Animals, Treatments, and Monitoring Chick Growth Performance
Broiler chicks used in this experiment were also utilized for the intestinal microbiota study reported by Fasina et al. (2016). Briefly, day-old male broiler chicks (400; Cobb x Cobb) were obtained from a commercial hatchery and subjected to a floor-pen trial at the Auburn University Poultry Research and Teaching Farm. Chicks were weighed, and randomly assigned to 4 treatments in a completely randomized design. Treatment 1 (CX) consisted of chicks not challenged with CP and fed an unmedicated corn-soybean meal (SBM) basal diet. Treatment 2 (CM) consisted of chicks not challenged with CP and fed a corn-SBM basal diet, with bacitracin methylene disalicylate (BMD) supplemented at 0.055 g/kg diet. Treatments 3 (PCX) and 4 (PCM) consisted of chickens challenged with CP on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks in treatments 1 and 2, respectively. The level of BMD antibiotic used in the CM and PCM diets was subtherapeutic and served as a prophylactic. Each treatment consisted of 4 replicate pens with 25 chickens per pen. The floor of each pen was covered with fresh pine shavings that served as litter. Experimental diets were formulated to meet the recommendations of the National Research Council (1994), and chickens were allowed ad libitum access to feed and water throughout the experiment. Duration of the experiment was 42 d. Growth performance of chickens was monitored throughout the experiment by recording body weight and feed conversion ratio on day 21 and 42.
CP Challenge and Confirmation of CP Infection
A cocktail of 3 CP (type A) strains obtained from commercial broiler chicken flocks were used to prepare a composite CP inoculum daily on day 14, 15, and 16 of the experiment, as described by Fasina et al. (2016). The concentration of CP cells in the inoculum prepared each day was estimated spectrophotometrically at 600 nm with the aid of a standard curve. Actual CP concentration in the inoculum was confirmed by colony-plating on tryptose sulfite cycloserine agar (Oxoid Ltd, Hampshire, UK) plates, incubating the plates anaerobically at 37°C for 18 h, and counting the numbers of black presumptive CP colonies. The concentration of CP cells in the inoculum prepared on day 14, 15, and 16 was 8.6 × 108, 8.9 × 108, and 9.1 × 108 CFU/mL, respectively. Furthermore, the black presumptive colonies were confirmed as CP by the Rapid ID 32 A test kit (BioMerieux, Durham, NC). On each day of CP challenge (day 14, 15, and 16), a round-tipped animal feeding needle (15 G, 78 mm; Solomon Scientific, PA) attached to a repeating syringe (Popper & Sons, Inc., New Hyde Park, NY) was used to orally deliver 3 mL of the freshly prepared composite CP inoculum into the crop of each chick in the PCX and PCM treatments. Chickens in the control CX and CM treatments were mock-challenged with freshly prepared sterile fluid thioglycolate broth (FTG, Oxoid Ltd).
The establishment of CP infection was confirmed by estimating the intestinal CP concentration on day 13 (day 1 pre-challenge), 17 (day 1 post-challenge), 23 (day 7 post-challenge), and 37 (day 21 post-challenge) of experiment. On each day, 2 chicks were randomly taken from each pen (totaling 8 chicks per treatment) and euthanized by CO2 asphyxiation. Thereafter, the intestine of each chick was aseptically excised, and a 15-cm long segment from the mid-jejunum to the mid-ileum was removed, placed in a Whirl-Pak sample bag (Nasco, Fort Atkinson, WI), and kept on ice until time for transportation to the laboratory. From each sample, approximately 5 g of intestinal digesta was weighed and transferred into 10 mL of anaerobic FTG broth in a sterile filter-containing Whirl-Pak bag (Nasco, Fort Atkinson, WI). The Whirl-Pak filter bag was stomached for 30 s (Stomacher 400 Circulator, Seward Limited, London, UK), after which 1 mL of the resulting filtrate was transferred into a test tube containing 9 mL of FTG broth that was subjected to a 10-fold serial dilution. The dilutions for each sample were spiral-plated on tryptose sulfite cycloserine agar, and then incubated anaerobically at 37°C for 24 h. The number of characteristic black presumptive CP colonies was then counted for each sample. The CP concentration was expressed as log10 CFU/g intestinal content. Results obtained affirmed that CP infection was successfully established in PCX and PCM chicks (Table 1).
Table 1.
Intestinal concentration of C. perfringens in broiler chicks.1
| Log10 CFU of C. perfringens/g intestinal contents | ||||
|---|---|---|---|---|
| Treatments2 | Baseline3 | Day 1 PC | Day 7 PC | Day 21 PC |
| CX | ND | 1.26 ± 0.01b | 2.03 ± 0.29c | 3.09 ± 0.77a,b |
| CM | ND | 1.26 ± 0.01b | 1.43 ± 0.16c | 1.47 ± 0.19b |
| PCX | ND | 4.19 ± 0.69a | 4.37 ± 0.38a | 3.89 ± 0.53a |
| PCM | ND | 2.85 ± 0.75a,b | 3.45 ± 0.24b | 2.70 ± 0.33a,b |
| Sources of variation | Probability | |||
| Dietary treatment | — | NS | 0.0252 | 0.0170 |
| CP challenge | — | 0.0007 | <0.0001 | NS |
| Diet x CP challenge | — | NS | NS | NS |
a-cMean values bearing different superscript letters within a column are significantly different (P < 0.05).
1Adapted from Fasina et al. (2016).
2Treatment 1 (CX) consisted of unchallenged chicks fed corn-soybean meal (SBM) basal without antibiotic supplementation; Treatment 2 (CM) consisted of unchallenged chicks fed corn-SBM basal into which bacitracin methylene disalicylate antibiotic was supplemented at 0.055 g/kg diet; treatments 3 (PCX) and 4 (PCM) consisted of chicks challenged with C. perfringens on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks treatments 1 and 2, respectively.
3Baseline intestinal C. perfringens concentration determined at 1 d pre-challenge (i.e., day 13 of experiment). ND = not detectable, with the detection limit of the recovery assay being ≥ 0.10 Log10 CFU of C. perfringens/g intestinal contents.
PC = post-challenge; CP = Clostridium perfringens; NS = not significant.
Collection of Intestinal Tissue and Blood for Downstream Analysis, and Lesion Scoring
Intestinal tissue sections were collected on day 1 and 7 post-CP challenge for the determination of expression levels of proinflammatory, Th1, Th2, and cytokine genes. On each day, 2 chickens were randomly taken from each pen (totaling 8 chickens per treatment) and euthanized by CO2 asphyxiation. Thereafter, the small intestine of each chicken was aseptically excised and placed on ice. The mid-portion of the jejunum (from the pancreo-biliary ducts to the yolk stalk) was cut, rinsed in cold saline (0.9% NaCl) to remove gut contents, placed in a DNase- and RNase-free cryovial, snap-frozen in liquid N2, and kept at –70°C until time for analysis. The cecal tonsil was collected only on day 7 post-CP challenge, and frozen as previously described for jejunal segments. For lesion scoring, the duodenum of each chicken sampled was scored on day 1 post-challenge. Assessing the presence and severity of necrotic lesions in the presence of CP infection indicates if a subclinical or clinical NE disease has been established in the birds (McDevitt et al., 2006). Accordingly, the presence of macroscopic lesions in the duodenum was assessed using a scale described by Prescott et al. (1978). Lesions were scored on a 0 to 4 scale, where 0 = no apparent lesions, 1 = thin, friable small intestine, 2 = focal necrosis, ulceration, or both, 3 = patchy necrosis, and 4 = severe, extensive mucosal necrosis.
On day 14 post-CP challenge, 2 chickens were randomly selected from each pen (totaling 8 chickens/treatment) and bled. Specifically, a 23-G needle (1 inch long) attached to a 5 mL syringe pre-coated with ethylenediaminetetraacetic acid (EDTA) was used to collect 3 mL of blood from the brachial vein of each chick. Thereafter, the needle was removed, and the syringe was inverted 3 times to ensure adequate mixing of blood with the EDTA anticoagulant. Each blood sample was then transferred into an appropriately labeled 15 mL plastic centrifuge tube and kept on ice until all birds were bled. Tubes were then transported to the laboratory and centrifuged at 4,000 × g for 10 min. The resulting supernatant, which is the plasma, was decanted into appropriately labeled 1.5 mL microcentrifuge tubes and stored at −70°C until time to determine CP-specific antibody levels.
RNA Extraction and cDNA Synthesis for Gene Expression Analyses
Jejunal tissue and cecal tonsils (30 μg each) were homogenized with a MiniBeadbeater-96TM (Biospec Products, Inc., Bartlesville, OK). Total RNA was isolated from homogenates using RNeasy mini kit (Qiagen, Waltham, MA) following the manufacturer's protocol, and then eluted in 100 μL RNase-free water. To determine RNA concentration in each sample, a 1:100 dilution of the eluted RNA in 10 mM Tris-HCl (pH 7.5) was prepared and measured spectrophotometrically at 260 nm. RNA purity was determined by measuring the ratio of absorbance readings of RNA samples at 260 and 280 nm (i.e., A260/A280). The A260/A280 ratio for all RNA samples was between 1.8 and 2.0, indicating that the RNA samples are pure. Remaining RNA for each sample were then stored at –70°C until time for cDNA synthesis.
Synthesis of cDNA was done as previously described by Hong et al. (2006). Briefly, 5 μg of each RNA sample was treated with 1.0 unit of DNase I and 1.0 μL of 10X reaction buffer (Sigma), and then incubated for 15 min at room temperature. Stop solution (1.0 μL) was then added to inactivate DNase I, and the mixture was heated at 70°C for 10 min. RNA was reverse transcribed using StrataScript first-strand synthesis system (Stratagene, La Jolla, CA) according to the manufacturer's recommendations. For each sample, 5.0 μg of RNA was combined with 10X first strand buffer, 1.0 μL of oligo(dT) primer (5 μg/μL), 0.8 μL of dNTP mix (25 mM of each dNTP), and RNase-free water to total volume 19 μL. The mixture was incubated at 65°C for 5 min, cooled to room temperature, and 50 units of StrataScript reverse transcriptase was added. The mixture was then incubated at 42°C for 1 h, and the reaction stopped by heating at 70°C for 5 min.
Quantitative Real-Time RT-PCR
The oligonucleotide primers used for quantitative RT-PCR analyses of chicken cytokines and GAPDH housekeeping gene are listed in Table 2. Briefly, the Mx3000P system and Brilliant SYBR Green QPCR master mix (Stratagene) were used in the amplification and detection of equivalent amounts of total RNA from each sample as described by Hong et al. (2006). Standard curves were generated using log10 diluted standard RNA, and levels of individual transcripts were normalized to those of GAPDH analyzed by the Q-gene program (Muller et al., 2002). Each analysis was performed in triplicate. To normalize RNA levels between samples within an experiment, the mean threshold cycle (Ct) values for the amplification products were calculated by pooling values from all samples in that experiment.
Table 2.
Sequence of primers used for real-time quantitative RT-PCR.1
| Primer sequence (5΄-3΄) | ||||
|---|---|---|---|---|
| Target gene | Forward primer | Reverse primer | PCR product size (base pairs) | Accession number |
| GAPDH | 5΄-GGTGGTGCTAAGCGTGTTAT-3΄ | 5΄-ACCTCTGTCATCTCTCCACA-3΄ | 264 | K01458 |
| IL-1β | 5΄-TGGGCATCAAGGGCTACA-3΄ | 5΄-TCGGGTTGGTTGGTGATG-3΄ | 244 | Y15006 |
| IL-17 | 5΄-CTCCGATCCCTTATTCTCCTC-3΄ | 5΄-AAGCGGTTGTGGTCCTCAT-3΄ | 292 | AJ493595 |
| IFN-γ | 5΄-AGCTGACGGTGGACCTATTATT-3΄ | 5΄-GGCTTTGCGCTGGATTC-3΄ | 259 | Y07922 |
| IL-2 | 5΄-TCTGGGACCACTGTATGCTCT-3΄ | 5΄-ACACCAGTGGGAAACAGTATCA-3΄ | 256 | AF000631 |
| IL-13 | 5΄-CCAGGGCATCCAGAAGC-3΄ | 5΄-CAGTGCCGGCAAGAAGTT-3΄ | 256 | AJ621735 |
| IL-10 | 5΄-CGGGAGCTGAGGGTGAA-3΄ | 5΄-GTGAAGAAGCGGTGACAGC-3΄ | 272 | AJ621614 |
| TGF-β4 | 5΄-CGGGACGGATGAGAAGAAC-3΄ | 5΄-CGGCCCACGTAGTAAATGAT-3΄ | 258 | M31160 |
Enzyme-Linked immunosorbent Assay (ELISA)
Frozen plasma samples were thawed on ice and subjected to an enzyme-linked immunosorbent assay (ELISA) procedure for the determination of antibody levels to recombinant NetB CP protein as described by Lee et al. (2011). Briefly, flat-bottom, 96-well microtiter plates (Corning Costar, Corning, NY) were coated overnight at 4°C with 2.5 μg/well of purified recombinant NetB toxin protein in 0.1 M carbonate buffer, pH 9.6. The plates were washed twice with PBS containing 0.05% Tween 20 (PBS-T), blocked for 1 h at 22°C with 100 μL/well of PBS containing 1% bovine serum albumin, washed with PBS-T, and incubated for 2 h at 22°C with 100 μL/well of appropriately diluted plasma samples. Thereafter, plates were washed with PBS-T, incubated for 2 h at 22°C with peroxidase-conjugated goat anti-chicken IgY secondary antibody (1:1000; Sigma), and for 10 min with 0.01% tetramethylbenzidine substrate (Sigma) in 0.05 M phosphate-citrate buffer, pH 5.0. Bound antibodies were detected by measuring optical density at 450 nm (OD450) using a microplate reader (Bio-Rad).
Statistical Analyses
All quantitative RT-PCR cytokine gene expression data and antibody ELISA data were subjected to 1-way ANOVA using the general linear models (GLM) procedure of SAS (SAS Inc., 2004). Significant differences among means were determined using the Duncan option of the GLM procedure as a post hoc test, and statements of statistical significance were based upon P < 0.05. Data are presented as means ± SEM.
RESULTS
There were no differences (P > 0.05) in the body weight and feed conversion ratio of birds in all treatments as previously reported by Fasina et al. (2016). However, the establishment of CP infection in experimental chickens was confirmed, with the treatment groups exposed to CP having a 1.5 to 2-fold higher CP levels (P < 0.05) compared to the non-exposed groups (Table 1; Fasina et al., 2016). In addition, macroscopic lesion scores for treatments exposed to CP challenge were numerically higher (PCX = 1.52; PCM = 1.25) compared to their corresponding controls (CX = 1.22; CM = 0.90), and these differences approached significance (P = 0.0791). Presence of CP infection was further corroborated by the higher mortality observed in the treatment groups exposed to CP (P = 0.0003; PCX = 4.25%; PCM = 5.50), compared to their corresponding controls (CX = 1.25%; CM = 1.25%). Total mortality in this experiment was 12.25%.
Intestinal Cytokine Gene Expression
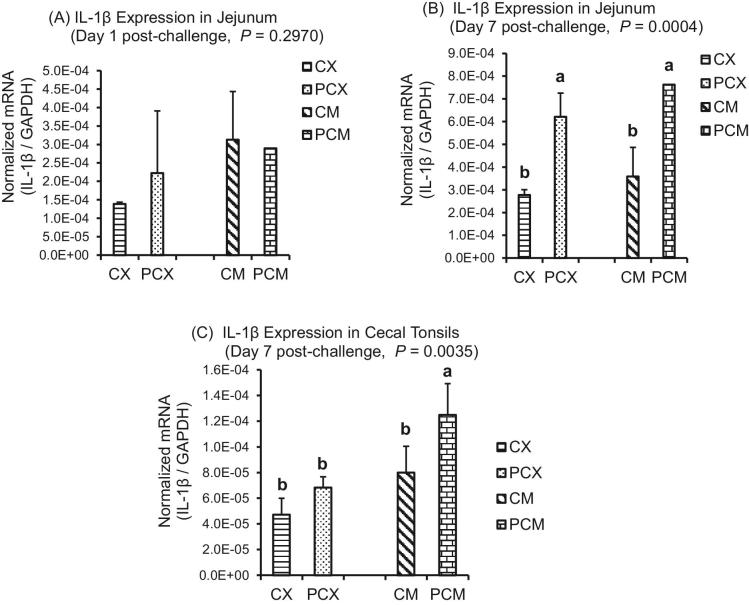
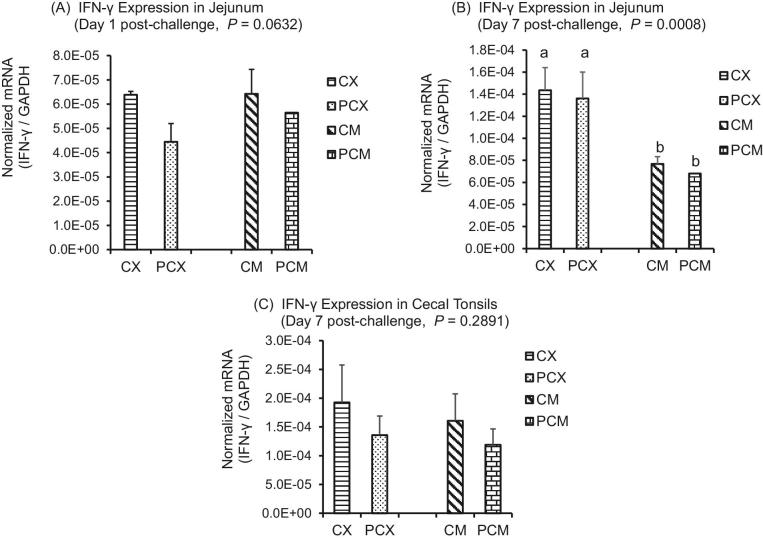
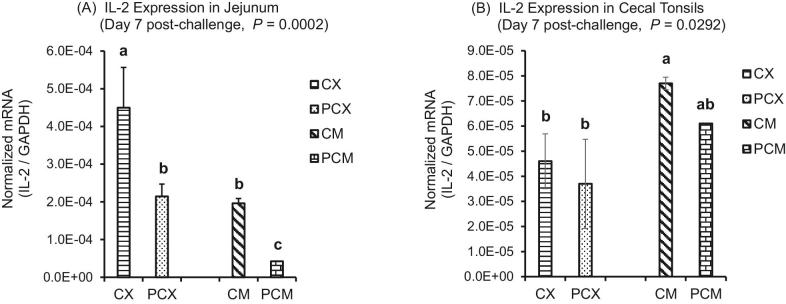
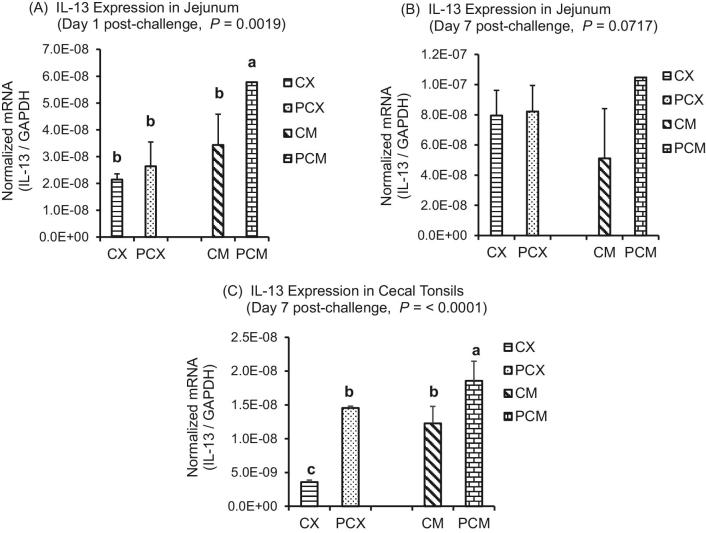
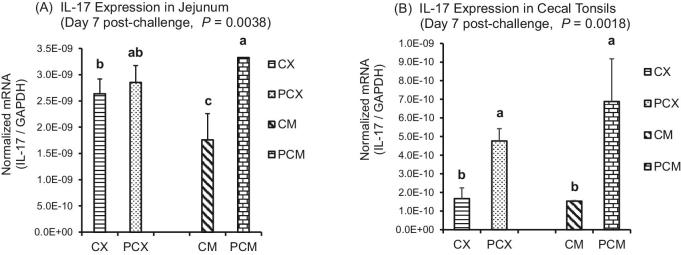
The expression levels of genes of proinflammatory IL-1β, Th1 cytokines (IFN-γ; IL-2), Th2 cytokines (IL-13), Th17 cytokine (IL-17), and Treg cytokines (IL-10 and TGF-β) are presented in Figures 1 to 7. On day 1 post-challenge, IL-1β gene was not upregulated in the jejunum of CP-challenged PCX and PCM treatments (Figure 1A; P > 0.05). However, by day 7 post-challenge, expression of IL-1β gene was upregulated in the jejunum of PCM treatment (P < 0.05), compared to the control (unchallenged) CX and CM treatments (Figure 1B and C). Expression of IFN-γ gene approached downregulation (P = 0.0632) on day 1 post-challenge in the jejunum of challenged PCX and PCM treatments (Figure 2A). However, this apparent downregulation was not sustained through day 7 post-challenge in the jejunum (Figure 2B) and cecal tonsils (Figure 2C). Another interesting observation on day 7 was that the unmedicated treatments (CX and PCX) had higher expression levels of IFN-γ gene (P < 0.05) compared to the medicated BMD-treated CM and PCM (Figure 2B). IL-2 was downregulated in the jejunum of PCX and PCM treatments on day 7 post-challenge, compared to their corresponding control treatments CX and CM (P < 0.05; Figure 3A). A similar trend was observed in the cecal tonsils (Figure 3B). In the jejunum, IL-13 was upregulated in the challenged PCM treatment on day 1 post-challenge, compared to the control CX and CM treatments (P < 0.05; Figure 4A), and this upregulation diminished by day 7 post-challenge in the jejunum (Figure 4B), but was sustained in the cecal tonsil (Figure 4C). IL-17 gene was significantly upregulated (P < 0.05) in the jejunum and cecal tonsils of challenged PCX and PCM treatments on day 7 post-challenge (Figure 5A and B).
Figure 1.
Expression of IL-1β gene in the intestine and cecal tonsils of Clostridium perfringens (CP)-challenged broiler chickens. Treatment 1 (CX) consisted of unchallenged chicks fed corn-soybean meal (SBM) basal only; treatment 2 (CM) consisted of unchallenged chicks fed corn-SBM basal into which bacitracin methylene disalicylate antibiotic was supplemented at 0.055 g/kg diet; Treatments 3 (PCX) and 4 (PCM) consisted of chicks challenged with CP on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks treatments 1 and 2, respectively. Bars with different letters within each figure differ significantly (P < 0.05).
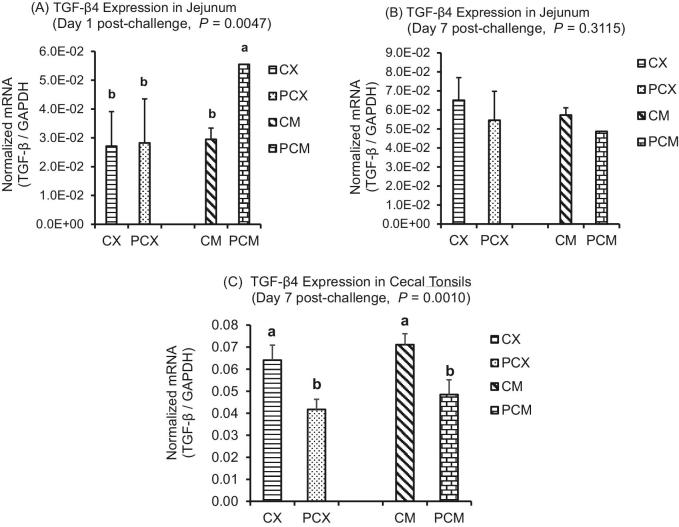
Figure 7.
Expression of TGF-β4 gene in the intestine and cecal tonsils of Clostridium perfringens (CP)-challenged broiler chickens. Treatment 1 (CX) consisted of unchallenged chicks fed corn-soybean meal (SBM) basal only; treatment 2 (CM) consisted of unchallenged chicks fed corn-SBM basal into which bacitracin methylene disalicylate antibiotic was supplemented at 0.055 g/kg diet; treatments 3 (PCX) and 4 (PCM) consisted of chicks challenged with CP on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks treatments 1 and 2, respectively. Bars with different letters within each figure differ significantly (P < 0.05).
Figure 2.
Expression of IFN-γ gene in the intestine and cecal tonsils of Clostridium perfringens (CP)-challenged broiler chickens. Treatment 1 (CX) consisted of unchallenged chicks fed corn-soybean meal (SBM) basal only; treatment 2 (CM) consisted of unchallenged chicks fed corn-SBM basal into which bacitracin methylene disalicylate antibiotic was supplemented at 0.055 g/kg diet; treatments 3 (PCX) and 4 (PCM) consisted of chicks challenged with CP on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks treatments 1 and 2, respectively. Bars with different letters within each figure differ significantly (P < 0.05).
Figure 3.
Expression of IL-2 gene in the intestine and cecal tonsils of Clostridium perfringens (CP)-challenged broiler chickens. Treatment 1 (CX) consisted of unchallenged chicks fed corn-soybean meal (SBM) basal only; treatment 2 (CM) consisted of unchallenged chicks fed corn-SBM basal into which bacitracin methylene disalicylate antibiotic was supplemented at 0.055 g/kg diet; treatments 3 (PCX) and 4 (PCM) consisted of chicks challenged with CP on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks treatments 1 and 2, respectively. Bars with different letters within each figure differ significantly (P < 0.05).
Figure 4.
Expression of IL-13 gene in the intestine and cecal tonsils of Clostridium perfringens (CP)-challenged broiler chickens. Treatment 1 (CX) consisted of unchallenged chicks fed corn-soybean meal (SBM) basal only; treatment 2 (CM) consisted of unchallenged chicks fed corn-SBM basal into which bacitracin methylene disalicylate antibiotic was supplemented at 0.055 g/kg diet; treatments 3 (PCX) and 4 (PCM) consisted of chicks challenged with CP on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks treatments 1 and 2, respectively. Bars with different letters within each figure differ significantly (P < 0.05).
Figure 5.
Expression of IL-17 gene in the intestine and cecal tonsils of Clostridium perfringens (CP)-challenged broiler chickens. Treatment 1 (CX) consisted of unchallenged chicks fed corn-soybean meal (SBM) basal only; treatment 2 (CM) consisted of unchallenged chicks fed corn-SBM basal into which bacitracin methylene disalicylate antibiotic was supplemented at 0.055 g/kg diet; treatments 3 (PCX) and 4 (PCM) consisted of chicks challenged with CP on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks treatments 1 and 2, respectively. Bars with different letters within each figure differ significantly (P < 0.05).
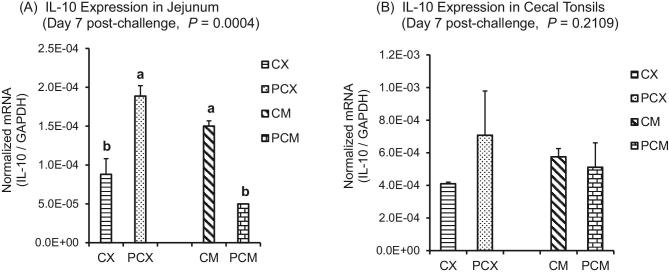
For Treg cytokines, there was no consistent trend in the expression level of IL-10 between the non-medicated (CX and PCX) and medicated treatments (CM and PCM). Specifically, IL-10 was upregulated (P < 0.05) in the jejunum of PCX compared to CX, but was downregulated (P < 0.05) in PCM compared to CM (Figure 6A). A similar trend was observed for cecal tonsils, except that the differences in IL-10 expression between treatments were not significant (P > 0.05; Figure 6B). Among the medicated treatments, TGF-β4 was significantly upregulated (P < 0.05) in the jejunum of PCM treatment compared to CM (Figure 7A), while TGF-β4 expression level was similar for the unmedicated CX and PCX at day 1 post-challenge. However, by day 7 post-challenge, the expression levels of TGF-β4 gene in both PCX and PCM treatments were consistently downregulated in the jejunum (Figure 7B; P > 0.05) and cecal tonsils (Figure 7C; P < 0.05) compared to their corresponding controls.
Figure 6.
Expression of IL-10 gene in the intestine and cecal tonsils of Clostridium perfringens (CP)-challenged broiler chickens. Treatment 1 (CX) consisted of unchallenged chicks fed corn-soybean meal (SBM) basal only; treatment 2 (CM) consisted of unchallenged chicks fed corn-SBM basal into which bacitracin methylene disalicylate antibiotic was supplemented at 0.055 g/kg diet; treatments 3 (PCX) and 4 (PCM) consisted of chicks challenged with CP on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks treatments 1 and 2, respectively. Bars with different letters within each figure differ significantly (P < 0.05).
The expression of most of the cytokine genes evaluated in this study followed a similar trend for both BMD-free (CX and CPX) and BMD-supplemented (CM and PCM) treatments. In contrast, the expression of IFN-γ in the jejunum was higher (P < 0.05) for the BMD-free treatments on day 7 post-challenge, compared to the BMD-supplemented treatments. The reverse was observed for IL-2 in the cecal tonsils. Specifically, the expression level of IL-2 in the cecal tonsils was lower (P < 0.05) for BMD-free treatments compared to BMD-supplemented treatment.
In summary, the results of cytokine gene expression in this study indicated that CP-induced subclinical NE activated inflammatory immune response via Th2 and Th17 cytokines. This is because, proinflammatory IL-1β was upregulated, IFN-γ (Th1 cytokine) was not affected, IL-2 (Th1 cytokine) was downregulated, IL-13 (Th2 cytokine) was upregulated, IL-17 (Th17 cytokine) was upregulated, IL-10 (Treg cytokine) response was pleiotropic, and anti-inflammatory TGF-β4 was downregulated. In addition, humoral adaptive immune response indicated that higher levels of antibodies to CP recombinant proteins were detected in the plasma of challenged chicks (PCX and PMX; P < 0.05) compared to the control chicks in CX and CM (Figure 8).
Figure 8.
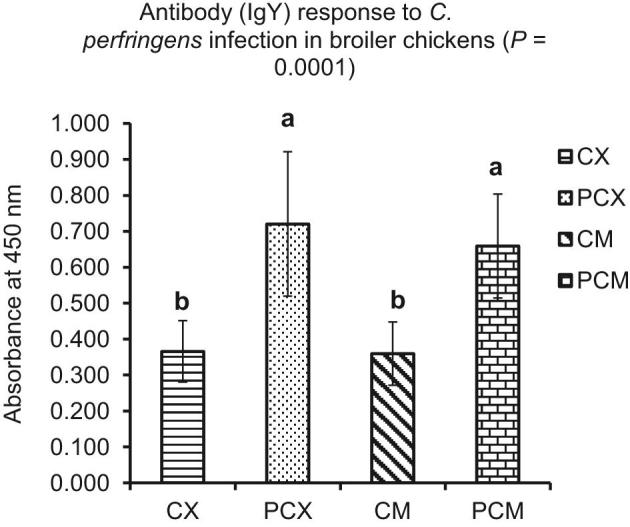
Plasma antibody (IgY) response to Clostridium perfringens (CP) infection in broiler chickens. Treatment 1 (CX) consisted of unchallenged chicks fed corn-soybean meal (SBM) basal only; treatment 2 (CM) consisted of unchallenged chicks fed corn-SBM basal into which bacitracin methylene disalicylate antibiotic was supplemented at 0.055 g/kg diet; treatments 3 (PCX) and 4 (PCM) consisted of chicks challenged with CP on 3 consecutive days (day 14, 15, and 16), and fed diets similar to those given to chicks treatments 1 and 2, respectively. Bars with different letters within each figure differ significantly (P < 0.05).
DISCUSSION
An experiment was conducted to determine the expression levels of selected proinflammatory, Th1, Th2, Th17, and Treg cytokine genes in the jejunum and cecal tonsils of CP-challenged broiler chickens. The CP-challenged chickens used in this study were confirmed to have an established CP infection (Table 1), without an apparent effect on growth performance (Fasina et al., 2016). Similarly, Du et al. (2016) found no effect on growth performance of broiler chickens challenged with CP between 14 and 20 d of age, even though changes in cytokine expression and an elevated mucosal secretory IgA were observed.
Assessment of mortality levels along with the presence and severity of focal intestinal macroscopic lesions are accepted indicators of an established subclinical or clinical NE disease in the presence of CP infection in broiler chickens (McDevitt et al., 2006; Prescott et al., 2016; Smyth, 2016; Hofacre et al., 2018). The moderately high total mortality (12.25%) in this experiment, along with the relatively low lesion scores observed for the CP-challenged PCX (1.52) and PCM (1.25) treatments are indicative of an established subclinical NE in the PCX and PCM chickens. It is not uncommon for broiler chickens experiencing subclinical NE to have low levels of focal macroscopic lesions in the small intestine (Lovland and Kaldhusdal, 2001; Van Immerseel et al., 2004). In a similar study, Li et al. (2018) orally challenged broiler chickens with CP (2.0 × 108 CFU/mL, 1.0 mL per bird) or sterile media from day 14 to 20 of experiment. They assessed intestinal lesions on day 1 post-challenge and found that CP-challenged birds had a lesion score of 2.0, while no lesions were found in the control birds (P = 0.003). In addition, they reported that the CP-challenged birds had 20% mortality, while no birds died in the control group (P = 0.061). Broiler flocks that have clinical NE may experience up to 45% mortality (McDevitt et al., 2006; Hofacre et al., 2018). Accordingly, in this study, CP infection was successfully established in CP-challenged PCX and PCM birds as indicated by their moderately high mortality and low lesion scores. We further speculate that the CP infection perhaps induced subclinical NE in PCX and PCM birds.
The diverse functions and characteristics of cytokines and immunoregulatory molecules secreted by the different subsets of T-helper cells have been described by various authors (Romagnani, 1994; Vignali et al., 2008; Cosmi et al., 2014; Oh and Lillehoj, 2016; Narsale et al., 2018). In general, when activated by macrophages and/or dendritic cells, naïve T-helper cells characteristically produce IL-1 and other pro-inflammatory cytokines that promote their differentiation into Th1, Th2, Th17, or Treg cells. In this study, IL-1β was upregulated in the jejunum and cecal tonsils of CP-challenged chickens, indicating that CP infection induced inflammation. Activated Th1 cells principally produce IFN- γ, tumor necrosis factor-α, and sometimes IL-2, and are important in providing protection against intracellular pathogens through activation of phagocytes and the production of opsonizing and complement-fixing antibodies (Cosmi et al., 2014). In this study, IFN-γ was not affected, while IL-2 was significantly downregulated in the jejunum. This may indicate that Th1 cells play limited role in mounting immune response to intestinal CP during the early stage of infection.
Th2 cells function to promote acute and chronic inflammatory responses against a variety of pathogens, particularly helminths, and characteristically produce IL-4 and IL-13 cytokines upon activation (Cosmi et al., 2014; Narsale et al., 2018). In this study, IL-13 was upregulated in the jejunum and cecal tonsils of CP-challenged chickens, indicating that Th2 cells are involved in mediating immune response to intestinal CP. Th17 cells are primarily involved in the clearance of extracellular bacteria and fungi through the activation of granulocytes, and they characteristically produce IL-17 and IL-22 cytokines when activated. In this study, IL-17 was upregulated, indicating its involvement in mounting the immune response to intestinal CP. The Treg cells primarily produce a variety of anti-inflammatory cytokines such as TGF-β, IL-10, and IL-35. In this study, TGF-β was downregulated in the cecal tonsils of CP-challenged chicks on day 7 (P < 0.05; Figure 7C), but not in the jejunum. On the other hand, IL-10 response was inconsistent (Figure 6A and B), reinforcing its pleiotropic and contradictory effect on the immune system (Mannino et al., 2015). Furthermore, the higher levels of plasma antibodies to CP recombinant proteins observed in challenged chicks (PCX and PCM; P < 0.05) confirm the involvement of the adaptive humoral immune system in the immune response to CP.
Previous work by Du et al. (2016) similarly reported an upregulation of the expression of IL-1β gene, and an elevated level of mucosal secretory IgA (P < 0.05) in orally gavaged CP-challenged broiler chickens. Alizadeh et al. (2016) also reported similar results to ours for IFN-γ. Specifically, they found no difference (P > 0.05) in the expression of IFN-γ in the ileum and cecal tonsils of broiler chickens on day 7 post-challenge. The similarity in IFN-γ results is interesting because Alizadeh et al. (2016) used a different CP challenge model in which the CP inoculum was added into the feed. In this study, CP challenge was done by orally gavaging the CP inoculum into the crop of the birds. Intestinal cytokine responses to CP infection can be modulated by differences in existing predisposable factors, differences in CP challenge methods used (oral gavage vs. inoculating CP in feed), differences in CP challenge dose administered to birds, and day of CP challenge (Prescott et al., 2016).
An important observation in this study was the differences between BMD-free (CX and CPX) and BMD-supplemented (CM and PCM) treatments in the expression levels of jejunal IFN-γ and cecal IL-2 genes. BMD is one of the most commonly used antibiotic in commercial poultry production, and it is particularly active against Gram-positive bacteria such as CP (Marshall and Levy, 2011; Crisol-Martínez et al., 2017). Specifically, BMD suppresses the synthesis of the bacterial cell wall by inhibiting the lipid phosphorylase enzyme involved in the formation of late-stage peptidoglycan intermediates (Sugimoto et al., 2017). Because intestinal microbial populations are interdependent, antibiotics may perturb the microbial composition, thereby altering immune responses (Ubeda and Pamer, 2012). In this study, BMD may have triggered the differences in jejunal IFN-γ and cecal IL-2 gene expression levels by decreasing the number of Gram-positive bacteria, increasing the population of Enterobacteriaceae, and decreasing the number of Treg cells in the cecal tonsils (Atarashi et al., 2011; Ubeda and Pamer, 2012; Furusawa et al., 2015).
In summary, downregulation of TGF-β along with a concurrent upregulation in IL-1β, IL-13, and IL-17 indicates that CP-induced subclinical NE triggered an inflammatory immune response in the intestine of broiler chicks. In addition, the presence of higher levels of antibodies to recombinant CP proteins in challenged PCX and PCM treatments (P < 0.05) compared to the unchallenged CX and CM corroborates the cytokine gene expression results. It was concluded that the signaling mechanisms underlying the inflammatory response to intestinal CP infection are probably modulated by Th2 and Th17 cells. Furthermore, these results suggest that any future strategies to reduce the negative effects of NE should be selected based on their potential to induce Th2 and Th17 cytokine secretion.
ACKNOWLEDGMENTS
This research was funded, in part, by NIFA through the Agricultural Research Program at North Carolina Agricultural and Technical State University (Evans-Allen Program, project number NC.X-305-5-17-120-1).
REFERENCES
- Alizadeh M., Rogiewicz A., McMillan E., Rodriguez-Lecompte J. C., Patterson R., Slominski B. A.. 2016. Effect of yeast-derived products and distillers dried grains with solubles (DDGS) on growth performance and local innate immune response of broiler chickens challenged with Clostridium perfringens. Avian Pathol. 45:334–345. [DOI] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K., Hori S., Ivanov II, Umesaki Y., Itoh K., Honda K.. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341. [PubMed: 21205640] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Cheng A., Wang M.. 2013. Innate sensing of viruses by pattern recognition receptors in birds. Vet. Res. 44:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. K., Songer J. G., Uzal F. A.. 2013. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Invest. 25:314–327. [DOI] [PubMed] [Google Scholar]
- Cosmi L., Maggi L., Santarlasci V., Liotta F., Annunziato F.. 2014. T helper cells plasticity in inflammation. Cytometry 85:36–42. [DOI] [PubMed] [Google Scholar]
- Crisol-Martínez E., Stanley D., Geier M. S., Hughes R. J., Moore R. J.. 2017. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 101:4547–4559. [DOI] [PubMed] [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y.. 2016. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasina Y. O., Newman M. M., Stough J. M., Liles M. R.. 2016. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 95:247–260. [DOI] [PubMed] [Google Scholar]
- Furusawa Y., Obata Y., Hase K.. 2015. Commensal microbiota regulates T cell fate decision in the gut. Semin. Immunopathol. 37:17–25. [DOI] [PubMed] [Google Scholar]
- Hofacre C. L., Smith J. A., Mathis G. F.. 2018. An optimist's view on limiting necrotic enteritis and maintaining broiler gut health and performance in today's marketing, food safety, and regulatory climate. Poult. Sci. 97:1929–1933. [DOI] [PubMed] [Google Scholar]
- Hong Y. H., Lillehoj H. S., Lillehoj E. P., Lee S. H.. 2006. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 114:259–272. [DOI] [PubMed] [Google Scholar]
- Lee K., Lillehoj H. S., Li G., Park M. S., Jang S. I., Jeong W., Jeoung H. Y., An D. J., Lillehoj E. P.. 2011. Identification and cloning of two immunogenic Clostridium perfringens proteins, elongation factor Tu (EF-Tu) and pyruvate:ferredoxin oxidoreductase (PFO) of C. perfringens. Res. Vet. Sci. 91:e80–e86. [DOI] [PubMed] [Google Scholar]
- Li G., Lillehoj H. S., Lee K. W., Lee S. H., Park M. S., Jang S. I., Bauchan G. R., Gay C. G., Ritter G. D., Bautista D. A., Siragusa G. R.. 2010. Immunopathology and cytokine responses in commercial broiler chickens with gangrenous dermatitis. Avian Pathol. 39:255–264. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y.. 2018. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovland A., Kaldhusdal M.. 2001. Severely impaired production performance in broiler flocks with high incidence of Clostridium perfringens-associated hepatitis. Avian Pathol. 30:73–81. [DOI] [PubMed] [Google Scholar]
- Mannino M. H., Zhu Z., Xiao H., Bai Q., Wakefield M. R., Fang Y.. 2015. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 367:103–107. 10.1016/j.canlet.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Marshall B. M., Levy S. B.. 2011. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24:718–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt R. M., Brooker J. D., Acamovic T., Sparks N. H. C.. 2006. Necrotic enteritis; a continuing challenge for the poultry industry. Worlds Poult. Sci. J. 62:221–247. [Google Scholar]
- Miller R. W., Skinner J., Sulakvelidze A., Mathis G. F., Hofacre C. L.. 2010. Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 54:33–40. [DOI] [PubMed] [Google Scholar]
- Muller P. Y., Janovjak H., Miserez A. R., Dobbie Z.. 2002. Processing of gene expression data generated by quantitative real-time RT-PCR. BioTechniques 32:1372–1374, 1376, 1378–1379. [PubMed] [Google Scholar]
- Narsale A., Moya R., Davies J. D.. 2018. Human CD4 + CD25 + CD127 hi cells and the Th1/Th2 phenotype. Clin. Immunol. 188:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council 1994. Nutrient Requirements of Poultry. 9th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Oh S. T., Lillehoj H. S.. 2016. The role of host genetic factors and host immunity in necrotic enteritis. Avian Pathol. 45:313–316. [DOI] [PubMed] [Google Scholar]
- Prescott J. F., Smyth J. A., Shojadoost B., Vince A.. 2016. Experimental reproduction of necrotic enteritis in chickens: a review. Avian Pathol. 45:317–322. [DOI] [PubMed] [Google Scholar]
- Prescott J. F., Sivendra R., Barnum D. A.. 1978. The use of bacitracin in the prevention and treatment of experimentally induced necrotic enteritis in the chicken. Can. Vet. J. 19:181–183. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. 1994. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 12:227–257. [DOI] [PubMed] [Google Scholar]
- SAS Institute 2004. SAS/STAT User's Guide. Version 9.1 for Windows. SAS Inst. Inc., Cary, NC. [Google Scholar]
- Smyth J. A. 2016. Pathology and diagnosis of necrotic enteritis: is it clear-cut? Avian Pathol. 45:282–287. 10.1080/03079457.2016.1158780 [DOI] [PubMed] [Google Scholar]
- St. Paul M., Brisbin J. T., Abdul-Careem M. F., Sharif S.. 2013. Immunostimulatory properties of Toll-like receptor ligands in chickens. Vet. Immunol. Immunopathol. 152:191–199. [DOI] [PubMed] [Google Scholar]
- Sugimoto A., Maeda A., Itto K., Arimoto H.. 2017. Deciphering the mode of action of cell wall-inhibiting antibiotics using metabolic labeling of growing peptidoglycan in Streptococcus pyogenes. Sci. Rep. 7:1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Lanckriet A., Gholamiandehkordi A. R., Pasmans F., Martel A., Haesebrouck F., Ducatelle R., Immerseel F. V.. 2009. Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers. Comp. Immunol. Microbiol. Infect. Dis. 32:503–512. [DOI] [PubMed] [Google Scholar]
- Ubeda C., Pamer E. G.. 2012. Antibiotics, microbiota, and immune defense. Trends Immunol. 33:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R.. 2004. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 33:537–549. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Rood J. I., Moore R. J., Titball R. W.. 2009. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 17:32–36. [DOI] [PubMed] [Google Scholar]
- Van Waeyenberghe L., De Gussem M., Verbeke J., Dewaele I., De Gussem J.. 2016. Timing of predisposing factors is important in necrotic enteritis models. Avian Pathol. 45:370–375. [DOI] [PubMed] [Google Scholar]
- Vignali D. A., Collison L. W., Workman C. J.. 2008. How regulatory T cells work. Nat. Rev. Immunol. 8:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade B., Keyburn A. L.. 2015. The true cost of necrotic enteritis. World Poult. 31:16–17. [Google Scholar]
- Williams R. B. 2005. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 34:159–180. [DOI] [PubMed] [Google Scholar]
- Wu S. B., Stanley D., Rodgers N., Swick R. A., Moore R. J.. 2014. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 169:188–197. [DOI] [PubMed] [Google Scholar]
- Yitbarek A., Echeverry H., Brady J., Hernandez-Doria J., Camelo-Jaimes G., Sharif S., Guenter W., House J. D., Rodriguez-Lecompte J. C.. 2012. Innate immune response to yeast-derived carbohydrates in broiler chickens fed organic diets and challenged with Clostridium perfringens. Poult. Sci. 91:1105–1112. [DOI] [PubMed] [Google Scholar]
- Zhou H., Lepp D., Pei Y., Liu M., Yin X., Ma R., Prescott J. F., Gong J.. 2017. Influence of pCP1NetB ancillary genes on the virulence of Clostridium perfringens poultry necrotic enteritis strain CP1. Gut Pathog. 9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]