Abstract
Background
Gout is a common inflammatory arthritis associated with adverse clinical outcomes. Under treatment is common in the general population. The aim of this study was to determine the prevalence of gout and its treatment among patients with chronic kidney disease (CKD).
Methods
We conducted a multi-centre cross sectional study of patients (n = 522) who attended specialist nephrology clinics in Ireland. Standardized data collection tool recorded clinical characteristics and medication use at clinic visits and kidney function was assessed with standardised creatinine measurements and Estimated Glomerular Filtration Rate (eGFR). The prevalence of gout and the corresponding use of urate lowering therapies (ULT) were determined. Multivariate logistic regression explored correlates of gout expressed as Odds Ratios (OR) and 95% Confidence Intervals (CI) adjusting for demographic and clinical characteristics.
Results
Overall prevalence of gout was 16.6% and increased significantly from 7.5% in Stage 1–2 CKD to 22.8% in stage 4–5 CKD, P< 0.005. Prevalence increased with age (P < 0.005) and was higher in men than women (19.1% versus 10.3% P< 0.005). Overall, 67.9% of gout patients with CKD were treated with ULT, and the percentage increased with advancing stage of CKD from 55.6% in Stage 1–2 to 77.4% in Stage 4–5, P<0.005. Multivariable modelling identified men (vs women), OR, 1.95 (0.95–4.03), serum albumin, OR 1.09 (1.02–1.16) per 1 g/L lower, poorer kidney function, OR 1.11 (1.01–1.22) per 5 ml/min/1.73m2 lower, and rising parathyroid hormone levels, OR 1.38 (1.08–1.77) per 50 pg/ml higher as disease correlates.
Conclusions
Gout is common in CKD and increases with worsening kidney function in the Irish health system. Over two thirds of patients with gout were receiving ULT, increasing to 77% of patients with advanced CKD. Greater awareness of gout in CKD, its treatment and the effectiveness of treatment strategies should be vigorously monitored to improve patient outcomes.
Introduction
Gout is a common inflammatory arthropathy caused by the deposition of monosodium urate crystals in joints and soft tissues. In the general population, the prevalence of gout varies worldwide from 0.1% to approximately 10% and incidence rates vary from 0.3 to 6 cases per 1,000 person-years [1]. In addition to causing excruciating arthritic pain, gout is associated with premature death, classically explained by a high frequency of comorbid conditions, especially renal and cardiovascular diseases [2–4]. Gout is associated with a progressive functional impairment, reduced quality of life, lost productivity and increased mortality [5,6]. Recent observational studies implicate both hyperuricaemia and gout as possible risk factors for progression of chronic kidney disease (CKD) suggesting that the treatment of these conditions may lead to measurable clinical benefits [7,8]. Several small clinical trials have found that treatment with Urate-Lowering Therapy (ULT) reduced the progression of kidney disease [9,10]. Furthermore, a recent meta-analysis of clinical trials with over 1, 200 patients found that treatment with ULT significantly reduced the risk of major renal and cardiovascular events [11]. This emerging evidence would suggest that treatment and control of gout is especially important among patients with impaired kidney function.
Few studies have examined the burden of gout among individuals with impaired kidney function in the general population or with CKD within the health system. A report from Krishnan using data from the 2009–2010 National Health and Nutrition Examination Survey (NHANES) in the US found that the prevalence of self-reported gout increased from 2.9% in patients with normal renal function to 33.3% among those with glomerular filtration rate (eGFR) < 30 ml/min/1.73m2. Adjusting for confounding, individuals with severe renal impairment had a 6-fold higher prevalence of gout compared to those with normal kidney function [12]. Data from the German Chronic Kidney Disease (GCKD) cohort, a prospective observational study of 5,085 patients, found a prevalence of 24.3% among patients with pre-existing CKD which increased to 35.6% among those with GFR < 30 ml/min/1.73m2 [13]. These studies would suggest that gout is highly prevalent among patients with pre-existing CKD and may contribute significantly to morbidity from arthropathy and accelerated kidney disease progression. Despite these studies there are several unanswered questions with regard to the burden of gout and its management among patients with CKD in the health system. For example, it is unclear to what extent patients with gout who attend specialist renal clinics are treated with ULT and whether treatment rates vary across stage of CKD.
Given the paucity of data on the burden and management of gout in health systems we conducted a multicentre cross–sectional study to determine the prevalence of gout and concurrent treatment strategies among CKD patients within the Irish health system.
Materials and methods
Study design
This study was a multicentre cross-sectional study of adult patients with CKD treated at 18 adult specialist nephrology clinics during the first 2 weeks of December 2012 and 2013. All nephrology clinics were invited to participate in the audit and a consecutive sampling approach for patient selection was adopted. The clinics were geographically dispersed across six health regions in the Republic of Ireland (West, Midwest, Northwest, Midlands, East and Southeast). Patients less than 18 years of age or receiving dialysis were excluded. A standardised data collection tool was used to capture anonymised clinical information from medical case records, laboratory information systems and physician clinic letters. Demographic and clinical characteristics were captured including primary cause of kidney disease, comorbid medical conditions, prescribed medications and laboratory values recorded within the previous 3 months [or within 6 months for specific laboratory values for iron indices, parathyroid hormone (PTH), lipids and haemoglobin A1c (HbA1c)]. The study was approved by the Ethics Committee of University Hospital Limerick.
Definition of gout
Gout was defined as the presence of a documented clinical diagnosis of gout on medical case records and/or physician clinic letters or if the patient was receiving ULT. The Estimated Glomerular Filtration Rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [14] and CKD was classified according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines [15]. The following eGFR categories were defined: ≥ 60 ml/min/1.73 m2 (Stages 1–2), 30–59 (Stage 3) and <30 (Stages 4–5).
Statistical methods
Descriptive statistics were calculated for continuous variables (reported as mean values and standard deviations or median and IQR where appropriate) and categorical variables (reported as numbers and percentages). Comparisons across groups were made using Fisher’s exact tests for categorical variables and Kruskal-Wallis test for continuous variables. Multivariable logistic regression models were fitted to explore the associations of demographic and clinical factors with prevalent gout. Sequential age, and age and sex adjusted models were developed to examine relationships. The explanatory variables included age modelled as 5-year intervals, sex, comorbid medical conditions, laboratory values recorded prior to clinic visit, and prescribed medications. A final multivariate model was constructed to identify the relative contributions of demographic, clinical and treatment factors with the presence of gout. Model performance was assessed using the c-statistic and the adequacy of the logistic models was tested using the Hosmer and Lemeshow goodness-of-fit test. Associations were expressed as odds ratios (ORs) and 95% confidence intervals (CIs) and all analyses were performed using R statistical software [16].
Results
Baseline characteristics of the study population
Table 1 outlines the basic characteristics of the study population by CKD stage. The majority were men (55.2%), white Irish (95%) and the average age was 58.2 (SD 16.9). The average eGFR was 48.4 (SD 27.7) ml/min/1.73 m2. The principal causes of CKD were hypertension (26.8%), glomerulonephritis (18.1%) and diabetes (12%), although for a large proportion the primary cause was classified as unknown in 13.6%.
Table 1. Baseline characteristics of the study population by stage of CKD.
| Variable | n | Overall Cohort (n = 522) |
Stage 1–2 (n = 142) |
Stage 3 (n = 176) |
Stage 4–5 (n = 139) |
P-value |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age mean (SD) | 515 | 58.2 (16.9) | 48.8(14.5) | 60.3(15.1) | 66.3(15.7) | <0.001 |
| Sex | ||||||
| Men (%) | 283 | (55.2) | 78.0 (54.9) | 106.0 (60.2) | 77.0 (55.4) | |
| Women (%) | 230 | (44.8) | 64.0 (45.1) | 70.0 (39.8) | 62.0 (44.6) | 0.568 |
| Race (%) | ||||||
| White Irish | 479 | (95.0) | 126.0 (91.3) | 168.0 (96.6) | 132.0 (96.4) | 0.051 |
| White Irish traveller | 2 | (0.4) | 1.0 (0.7) | 0.0 (0.0) | 0.0 (0.0) | 0.054 |
| White other | 12 | (2.4) | 2.0 (1.4) | 5.0 (2.9) | 4.0 (2.9) | 0.054 |
| Asian | 2 | (0.4) | 1.0 (0.7) | 1.0 (0.6) | 0.0 (0.0) | 0.051 |
| Other | 9 | (1.8) | 8.0 (5.8) | 0.0 (0.0) | 1.0 (0.7) | 0.052 |
| Cause of CKD (%) | ||||||
| Hypertension | 138 | (26.8) | 31.0 (21.8) | 44.0 (25.0) | 48.0 (34.5) | 0.045 |
| Diabetes | 62 | (12.0) | 12.0 (8.5) | 25.0 (14.2) | 23.0 (16.5) | 0.105 |
| Glomerulonephritis | 93 | (18.1) | 35.0 (24.6) | 26.0 (14.8) | 21.0 (15.1) | 0.052 |
| Autosomal dominant PKD | 33 | (6.4) | 7.0 (4.9) | 16.0 (9.1) | 7.0 (5.0) | 0.253 |
| Hereditary nephritis | 13 | (2.5) | 5.0 (3.5) | 6.0 (3.4) | 1.0 (0.7) | 0.257 |
| Other cause of CKD | 168 | (32.6) | 53.0 (37.3) | 58.0 (33.0) | 43.0 (30.9) | 0.496 |
| Not known | 70 | (13.6) | 16.0 (11.3) | 18.0 (10.2) | 23.0 (16.5) | 0.231 |
| Biopsy (%) | ||||||
| Native Kidney biopsy | 75 | (14.4) | 27.0 (19.0) | 26.0 (14.8) | 18.0 (12.9) | 0.363 |
| Comorbid Conditions (%) | ||||||
| Gout | 78 | (16.6) | 9.0 (7.5) | 29.0 (17.8) | 31.0 (22.8) | 0.003 |
| Diabetes | 106 | (22.6) | 21.0 (17.6) | 38.0 (23.2) | 38.0 (27.9) | 0.148 |
| Hypertension | 370 | (78.7) | 87.0 (73.1) | 131.0 (79.9) | 114.0 (83.8) | 0.108 |
| Cancer | 33 | (7.0) | 9.0 (7.6) | 13.0 (7.9) | 9.0 (6.6) | 0.919 |
| Heart failure | 22 | (4.7) | 2.0 (1.7) | 7.0 (4.3) | 10.0 (7.4) | 0.105 |
| Thyroid disease | 48 | (10.2) | 7.0 (5.9) | 19.0 (11.6) | 18.0 (13.2) | 0.124 |
| Stroke or Transient ischaemic attack | 22 | (4.7) | 4.0 (3.4) | 7.0 (4.3) | 9.0 (6.6) | 0.483 |
| Chronic obstructive airways disease | 22 | (4.7) | 4.0 (3.4) | 7.0 (4.3) | 8.0 (5.9) | 0.607 |
| Peripheral vascular disease | 38 | (8.1) | 4.0 (3.4) | 17.0 (10.4) | 15.0 (11.0) | 0.042 |
| Coronary heart disease | 75 | (16.0) | 11.0 (9.2) | 26.0 (16.0) | 33.0 (24.3) | 0.005 |
| Obesity | 28 | (6.0) | 5.0 (4.2) | 8.0 (4.9) | 12.0 (8.8) | 0.262 |
| Hypercholesterolemia | 127 | (27.1) | 28.0 (23.3) | 48.0 (29.4) | 43.0 (32.1) | 0.287 |
| Depression | 20 | (4.3) | 6.0 (5.0) | 6.0 (3.7) | 7.0 (5.1) | 0.791 |
| Arthritis | 27 | (5.7) | 6.0 (5.0) | 11.0 (6.7) | 6.0 (4.4) | 0.689 |
| Osteoporosis | 30 | (6.4) | 7.0 (5.9) | 16.0 (9.8) | 4.0 (2.9) | 0.053 |
| Current or ex-smoker | 52 | (11.1) | 15.0 (12.6) | 20.0 (12.2) | 12.0 (8.8) | 0.583 |
| Physical Measurements | ||||||
| Weight (kg) | 397 | 80.7 (17.5) | 79.8 (15.9) | 79.8 (17.6) | 82.2 (19.5) | 0.748 |
| Pulse (beats/min) | 293 | 75.2 (16.3) | 75.6 (13.1) | 73.6 (13.6) | 75.7 (19.3) | 0.473 |
| Systolic BP (mmHg) | 481 | 137.7 (19.6) | 131.8 (16.6) | 138.5 (19.0) | 141.7 (21.7) | <0.001 |
| Diastolic BP (mmHg) | 481 | 77.7 (13.3) | 78.7 (13.8) | 77.7 (12.9) | 76.7 (12.3) | 0.302 |
| Urine tests | ||||||
| Protein: creatinine ratio | 136 | 136.0 (246.8) | 49.0 (71.3) | 150.1 (348.0) | 209.7 (226.3) | <0.001 |
| Albumin: creatinine ratio | 51 | 101.9 (214.9) | 22.7 (28.1) | 93.4 (239.2) | 208.2 (280.0) | 0.004 |
| Prescribed Medications (%) | ||||||
| ACE-I | 114 | (21.8) | 37.0 (26.1) | 45.0 (25.6) | 21.0 (15.1) | 0.040 |
| ARB | 93 | (17.8) | 27.0 (19.0) | 34.0 (19.3) | 26.0 (18.7) | 0.999 |
| ACE-I & ARB | 11 | (2.1) | 3.0 (2.1) | 6.0 (3.4) | 1.0 (0.7) | 0.290 |
| ACE-I or ARB | 196 | (37.5) | 61.0 (43.0) | 73.0 (41.5) | 46.0 (33.1) | 0.187 |
| Aspirin | 169 | (32.4) | 34.0 (23.9) | 59.0 (33.5) | 63.0 (45.3) | 0.001 |
| Beta- blocker | 147 | (28.2) | 36.0 (25.4) | 52.0 (29.5) | 47.0 (33.8) | 0.297 |
| Calcium blocker | 152 | (29.1) | 32.0 (22.5) | 52.0 (29.5) | 50.0 (36.0) | 0.045 |
| Diuretic | 121 | (23.2) | 17.0 (12.0) | 37.0 (21.0) | 54.0 (38.8) | <0.001 |
| Statin | 169 | (32.4) | 36.0 (25.4) | 64.0 (36.4) | 57.0 (41.0) | 0.016 |
| Vasodilator | 18 | (3.4) | 2.0 (1.4) | 7.0 (4.0) | 8.0 (5.8) | 0.134 |
| Gout-specific medications (%) | ||||||
| Urate lowering therapies | 53 | (10.2) | 5.0 (3.5) | 17.0 (9.7) | 24.0 (17.3) | <0.001 |
| Allopurinol | 49 | (9.4) | 4.0 (2.8) | 15.0 (8.5) | 23.0 (16.5) | 0.001 |
| Febuxostat | 4 | (0.8) | 1.0 (0.7) | 2.0 (1.1) | 1.0 (0.7) | 0.999 |
| Acute Flare /flare prophylaxis | ||||||
| Colchicine | 4 | (0.8) | 0.0 (0.0) | 2.0 (1.1) | 0.0 (0.0) | 0.340 |
| Corticosteroid | 21 | (4.0) | 5.0 (3.5) | 11.0 (6.2) | 2.0 (1.4) | 0.093 |
| NSAIDs | 4 | (0.8) | 2.0 (1.4) | 1.0 (0.6) | 0.0 (0.0) | 0.507 |
PKD, polycystic kidney disease; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; NSAID, non-steroidal anti-inflammatory medication
Prevalence of gout
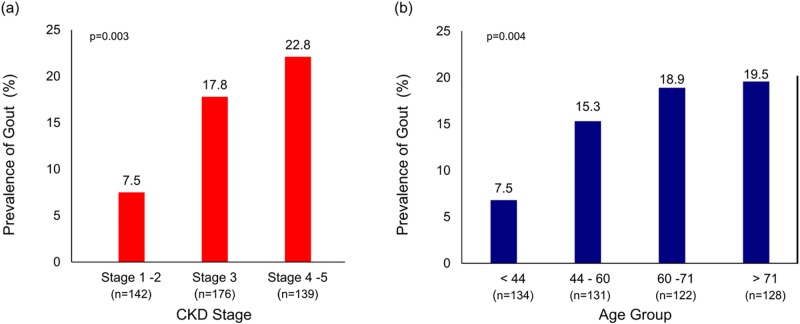
The overall prevalence of gout in the entire cohort was 16.6% and increased significantly from 7.5% in CKD stages 1–2 to 22.8% in CKD stages 4–5, p < 0.005 (Fig 1). When gout was defined solely by a medical record diagnosis, the prevalence of gout and by CKD stage followed a similar trend (S1 Appendix). The baseline characteristics of the study population by presence or absence of gout are shown in Table 2. Patients with gout were significantly older, predominantly men, and had significantly higher prevalence of coronary disease than those without gout (p<0.005). Overall frequency of gout increased with advancing age; from 7.5% in patients age < 44 years to 19.5% for patients age > 71 years, p<0.005. Compared to patients without gout, those with gout had significantly lower eGFR values [36.3 (19.8) versus 48.1 (26.9) ml/min/1.73m2 (p < 0.005) and significantly greater proteinuria [protein/creatinine ratio 323.4 (571) mg/mmol versus 116.1 (174) respectively, all p< 0.01]. Gout patients also experienced significantly lower serum albumin concentrations and significantly higher parathyroid hormone concentrations than those without gout, all p<0.001.
Fig 1. Prevalence of gout by CKD stage and age group.
(a) Prevalence of gout by CKD stage groups (b) Prevalence of gout by age group; a proportional trend test was used to assess the linear trend in the proportion of gout cases across age categories.
Table 2. Baseline characteristics of the study population by presence or absence of Gout.
| Variable | n | Overall Cohort (n = 522) |
No Gout (n = 393) |
Gout (n = 78) |
P-value |
|---|---|---|---|---|---|
| Demographic factors | |||||
| Age mean (SD) | 515 | 58.2(16.9) | 58.8(16.6) | 63.9(13.6) | 0.021 |
| Sex | |||||
| Men (%) | 283 | (55.2) | 214 (54.7) | 54 (69.2) | |
| Women (%) | 230 | (44.8) | 177 (45.3) | 24 (30.8) | 0.024 |
| Race (%) | |||||
| White Irish | 479 | (95.0) | 368 (95.8) | 74 (96.1) | 0.756 |
| White Irish traveller | 2 | (0.4) | 2 (0.5) | 0 (0.0) | 0.761 |
| White other | 12 | (2.4) | 5 (1.3) | 3 (3.9) | 0.756 |
| Asian | 2 | (0.4) | 2 (0.5) | 0 (0.0) | 0.76 |
| Other | 9 | (1.8) | 7 (1.8) | 0 (0.0) | 0.755 |
| Cause of CKD (%) | |||||
| Hypertension | 138 | (26.8) | 117 (29.8) | 18 (23.1) | 0.273 |
| Diabetes | 62 | (12.0) | 49 (12.5) | 13 (16.7) | 0.358 |
| Glomerulonephritis | 93 | (18.1) | 66 (16.8) | 17 (21.8) | 0.328 |
| Autosomal dominant PKD | 33 | (6.4) | 26 (6.6) | 6 (7.7) | 0.805 |
| Hereditary nephritis | 13 | (2.5) | 12 (3.1) | 1 (1.3) | 0.704 |
| Other cause of CKD | 168 | (32.6) | 129 (32.8) | 22 (28.2) | 0.507 |
| Not known | 70 | (13.6) | 49 (12.5) | 12 (15.4) | 0.464 |
| Biopsy (%) | |||||
| Native kidney biopsy | 75 | (14.4) | 60 (15.3) | 9 (11.5) | 0.485 |
| Comorbid Conditions (%) | |||||
| Diabetes | 106 | (22.6) | 87 (22.2) | 19 (24.7) | 0.656 |
| Hypertension | 370 | (78.7) | 306 (78.1) | 63 (81.8) | 0.544 |
| Cancer | 33 | (7.0) | 27 (6.9) | 6 (7.8) | 0.807 |
| Heart failure | 22 | (4.7) | 19 (4.9) | 3 (3.9) | 0.999 |
| Thyroid disease | 48 | (10.2) | 42 (10.7) | 6 (7.8) | 0.54 |
| Stroke or Transient ischaemic attack | 22 | (4.7) | 21 (5.4) | 1 (1.3) | 0.149 |
| Chronic obstructive airways disease | 22 | (4.7) | 18 (4.6) | 4 (5.2) | 0.77 |
| Peripheral vascular disease | 38 | (8.1) | 32 (8.2) | 6 (7.8) | 0.999 |
| Coronary heart disease | 75 | (16.0) | 52 (13.3) | 22 (28.6) | 0.002 |
| Obesity | 28 | (6.0) | 22 (5.6) | 6 (7.8) | 0.435 |
| Hypercholesterolaemia | 127 | (27.1) | 106 (27.1) | 21 (27.3) | 0.999 |
| Depression | 20 | (4.3) | 20 (5.1) | 0 (0.0) | 0.057 |
| Arthritis | 27 | (5.7) | 23 (5.9) | 4 (5.2) | 0.999 |
| Osteoporosis | 30 | (6.4) | 25 (6.4) | 5 (6.5) | 0.999 |
| Current or ex-smoker | 52 | (11.1) | 44 (11.2) | 8 (10.4) | 0.999 |
| Obesity | 28 | (6.0) | 22 (5.6) | 6 (7.8) | 0.435 |
| Physical Measurements | |||||
| Weight (kg) | 397 | 80.7(17.5) | 80.1(16.0) | 86.7(23.1) | 0.094 |
| Pulse (beats/min) | 293 | 75.2(16.3) | 74.7(16.2) | 76.8(18.2) | 0.569 |
| Systolic BP (mmHg) | 481 | 137.7(19.6) | 137.8(18.8) | 140.2(22.5) | 0.576 |
| Diastolic BP (mmHg) | 481 | 77.7(13.3) | 77.6(13.7) | 78.6(12.1) | 0.826 |
| Laboratory Measures | |||||
| eGFR (mL/min/1.73m2) | 457 | 48.4 (27.7) | 48.1 (26.9) | 36.3 (19.8) | 0.001 |
| Haemoglobin (g/dL) | 448 | 12.6 (1.9) | 12.6 (1.9) | 12.5 (1.8) | 0.289 |
| Ferritin (ng/L) | 157 | 253.1 (339.4) | 268.6 (361.3) | 205.8 (194.9) | 0.799 |
| TSAT ratio (%) | 126 | 26.7 (13.4) | 27.6 (13.9) | 22.9 (8.5) | 0.184 |
| Folate (nmol/L) | 95 | 40.2 (143.7) | 38.1 (137.8) | 58.6 (190.4) | 0.215 |
| Vitamin B12 (nmol/L) | 119 | 460.1 (272.1) | 471.4 (287.1) | 451.2 (160.2) | 0.617 |
| Corrected Calcium (mmol/L) | 144 | 2.3 (0.2) | 2.3 (0.1) | 2.3 (0.3) | 0.528 |
| Calcium (mmol/L) | 405 | 2.4 (0.2) | 2.4 (0.2) | 2.3 (0.1) | 0.701 |
| Albumin (g/L) | 354 | 41.1 (5.6) | 41.4 (5.8) | 38.6 (4.6) | <0.001 |
| Phosphate (mmol/L) | 376 | 1.1 (0.3) | 1.1 (0.3) | 1.2 (0.3) | 0.068 |
| Parathyroid hormone (PTH) (pg/mL) | 162 | 130.6(129.1) | 109.8 (95.1) | 230.3 (199.6) | <0.001 |
| Total cholesterol (mmol/L) | 192 | 4.6 (1.4) | 4.6 (1.4) | 4.5 (1.1) | 0.783 |
| LDL cholesterol (mmol/L) | 124 | 2.6(1.2) | 2.6(1.2) | 2.1(0.6) | 0.106 |
| HDL cholesterol (mmol/L) | 126 | 1.4(0.5) | 1.4(0.5) | 1.3(0.4) | 0.728 |
| Triglycerides (mmol/L) | 190 | 4.0(21.6) | 3.1(17.3) | 2.6(1.7) | 0.005 |
| HbA1c (mmol/mol) | 79 | 51.6(21.1) | 52.5(22.0) | 52.5(17.8) | 0.850 |
| Urine tests | |||||
| Protein: creatinine ratio | 136 | 136.0(246.8) | 116.1(173.8) | 323.4(571.3) | 0.009 |
| Albumin: creatinine ratio | 51 | 101.9(214.9) | 116.8(233.9) | 38.4(33.2) | 0.999 |
| Medications (%) | |||||
| ACE-I | 114 | (21.8) | 93 (23.7) | 17 (21.8) | 0.772 |
| ARB | 93 | (17.8) | 75 (19.1) | 15 (19.2) | 0.999 |
| ACE-I & ARB | 11 | (2.1) | 9 (2.3) | 2 (2.6) | 0.999 |
| ACE-I or ARB | 196 | (37.5) | 159 (40.5) | 30 (38.5) | 0.801 |
| Aspirin | 169 | (32.4) | 133 (33.8) | 31 (39.7) | 0.363 |
| Alpha Blocker | 18 | (3.4) | 17 (4.3) | 0 (0.0) | 0.089 |
| Beta Blocker | 147 | (28.2) | 108 (27.5) | 34 (43.6) | 0.007 |
| Calcium blocker | 152 | (29.1) | 126 (32.1) | 21 (26.9) | 0.423 |
| Diuretic | 121 | (23.2) | 84 (21.4) | 35 (44.9) | <0.001 |
| Statin | 169 | (32.4) | 140 (35.6) | 26 (33.3) | 0.795 |
| Gout-specific Medications (%) | |||||
| Urate-lowering therapies | 53 | (10.2) | 0 (0.0) | 53 (67.9) | <0.001 |
| Allopurinol | 49 | (9.4) | 0 (0.0) | 49 (62.8) | <0.001 |
| Febuxostat | 4 | (0.8) | 0 (0.0) | 4 (5.1) | 0.001 |
| Probenecid | 0 | 0 | 0 | 0 | NA |
| Acute flare/flare prophylaxis | |||||
| Colchicine | 4 | (0.8) | 0 (0.0) | 4 (5.1) | 0.001 |
| Corticosteroid | 21 | (4.0) | 15 (3.8) | 6 (7.7) | 0.136 |
| NSAIDs | 4 | (0.8) | 3 (0.8) | 0 (0.0) | 0.999 |
PKD, polycystic kidney disease; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker: NSAIDs, Nonsteroidal anti-inflammatory drugs; eGFR, estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Gout-specific medications
Overall, 67.9% of patients with gout were receiving ULT as shown in Table 2 and the prevalence of ULT use increased from 55.6% in Stages 1–2 to 58.6 % in Stage 3 and to 77.4 % in Stages 4–5 (p = 0.002). Allopurinol was the most commonly prescribed ULT (62.8%) with a much smaller percentage treated with Febuxostat (5.1%). Colchicine use was recorded in 5.1% of patients while a further 7.7% of gout patients were receiving corticosteroids. None of the patients with gout were recorded as using non-steroidal anti-inflammatory drugs (NSAIDS).
Correlates of gout
The relationship of demographic and clinical factors with gout is illustrated in Table 3. With adjustment only for age and sex; advancing age [1.11 (95% CI 1.02–1.20) for every 5-year increase], male gender [OR 1.85 (1.09–3.12)] and patients with coronary disease [OR 1.94 (1.05–3.58)] were significantly more likely to have gout. Worsening kidney function increased the likelihood of having gout [OR 1.10 (1.03–1.17) for each 5 ml/min/1.73m2 decrease in eGFR]. Similarly, higher levels of proteinuria quantified by protein/creatinine ratio estimation were correlated with greater likelihood of gout [log2 PCR 1.45 (1.09–1.93), p<0.05). Lower serum albumin levels and worsening secondary hyperparathyroidism were also associated with greater likelihood of gout [OR 1.09 (1.03–1.14) per 1 g/dL decrease in albumin, and OR 1.33 (1.14–1.56) per 50 pg/mL increase in PTH, all p <0.005. Use of diuretics and beta-blockers also correlated with gout [OR 2.72 (1.61–4.60) and OR 1.81 (1.08–3.02) respectively.
Table 3. Unadjusted, and age- and sex-adjusted Odd Ratios and 95% confidence intervals for gout.
| Variable | N | OR (95% CI) | P-value | N | AOR (95% CI)1 | P-value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age per 5 year increase | 471 | 1.11 (1.02–1.2) | 0.012 | 469 | 1.11 (1.02–1.2) | 0.013 |
| Male vs Female | 469 | 1.86 (1.11–3.13) | 0.019 | 469 | 1.85 (1.09–3.12) | 0.022 |
| Comorbid Conditions | ||||||
| Diabetes | 469 | 1.15 (0.65–2.03) | 0.634 | 467 | 0.95 (0.52–1.71) | 0.851 |
| Hypertension | 469 | 1.26 (0.68–2.37) | 0.463 | 467 | 1.14 (0.6–2.16) | 0.682 |
| Cancer | 469 | 1.14 (0.46–2.87) | 0.777 | 467 | 0.89 (0.35–2.29) | 0.813 |
| Heart failure | 468 | 0.79 (0.23–2.75) | 0.716 | 466 | 0.66 (0.19–2.32) | 0.519 |
| Thyroid disease | 469 | 0.70 (0.29–1.72) | 0.441 | 467 | 0.67 (0.27–1.67) | 0.388 |
| Stroke or Transient ischaemic attack | 469 | 0.23 (0.03–1.75) | 0.157 | 467 | 0.21 (0.03–1.57) | 0.127 |
| Chronic obstructive airways disease | 469 | 1.14 (0.37–3.46) | 0.819 | 467 | 1.07 (0.35–3.3) | 0.910 |
| Peripheral vascular disease | 469 | 0.95 (0.38–2.36) | 0.913 | 467 | 0.69 (0.27–1.75) | 0.438 |
| Coronary heart disease | 468 | 2.61 (1.47–4.63) | 0.001 | 466 | 1.94 (1.05–3.58) | 0.034 |
| Obesity | 469 | 1.42 (0.56–3.63) | 0.462 | 467 | 1.21 (0.47–3.13) | 0.694 |
| Hypercholesterolaemia | 468 | 1.01 (0.58–1.75) | 0.977 | 466 | 0.90 (0.52–1.58) | 0.725 |
| Depression | 469 | 0.88 (0.3–2.62) | 0.817 | 467 | 0.80 (0.26–2.42) | 0.690 |
| Arthritis | 469 | 1.02 (0.38–2.75) | 0.970 | 467 | 0.97 (0.35–2.68) | 0.960 |
| Osteoporosis | 469 | 0.92 (0.41–2.03) | 0.831 | 467 | 0.95 (0.42–2.12) | 0.892 |
| Laboratory Variables | ||||||
| eGFR per 5 ml/min/1.73m2 decrease | 419 | 1.11 (1.04–1.18) | 0.001 | 419 | 1.10 (1.03–1.17) | 0.005 |
| Haemoglobin per 1 g/dL increase | 413 | 0.96 (0.83–1.1) | 0.530 | 411 | 0.98 (0.85–1.14) | 0.838 |
| Ferritin per 1 ng/L increase | 151 | 1.00 (1.00–1.00) | 0.450 | 151 | 1.00 (1.00–1.00) | 0.520 |
| TSAT ratio per 1% increase | 121 | 0.97 (0.92–1.01) | 0.167 | 121 | 0.97 (0.93–1.02) | 0.271 |
| Folate per 1 nmol/L increase | 92 | 1.00 (1.00–1.00) | 0.631 | 92 | 1.00 (1.00–1.00) | 0.499 |
| Vitamin B12 per 1 nmol/L increase | 114 | 1.00 (1.00–1.00) | 0.796 | 114 | 1.00 (1.00–1.00) | 0.892 |
| Calcium (mmol/L) | 373 | 0.56 (0.1–3.04) | 0.504 | 371 | 0.83 (0.14–4.73) | 0.830 |
| Serum Albumin per 1 g/L decrease | 327 | 1.08 (1.03–1.13) | 0.002 | 325 | 1.09 (1.03–1.14) | 0.001 |
| Phosphate per 1 mmol/L increase | 346 | 2.57 (0.97–6.83) | 0.059 | 344 | 2.43 (0.87–6.81) | 0.091 |
| PTH per 50 pg/mL increase | 157 | 1.34 (1.15–1.57) | <0.001 | 156 | 1.33 (1.14–1.56) | <0.001 |
| Total cholesterol per 1 mmol/L increase | 181 | 0.92 (0.64–1.32) | 0.652 | 180 | 1.01 (0.69–1.47) | 0.976 |
| LDL cholesterol per 1 mmol/L increase | 118 | 0.55 (0.27–1.15) | 0.112 | 117 | 0.55 (0.26–1.15) | 0.114 |
| HDL cholesterol per 1mmol/L increase | 120 | 0.63 (0.17–2.38) | 0.495 | 119 | 0.65 (0.16–2.66) | 0.545 |
| Triglycerides per 1 mmol/L increase | 179 | 1.00 (0.96–1.03) | 0.888 | 178 | 1.00 (0.96–1.03) | 0.837 |
| HbA1c per 1 mmol/mol increase | 75 | 1.00 (0.97–1.03) | 0.993 | 75 | 1.00 (0.97–1.03) | 0.961 |
| log2(PCR) | 125 | 1.43 (1.08–1.9) | 0.012 | 124 | 1.45 (1.09–1.93) | 0.011 |
| Medications | ||||||
| ACE-I | 471 | 0.90 (0.50–1.61) | 0.722 | 469 | 0.87 (0.48–1.6) | 0.665 |
| ARB | 471 | 1.01 (0.54–1.87) | 0.976 | 469 | 1.19 (0.63–2.25) | 0.594 |
| Aspirin | 471 | 1.29 (0.78–2.12) | 0.318 | 469 | 0.94 (0.55–1.61) | 0.829 |
| Beta Blocker | 471 | 2.04 (1.24–3.36) | 0.005 | 469 | 1.81 (1.08–3.02) | 0.023 |
| Calcium blocker | 471 | 0.78 (0.45–1.34) | 0.372 | 469 | 0.72 (0.41–1.25) | 0.243 |
| Diuretic | 471 | 2.99 (1.80–4.97) | <0.001 | 469 | 2.72 (1.61–4.60) | <0.001 |
| Statin | 471 | 0.90 (0.54–1.51) | 0.699 | 469 | 0.76 (0.45–1.29) | 0.306 |
LDL, low-density lipoprotein; HDL, high-density lipoprotein; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker: NSAIDs, Nonsteroidal anti-inflammatory drugs; eGFR, estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI).
1AOR: adjusted for age and sex only
In the fully adjusted model, lower eGFR and worsening serum albumin remained significant correlates of gout as illustrated in Table 4. Given that PTH values were available for a smaller percentage of patients (n = 162), an additional analysis was performed in which we restricted to the final model to those with valid PTH concentrations. In this restricted model, a rising PTH concentration was also significant [OR 1.38, (1.08–1.77) per 50 pg/mL increase, p = 0.01. This model had a C-statistic of 0.83.
Table 4. Multivariable Odds Ratio for gout among patients with CKD in the Irish health system.
| Variable | AOR (95% CI) | P-value | AOR (95% CI) | P-value |
|---|---|---|---|---|
| Model 1 (N = 286) | Model 2 (N = 126) | |||
| Age per 5 year increase | 1.09 (0.96–1.24) | 0.185 | 1.00 (0.82–1.21) | 0.962 |
| Male vs Female | 1.95 (0.95–4.03) | 0.070 | 2.99 (0.79–11.33) | 0.107 |
| Coronary Heart Disease | 1.57 (0.68–3.63) | 0.291 | 1.47 (0.37–5.76) | 0.583 |
| Diuretic use | 1.79 (0.87–3.68) | 0.111 | 0.78 (0.20–2.95) | 0.710 |
| eGFR per 5 ml/min/1/73m2 decrease | 1.11 (1.01–1.22) | 0.037 | 1.03 (0.85–1.23) | 0.792 |
| Serum albumin per 1 g/L decrease | 1.09 (1.02–1.16) | 0.008 | 1.15 (1.01–1.30) | 0.040 |
| Serum phosphate per 1 mmol/l increase | 0.34 (0.08–1.46) | 0.147 | 0.40 (0.04–4.20) | 0.445 |
| Parathyroid hormone per 50 pg/mL increase | – | 1.38 (1.08–1.77) | 0.011 |
Model 1: adjusted for continuous variables (age, eGFR, serum albumin, serum phosphate) and categorical variables (sex, history of coronary heart disease and diuretic use). The model had a C-statistic 0.77 and there was no evidence of poor fit from the Hosmer and Lemeshow goodness of fit test (p = 0.6). Model 2: adjusted for all variables as Model 1 in addition to serum parathyroid hormone (PTH). This model had a C-statistic of 0.83 and there was no evidence of poor fit from the Hosmer and Lemeshow goodness of fit test (p = 0.9).
Discussion
In this large multi-centre study, we found a substantial burden of gout among Irish patients with CKD (16.6%) that increased significantly from 7.5% in patients with mild CKD to 22.8% among patients with moderate to severe CKD (eGFR < 30 ml/min/1.73m2). Gout was more common in men than in women and rates increased with advancing age. Adjusting for differences in age and sex, we found that patients with lower kidney function, lower serum albumin, and worsening secondary hyperparathyroidism were more likely to have gout. Overall, almost 68% of gout patients with CKD were receiving ULT agents, with allopurinol being the most commonly prescribed ULT. Importantly and encouragingly, we observed a significant trend of increasing ULT use from 55.6% in Stage 1–2 to 77.4% in Stage 4–5, evidence that supports better recognition and treatment of gout in this high-risk population.
Recent studies have identified strong relationships between gout and CKD, which are not surprising given that hyperuricaemia, the principal driver of gout, is an inevitable consequence of worsening kidney function [17]. However, only few have quantified the burden of gout and corresponding rates of ULT treatment among patients with pre-existing CKD. In our study, we describe a substantial burden of gout in CKD patients attending specialist clinics in the Irish health system. Our estimate (16.6%) is somewhat lower than that reported by the German Chronic Kidney Disease study (24.3%), which may reflect underlying differences in prevalence in the general population from respective countries or differences in rates of death among gout patients with CKD [13]. However, the pattern of increase in gout prevalence from early CKD to advanced CKD followed near identical trends in both studies with an approximately 3-fold rise in prevalence. These results suggest that at the very least that gout is an extremely common but treatable comorbidity among CKD patients who are already under clinical surveillance in the health system.
Our study provides novel insights into the range and extent of treatment strategies for patients with gout managed by kidney specialists in the Irish health system. We report overall treatment rates of 68% for ULT among gout patients with CKD, which are identical to those reported by the German CKD cohort (67%). However, unlike Germany, the principal ULT in Ireland were uricostatic (predominantly allopurinol), as no patient was recorded as receiving uricosuric therapy. Encouragingly, we reveal that rates of ULT use have increased from 55.6% among patients in CKD stage 1–2 to 77.4% for patients with more advanced CKD 4–5 suggesting a propensity to greater ULT prescribing. This trend in prescribing patterns may reflect the increasing recognition by kidney specialists in Ireland that gout is eminently treatable or that gout and/or hyperuricaemia are emerging risk factors for CKD progression [7–11]. It is equally noteworthy, that colchicine (5.1%) and corticosteroids (7.7%) were the standard therapies for acute flare or flare prophylaxis with no recorded use of NSAIDs. This new data highlights a positive practice pattern that supports avoidance of nephrotoxic medications and kidney function preservation [15].
Despite the moderately high proportion of CKD patients with gout receiving ULT therapy in this study, a substantial proportion of patients (~32%) nevertheless were untreated. Studies have consistently shown that the treatment of gout is suboptimal in the general population [18]. Prescription rates of ULT vary substantially from as low as 23% (Taiwan), 38% (UK) and 42% (Sweden) to a spectacular 80% in South Korea [19–21]. Low treatment rates among patients with CKD may be partially explained by the presence of absolute and relative contraindications to the medications, as well as a lack of drug efficacy [22]. Additionally, dietary and lifestyle interventions may account for those not undertaking ULT. Dietary modification is typically suggested as part of the initial treatment for patients with gout, although some suggest it should be ancillary to ULT [23]. Prior to the availability of febuxostat, allopurinol was the principal ULT for patients with gout and impaired renal function. However, the risk of allopurinol hypersensitivity syndrome (AHS) is increased in renal impairment and consequently lingering concerns regarding this infrequent but potentially lethal event may contribute to ULT avoidance in CKD patients [24,25].
Prior studies have identified strong relationships between hyperuricaemia, the precursor to gout, and several metabolic markers [26]. In the current study, we observed for the first time an inverse relationship between serum albumin and gout. In the final adjusted model, for each 1 g/L lower serum albumin, the likelihood of gout increased by 9%. Low serum albumin may reflect protein-losing states, poor nutritional status, or systemic inflammation. Prior studies have also identified an independent relationship between serum uric acid and the extent of albuminuria [27–30]. Tseng and colleagues have reported positive increases in urinary albuminuria with increasing serum uric acid concentrations in type 2 diabetes [27] while prospectively designed cohorts have confirmed the predictive impact of hyperuricaemia on albuminuria [28–30]. Serum albumin is a negative acute phase reactant, and thus low serum albumin levels may also reflect ongoing systemic inflammation associated with gout.
We also uncovered a very strong independent association between elevated PTH and the occurrence of gout. This is an intriguing finding, which suggests that progressive secondary hyperparathyroidism serves to increase the likelihood of gout in the setting of CKD. Previous studies have suggested a strong biological influence of PTH on serum uric acid levels possibly mediated by reduced renal excretion of uric acid [31–35]. However, in virtually all of these studies, hyperparathyroidism was primary in nature, and correction of which led to a fall in uric acid concentrations [31,32]. Our study not only extends the observations of previous instigators but for the first time associates secondary hyperparathyroidism of CKD with clinical gout. Indeed in our analysis, a rising serum PTH level was a stronger correlate of gout than worsening eGFR, suggesting a strong pathobiological basis.
Our study is not with limitations. First, the design of the study was cross-sectional in nature with reliance on medical records for data extraction. Therefore, we cannot infer causality between explanatory covariates and gout. Second, although joint aspiration for monosodium urate crystals remains the gold standard for diagnosis in clinical practice, our study relied on a clinical diagnosis from medical records based on physician-diagnosis and/or use of ULT. Inclusion of ULT medications ensured greater capture of clinical gout that may not have been recorded on the patient’s medical record. Third, we lacked data on serum uric acid concentrations, and thus were unable to correlate gout or ULT use with target thresholds. Notwithstanding these limitations, the study was multicentre, with participation from a diverse range of nephrology clinics across Ireland, thus strengthening the generalisability. Although a probability sampling strategy would have been preferred, we argue that the application of consecutive sampling to a homogenous population, attending renal clinics across multiple sites reduces bias in relation to the representativeness of the study population. The large sample included a detailed description of patient characteristics including comorbid conditions, measures of kidney function including proteinuria. Finally, our analysis included a detailed description of current prescribed ULT medications as well as medications for acute flares and flare prophylaxis.
Conclusion
In conclusion, our findings demonstrate a substantial burden of gout among patients with CKD in the Irish health system that increases with advancing CKD. Male gender, worsening kidney function, malnutrition and secondary hyperparathyroidism were identified as independent correlates. Almost 68% of patients with CKD were receiving ULT that increased to 77% among patients with Stage 4–5 CKD. Patients with both gout and CKD represent a high-risk group for considerable morbidity and mortality, compounded by the individual contribution of each to adverse clinical outcomes. Given the substantial burden of gout in CKD patients, greater awareness, screening and treatment of gout are key to improving patient outcomes in this population. Future studies should examine dosing of ULT among CKD patients and the extent to which treatment targets are achieved in order to prevent the complications of gout and potentially slow the progression of CKD.
Supporting information
(DOC)
Acknowledgments
We thank all participating nephrologists from the Republic of Ireland and Northern Ireland. We are also grateful to the following individuals who assisted with data collection: Dr Liam Casserly, Dr Con Cronin, M.E., Dr Umair Sharif (University Hospital Limerick), Dr Sean Leavey, Dr Elizabeth Abernathy, Dr Louise Ryan (University Hospital Waterford), Dr Arif Mutwali, Dr Ann Marie Moran (University Hospital Letterkenny), Prof Peter Conlon, Dr Eoin Conlon, Dr Peter Conlon (Beaumont Hospital), D.N.R., Dr Dervla Connaughton (University Hospital Galway), Dr Eoin Bergin, Dr Katie Hanley, Dr Philip James, Dr Edward McMonagle, Dr Marie Connelly (Tullamore Regional Hospital).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
A.G.S. was supported by grants from the Irish Heart Foundation, Midwest Kidney Disease Research and Education Foundation (MKid), Limerick and the Health Research Institute, University of Limerick. A.G.S and L.D.B. were supported by grants from the Health Research Board of Ireland (HRA-2013-PHR-685 and HRA-2013-PHR-437).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kuo C-F, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015; 10.1038/nrrheum.2015.91 [DOI] [PubMed] [Google Scholar]
- 2.Stack AG, Donigiewicz U, Abdalla AA, Weiland A, Casserly LF, Cronin CJ, et al. Plasma fibrinogen associates independently with total and cardiovascular mortality among subjects with normal and reduced kidney function in the general population. QJM. 2014;107: 701–13. 10.1093/qjmed/hcu057 [DOI] [PubMed] [Google Scholar]
- 3.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131: 7–13. 10.7326/0003-4819-131-1-199907060-00003 [DOI] [PubMed] [Google Scholar]
- 4.Kuo C-F, Luo S-F. Risk of premature death in gout unchanged for years. Nat Rev Rheumatol. 2017;13: 200–201. 10.1038/nrrheum.2017.27 [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2008; 10.1136/ard.2007.081604 [DOI] [PubMed] [Google Scholar]
- 6.Edwards NL, Sundy JS, Forsythe A, Blume S, Pan F, Becker MA. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Econ. 2011; 10.3111/13696998.2010.540874 [DOI] [PubMed] [Google Scholar]
- 7.Sedaghat S, Hoorn EJ, van Rooij FJA, Hofman A, Franco OH, Witteman JCM, et al. Serum Uric Acid and Chronic Kidney Disease: The Role of Hypertension. PLoS One. 2013;8: e76827 10.1371/journal.pone.0076827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu KH, Kuo CF, Luo SF, See LC, Chou IJ, Chang HC, et al. Risk of end-stage renal disease associated with gout: A nationwide population study. Arthritis Res Ther. BioMed Central Ltd; 2012;14: R83 10.1186/ar3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goicoechea M, De Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5: 1388–1393. 10.2215/CJN.01580210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sircar D, Chatterjee S, Waikhom R, Golay V, Raychaudhury A, Chatterjee S, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: A 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. Elsevier Inc; 2015;66: 945–950. 10.1053/j.ajkd.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 11.Su X, Xu B, Yan B, Qiao X, Wang L. Effects of uric acid-lowering therapy in patients with chronic kidney disease: A meta-analysis. PLoS One. 2017; 10.1371/journal.pone.0187550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan E. Reduced Glomerular Function and Prevalence of Gout: NHANES 2009–10. PLoS One. 2012; 10.1371/journal.pone.0050046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing J, Kielstein JT, Schultheiss UT, Sitter T, Titze SI, Schaeffner ES, et al. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant. 2015; 10.1093/ndt/gfu352 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF III, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate Disclosure of conflicts of interest: We have received confirmation from Drs. Ann Intern Med. 2009;150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158: 825–30. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 16.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. R Foundation for Statistical Computing Vienna Austria; 2016. http://www.r-project.org/ [Google Scholar]
- 17.Krishnan E. Reduced glomerular function and prevalence of gout: NHANES 2009–10. Steinman TI, editor. PLoS One. 2012;7: e50046 10.1371/journal.pone.0050046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo C-F, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74: 661–7. 10.1136/annrheumdis-2013-204463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo C-F, Grainge MJ, See L-C, Yu K-H, Luo S-F, Zhang W, et al. Epidemiology and management of gout in Taiwan: a nationwide population study. Arthritis Res Ther. 2015;17: 13 10.1186/s13075-015-0522-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehlin M, Drivelegka P, Sigurdardottir V, Svärd A, Jacobsson LTH. Incidence and prevalence of gout in Western Sweden. Arthritis Res Ther. Arthritis Research & Therapy; 2016;18: 164 10.1186/s13075-016-1062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Kwak SG, Park S. Prescription pattern of urate-lowering therapy in Korean gout patients: data from the national health claims database. Korean J Intern Med. 2018;33: 228–229. 10.3904/kjim.2016.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan E. Chronic kidney disease and the risk of incident gout among middle-aged men: A seven-year prospective observational study. Arthritis Rheum. 2013; 10.1002/art.38171 [DOI] [PubMed] [Google Scholar]
- 23.Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology. 2018;57: i51–i58. 10.1093/rheumatology/kex421 [DOI] [PubMed] [Google Scholar]
- 24.Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol. 2006;33: 1646–50. 10.1097/RHU.0B013E3181B562F8 [DOI] [PubMed] [Google Scholar]
- 25.Dalbeth N, Stamp L. Allopurinol dosing in renal impairment: walking the tightrope between adequate urate lowering and adverse events. Semin Dial. Wiley/Blackwell (10.1111); 2007;20: 391–5. 10.1111/j.1525-139X.2007.00270.x [DOI] [PubMed] [Google Scholar]
- 26.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115: 2526–32. 10.1161/CIRCULATIONAHA.106.657627 [DOI] [PubMed] [Google Scholar]
- 27.Tseng C-H. Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int. 2005;68: 796–801. 10.1111/j.1523-1755.2005.00459.x [DOI] [PubMed] [Google Scholar]
- 28.Jalal DI, Rivard CJ, Johnson RJ, Maahs DM, McFann K, Rewers M, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: Findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010;25: 1865–1869. 10.1093/ndt/gfp740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hovind P, Rossing P, Tarnow L. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58: 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashino Y, Okamura S, Tsujii S, Ishii H. Association of serum uric acid levels with the risk of development or progression of albuminuria among Japanese patients with type 2 diabetes: a prospective cohort study [Diabetes Distress and Care Registry at Tenri (DDCRT 10)]. Acta Diabetol. 2016;53: 599–607. 10.1007/s00592-015-0825-x [DOI] [PubMed] [Google Scholar]
- 31.Mintz DH, Canary JJ, Carreon G, Kyle LH. Hyperuricemia in Hyperparathyroidism. N Engl J Med. Massachusetts Medical Society; 1961;265: 112–115. 10.1056/NEJM196107202650302 [DOI] [PubMed] [Google Scholar]
- 32.Hisatome I, Ishimura M, Sasaki N, Yamakawa M, Kosaka H, Tanaka Y, et al. Renal handling of urate in two patients with hyperuricemia and primary hyperparathyroidism. Intern Med. 1992;31: 807–11. Available: http://www.ncbi.nlm.nih.gov/pubmed/1392185 [DOI] [PubMed] [Google Scholar]
- 33.Hui JY, Choi JWJ, Mount DB, Zhu Y, Zhang Y, Choi HK. The independent association between parathyroid hormone levels and hyperuricemia: a national population study. Arthritis Res Ther. BioMed Central Ltd; 2012;14: R56 10.1186/ar3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paik JM, Farwell WR, Taylor EN. Demographic, dietary, and serum factors and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2012;23: 1727–36. 10.1007/s00198-011-1776-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chin K-Y, Ima-Nirwana S, Wan Ngah WZ. Significant association between parathyroid hormone and uric acid level in men. Clin Interv Aging. 2015; 1377 10.2147/CIA.S90233 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.