Abstract
Weather changes accompanied by decreases in barometric pressure are suggested to trigger meteoropathy, i.e., weather-related pain. We previously reported that neuropathic pain-related behavior in rats is aggravated by lowering barometric pressure, and that this effect is abolished by inner ear lesions. These results suggest that mechanisms that increase vestibular neuronal activity may parallel those that contribute to meteoropathy generation. However, it remains unknown whether changes in barometric pressure activate vestibular neuronal activity. To address this issue, we used expression of c-Fos protein as a marker for neural activation. Male and female mice were placed in a climatic chamber, and the barometric pressure was lowered by 40 hPa, from 1013 hPa, for 50 min (LP stimulation). The total number of c-Fos-positive cells in the vestibular nuclei was counted bilaterally after LP stimulation. We also video-recorded mouse behaviors and calculated the total activity score during the LP stimulation. LP stimulation resulted in significant c-Fos expression in the superior vestibular nucleus (SuVe) of male and female mice. There was no effect of LP stimulation on the total activity score. These data show that distinct neurons in the SuVe respond to LP stimulation. Similar mechanisms may contribute to the generation of meteoropathy in humans.
Introduction
It has long been assumed that weather changes can trigger episodes of meteoropathy, such as headache and other forms of chronic pain [1–6]. Meteorological factors that influence pain include barometric pressure, humidity, wind, precipitation, and temperature [6–9]. We have previously demonstrated that lowering barometric pressure (5–27 hPa lower than atmospheric pressure; LP stimulation) using a climatic chamber leads to increased pain-related behaviors in rats with chronic constriction injury (CCI) [10] and monoarthritic rats [11]. We have also reported that the LP-induced increase in pain-related behaviors vanishes after drug-induced destruction of the inner ear in CCI rats [12]. In another study, we extracellularly recorded neural activities in vestibular nuclei with a glass microelectrode and examined the effect of LP (40 hPa/ 8 min) in normal anesthetized rats. Seven out of 20 recorded vestibular neurons increased their discharge frequency upon LP stimulation [13]. These results suggest that the barometric sensor/sensing system influencing nociceptive behavior during LP in CCI rats is located in the inner ear. However, it is not known whether changes in barometric pressure activate vestibular neuronal activity in mice. If so, mechanisms that increase vestibular neuronal activity may parallel those that contribute to the development of meteoropathy. To investigate this issue, in this study, we used the expression of the immediate early-gene c-Fos, as a marker for neuronal activity in the vestibular nuclei and found that distinct vestibular neurons indeed respond to LP stimulation.
Materials and methods
Animals
Male (n = 18) and female (n = 16) C57BL/6J mice (14-weeks-old at the beginning of the experiments) were used in this study (Charles River Laboratories Japan, Kanagawa, Japan). The mice were housed in plastic cages and kept in a regulated environment (23 ± 1°C; 50 ± 5% relative humidity) with a 12-h light-dark cycle (lights on at 08:00). Food (Oriental MF; Oriental Yeast Co., Tokyo, Japan) and tap water were available ad libitum. All experiments were performed in accordance with the Guidelines for Animal Experiments of Chubu University and Aichi Medical University, and the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols for animal experiments were approved by the Animal Experiment Committee of Chubu University (No. 3010074) and Aichi Medical University (No. 2018–43).
Lowering of barometric pressure
In the present experiment, we used a pressure-controlled climatic chamber, which is able to lower barometric pressure at a variety of rates and ranges [14]. The chamber used can maintain its own barometric pressure independently of the atmospheric pressure changes outside.
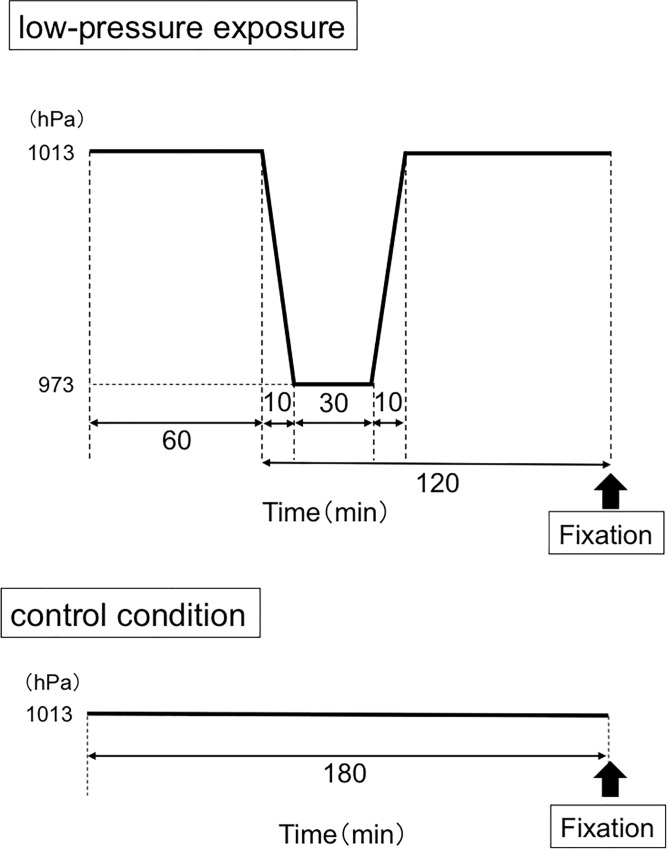
Before the experiments, animals were acclimated for 60 min in the chamber (barometric pressure: 1013 hPa, ambient temperature: 22 ± 2°C, relative humidity: 50 ± 10%) on 2 consecutive days. On the experimental day, mice were placed in the chamber set at the basal barometric pressure (1013 hPa) for 60 min (ambient temperature: 22 ± 2°C, relative humidity: 50 ± 10%). The barometric pressure was then lowered by 40 hPa over the course of 10 min, kept at this level for 30 min, and then returned to the normal level over the course of 10 min (LP stimulation). After returning to the basal pressure level (1013 hPa), mice were placed in the chamber for 70 min (Fig 1). A group of animals was placed in the chamber at 1013 hPa without pressure changes for 180 min and served as the control group.
Fig 1.
Time schedule of low-pressure exposure (upper panel) and control condition (lower panel). Barometric pressure was lowered from 1013 hPa to 973 hPa.
Animal activity
We tested the effect of LP stimulation on the activity of animals. The behavior of each mouse during the LP stimulation was recorded using a camera (Webcam C500; Logicool, Tokyo, Japan). As the total activity score, we calculated the total time that each animal spent walking, rearing, sniffing, and grooming during the 30 min before and 30 min after the beginning of LP stimulation. In control animals, the total activity score was calculated over a period of 60 min without LP stimulation. A researcher who was blind to the experimental conditions analyzed the behavior of the mice.
Animal treatment and tissue preparation
After LP stimulation or 180-min observation (control), mice were deeply anesthetized with sodium pentobarbital, and perfused transcardially with saline, followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4). The brains were removed, post-fixed overnight in the same fixative solution at 4°C, and then placed in 30% sucrose in 0.1 M phosphate-buffered saline (pH 7.4) for cryoprotection.
c-Fos immunocytochemistry
Serial coronal sections (40-μm thick) of each brain were cut on a cryostat. Every two sections that contained the vestibular nuclei were used for immunocytochemistry to detect c-Fos protein (17–22 sections/mouse), and the remaining sections were stained with cresyl violet (1% in water). Sections for immunocytochemistry were rinsed with 0.1 M phosphate-buffered saline (PBS, pH 7.4) and then treated with 3% hydrogen peroxide in PBS for 15 min. They were then washed with PBS for 20 min with one change and rinsed with 0.3% Triton X-100 in 0.1 M phosphate buffer (PBST, pH 7.4) for 20 min with one change; nonspecific binding sites were blocked by incubation in 25% Block Ace (DS Pharma Biomedical, Osaka, Japan) in PBST for 20 min at room temperature. The sections were subsequently incubated with an anti-human c-Fos antibody [rabbit monoclonal IgG: c-Fos (9F6) Rabbit mAb; Cell Signaling Technology, Beverly, MA, USA] diluted 10,000 times with 10% Block Ace in PBST for approximately 40 h at 4°C. After three 10-min washes with PBST, the sections were incubated with a biotinylated anti-rabbit secondary antibody (BA-1000; Vector Laboratories, Burlingame, CA, USA) diluted 500 times with PBST for 2 h at room temperature, and then processed with the ABC kit (VECTASTAIN Elite ABC kit PK-6100; Vector Laboratories) appropriately diluted with PBST. Each step was followed by three 10-min washes with PBST. After the last wash, the sections were immersed in 0.175 M sodium acetate buffer (pH 7.4) for 30 min with two changes, and then appropriately incubated with the chromogen solution [0.02% 3,3'-diaminobenzidine, 0.0025% hydrogen peroxide, and 0.25% nickel (II) chloride hexahydrate in 0.175 M sodium acetate buffer]. The reaction was stopped by transferring the sections to 0.175 M sodium acetate buffer, and finally washing them with PBS for 20 min, with one change, and mounting them on gelatin-coated glass slides.
Quantification of c-Fos immunoreactivity
The total number of c-Fos-immunopositive cells in the superior (SuVe), lateral (LVe), medial (MVe), and spinal (SpVe) vestibular nuclei was counted by an experimenter blind to the experimental conditions. c-Fos-positive cells were counted in each nucleus under a microscope at a magnification of 100×, with results expressed as the total number of c-Fos-positive cells in each nucleus, bilaterally. Each vestibular nucleus was identified by cresyl violet staining of sections adjacent to those used for c-Fos immunostaining, according to a mouse brain atlas [15].
Data analyses
Data are displayed as the mean ± standard error (SE). Statistical analyses were performed using a two-way analysis of variance (ANOVA) followed by a post hoc Tukey-Kramer test to determine the effect of the barometric pressure condition and sex on the number of c-Fos-immunoreactive cells in each vestibular nucleus and on the total activity scores. The criterion for statistical significance was set at p < 0.05 for all comparisons.
Results
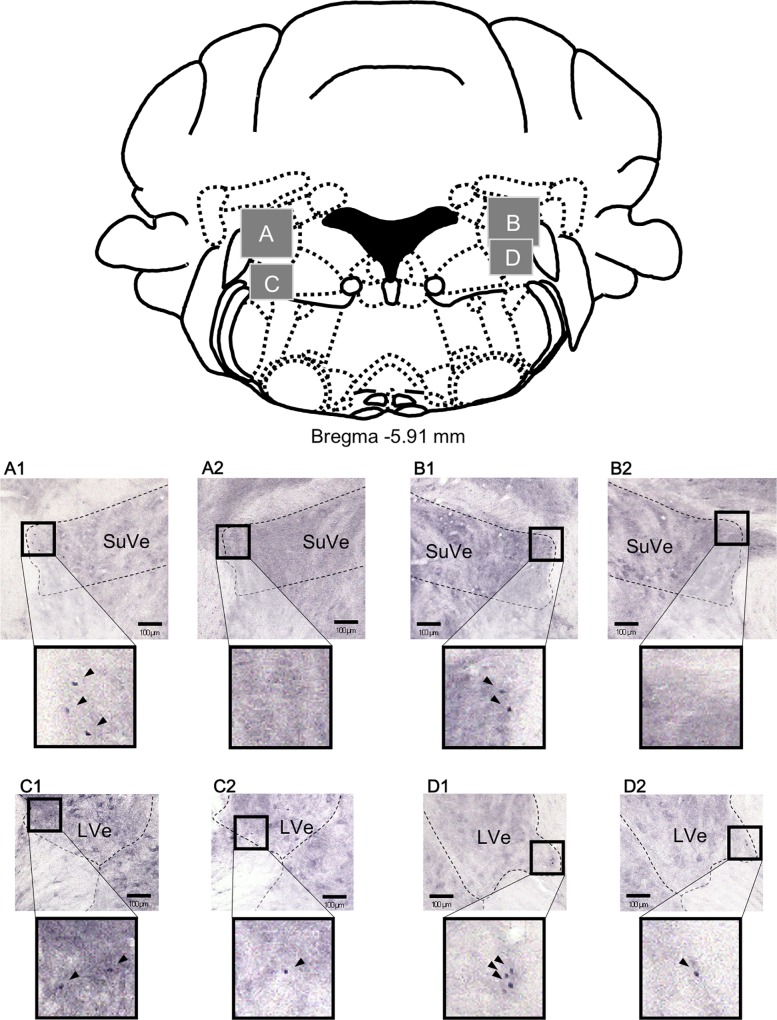
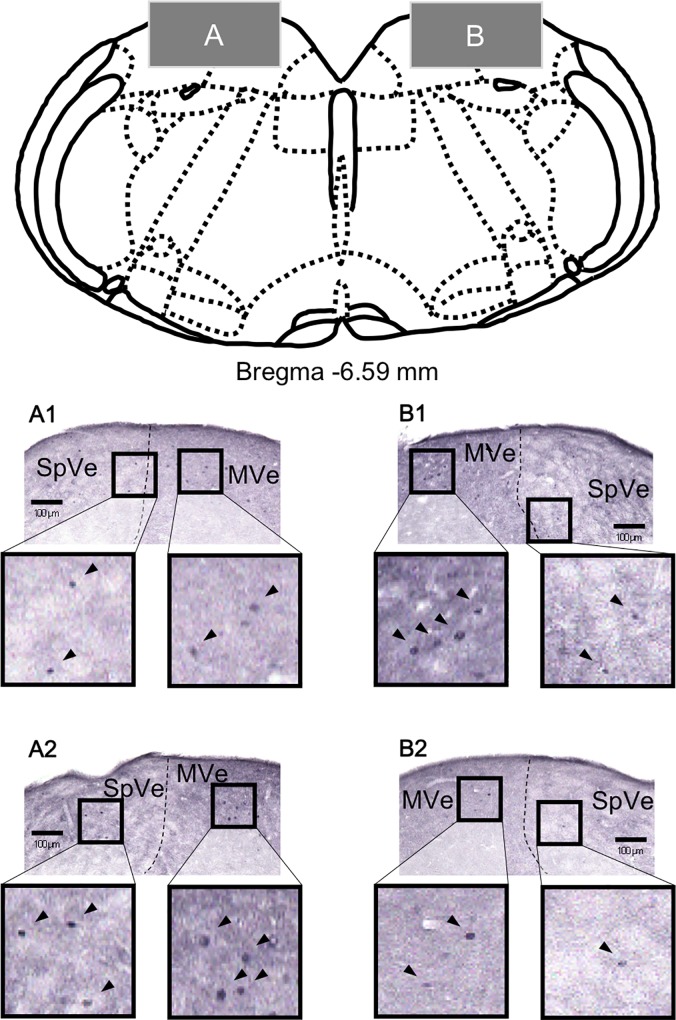
We examined whether LP stimulation induced neural activation in the vestibular nuclei. For this analysis, vestibular nuclear segments were immunohistologically examined for c-Fos protein, a marker of neuronal activation. The photomicrographs in Fig 2 were taken from SuVe and LVe sections bilaterally (SuVe: A and B, LVe: C and D) from male mice exposed to the LP stimulation (A1, B1, C1, and D1) or to control conditions (A2, B2, C2, and D2). The photomicrographs in Fig 3 were taken from SpVe and MVe sections bilaterally (A and B) from male mice exposed to the LP stimulation (A1 and B1) or to control conditions (A2 and B2). The tissues were processed for c-Fos immunostaining. As shown in Fig 2, we found c-Fos immunoreactivity in some SuVe cells after LP stimulation (A1 and B1), but little or no immunoreactivity was observed under control conditions (A2 and B2). Fig 4 shows the average number of c-Fos-immunopositive cells for each vestibular nucleus. This number was significantly increased in the SuVe of mice of both sexes exposed to LP stimulation. Two-way ANOVA revealed a significant effect of barometric pressure (F1, 30 = 9.76, p < 0.01) but not sex, and no significant interaction between the two factors. Post hoc analysis indicated that LP stimulation significantly increased the average number of c-Fos-positive cells in the SuVe (p < 0.01). In other vestibular nuclei, namely in the LVe, MVe, and SpVe, the number of c-Fos-immunopositive cells in each nucleus was not affected by barometric pressure or sex (two-way ANOVA, p > 0.05).
Fig 2.
Photomicrographs of the superior vestibular nucleus (SuVe) (A: left and B: right) and the lateral vestibular nucleus (LVe) (C: left and D: right) in male mice 2 h after low-pressure exposure (A1, B1, C1, and D1) and under control conditions (A2, B2, C2, and D2). Arrowheads indicate c-Fos-positive cells. The histological sections are shown as gray squares in the schematic drawing of the medulla oblongata (upper panel).
Fig 3.
Photomicrographs of the spinal vestibular nucleus (SpVe) and medial vestibular nucleus (MVe) (A: left and B: right) in male mice 2 h after low-pressure exposure (A1 and B1) and under control conditions (A2 and B2). Arrowheads indicate c-Fos-positive cells. The histological sections are shown as gray squares in the schematic drawing of the medulla oblongata (upper panel).
Fig 4. Number of c-Fos-positive cells in each vestibular nucleus.
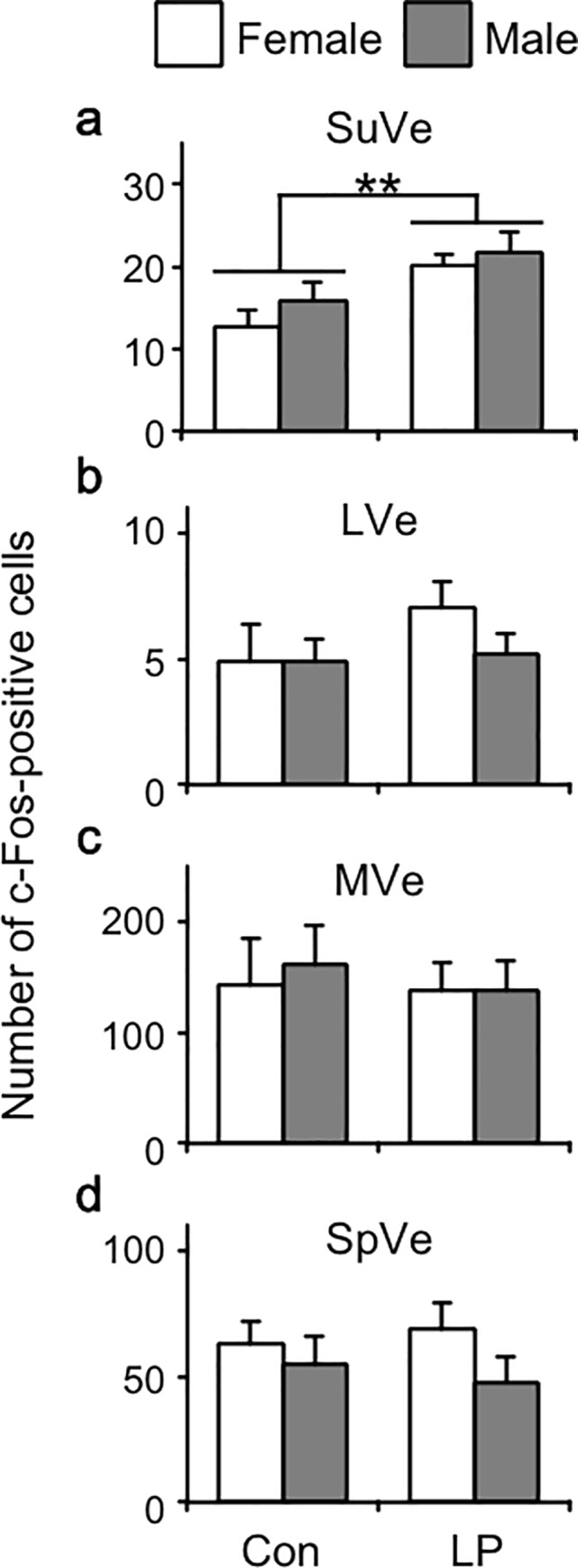
Quantification of c-Fos-positive cells in the (a) superior vestibular nucleus (SuVe), (b) lateral vestibular nucleus (LVe), (c) medial vestibular nucleus (MVe), (d) spinal vestibular nucleus (SpVe), in female (n = 8 per group) and male (n = 9 per group) mice 2 h after lowering barometric pressure stimulation (LP: 973 hPa) or under control conditions (Con: 1013 hPa). Each bar represents the mean + standard error; **, p < 0.01 (two-way ANOVA followed by Tukey-Kramer test).
Table 1 shows the mean total activity scores for female and male mice over a period of 60 min in the control condition (without LP stimulation) and those during the 30 min before and 30 min after the beginning of LP stimulation. In the control condition, the mean total activity scores of male mice were relatively larger than those of female mice, but the difference was not significant (p = 0.06). Moreover, there were no significant effects of LP stimulation and sex on the total activity scores.
Table 1. Total activity scores for female and male mice.
Behaviors of each mouse during the 30 min before and 30 min after the beginning of LP stimulation were recorded (LP exposure). In control animals, the total activity score was calculated over a period of 60 min without LP stimulation (Control condition). Each number represents the mean ± standard error of the activity score.
| Control condition | LP exposure | |
|---|---|---|
| Female (n = 8 per group) | 361 ± 58.3 | 383 ± 106 |
| Male (n = 9 per group) | 761 ± 333 | 608 ± 131 |
Discussion
This is the first study investigating the impact of barometric pressure changes on neuronal activity in the vestibular nuclei in mice. The rationale of the present study was to examine if changes in barometric pressure within a range of natural weather changes may influence the activity of second-order neurons in the vestibular nuclei of mice, particularly of neurons receiving vestibular afferent input. Our present data clearly demonstrate that distinct neurons in the SuVe respond to LP stimulation. These data are consistent with our previous observation that some vestibular neurons in rats increased their discharge frequency upon LP stimulation [13].
Nishihara et al. reported that the air volume in the middle ear cavity is important for the transmission of slowly changing atmospheric pressure to the perilymph in the inner ear [16]. It has also been reported that pressure changes in the middle ear cavity induce a pressure difference between the perilymph and endolymph, and that this difference causes an increase in vestibular neuronal activity [17–19]. These results, although obtained using a much larger and more rapid pressure change (200 mm H2O or -200 mm H2O within 8.5 s) than the one used in the present study, suggest that LP stimulation induces a relative overpressure in the middle ear cavity and, thus, results in vestibular nuclei activation. This hypothesis is supported by the fact that the activity levels of laboratory mice change along with natural barometric pressure fluctuations [20], indicating that mice can sense such changes. Taken together, our present data suggest that a barometric sensor or sensing system that detects atmospheric pressure is present in the inner ear of mice.
It has long been known that barometric pressure-sensing abilities may be common in wild vertebrates, especially ones that are small in size, for whom even individual storms can be life-threatening events. For example, birds have been reported to sense and respond to decreases in barometric pressure [21]. In addition, sensitivity to small barometric pressure changes as low as 1–2 hPa has also been reported in these animals [22]. Birds probably detect barometric pressure using the paratympanic organ (PTO), located in the middle ear, which is mechanoreceptive [23]. The PTO is thought to function as both a barometer and an altimeter, helping birds to detect changes in both weather and altitude during migration. In birds, the PTO is innervated by the facial nerve, projecting to the vestibular nuclei [24] and may mediate barometric perception [25]. In humans, no comparable system for sensing small barometric pressure changes is presently known. However, rapid and large pressure changes during diving or flight have occasionally been found to induce transient and reversible vertigo (alternobaric vertigo) [26,27]. Persistent alternobaric vertigo has also been reported in patients with nasal obstruction and obstructive sleep apnea at ground level [28,29]. We have previously reported that weather-sensitive patients suffering from pain show lowered thresholds for self-motion perception in response to galvanic vestibular stimulation [30].
The present findings show that LP stimulation increases c-Fos immunoreactivity in the SuVe but not in the other vestibular nuclei, with no significant differences between male and female mice. It is known that neurons in the SuVe receive input principally from the ampullae of the semicircular ducts [31–33] suggesting that a barometric sensor or sensing system might be located in the semicircular ducts in mice of both sexes. Further studies are needed in order to clarify the pressure sensing mechanism underlying this system.
How can vestibular neural activities be triggering factors for episodes of meteoropathy? It is possible that the influence of vestibular activation on autonomic functions, which occurs through modification of autonomic centers in the brain stem, also affects sympathetic nerve activities [34,35]. We have previously shown that LP increases blood pressure and heart rates of rats and mice [36] and in weather-sensitive patients [30]. These results indicate that changes in barometric pressure induce sympathetic activation both in rodents and humans. The influence of sympathetic nerve activities on nociceptive afferents after nerve injury is well documented [37–39]. In addition, we previously showed that sympathectomy inhibits LP-induced aggravation of mechanical hyperalgesia in nerve-injured rats, suggesting the involvement of sympathetic nerve activity in this phenomenon [10]. Based on these previous reports, we can assume that vestibular neuronal activation induced by LP stimulation increases pain through sympathetic nerve activities. Another possibility is that vestibular neural activities induce hormonal changes. This hypothesis is based on studies reporting that vestibular nuclear neurons in rats and humans project to the hypothalamus [40] [41], thus possibly modulating the hypothalamic-pituitary-adrenal axis (HPA-axis), one of the main bodily stress systems. It is well known that several chronic-pain conditions are related to the HPA-axis [42–44] and, thus, we can assume that hormones are released from the adrenal cortex in response to LP stimulation. These circulating hormones might directly activate the peripheral nociceptive fibers and induce vasoconstriction, thus increasing pain. Moreover, cutaneous nociceptive fibers have been reported to become responsive to adrenaline and noradrenaline after nerve injury [37,39,45].
In conclusion, the present study shows that distinct neurons in the SuVe respond to changes in barometric pressure. Similar mechanisms may contribute to the development of meteoropathy in humans, induced by changes in barometric pressure. How end organs in the inner ear sense barometric pressure changes remains to be elucidated.
Supporting information
(PDF)
(PDF)
Acknowledgments
We thank Drs. Y. Ohmichi, M. Ohmichi, H. Mizoguchi, and A. Sugiura for their technical assistance. We would like to thank Editage (www.editage.jp) for English language editing.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by a Chubu University Grant (30AM17) (https://www3.chubu.ac.jp/main/english/), and by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant numbers 23590710 and 15K08206) (http://www.jsps.go.jp/english/) to JS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Guedj D, Weinberger A. Effect of weather conditions on rheumatic patients. Ann Rheum Dis. 1990;49: 158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendler NH, Jamison RN, Morrison CH, Jenepher KP, Kahn Z. The relationship of diagnoses and weather sensitivity in chronic pain patients. J Neuromusc Syst. 1995;3: 10–15. [Google Scholar]
- 3.Jamison RN, Anderson KO, Slater MA. Weather changes and pain: perceived influence of local climate on pain complaint in chronic pain patients. Pain. 1995;61: 309–315. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell SW. The relations of pain to weather, being a study of the natural history of a case of traumatic neuralgia. Am J Med Sci. 1877;146: 305–329. [Google Scholar]
- 5.Okuma H, Okuma Y, Kitagawa Y. Examination of fluctuations in atmospheric pressure related to migraine. Springerplus. 2015;4: 790 10.1186/s40064-015-1592-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verges J, Montell E, Tomas E, Cumelles G, Castaneda G, Marti N, et al. Weather conditions can influence rheumatic diseases. Proc West Pharmacol Soc. 2004;47: 134–136. [PubMed] [Google Scholar]
- 7.Hollander JL, Yeostros SY. The effect of simultaneous variations of humidity and barometric pressure on arthritis. Bull Am Meteorol Soc. 1963;44: 389–393. [Google Scholar]
- 8.Sato J. Weather change and pain: a behavioral animal study of the influences of simulated meteorological changes on chronic pain. Int J Biometeorol. 2003;47(2): 55–61. 10.1007/s00484-002-0156-9 [DOI] [PubMed] [Google Scholar]
- 9.Scheidt J, Koppe C, Rill S, Reinel D, Wogenstein F, Drescher J. Influence of temperature changes on migraine occurrence in Germany. Int J Biometeorol. 2013;57(4): 649–654. 10.1007/s00484-012-0582-2 [DOI] [PubMed] [Google Scholar]
- 10.Sato J, Morimae H, Seino Y, Kobayashi T, Suzuki N, Mizumura K. Lowering barometric pressure aggravates mechanical allodynia and hyperalgesia in a rat model of neuropathic pain. Neurosci Lett. 1999;266: 21–24. [DOI] [PubMed] [Google Scholar]
- 11.Sato J, Aoyama M, Yamazaki M, Okumura S, Takahashi K, Funakubo M, et al. Artificially produced meteorological changes aggravate pain in adjuvant induced arthritic rats. Neurosci Lett. 2004;354: 46–49. [DOI] [PubMed] [Google Scholar]
- 12.Funakubo M, Sato J, Honda T, Mizumura K. The inner ear is involved in the aggravation of nociceptive behavior induced by lowering barometric pressure of nerve injured rats. Eur J Pain. 2010;14: 32–39. 10.1016/j.ejpain.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 13.Funakubo M, Sato J, Mizumura K. Existence of the neurons responding to barometric pressure decrease in the vestibular nuclei of rats. J Physiol Sci. 2009;59(Suppl.1): 162. [Google Scholar]
- 14.Funakubo M, Sato J, Obata K, Mizumura K. The rate and magnitude of atmospheric pressure change that aggravate pain-related behavior of nerve injured rats. Int J Biometeorol. 2011;55(3): 319–326. 10.1007/s00484-010-0339-8 [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 4th ed London: Academic Press; 2013. [Google Scholar]
- 16.Nishihara S, Gyo K, Yanagihara N. Transmission of change in the atmospheric pressure of the external ear to the perilymph. Am J Otol. 1992;13(4): 364–368. [PubMed] [Google Scholar]
- 17.Suzuki M, Kitahara M, Kitano H. The influence of middle ear pressure changes on the primary vestibular neurons in guinea pigs. Acta Otolaryngol. 1994;114(sup510): 9–15. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M, Kitano H, Yazawa Y, Kitajima K. The influence of rates of pressure change on pressure-induced vestibular response in guinea pigs. J Otolaryngol Jpn. 1995;98: 820–824. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M, Kitano H, Yazawa Y, Kitajima K. Involvement of round and oval windows in the vestibular response to pressure changes in the middle ear of guinea pigs. Acta Otolaryngol. 1998;118(5): 712–716. [DOI] [PubMed] [Google Scholar]
- 20.Sprott RL. Barometric Pressure Fluctuations: Effects on the activity of laboratory mice. Science. 1967;157(3793): 1206–1207. [DOI] [PubMed] [Google Scholar]
- 21.Breuner CW, Sprague RS, Patterson SH, Woods HA. Environment, behavior and physiology: do birds use barometric pressure to predict storms? J Exp Biol. 2013;216(11): 1982–1990. [DOI] [PubMed] [Google Scholar]
- 22.Kreithen ML, Keeton WT. Detection of changes in atmospheric pressure by the homing pigeon, Columba livia. J Comp Physiol. 1974;89: 73–82. [Google Scholar]
- 23.von Bartheld CS, Giannessi F. The paratympanic organ: a barometer and altimeter in the middle ear of birds? J Exp Zool B Mol Dev Evol. 2011;316(6): 402–408. 10.1002/jez.b.21422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Bartheld CS. Development and innervation of the paratympanic organ (Vitali organ) in chick embryos. Brain Behav Evol. 1990;35: 1–15. 10.1159/000115851 [DOI] [PubMed] [Google Scholar]
- 25.von Bartheld CS. Functional morphology of the paratympanic organ in the middle ear of birds. Brain Behav Evol. 1994;44: 61–73. 10.1159/000113570 [DOI] [PubMed] [Google Scholar]
- 26.Lundgren CE. Alternobaric vertigo–A diving hazard. Br Med J. 1965;2(5460): 511–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundgren CE, Malm LU. Alternobaric vertigo among pilots. Aerosp Med. 1966;37(2): 178–180. [PubMed] [Google Scholar]
- 28.Bluestone CD, Swarts JD, Furman JM, Yellon RF. Persistent alternobaric vertigo at ground level. Laryngoscope. 2012;122(4): 868–872. 10.1002/lary.22182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endara-Bravo A, Ahoubim D, Mezerhane E, Abreu RA. Alternobaric vertigo in a patient on positive airway pressure therapy. J Clin Sleep Med. 2013;9(12): 1347–1348. 10.5664/jcsm.3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato J, Aono S, Sakurai H, Saito A, Toda M, Ushida T. Lowered threshold for self-motion perception to galvanic vestibular stimulation in patients suffering from weather-related pain. 16th World Congress of Pain 2016, Abstract No. 1643.
- 31.Imagawa M, Isu N, Sasaki M, Endo K, Ikegami H, Uchino Y. Axonal projections of utricular afferents to the vestibular nuclei and the abducens nucleus in cats. Neurosci Lett. 1995;186(2–3): 87–90. [DOI] [PubMed] [Google Scholar]
- 32.Imagawa M, Graf W, Sato H, Suwa H, Isu N, Izumi R, et al. Morphology of single afferents of the saccular macula in cats. Neurosci Lett. 1998;240(3): 127–130. [DOI] [PubMed] [Google Scholar]
- 33.Sato F, Sasaki H, Ishizuka N, Sasaki S, Mannen H. Morphology of single primary vestibular afferents originating from the horizontal semicircular canal in the cat. J Comp Neurol. 1989;290(3): 423–439. 10.1002/cne.902900310 [DOI] [PubMed] [Google Scholar]
- 34.Porter JD, Balaban CD. Connections between the vestibular nuclei and brain stem regions that mediate autonomic function in the rat. J Vestib Res. 1997;7: 63–76. [PubMed] [Google Scholar]
- 35.Yates BJ, Balaban CD, Miller AD, Endo K, Yamaguchi Y. Vestibular inputs to the lateral tegmental field of the cat: potential role in autonomic control. Brain Res 1995;689: 197–206. [DOI] [PubMed] [Google Scholar]
- 36.Sato J, Takanari K, Omura S, Mizumura K. Effects of lowering barometric pressure on guarding behavior, heart rate and blood pressure in a rat model of neuropathic pain. Neurosci Lett. 2001;299: 17–20. [DOI] [PubMed] [Google Scholar]
- 37.Bossut DF, Perl ER. Effects of nerve injury on sympathetic excitation of A delta mechanical nociceptors. J Neurophysiol. 1995;73: 1721–1723. 10.1152/jn.1995.73.4.1721 [DOI] [PubMed] [Google Scholar]
- 38.Devor M, Janig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71: 38–47. 10.1152/jn.1994.71.1.38 [DOI] [PubMed] [Google Scholar]
- 39.Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251: 1608–1610. [DOI] [PubMed] [Google Scholar]
- 40.Markia B, Kovács ZI, Palkovits M. Projections from the vestibular nuclei to the hypothalamic paraventricular nucleus: morphological evidence for the existence of a vestibular stress pathway in the rat brain. Brain Struct Funct. 2008;213(1–2): 239–245. 10.1007/s00429-008-0172-6 [DOI] [PubMed] [Google Scholar]
- 41.Jang SH, Lee MY, Yeo SS, Kwon HG. Structural neural connectivity of the vestibular nuclei in the human brain: a diffusion tensor imaging study. Neural Regen Res. 2018;13(4): 727–730. 10.4103/1673-5374.230304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aloisi AM, Buonocore M, Merlo L, Galandra C, Sotgiu A, Bacchella L, et al. Chronic pain therapy and hypothalamic-pituitary-adrenal axis impairment. Psychoneuroendocrinology. 2011;36(7): 1032–1039. 10.1016/j.psyneuen.2010.12.017 [DOI] [PubMed] [Google Scholar]
- 43.Eller-Smith OC, Nicol AL, Christianson JA. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci. 2018;12: 35 10.3389/fncel.2018.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Generaal E, Vogelzangs N, Macfarlane GJ, Geenen R, Smit JH, Penninx BW, et al. Reduced hypothalamic-pituitary-adrenal axis activity in chronic multi-site musculoskeletal pain: partly masked by depressive and anxiety disorders. BMC Musculoskelet Disord. 2014;15: 227 10.1186/1471-2474-15-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Halloran KD, Perl ER. Effects of partial nerve injury on the responses of C-fiber polymodal nociceptors to adrenergic agonists. Brain Res. 1997;759(2): 233–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.