Abstract
Sepsis is a life threatening condition which produces multi-organ dysfunction with profound circulatory and cellular derangements. Administration of E.Coli endotoxin (LPS) produces systemic inflammatory effects of sepsis including disruption of endothelial barrier, and if severe enough death. Whole body periodic acceleration (pGz) is the headward-footward motion of the body. pGz has been shown to induce pulsatile shear stress to the endothelium, thereby releasing vascular and cardio protective mediators. The purpose of this study was to determine whether or not pGz performed as a pre-treatment or post-treatment strategy improves survival in a lethal murine endotoxin model.This study was designed as a prospective randomized controlled study in mice. pGz was performed in mice as pre-treatment (pGz-LPS, 3 days prior to LPS), post-treatment (LPS- pGz, 30 min after LPS) strategies or Control (LPS-CONT), in a lethal murine model of endotoxemia. Endotoxemia was induced with intraperitoneal injection of E.Coli LPS (40mg/kg). In a separate group of mice, a nonspecific nitric oxide synthase inhibitor (L-NAME) was provided in their drinking water and pGz-LPS and LPS-pGz performed to determine the effect of nitric oxide (NO) inhibition on survival. In another subset of mice, micro vascular leakage was determined. Behavioral scoring around the clock was performed in all mice at 30 min intervals after LPS administration, until 48 hrs. survival or death. LPS induced 100% mortality in LPS-CONT animals by 30 hrs. In contrast, survival to 48 hrs. occurred in 60% of pGz-LPS and 80% of LPS-pGz. L-NAME abolished the survival effects of pGz. Microvascular leakage was markedly reduced in both pre and post pGz treated animals and was associated with increased tyrosine kinase endothelial-enriched tunica interna endothelial cell kinase 2 (TIE2) receptor and its phosphorylation (p-TIE2). In a murine model of lethal endotoxemia, pGz performed as a pre or post treatment strategy significantly improved survival, and markedly reduced microvascular leakage. The effect was modulated, in part, by NO since a non-selective inhibitor of NO abolished the pGz survival effect.
Introduction
Sepsis is a life-threatening condition of multi-organ dysfunction caused by a dysregulated host response to infection. Septic shock is a subset of sepsis in which profound circulatory cellular and metabolic abnormalities are associated with a greater risk of mortality than sepsis alone [1]. Sepsis affects more than 1.5 million humans in the USA with mortality rates of 15–30% [2]. The economic burden of sepsis is highly significant. The Agency for Healthcare Research and Quality lists sepsis as the most expensive condition treated in U.S. hospitals, costing nearly $24 billion in 2013, and accounting for 6.2% of the aggregate costs for hospitalization in the USA [3]. Despite hundreds of treatment trials dating to the 1960s, interventions to improve survival from sepsis have not significantly lowered mortality.
Administration of lipopolysaccharide (LPS), the endotoxin derived from the purified outer membrane of E. Coli, produces systemic inflammatory effects of sepsis in mice. Exposure to LPS causes a dose-dependent activation of a widespread cascade of inflammatory mediators that disrupts the endothelial barrier. This leads to intracellular hyperpermeability, multiple organ dysfunction and if sufficiently severe, death [4]. Such a situation calls for an effective, prompt, endothelial repair strategy that has not yet been promulgated. Large quantities of nitric oxide that are released into the circulation through the action of inducible nitric oxide synthase (iNOS) are an important component of this inflammatory cascade in sepsis disruption to the endothelial barrier. In contrast, small quantities of nitric oxide normally released from endothelial nitric oxide (eNOS) by flowing blood are a crucial determinate of inter-endothelial junctions [5]. The potential effectiveness of eNOS was reported by Yamashita et al almost 20 years ago in which chronic overexpression of endothelial derived NO by transgenic mice resulted in resistance to LPS-induced hypotension, lung injury and death [6].
In this paper, we employed non-invasive, periodic acceleration (pGz), a means to increase pulsatile shear stress to the endothelium a phenomenon that also takes place during exercise to stimulate increase release of NO into the circulation as an alternative to overexpression by transgenic mice. This was accomplished by rapidly and repetitively moving a mouse in headward-footward direction to induce pulsatile shear stress to the endothelium [7–9]. We and others reported that pulsatile shear increases expression of both endothelial derived nitric oxide synthase (eNOS) and neuronal derived nitric oxide synthase (nNOS) both which are produced in nanomolar concentrations, and are important in modulating the anti-inflammatory response in sepsis [9–11]. We have previously shown in animal models of whole body and focal ischemia reperfusion injury of the heart, brain, and skeletal muscles, that pGz improves outcomes, [12–19]. In part, the effects of pGz are related to increased release of eNOS into the circulation as well as prostaglandins, adrenomedullin, and signaling via Phosphoinositide 3-kinase protein kinase B pathway (PI3K-AKT). pGz also reduces intracellular calcium overload at the cellular level. [10, 11, 19–22].
We hypothesized that pGz performed as a pre-treatment or post-treatment strategy in a lethal murine endotoxin model might confer improved survival.
Materials and methods
Animal preparation & pGz
This study protocol was approved by the Institutional Animal Care and Use Committee of Mount Sinai Medical Center (which maintains accreditation with AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International) and Office for Laboratory Animal Welfare (Assurance # A3044-01)
The protocol conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85–23, revised 1996) Protocol No. 17-20-A-04.
The motion platform that imparts pGz has been previously described [18, 23]. The platform moves the horizontally placed body in the z-plane. The frequency of periodic acceleration has been previously determined in our laboratory for mice to be 480cpm. The mice were acclimated to a mouse holder (Kent Scientific Design, Torrington, CT) for 2 days and thereafter the mice voluntarily walked into the mice holder. The mice holder was placed on the pGz motion platform. pGz was carried out at a frequency of 480 cycles/minute and acceleration in the z-plane (Gz) of ± 3.9 m/sec2
Experimental design
Survival experiments
Prior to LPS inoculation, mice were randomly assigned to one of three groups; a) Pre-treatment (pGz-LPS) (n = 12) pGz was performed 1 hrs. per day for 3 days, b) Post-treatment (LPS-pGz) (n = 12) pGz was performed starting 30 min after LPS and continued for 1 hrs., c) LPS Control (LPS-CONT) (n = 12). Mice received an intraperitoneal injection of E.Coli lipopolysaccharide (Sigma Aldrich, St. Luis, MO) at a lethal dose of 40mg/kg diluted in phosphate buffered saline, total volume 0.1ml. A separate group of mice received same volume of phosphate buffered saline but did not receive LPS or pGz and were used as Sham (n = 8) controls. After LPS injection animals were returned to their cage with access to food and water ad libitum.
Behavioral scoring was performed in all animals for the initial 48 hrs. after LPS injection. The Behavioral Scoring criteria utilized has been described by Shrum et al [24]. The Behavioral Scoring was amended to include stool quality as additional criteria, with a maximum score of 32 Table A in S1 File. Animals were humanely euthanized within 15 min once a score of 28 was reached. Behavioral Scoring was performed every 30 min after LPS for the first 2 hrs, thereafter every 1hr for until 10hrs, and thereafter every 2 hrs until 48 hrs.
Nitric oxide inhibition
A separate group of animals received a non-specific nitric oxide synthase inhibitor (L-NAME, 1.5mg/ml) in their drinking water for 5 days and were randomized prior to LPS injection (same dose as above) to receive pGz pre-treatment (n = 8) post-treatment(n = 8) or LNAME-Control (n = 8).
Determination of microvascular leakage
LPS increases microvascular permeability, with endothelial tight junction disruption, and increased endothelium permeability. To determine the severity of microvascular leakage and the effects of pGz on such, a separate group of animals was injected intravenously with 0.5% sterile filtered Evans Blue at a dose of 8ml/kg (total volume 0.2ml) 5 hrs. after LPS. The animals were randomized to LPS-CONT (n = 8) pGz-LPS (n = 8), LPS-pGz (n = 8), or Sham (received Evans Blue but did not received LPS or pGz, n = 4). At 6 hrs., the animals were sacrificed and Evans Blue extravasation was quantitated using SpectraMax Plate Reader (Molecular Devices, Sunnyvale, CA) [25]. Microvascular leakage was determined in the lungs, liver and mesenteric circulation (testis).
Protein expression
Protein extraction was performed as previously described. Protein analysis was performed using Western Blot method and visualized by enhanced chemifluorescence S1 File.
Euthanasia
After completion of each of the experimental protocols animals were euthanized by a method approved by the American Veterinary Medical Association Guidelines on Euthanasia S1 File.
Statistical analysis
All values are reported as means ± SEM. Continuous variables were evaluated by analysis of variance for repeated measures. For variables with significant differences, post hoc analysis was done using Tukey HSD for equal or unequal sample size. Comparisons of discrete variables were evaluated by Fisher’s exact test. Statistical analyses were performed using STATISTICA (StatSoft Inc., Tulsa, OK). Sample size was calculated using Statistica based on power analysis with α = 0.05 and power 0.80. A p value of < 0.05 was considered statistically significant.
Results
Behavioral scores & survival
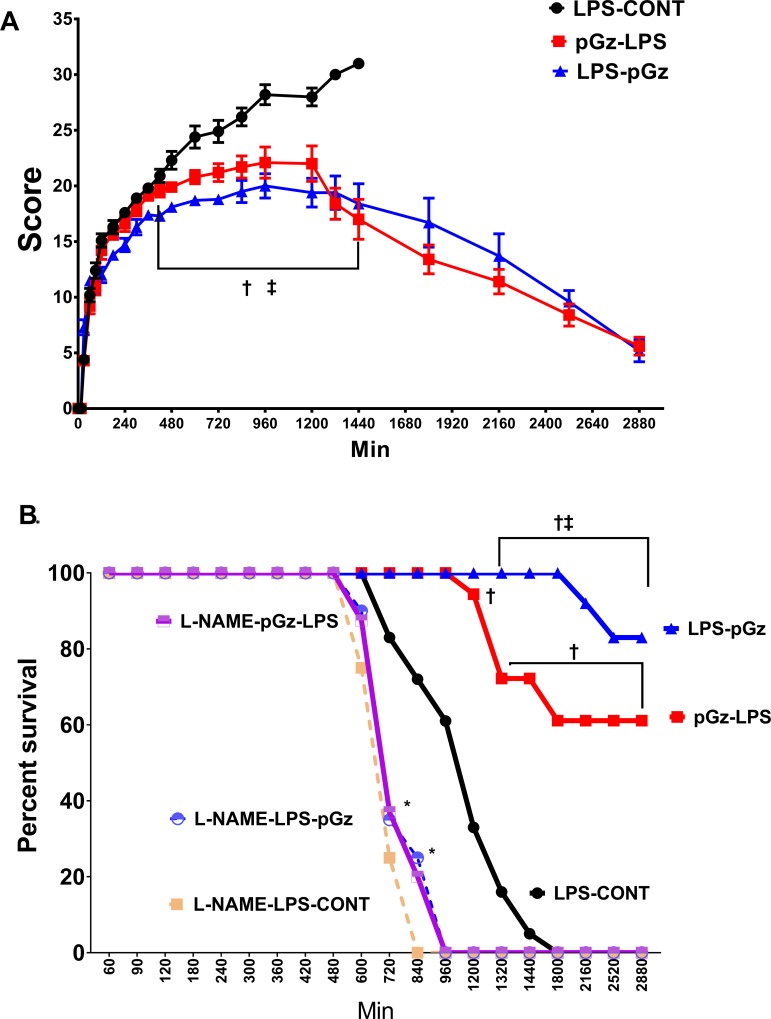
Using the behavioral scoring criteria of Shrum et al [24], there were significant differences in outcomes between LPS-CONT compared to pre-treated (pGz-LPS) and post-treated (LPS-pGz) 8 hrs. after LPS (Fig 1). At 30 hrs. after LPS injection, nontreated animal had 100% mortality. In contrast, pGz-LPS and LPS-pGz had 60 and 80% survival (p < 0.001). The survival benefit of pGz persisted beyond 48 hrs. without additional pGz treatment. L-NAME (non-selective NO inhibitor) significantly reduced survival for both non treated and pGz treated animals. Survival for LNAME animals did not surpass 16 hrs. (Fig 1). pGz-LPS and LPS-pGz animals which survived beyond 48 hrs., appeared normal 14 days after completion of the study. Their weight gain feeding and grooming habits were not different than normal animals. We did not extend animal behavior observations beyond these 14 days. None of the surviving animals where treated with pGz after completion of the study protocol.
Fig 1. Behavioral scoring and survival Kaplan-Meyers curve in mice after lethal dose of LPS.
(A) Mean behavioral scores in mice every 30 mins, using scoring criteria of Schrum et al [24] in LPS-CONT, pGz pre-treatment (pGz-LPS) and pGz post-treatment (LPS-pGz). Scoring was begun 30 min after LPS injection and continued until death or survival for up to 48 hrs. (B) Survival in mice exposed to a lethal dose of LPS over the initial 48 hrs. Fifty percent survival in LPS treated mice was reached at 20 hrs. after LPS in control animals (LPS-CONT), in contrast all LNAME pretreated animals reached 50% survival at 12 hrs. 80% of LPS-pGz and 60% of pGz-LPS animals survived beyond 48 hrs. Data are mean ± SEM (†p<0.01 pGz-LPS or LPs-pGz vs. LPS-CONT ǂ p< 0.01 LPS-pGz vs. pGz-LPS, * p< 0.01 L-NAME-LPS-pGz or L-NAME-pGz-LPS or L-NAME-LPS-CONT vs LPS-CONT).
Microvascular leakage and TIE2 expression
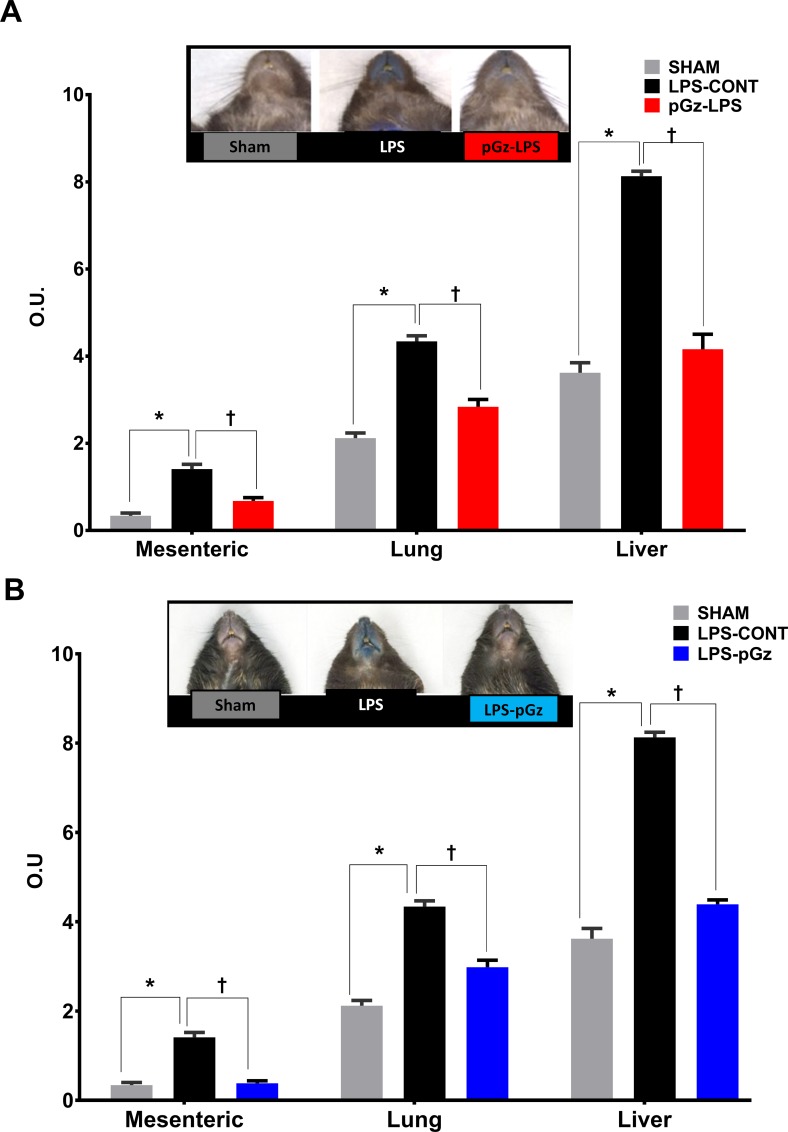
LPS induced a significant increase of at least double the amount of microvascular leakage determined in Sham animals (which received Evans Blue, but did not receive LPS or pGz) in mesenteric, lung and liver vasculature. pGz pre and post treatments reduced microvascular leakage in all three vascular beds by 50% (Fig 2).
Fig 2. Microvascular leakage after LPS in mesenteric, lung and liver vasculature.
Mesenteric, lung and liver microvascular leakage determined by spectrophotometric optical density (OD) of Evans Blue, in Sham, LPS-CONT and pGz pretreated (pGz-LPS) (A) and (B) post-treated mice (LPS-pGz) mice. LPS-CONT (n = 8) pGz-LPS (n = 8), LPS-pGz (n = 8), or Sham (received Evans Blue but did not received LPS or pGz, n = 4). Data are mean ± SEM. (* p<0.01 vs. Sham, Ɨ p<0.01 vs. LPS-CONT).
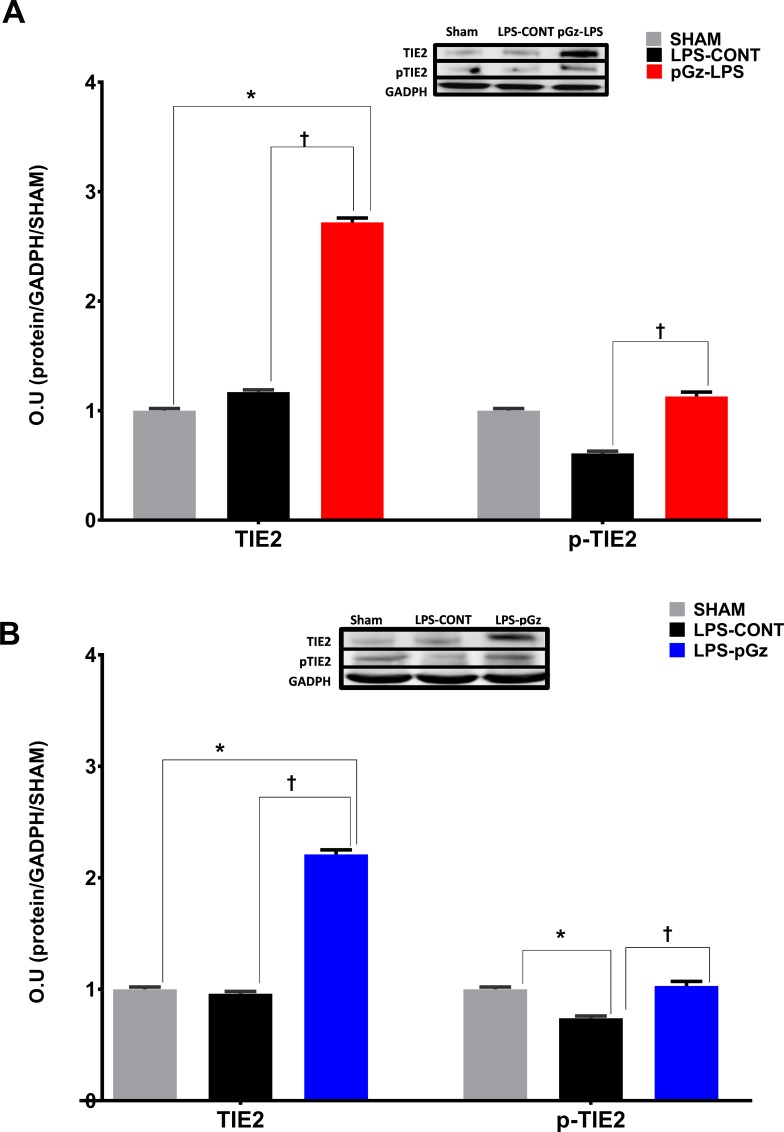
Expression of the tyrosine kinase endothelial-enriched tunica interna endothelial cell kinase 2 (TIE2) receptor was unchanged with LPS but both pre and post treatment with pGz increased TIE2 by 150%. In contrast, p-TIE2 decreased after LPS by 50% and pre and post treatment with pGz increased the latter to Sham levels (Fig 3).
Fig 3. Expression and phosphorylation of TIE2 in mice after LPS.
Expression of the tyrosine kinase receptor tunica interna endothelial cell kinase 2 (TIE2) and its phosphorylation in mice, 6 hrs. after LPS. LPS did not significantly change expression of TIE2, but significantly decreased TIE2 phosphorylation. (A) pGz pre-treatment (pGz-LPS) and (B) pGz post-treatment (LPS-pGz) significantly increased both TIE2 expression and phosphorylation. Inserts are representative western blots of protein expression of TIE2, p-TIE and GADPH in Sham, LPS-Control, and pGz-LPS and LPS-pGz. LPS-CONT (n = 8) pGz-LPS (n = 8), LPS-pGz (n = 8), or Sham (received equal volume of phosphate buffer but did not received LPS or pGz, n = 4). Data are mean ± SEM (* p<0.01 vs Sham, Ɨ p<0.01 vs. LPS-CONT).
Discussion
The present study demonstrates that pGz as a pre or post treatment strategy significantly improves survival in a lethal model of LPS induced endotoxin shock. To our knowledge, our study is the first to show that repetitive passive motion of the body as produced by pGz, markedly improves endotoxin shock survival, accompanied by a decrease of microvascular leakage in lungs, liver and mesenteric vasculature.
Interventions such as exercise and remote ischemic pre and post conditioning have been used as therapeutic modalities in mice models of LPS induced inflammation. Table 1 summarizes exercise or remote ischemic pre or post treatment interventions with doses of LPS and survival outcomes in all studies which have reported at least 24 hrs. survival in mice.
Table 1. Comparison of studies using non pharmacological pre or post treatment strategies in LPS induced sepsis in mice.
| Author | Year | Species | Dose of LPS | Pre or Post Treatment | Mode | 24 hr Survival |
48 hr Survival |
|---|---|---|---|---|---|---|---|
| Ishizashi et al(28) | 1995 | 5 week old C57BL/6J |
IV LPS 20mg/kg | Pre-Treated (P. acnes, IP 7 days before exercise). Exercise 120 min prior to LPS |
Voluntary Wheel Running | Control = 0 Exerc = 22% |
|
| Martin et al (27) | 2013 | 22 month C57BL/6J | Max Dose IP 0.33mg/kg | Pre treatment 10 weeks | Voluntary wheel running | Control = 73% Exerc = 100% |
|
| Kim et al (29) | 2014 | 6 weeks mice BALB/c |
IP 20 mg/kg | Remote Ischemic Pre (RIPC) & Post Conditioning (RpostC), immediately before or after LPS |
Hind-limb tourniquet 3 cycles (10min Ischemia/10min reperfusion) |
Control = 80% RIPC = 100% RpostC = 90% |
Control = 15% RIPC = 65% Rpostc = 65% |
| Irahara et al (26) | 2016 | 11 weeks C57BL/6J |
Max Dose IP 10mg/kg | Post Treatment exercise |
Treadmill 30 min X 3 after LPS and 30 min X 2 next day | Control = 80% Exercise = 100% |
Control = 60% Exercise = 100% |
| Adams et al Current work |
2018 | 6–10 weeks C57BL/6J | IP 40mg/kg | Pre and Post Treatment | pGz Pre Treatment = 3 days Post Treatment = 30 min after LPS |
Control = 6% pGz-LPS = 75% LPS-pGz = 100% |
LPS-Control = 0% pGz-LPS = 61% LPS-pGz = 83% |
Compendium of mice studies using non pharmacological interventions pre or post treatment in models of LPS induced endotoxin in mice published from 1995 to 2018 with at least 24 hrs. survival. Each study shows the dose of LPS, method of administration (IP = intraperitoneal, IV = intravenous) the pre or post treatment strategy, the modality and 24 and 48 hrs. survival.
Only one study utilized exercise as a post-treatment strategy, viz., treadmill exercise during the acute phase after LPS up to 72 hrs. [26]. Survival in sedentary controls at the highest dose of LPS (10mg/kg) was 50%, whereas 100% survived in low dose LPS control and exercise groups. The highest dose used by this investigation was 25% of the LPS dose used in our study. Based on our behavioral score data it is obvious that exercise after a lethal dose of LPS such as the one used in our experiments cannot be performed because of the severity of symptoms. Our data is consistent with other investigators who have also shown improved survival when animals are pre-treated with an active exercise intervention [27–29].
Survival was significantly decreased in L-NAME treated animals. The latter was not surprising since other animal studies have also shown similar findings with non-selective NOS inhibition [30, 31]. Human studies performed with nonselective NOS inhibition also support the latter [32, 33]. We have previously shown the time course of eNOS upregulation and phosphorylation in both animal models and cells under pGz [11, 23]. Data are conflicting with regards to eNOS and survival and outcomes from LPS inflammatory response and sepsis induced by cecal ligation. Some studies using eNOS deficient mice demonstrate that eNOS deficiency decreases mortality and improves outcomes from LPS sepsis [34, 35] while others suggest the opposite [6, 36–38]. All three NOS isoforms play a role in sepsis, however, the extent timing, tissue location and eNOS uncoupling are all important to take into consideration [6, 34–37, 39–41]. eNOS has been shown to modulate inflammatory response, and particularly that of NFĸβ and its crosstalk with iNOS [42]. The effects of eNOS on other cytokines also remains to be determined. It is important to acknowledge that a single plausible mechanism for our findings is not possible, since pGz produces a multifaceted response of cytoprotective proteins, including antioxidant defense [23, 43].
Microvascular leakage has been described in various models of sepsis including LPS and its etiology multifactorial, with endothelial dysfunction and disruption of tight junctions via various mechanisms [44, 45]. We have shown that pGz both pre and post treatment decreases microvascular leakage induced by LPS. The decrease in microvascular leakage by pGz is also likely multifactorial. Microvascular leakage has been shown by others to be decreased by eNOS. [46]. We also found that pGz produces a significant increase in Tie2 and restoration of p-Tie2. Tie2 is a tyrosine receptor kinase which has been shown to be important in maintenance of tight junctions and decreasing vascular hyperpermeability and specifically during sepsis [47, 48]. Tie2 stimulation can promote a broad range of microvascular anti-leakage including endothelial activation. David et al showed a decrease in total Tie2 expression and phosphorylation in LPS treated mice and a restoration and improved survival in mice pretreated with a known Tie2 agonist (vaculotide) [49]. The latter has been shown to improve endothelial tight junctions. Oxidative stress has been shown by others to be a hallmark of the inflammatory response, and to play a role in microvascular leakage. We did not specifically studied ROS production in this study but we have previously shown that pGz in mice also increases antioxidant enzymes (SOD, Catalase) and total antioxidant capacity [23].
Potential clinical application
Increase pulsatile shear stress to the vascular endothelium by way of mechanical addition of pulsations is a novel method with clinical applicability. A motion platform to produce Whole Body Periodic Acceleration in humans was formerly available but is no longer being marketed (NIMS, Miami FL 33137) However, it was not portable because it weighed 179 kg and was difficult to perform nursing care while subject was moving back and forth.
A new non-invasive, portable, passive simulated jogging device (JD) has recently been fabricated and tested in normal and hypertensive humans as a clinical trial with favorable benefits. [50, 51] This device utilizes microprocessor controlled, DC motorized movements of foot pedals placed within a chassis to repetitively tap against a semi-rigid surface for simulation of locomotion activities while the subject is seated or lying in a bed (Gentle Jogger, Sackner Wellness Products LLC, Miami FL 33132). It weighs about 4.5 kg with chassis dimensions of 34 x 35 x 10 cm. It is placed on the floor for seated applications and secured to the footplate of a bed for supine applications. Its foot pedals rapidly and repetitively alternate between right and left pedal movements to actively lift the forefeet upward about 2.5 cm followed by active downward tapping against a semi-rigid bumper placed within the chassis. In this manner, it simulates feet impacting against the ground during selective speeds of locomotor activities. Each time the moving foot pedals strike the bumper, a small pulse is added to the circulation as a function of pedal speed that produce up to 190 steps per minute, thereby increasing pulsatile shear stress to the endothelium with its benefits as in the current mice study.
The maximum acceleration forces in seated posture from a triaxial accelerometer during run speed operation of JD was ±5.4 m/s2 over tibia, ±5.1 m/s2 over femur, and ± 1.0 m/s2 over forehead. The maximum acceleration forces in supine posture from a triaxial accelerometer during run speed operation of JD was ±2.9 m/s2 over tibia, ±6.3 m/s2 over femur and ±3.6 m/s2 over the forehead. Clinical trials for prevention and treatment of human sepsis will be needed to demonstrate its effectiveness of this safe low risk modality according to FDA wellness guidelines.
Study limitations
We must also acknowledge what others have previously recognized and thoroughly reviewed about the LPS murine model and its differences between it and other murine models of sepsis and clinical sepsis [52–54]. This study addresses short term survival and microvascular leakage, in LPS induced septic shock and long term outcomes could not be addressed The LPS dose utilized in our experiments was specifically selected due to the severity of injury produced, thereby providing a model with very high mortality. We utilized an orally administered dose of L-NAME, a nonspecific NO inhibitor, since specific eNOS inhibitors in mice are lacking. It can be argued that the exact dose of L-NAME each animal received may be slightly different, however the effects of NO inhibition on survival from LPS were uniformly dismal. We also did not compare pGz to any other intervention, since there are no other passive exercise strategies suitable for comparison in mice with this level of LPS induced injury. The translation of these findings to human sepsis, must be done with caution. There are important differences between species (rodents and humans) as to their nitric oxide response to LPS or sepsis, including genomic differences for arginine metabolism and regulation of arginase. [55–57]
Notwithstanding the above limitations, we conclude that pGz is a simple method which increases survival, and reduces microvascular leakage in a lethal mouse model of LPS induced endotoxin shock. pGz may serve as an adjunctive therapeutic strategy to current pharmacological modalities to ameliorate or improve outcomes from sepsis. Human clinical trials are needed to confirm efficacy in human sepsis.
Supporting information
A table also contains Behavioral Scoring Criteria in Mice, amended to include stool quality criteria, a maximal worse score is 32.
(PDF)
Acknowledgments
This study was supported in part by a grant from the Florida Heart Research Institute.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded in part by a grant to JAA from the Florida Heart Research Institute (https://www.floridaheart.org). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. 10.1001/jama.2016.0287 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershey TB, Kahn JM. State Sepsis Mandates—A New Era for Regulation of Hospital Quality. The New England journal of medicine. 2017;376(24):2311–3. 10.1056/NEJMp1611928 . [DOI] [PubMed] [Google Scholar]
- 3.Martin AB, Hartman M, Benson J, Catlin A, National Health Expenditure Accounts T. National Health Spending In 2014: Faster Growth Driven By Coverage Expansion And Prescription Drug Spending. Health Aff (Millwood). 2016;35(1):150–60. 10.1377/hlthaff.2015.1194 . [DOI] [PubMed] [Google Scholar]
- 4.Di Lorenzo A, Lin MI, Murata T, Landskroner-Eiger S, Schleicher M, Kothiya M, et al. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J Cell Sci. 2013;126(Pt 24):5541–52. 10.1242/jcs.115972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289(3):L371–81. 10.1152/ajplung.00175.2004 . [DOI] [PubMed] [Google Scholar]
- 6.Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Ueyama T, Ishida T, et al. Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation. 2000;101(8):931–7. . [DOI] [PubMed] [Google Scholar]
- 7.Adams JA, Mangino MJ, Bassuk J, Sackner MA. Hemodynamic effects of periodic G(z) acceleration in meconium aspiration in pigs. J Appl Physiol (1985). 2000;89(6):2447–52. 10.1152/jappl.2000.89.6.2447 . [DOI] [PubMed] [Google Scholar]
- 8.Adams JA, Mangino MJ, Bassuk J, Kurlansky P, Sackner MA. Regional blood flow during periodic acceleration. Critical care medicine. 2001;29(10):1983–8. . [DOI] [PubMed] [Google Scholar]
- 9.Uryash A, Wu H, Bassuk J, Kurlansky P, Sackner MA, Adams JA. Low-amplitude pulses to the circulation through periodic acceleration induces endothelial-dependent vasodilatation. J Appl Physiol (1985). 2009;106(6):1840–7. Epub 2009/03/28. 10.1152/japplphysiol.91612.2008 . [DOI] [PubMed] [Google Scholar]
- 10.Wu H, Jin Y, Arias J, Bassuk J, Uryash A, Kurlansky P, et al. In vivo upregulation of nitric oxide synthases in healthy rats. Nitric Oxide. 2009;21(1):63–8. 10.1016/j.niox.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H, Uryash A, Bassuk J, Kurlansky P, Giridharan GA, Shakeri M, et al. Mechanisms of Periodic Acceleration Induced Endothelial Nitric Oxide Synthase (eNOS) Expression and Upregulation Using an In Vitro Human Aortic Endothelial Cell Model. Cardiovascular Engineering and Technology. 2012;3(3):292–301. 10.1007/s13239-012-0096-4 [DOI] [Google Scholar]
- 12.Adams JA, Bassuk J, Aria J, Wu H, Jorapur V, Lamas G, et al. Acute Effects of “Delayed Postconditioning” With Periodic Acceleration After Asphyxia Induced Shock in Pigs. Pediatric Research. 2008;64(5):533–7. 10.1203/PDR.0b013e318183f147 [DOI] [PubMed] [Google Scholar]
- 13.Adams JA, Wu H, Bassuk JA, Arias J, Uryash A, Jorapur V, et al. Periodic acceleration (pGz) prior to whole body ischemia reperfusion injury provides early cardioprotective preconditioning. Life Sci. 2010;86(19–20):707–15. Epub Epub 2010 Mar 6. 10.1016/j.lfs.2010.02.022 . [DOI] [PubMed] [Google Scholar]
- 14.Bassuk JI, Wu H, Arias J, Kurlansky P, Adams JA. Whole body periodic acceleration (pGz) improves survival and allows for resuscitation in a model of severe hemorrhagic shock in pigs. The Journal of surgical research. 2010;164(2):e281–9. Epub 2010/09/28. 10.1016/j.jss.2010.07.047 . [DOI] [PubMed] [Google Scholar]
- 15.Uryash A, Wu H, Bassuk J, Kurlansky P, Adams JA. Preconditioning with periodic acceleration (pGz) provides second window of cardioprotection. Life Sci. 2012;91(5–6):178–85. Epub 2012/07/14. 10.1016/j.lfs.2012.06.031 . [DOI] [PubMed] [Google Scholar]
- 16.Serravite DH, Perry A, Jacobs KA, Adams JA, Harriell K, Signorile JF. Effect of whole-body periodic acceleration on exercise-induced muscle damage after eccentric exercise. International journal of sports physiology and performance. 2014;9(6):985–92. 10.1123/ijspp.2013-0512 . [DOI] [PubMed] [Google Scholar]
- 17.Uryash A, Bassuk J, Kurlansky P, Altamirano F, Lopez JR, Adams JA. Non-invasive technology that improves cardiac function after experimental myocardial infarction: Whole Body Periodic Acceleration (pGz). PLoS One. 2015;10(3):e0121069 10.1371/journal.pone.0121069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez JR, Mijares A, Kolster J, Henriquez-Olguin C, Zhang R, Altamirano F, et al. Whole Body Periodic Acceleration Improves Muscle Recovery after Eccentric Exercise. Medicine and science in sports and exercise. 2016;48(8):1485–94. 10.1249/MSS.0000000000000932 . [DOI] [PubMed] [Google Scholar]
- 19.Lopez JR, Uryash A, Kolster J, Esteve E, Zhang R, Adams JA. Enhancing Endogenous Nitric Oxide by Whole Body Periodic Acceleration Elicits Neuroprotective Effects in Dystrophic Neurons. Molecular neurobiology. 2018;55(11):8680–94. Epub 2018/03/28. 10.1007/s12035-018-1018-8 . [DOI] [PubMed] [Google Scholar]
- 20.Bassuk JA, Wu D, Lozano H, Arias J, Kurlansky P, Lamas GA, et al. Non-selective cyclooxygenase inhibition before periodic acceleration (pGz) cardiopulmonary resuscitation (CPR) in a porcine model of ventricular fibrillation. Resuscitation. 2008;77(2):250–7. 10.1016/j.resuscitation.2007.11.018 . [DOI] [PubMed] [Google Scholar]
- 21.Martinez A, Arias J, Bassuk JA, Wu H, Kurlansky P, Adams JA. Adrenomedullin is increased by pulsatile shear stress on the vascular endothelium via periodic acceleration (pGz). Peptides. 2008;29(1):73–8. 10.1016/j.peptides.2007.10.021 . [DOI] [PubMed] [Google Scholar]
- 22.Lopez JR, Kolster J, Zhang R, Adams J. Increased constitutive nitric oxide production by whole body periodic acceleration ameliorates alterations in cardiomyocytes associated with utrophin/dystrophin deficiency. Journal of molecular and cellular cardiology. 2017;108:149–57. 10.1016/j.yjmcc.2017.06.004 . [DOI] [PubMed] [Google Scholar]
- 23.Uryash A, Bassuk J, Kurlansky P, Altamirano F, Lopez JR, Adams JA. Antioxidant Properties of Whole Body Periodic Acceleration (pGz). PLoS One. 2015;10(7):e0131392 10.1371/journal.pone.0131392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SM, McCormick JK, et al. A robust scoring system to evaluate sepsis severity in an animal model. BMC research notes. 2014;7:233 10.1186/1756-0500-7-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. Journal of visualized experiments: JoVE. 2013;(73):e50062 10.3791/50062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irahara T, Sato N, Inoue K, Otake K, Ohtsuru S, Koike K, et al. Low-intensity exercise in the acute phase of lipopolysaccharide-induced sepsis improves lipid metabolism and survival in mice by stimulating PGC-1α expression. Journal of Trauma and Acute Care Surgery. 2016;80(6):933–40. 10.1097/TA.0000000000001023 [DOI] [PubMed] [Google Scholar]
- 27.Martin SA, Pence BD, Greene RM, Johnson SJ, Dantzer R, Kelley KW, et al. Effects of voluntary wheel running on LPS-induced sickness behavior in aged mice. Brain, behavior, and immunity. 2013;29:113–23. 10.1016/j.bbi.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizashi H, Yoshimoto T, Nakanishi K, Tsujita J, Hori S. Effect of exercise on endotoxin shock with special reference to changes in concentration of cytokines. The Japanese journal of physiology. 1995;45(4):553–60. . [DOI] [PubMed] [Google Scholar]
- 29.Kim YH, Yoon DW, Kim JH, Lee JH, Lim CH. Effect of remote ischemic post-conditioning on systemic inflammatory response and survival rate in lipopolysaccharide-induced systemic inflammation model. Journal of inflammation. 2014;11:16 10.1186/1476-9255-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liaudet L, Rosselet A, Schaller MD, Markert M, Perret C, Feihl F. Nonselective versus selective inhibition of inducible nitric oxide synthase in experimental endotoxic shock. The Journal of infectious diseases. 1998;177(1):127–32. . [DOI] [PubMed] [Google Scholar]
- 31.Wu CC, Chen SJ, Szabo C, Thiemermann C, Vane JR. Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock. Br J Pharmacol. 1995;114(8):1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Critical care medicine. 2004;32(1):21–30. 10.1097/01.CCM.0000105581.01815.C6 . [DOI] [PubMed] [Google Scholar]
- 33.Watson D, Grover R, Anzueto A, Lorente J, Smithies M, Bellomo R, et al. Cardiovascular effects of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) in patients with septic shock: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144–002). Critical care medicine. 2004;32(1):13–20. 10.1097/01.CCM.0000104209.07273.FC . [DOI] [PubMed] [Google Scholar]
- 34.van de Sandt AM, Windler R, Godecke A, Ohlig J, Zander S, Reinartz M, et al. Endothelial NOS (NOS3) impairs myocardial function in developing sepsis. Basic research in cardiology. 2013;108(2):330 10.1007/s00395-013-0330-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross CM, Rafikov R, Kumar S, Aggarwal S, Ham Iii PB, Meadows ML, et al. Endothelial nitric oxide synthase deficient mice are protected from lipopolysaccharide induced acute lung injury. PLoS One. 2015;10(3):e0119918 10.1371/journal.pone.0119918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Mitra A, Poole B, Falk S, Lucia MS, Tayal S, et al. Endothelial nitric oxide synthase-deficient mice exhibit increased susceptibility to endotoxin-induced acute renal failure. Am J Physiol Renal Physiol. 2004;287(5):F1044–8. 10.1152/ajprenal.00136.2004 . [DOI] [PubMed] [Google Scholar]
- 37.Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circulation research. 2007;100(1):130–9. 10.1161/01.RES.0000253888.09574.7a . [DOI] [PubMed] [Google Scholar]
- 38.Bougaki M, Searles RJ, Kida K, Yu J, Buys ES, Ichinose F. Nos3 protects against systemic inflammation and myocardial dysfunction in murine polymicrobial sepsis. Shock. 2010;34(3):281–90. 10.1097/SHK.0b013e3181cdc327 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang B, et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell research. 2013;23(2):201–12. 10.1038/cr.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farah C, Michel LYM, Balligand J-L. Nitric oxide signalling in cardiovascular health and disease. Nature Reviews Cardiology. 2018. 10.1038/nrcardio.2017.224 https://www.nature.com/articles/nrcardio.2017.224#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 41.Duma D, Fernandes D, Bonini MG, Stadler K, Mason RP, Assreuy J. NOS-1-derived NO is an essential triggering signal for the development of systemic inflammatory responses. Eur J Pharmacol. 2011;668(1–2):285–92. 10.1016/j.ejphar.2011.05.065 . [DOI] [PubMed] [Google Scholar]
- 42.PERSICHINI T, CANTONI O. Cross-Talk Between Constitutive and Inducible NO Synthase An Update. Antioxidants & redox signaling. 2006;8(5 & 6). [DOI] [PubMed] [Google Scholar]
- 43.Adams JA, Pastuszko P, Uryash A, Wilson D, Lopez Padrino JR, Nadkarni V, et al. Whole Body Periodic Acceleration (pGz) as a non-invasive preconditioning strategy for pediatric cardiac surgery. Medical hypotheses. 2018;110:144–9. 10.1016/j.mehy.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 44.Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascon GA, et al. The Endothelium in Sepsis. Shock. 2016;45(3):259–70. 10.1097/SHK.0000000000000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng H, He X, Tuo QH, Liao DF, Zhang GQ, Chen JX. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2alpha/Notch3 pathways. Sci Rep. 2016;6:20931 10.1038/srep20931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stark RJ, Koch SR, Choi H, Mace EH, Dikalov SI, Sherwood ER, et al. Endothelial nitric oxide synthase modulates Toll-like receptor 4-mediated IL-6 production and permeability via nitric oxide-independent signaling. FASEB J. 2018;32(2):945–56. 10.1096/fj.201700410R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han S, Lee SJ, Kim KE, Lee HS, Oh N, Park I, et al. Amelioration of sepsis by TIE2 activation-induced vascular protection. Science translational medicine. 2016;8(335):335ra55 10.1126/scitranslmed.aad9260 . [DOI] [PubMed] [Google Scholar]
- 48.Leligdowicz A, Richard-Greenblatt M, Wright J, Crowley VM, Kain KC. Endothelial Activation: The Ang/Tie Axis in Sepsis. Frontiers in immunology. 2018;9:838 10.3389/fimmu.2018.00838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.David S, Ghosh CC, Kumpers P, Shushakova N, Van Slyke P, Khankin EV, et al. Effects of a synthetic PEG-ylated Tie-2 agonist peptide on endotoxemic lung injury and mortality. Am J Physiol Lung Cell Mol Physiol. 2011;300(6):L851–62. 10.1152/ajplung.00459.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams JA, Patel S, Lopez JR, Sackner MA. The Effects of Passive Simulated Jogging on Short-Term Heart Rate Variability in a Heterogeneous Group of Human Subjects. Journal of Sports Medicine. 2018;2018:1–9. 10.1155/2018/4340925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sackner MA, Patel S, Adams JA. Changes of blood pressure following initiation of physical inactivity and after external addition of pulses to circulation. Eur J Appl Physiol. 2018. 10.1007/s00421-018-4016-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Remick DG, Ward PA. Evaluation of Endotoxin Models for the Study of Sepsis. Shock. 2005;24(Supplement 1):7–11. 10.1097/01.shk.0000191384.34066.85 [DOI] [PubMed] [Google Scholar]
- 53.Fink MP. Animal models of sepsis. Virulence. 2014;5(1):143–53. 10.4161/viru.26083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis AJ, Seymour CW, Rosengart MR. Current Murine Models of Sepsis. Surg Infect (Larchmt). 2016;17(4):385–93. 10.1089/sur.2016.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. British journal of anaesthesia. 2003;90(2):221–32. 10.1093/bja/aeg034 . [DOI] [PubMed] [Google Scholar]
- 56.Reade MC, Young JD. Editorial I. British journal of anaesthesia. 2003;90(2):115–8. 10.1093/bja/aeg033 [DOI] [PubMed] [Google Scholar]
- 57.Young R, Bush SJ, Lefevre L, McCulloch MEB, Lisowski ZM, Muriuki C, et al. Species-Specific Transcriptional Regulation of Genes Involved in Nitric Oxide Production and Arginine Metabolism in Macrophages. Immunohorizons. 2018;2(1):27–37. 10.4049/immunohorizons.1700073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A table also contains Behavioral Scoring Criteria in Mice, amended to include stool quality criteria, a maximal worse score is 32.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.