Abstract
Biological aging is associated with progressive damage accumulation, loss of organ reserves, and systemic inflammation ('inflammaging'), which predispose for a wide spectrum of chronic diseases, including several types of cancer. In contrast, aerobic exercise training (AET) reduces inflammation, lowers all-cause mortality, and enhances both health and lifespan. In this study, we examined the benefits of early-onset, lifelong AET on predictors of health, inflammation, and cancer incidence in a naturally aging mouse model (C57BL/J6). Lifelong, voluntary wheel-running (O-AET; 26-month-old) prevented age-related declines in aerobic fitness and motor coordination vs. age-matched, sedentary controls (O-SED). AET also provided partial protection against sarcopenia, dynapenia, testicular atrophy, and overall organ pathology, hence augmenting the ‘physiologic reserve’ of lifelong runners. Systemic inflammation, as evidenced by a chronic elevation in 17 of 18 pro- and anti-inflammatory cytokines and chemokines (P < 0.05 O-SED vs. 2-month-old Y-CON), was potently mitigated by lifelong AET (P < 0.05 O-AET vs. O-SED), including master regulators of the cytokine cascade and cancer progression (IL-1β, TNF-α, and IL-6). In addition, circulating SPARC, previously known to be upregulated in metabolic disease, was elevated in old, sedentary mice, but was normalized to young control levels in lifelong runners. Remarkably, malignant tumours were also completely absent in the O-AET group, whereas they were present in the brain (pituitary), liver, spleen, and intestines of sedentary mice. Collectively, our results indicate that early-onset, lifelong running dampens inflammaging, protects against multiple cancer types, and extends healthspan of naturally-aged mice.
Introduction
In the aftermath of the sociopolitical, scientific, and medical advances of the 20th century, global fertility and mortality rates have steadily declined and average life-expectancies risen [1–3]. Albeit a remarkable human achievement per se, population demographics are also shifting in favor of older adults (≥60 y), and this group is expected to increase from 800 million to 2 billion in the next four decades, representing 22% of the total world population by 2050 [4]. Age-related disorders currently account for ≈25% of the global burden of disease with the leading contributors being the chronic, non-communicable diseases (NCDs), such as cardiovascular disease (CVD), cancer, musculoskeletal disorders (MSD), and mental/neurological conditions [5]. Because population aging is expected to impose a formidable challenge in terms of escalating health-care costs, and NCDs are the principal preventable cause of death and disability, a greater emphasis must be put on preventive medicine in the 21st century [4].
Sedentary living and physical inactivity are highly prevalent and strongly related to old age [6]. As accelerants of the biological aging process, they predispose for chronic disease and are estimated to underlie 3.2–5.3 million deaths per year globally (i.e. ≈ 9% of all-cause mortality), with associated costs exceeding $67.5 billion [7, 8]. In contrast, physical activity (PA) protects against CVDs, cancers, MSDs, metabolic syndrome, depression, anxiety, and cognitive/neurodegenerative disorders; collectively, reducing all-cause mortality risk by ≈30–40% [9–15]. Specifically, aerobic exercise training (AET) delays the onset of morbidity and enhances both health and lifespan [16]. Epidemiological evidence suggest that as little as 10 to 20 minutes of leisure PA per day is sufficient to extend life expectancy [17], while vigorous AET (e.g., running) provides additional survival benefits up to 3–5 times the recommended PA minimum (75–150 min/wk), with up to 10-fold higher training volumes generally considered safe and well-tolerated [9, 10, 12, 18]. Runners have ≈45–70% and ≈30–50% reduced risks of CVD- and cancer-related mortality, respectively, and live ≈3–10% (2–8 y) longer than non-runners, even after adjusting for potential confounders such as other types of PA [19, 20]. Epidemiological studies have consistently verified significant pro-longevity effects of AET, and an ongoing longitudinal trial suggests morbidity compression in lifelong runners [21].
The multi-systemic benefits of AET are well-documented in the discipline of oncology and exercise has been shown to be protective against breast, endometrial, colon and prostate cancers [21–27]. Numerous other malignancies, including liver, lung, kidney, bladder, rectal, brain, neck, esophageal adenocarcinoma, gastric cardia, myeloid leukemia, and myeloma, were recently added to this list of cancers favourably influenced by AET [15]. For example, walking (19–37 km/wk) and running (12–25 km/wk) may lower the risk of fatal brain cancers by > 40% [28]. Although the underlying mechanisms behind the oncoprotective benefits of regular exercise remain largely unknown, AET may mitigate several distinct stages in cancer progression, such as tumor initiation, growth, and metastasis [29–32].
Many cellular processes associated with aging predispose to oncogenic transformation, including mitochondrial dysfunction, oxidative stress, somatic mutation burden, cell senescence, and systemic inflammation [33–38]. Age-related chronic, low-grade inflammation (‘inflammaging’) is characterized by a two- to four-fold increase in circulating cytokines, chemokines, growth factors, and proteases, henceforth termed ‘gerokines’. Depending upon the biological context, gerokines can be broadly classified into pro-inflammatory (TNF-α, IL-1α/β, IL-6, IL-8, IFN-γ, VEGF etc.) and/or anti-inflammatory (IL-2, IL-4, IL-10, IL-13, TGF-β etc.), both of which are predicted to contribute to the aging secretome [39–41]. Several gerokines are capable of modulating all stages of oncogenesis, from tumor initiation and growth to cancer invasion and metastasis [33, 42], particularly TNF-α and IL-6. Virtually all solid malignancies exhibit extensive immune cell infiltration and contain high levels of pro-inflammatory cytokines, growth factors, and remodeling proteins, making them attractive targets for adjuvant cancer therapies, such as cytokine blockade and exercise interventions [32, 43, 44].
Strategies to rejuvenate pivotal systems that govern the basic survival needs of the cell, including energy production, growth, and quality control (repair, folding, recycling/degradation, and synthesis), may significantly extend health- and lifespan. Acute aerobic exercise is a stress stimulus characterized by pulsatile changes in intracellular danger signals (Ca2+, ROS, pH, and hypoxia), metabolic intermediates (NAD+/NADH and AMP/ATP), and circulating factors (termed ‘exerkines’), that coordinately stimulate mitochondrial biogenesis, antioxidant defense, cellular repair and recycling mechanisms, and immunity [45–50]. Over time, AET results in physiological adaptations into a more stress-resistant, homeostatic level [51, 52], which likely protects against systemic inflammation and cancer development.
In the current study, we report that lifelong, voluntary wheel-running with onset at sexual maturity alleviated senescence and inflammaging, including the key drivers of the cytokine cascade and tumor progression (TNF-α, IL-6, and IL-1β), and protected against several different types of cancer (e.g., brain, liver, spleen, and intestinal) in naturally-aged mice. Although lifelong AET provided a pleiotropic stimulus, which attenuated the biological aging process of multiple organ systems (for example, heart, skeletal muscle, and testicles) and improved overall health, the median lifespan was only modestly enhanced in the runners. These results are integrated and discussed within the framework of major biological aging hypotheses, namely the entropic, garbage catastrophe, and inflammaging/remodelling models by Hayflick et al., Terman, and Franceschi, respectively [39, 53, 54].
Materials and methods
Ethics
All methods and procedures described herein followed the guidelines published by the Canadian Council of Animal Care and were approved by McMaster University’s Animal Research and Ethics Board under the Animal Utilization Protocol 12-03-09.
Animals and study design
Four-week-old C57BL/J6 mice were purchased from Jackson Laboratories (Bar Harbor, ME), matched according to sex and bodyweight, and assigned to either young baseline control (Y-CON; N = 40), old sedentary (O-SED; N = 32), or old lifelong aerobic exercise (O-AET; N = 36) conditions. Following a 3-week acclimation period, O-AET animals were independently housed in activity wheel chambers and engaged in lifelong voluntary wheel-running, while baseline and sedentary cohorts were kept in separate microisolator cages with standard environmental enrichment. All mice were maintained on a 12-h light/dark cycle in a temperature and humidity-controlled room with water and chow available ad libitum (Harlan Teklad 8640 22/5). Sexual maturity (56 d) and old age (806 d) were used as endpoints for young and old cohorts, respectively, with the latter representing the lower limit of the 95% confidence interval for the median lifespan of C57BL/J6 mice [55, 56]. One week prior to necropsies, animals underwent a health examination and a battery of functional tests over two days to determine aerobic fitness, muscular strength and endurance, and motor coordination. A sub-set of mice from each group was terminally bled at rest or post exercise for assessment of age- and exercise-induced circulatory factors (Y-CON; n = 7, Y-CON-EX; n = 8, O-SED; n = 8, O-SED-EX; n = 6, O-AET; n = 9, and O-AET-EX; n = 8). To ensure a robust exercise stimulus in the young cohort, additional C57BL/J6 mice were obtained and exercised separately from the main-study animals until complete exhaustion and assessed independently for the secretory response (Y-CON2; n = 5 and Y-CON-EX2; n = 5).
Necropsies and histopathology
All experimental conditions were equally represented each day of sacrifice, and the necropsy order was balanced for time-point between groups. Complete, macroscopic post-mortems were performed by a veterinary pathologist blinded to the group allocations. Carcasses were first evaluated externally prior to internal examination and collection of major organs of the respiratory (lungs), cardiovascular (heart), digestive (liver, gallbladder, and intestine), lymphatic (spleen), endocrine (pancreas), urogenital (kidney, urinary bladder, and gonads), integumentary (skin), musculoskeletal (skeletal muscle), and central nervous (brain and spinal cord) systems. Any suspected tumor or mass was formalin-fixed, embedded in paraffin, sectioned, and stained with haematoxylin and eosin (H&E) for histological examination. The H&E-stained slides were then sent to an anatomical pathologist for blinded microscope analyses. The tumors, based on histology, were classified as malignant, benign, or unclassified.
Voluntary running-wheel training
O-AET mice were singly housed in 35.3L x 23.5W x 20H cm chambers equipped with free-spinning exercise wheels (40 cm/revolution) and counters connected to an activity monitoring software (Lafayette Instruments, Model 80820). Age-related changes in activity patterns were similar to those previously observed in C57BL/J6 mice [57, 58], and daily running distances were significantly reduced from middle adulthood onwards (S1 Fig). In a similar fashion to recent published work [59, 60], sedentary animals were independently housed without running-wheels to limit the complexity of the environment, spontaneous physical activity, and escape behaviors (e.g., climbing). Assessment of non-wheel cage activity during light and dark cycles using the Comprehensive Lab Monitoring System indicated no differences between O-SED and O-AET, while old animals were significantly less active versus young (S1 Table).
Functional testing and maximal run protocol
One week prior to sacrifice, a battery of in vivo functional tests was administered to predict health and morbidity status of the mice, including muscular strength (‘dynapenia 1’) and endurance (‘dynapenia 2’), motor coordination, and aerobic fitness. Our group has validated these outcomes extensively in various mouse models, and performance variables generally reflect organ dysfunction and total disease burden [61, 62]. In brief, forelimb maximal strength (N) was tested using a digital grip strength meter equipped with a pull bar and a force gauge (Columbus Instruments, Columbus, OH). Motor coordination and dynamic balance were assessed using a conventional RotaRod system (AccuScan Instruments, Inc., Columbus, OH), in which the speed (RPM) and time (s) at fall were recorded. Muscular endurance was evaluated by a four limb, inverted grid-hang test until failure (s) (Paw Grip Endurance, PaGE). Lastly, maximal aerobic capacity was predicted in all mice using an incremental running test, which consisted of a ramped exercise protocol on a motorized treadmill starting at 10 m/min and increasing 1 m/min until volitional exhaustion (e.g., sitting/lying on the electrical grid). A separate cohort of young mice was exercised until complete exhaustion, defined as the inability to run on the treadmill despite 10 sec of mechanical prodding, to provide a robust stimulus for exercise factor release. All other parameters remained the same between tests.
Serum secretome analyses
Blood was drawn from the retro-orbital sinus, clotted for 1 hr at room temperature (RT), spun at 2,500 g for 15 min at 4°C, and serum was retained for assessment of age- and exercise-induced cytokines, chemokines, and myokines. Serum samples were immediately frozen in liquid N2 and stored at -80°C until shipped on dry ice to the Analytical Facility for Bioactive Molecules (The Hospital for Sick Children, Toronto, Canada). Pro- and anti-inflammatory gerokines (GM-CSF, IFN-γ, IL-1α, IL-1β, IL-5, IL-6, IL-7, IL-12, IL-17A, CXCL1/KC, CXCL5/LIX, CCL2/MCP-1, CXCL2/MIP-2, and TNF-α vs. L-2, IL-4, IL-10, and IL-13, respectively) were analyzed by a research technologist using EMD Millipore’s (Temecula, CA, USA) high-sensitivity 18-plex plate (MHSTCMAG-70K) and were readily detected in all serum samples. Myokines were assessed on a separate 12-plex plate (MMYOMAG-74K) and a majority were above the lower limit of quantitation (LOQ), including FGF-21, FSTL-1, FKN/CX3CL1, IL-15, MSTN/GDF8, OSTN, and SPARC. Preparation, storage, and analyses were standardized between experimental conditions. Each sample consisted of serum from one or two mice within the same group and samples were run in duplicate on each plate.
Cumulative health index rank
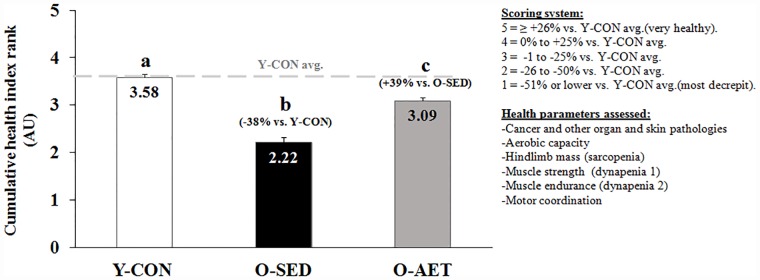
Predictors of successful aging, including cancer incidence, skin abscesses and lesions, other organ pathology, aerobic capacity, muscle mass (sarcopenia), grip strength (dynapenia 1), muscular endurance (dynapenia 2), and motor coordination were individually ranked on a 5-grade scale as follows: 1 = -51% or lower vs. Y-CON, 2 = -26% to -50% vs. Y-CON, 3 = -1% to -25% vs. Y-CON, 4 = 0% to +25% vs. Y-CON, and 5 = ≥ +26% vs. Y-CON. A cumulative health index rank was obtained by averaging the individual ranks, with indexes 1 and 5 representing most decrepit (1–2 = pathological aging) and excellent health status (3–5 = healthy aging), respectively.
Statistical analyses
Parametric data
All parametric data were tested for normality and homogeneity of variance by Shapiro-Wilk and Levene’s tests, respectively. Two balanced, three-way ANOVAs were used to determine the independent and interactive effects of age, AET, acute exercise, and gender on all parametric data, including functional, morphometric, and secretory outcomes. Specifically, the 2 x 2 x 2 ANOVA designs were: 1. AGE x ACUTE EXERCISE x GENDER (young and old mice) and, 2: AET x ACUTE EXERCISE x GENDER (old mice). If omnibus F-test(s) were significant, a Fisher’s LSD post hoc test was used to specify group differences. Independent T-tests were used to assess the exercise-induced secretory response in a sub-set of young animals. Statistical significance was set at P ≤ 0.05 for all parametric tests.
Non-parametric data
For organ and skin pathology, frequencies were coded as binary data (i.e. present vs. non-present), evaluated by either Chi-square or Fisher’s Exact tests, and graphed as % incidences for each cohort ([total frequency of pathology/number of necropsies]*100). Cumulative health index ranks were analyzed by Kruskal Wallis and Dunn’s multiple comparison tests. Significance was set at P ≤ 0.05 for all non-parametric tests.
Graphs, tables, and denotations
For conciseness, groups were collapsed across gender and acute exercise and presented as Y-CON, O-SED, and O-AET when appropriate, with the complete statistical designs available in table format in the supplementary data section. Statistical differences are denoted alphabetically (a, b, and/or c) or symbolically (*, †, and ‡) and if group means are significantly different they do not share the same letter(s) or symbol(s) (P > 0.05).
Results and discussion
Aging, organ deterioration, and disability
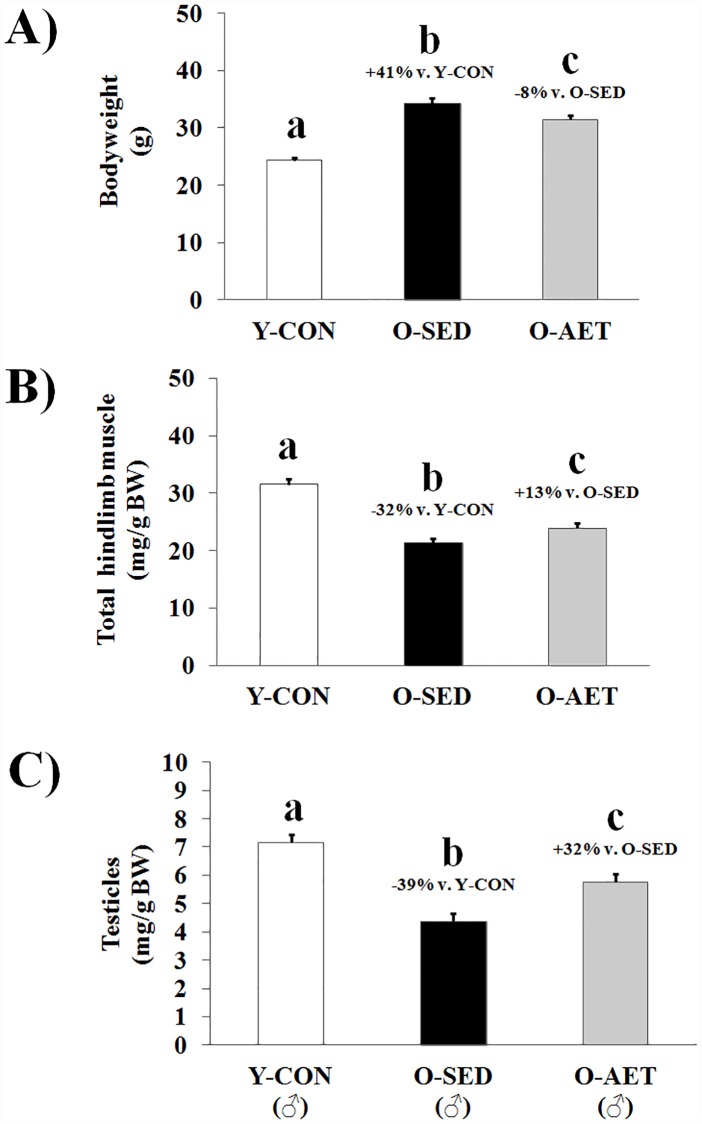
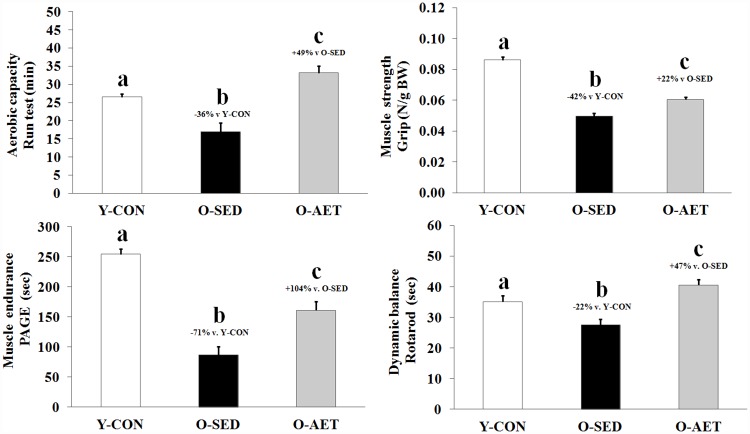
Skeletal muscle (SM) comprises 30–50% of total bodyweight in mature adults and is lost at 3.7–4.7% of peak mass per decade between 18 and 80 years of age [63, 64]. Age-related muscle loss is classified as sarcopenia (‘flesh-deficiency’) when falling two standard deviations below the young adult mean [65], and is estimated to affect more than 50 million individuals worldwide [66]. Sarcopenia and obesity are independent risk factors of NCDs, and act synergistically to worsen prognostic outcomes and disability [67]. Analogous to the human condition, aging in C57BL/J6 mice is characterized by a relative increase in fat mass versus lean mass [68]. Consistent with these observations, the O-SED cohort exhibited significant bodyweight gain concurrent with SM wasting (Fig 1A and 1B), which primarily affected the fast-twitch muscles of the lower leg, including the quadriceps and anterior crural muscle groups (S2 Table). As shown previously [56], whole brain (specific regions not assessed), heart, liver, kidney, and spleen were enlarged with aging, while testicles underwent significant atrophy (Fig 1C). Skeletal muscle deterioration was coupled with reduced locomotion, aerobic deconditioning, lower muscle strength and endurance (dynapenia; ‘power deficiency’), and impaired motor coordination (Fig 2 and S1 Table). The disproportionate loss of functional capacity (-32%, -42%, and -71% in muscle mass vs. strength vs. endurance, respectively) likely reflects age-related degeneration of neural components (α-motor neurons, motor units etc.) which are integral for force/power production, and have previously been shown to contribute significantly to weakness and frailty at old age [63].
Fig 1. Lifelong aerobic exercise mitigates age-associated bodyweight gain, sarcopenia, and testicular atrophy.
A) Bodyweight, B) hindlimb muscle mass, and C) testicle mass of young control (Y-CON), old sedentary (O-SED), and lifelong aerobically trained (O-AET) C57BL/J6 mice. Major thigh (quadriceps complex) and lower leg (anterior and posterior crural) muscles were summed for total hindlimb muscle mass. Complete data-sets are shown in S1 and S2 Tables. Group means (bars) that do not share the same alphabetical letter(s) are statistically different at P < 0.05.
Fig 2. Lifelong aerobic exercise mitigates dynapenia and preserves aerobic fitness and motor function in old C57BL/J6 mice.
Top left) Maximal aerobic capacity (min). Top right) Maximal forelimb grip-strength (N). Bottom left) Muscle endurance/grid-hang (PaGE; sec). Bottom right) Dynamic balance/motor coordination (Rotarod; sec). Complete data-sets are shown in S1 and S2 Tables. Group means (bars) that do not share the same alphabetical letter(s) are statistically different at P < 0.05.
Lifelong AET preserves organ reserves
A sedentary lifestyle is very common among elderly and a globally occurring phenomenon, regardless of geographic area and social development index (6). This age-related decrease in PA levels also appears to be conserved across many species on Earth (from worms to humans), and accelerates the rate of organ deterioration in a positive feedback manner. Predictably, regular PA maintains organ reserves and prolongs health- and lifespan, particularly in modern humans [9–15]. Widely recognized as the foundation of cardiometabolic disease prevention, aerobic exercise training (AET) protects against obesity, T2DM, and death from cardiovascular events by enhancing oxidative capacity and maximal aerobic power [69]. Additional benefits include pro-anabolic effects in SM, enhanced musculoskeletal integrity, and improvements in organs that are not classically associated with exercise (such as skin, kidney, and brain) [70]. Our results are in general agreement with those of Garcia-Valles and colleagues (2015), who demonstrated that lifelong wheel running prevented several indices of frailty and improved overall health in C57BL/J6 mice, while it did not enhance median or maximal lifespan significantly [58]. Specifically, we report that lifelong AET mitigated bodyweight gain, muscle loss, strength deficits, and testicular atrophy, and even elevated motor function and aerobic fitness above young control levels (Figs 1 and 2). However, despite many favorable adaptations predicted to extend lifespan, the survival rate at 26 months (i.e. median lifespan; equivalent to 70–75 human years) was only modestly improved in lifelong runners vs. sedentary controls (+6% vs. O-AET vs. O-SED; NS). Notwithstanding, a 3–10% extension in average life-expectancy is consistent with large population-based studies in human runners [19, 20].
Aging and systemic inflammation
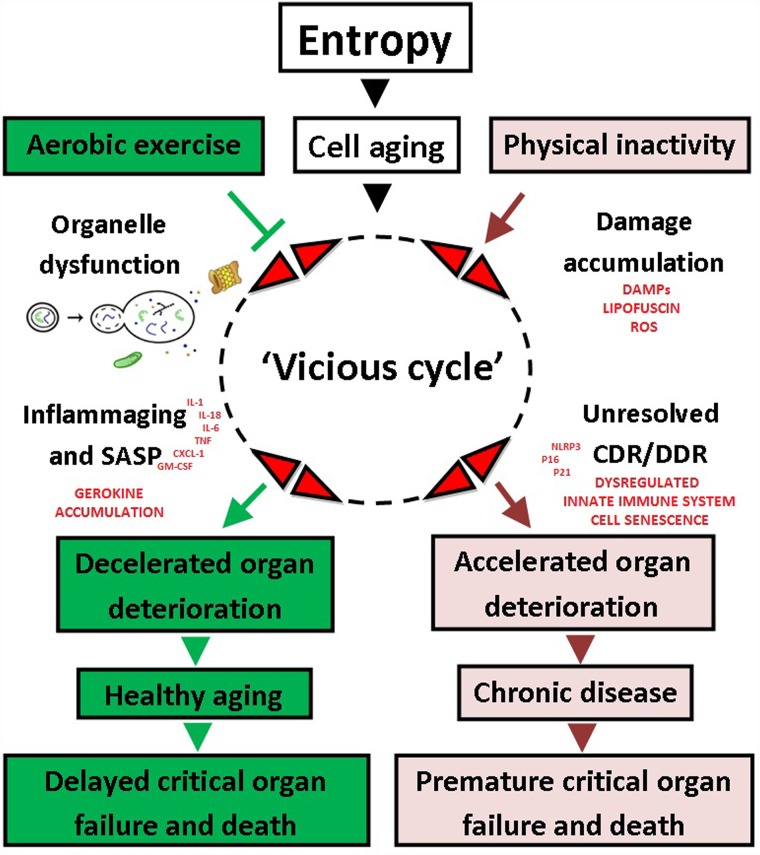
In parallel with age-related declines in organ reserves, glucose tolerance, and sex-hormones, systemic inflammatory factors steadily rise from the third decade onwards in humans [71, 72]. Imperfect repair, recycling, and removal of danger-associated molecular patterns (DAMPs) are postulated to trigger activation of evolutionary conserved cell danger response programs (CDR and DDR, respectively), which fuel a vicious cycle of unresolved cell damage, build-up of toxic debris, senescence, and inflammation [52]. Inflammaging is thereby multi-factorial, but principally attributed to DAMP-activation of the innate immune system, immunosenescence, and the senescence-associated secretory phenotype (SASP) [73].
Pro-inflammatory gerokines, such as TNF, IL-1, and IL-6, are positively correlated with morbidity and mortality in elderly [71, 74], and predispose to a wide spectrum of NCDs, including cardiovascular diseases, neurodegenerative disorders, and cancers [33–37, 42, 75, 76]. However, the biological effects of any cytokine in vivo depends on the presence of other cytokines with additive, synergistic, or antagonistic modes of action [77]. For example, IL-1 and TNF-α work in synergy to initiate and amplify the cytokine cascade, and blockade of either master regulator improves survival following infection [78, 79]. The remodeling theory of aging, as outlined by Franceschi et al., stipulates a progressive accumulation of both pro- and anti-inflammatory gerokines occurring simultaneously with aging [39, 41].
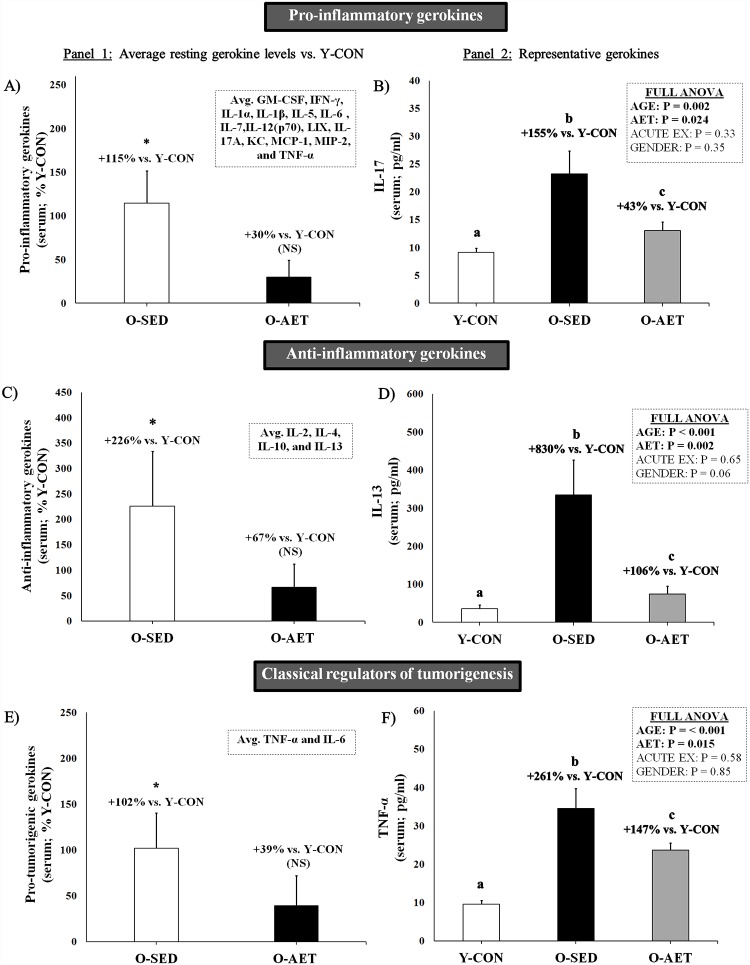
In support of the remodeling theory, we found that a vast majority of pro- and anti-inflammatory serum markers were chronically elevated by aging (Fig 3A and 3C; pro-inflammatory: + 115% and anti-inflammatory: +226% vs. Y-CON), with a main effect of age in 17 of 18 targets (S3 and S4 Tables). The full ANOVA design indicated that IL-17a (Fig 3B; +155% vs. Y-CON), IL-13 (Fig 3D; +830%), TNF-α (Fig 3F; +261%), IFN-γ (+534%), IL1-β (+360%), GM-CSF (+262%), CCL-2/MCP-1 (+235%), and CXCL1/KC (+46.3%) were the most robust markers of inflammaging in the C57BL/J6 model. Notably, IL-1β and TNF-α, and to a lesser extent IL-6, were significantly elevated in O-SED as compared to Y-CON (Fig 3E and 3F), and are considered key drivers of the cytokine cascade and cancer progression [42, 43]. Other gerokines, such as IL-1α (+61%, IL-2 (+107%), IL-4 (+901%), IL-6 (+373%), IL-10 (+1006%), and IL-12(p70) (+1074%), also contributed to the overall inflammatory state in old mice, but statistical significance was partly driven by the acute exercise response in O-SED and O-AET groups.
Fig 3. Aging and lifelong aerobic exercise effects on pro-inflammatory, anti-inflammatory, and pro-tumorigenic serum gerokines.
Panel 1) Average resting serum gerokine levels in 26-mo-old sedentary (O-SED) and lifelong aerobically trained (O-AET) C57BL/J6 mice vs. 2-mo-old controls (% Y-CON). Acute exercise groups were omitted from these analyses. Panel 2) Representative serum gerokines (pg/mL) collapsed within main groups. Full data sets are available in the supplementary data section. For panel 1, NS = not significantly different from Y-CON. *Significantly higher vs. Y-CON (P ≤ 0.05). For panel 2, group columns that are significantly different do not share the same letter(s) (P ≤ 0.05).
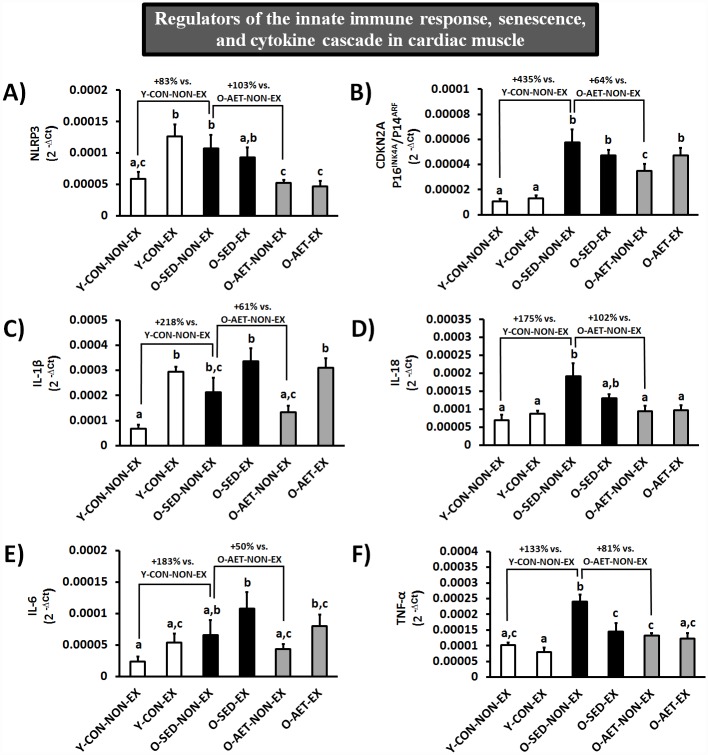
Although multiple tissue- and cell-types contribute to the aging secretome in vivo, we analyzed the expression of regulators of the innate immune system (NLRP3), cell-cycle progression (CDKN2A/P16INK4A), and the cytokine cascade (IL-1β, IL-6, TNF-α, and IL-18) in cardiac and skeletal muscles of young and old mice (Fig 4, S2 Fig, and S8 Table, respectively). Our findings largely support the contention that the innate immune system is constitutively primed and/or activated at old age, while the cell cycle is progressively inhibited (i.e. cellular senescence), both of which may contribute to inflammaging and SASP [73]. Specifically, basal mRNA levels of NLRP3 (+83%), CDKN2A (+435%), IL-1β (+218%), IL-18 (+175%), IL-6 (+183%), and TNF-α (+133%) were substantially elevated in unexercised O-SED vs. Y-CON mice (Fig 4A–4F).
Fig 4. Cardiac muscle mRNA expression of regulators of the innate immune response and cell cycle progression.
Group columns that are significantly different do not share the same letter(s) (P ≤ 0.05).
Aging and myokines
In principle, all cells that undergo exchange with the extracellular environment may contribute to the myriad of blood-borne factors that exert autocrine, paracrine, and endocrine effects. For example, immune, muscle, and fat cells each provide a unique spectrum of proteins to the circulatory system (cytokines, myokines, and adipokines, respectively), while also exhibiting significant overlap between their secretomes (e.g., myocytokines, adipocytokines, adipomyokines etc.).
Skeletal muscle may be considered the largest endocrine organ in the body that actively secretes myokines at rest and in response to various stressors (pathological, contractile, metabolic etc.). To date, over 300 factors secreted by primary SM cells have been identified using targeted and exploratory proteomic approaches [64, 80–82] including a substantial number of cytokines and chemokines (e.g., myocytokines; GM-CSF, IFN-γ, IL-1α/β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, CXCL1/KC, LIF, CCL2/MCP-1, and TNF-α). Many other secretory proteins, such as osteonectin (SPARC), ostecrin (musclin), follistatin-like protein 1 (FSTL-1), myostatin (MSTN), FGF-21, fractalkine (CXC3CL1), VEGF-A, BDNF, metorin-like (Metrnl), and irisin, are either confirmed myokines or promising candidates.
Aging has well-documented adverse effects on SM cells, including build-up of DAMPs, chronic immune activation, and myofibrillar atrophy, all of which may alter the muscle-specific secretome. Resident and invading immune cells, adipocytes, and fibroblasts, also contribute to the SM secretory spectrum in vivo, especially during remodelling and pathological processes. Because muscle fiber degeneration may fuel systemic inflammation independently (in muscular dystrophies, for example), it is likely that age-related SM deterioration partly underlies inflammaging. Regardless of the tissue/cell origins, the key drivers of the cytokine cascade, TNF-α, IL-1β, and IL-6 (+128%, +159%, and +75%, respectively, O-SED vs. Y-CON), were chronically elevated at old age, and may have contributed to SM wasting in this study. Further indicative of anabolic dysregulation and an overall pro-catabolic systemic environment, myostatin, follistatin-like protein 1, FGF-21, and osteocrin/musclin, and to a lesser extent IL-15 and fractaline/CX3CL1, were significantly lower in O-SED vs. Y-CON cohorts (S5 and S6 Tables).
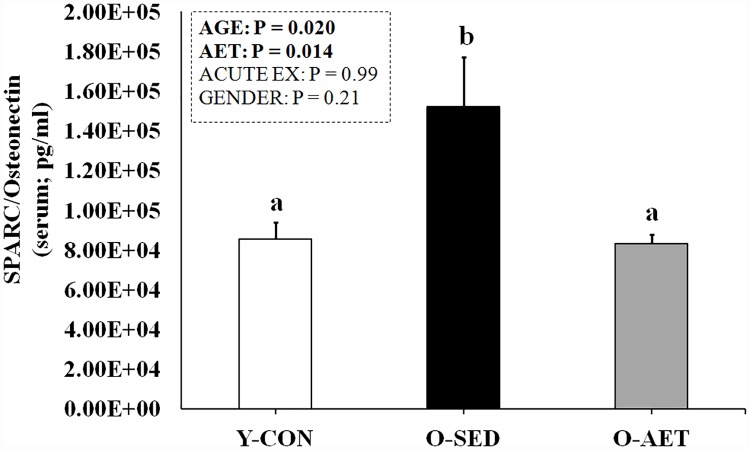
Conversely, the matricellular protein SPARC/osteonectin followed the same general pattern as pro- and anti-inflammatory gerokines and was elevated with aging (Fig 5). SPARC is expressed during remodelling and repair in intestinal, glandular, skin, muscle (cardiac, smooth and skeletal), adipose, and neoplastic tissues [83]. In aggressive tumor phenotypes, SPARC may act as a positive regulator of metastatic potential by augmenting vascular permeability [84]. Persistent induction of SPARC promotes tissue fibrosis, nephropathy, retinopathy, and non-alcoholic fatty liver disease, while its deletion/inhibition protects against the same [85–87]. Considering that circulating SPARC is up-regulated in metabolic disease, accelerated aging conditions (Werner Syndrome etc.), and in sarcopenia [85, 88–93], it is conceivable that it may be used as a general biomarker of advancing age and/or pathological aging processes.
Fig 5. Basal serum levels of SPARC in 26-mo-old sedentary (O-SED) and lifelong aerobically trained (O-AET) C57BL/J6 mice vs. 2-mo-old controls (% Y-CON).
Group columns that are significantly different do not share the same letter(s) (P ≤ 0.05).
Lifelong AET mitigates inflammaging
In contrast to chronic stress, the intermittent application of a physiological challenge, such as AET, minimizes perturbations to the intracellular milieu and allows for sufficient recovery for the cell to evolve into a more stress-resistant, homeostatic level, thereby attenuating biological aging and protecting against inflammation [51, 52]. The anti-inflammatory effects of exercise training are well-documented (with higher IL-10, and concomitantly lower TNF-α, IL-6 and CRP levels), and benefits appear to be independent of age and chronic disease [94]. As expected, lifelong AET effectively dampened inflammaging in the C57BL/J6 model, with a restoration of both pro- and anti-inflammatory gerokines to young control levels (Fig 3A and 3C; NS vs. Y-CON), including the pivotal regulators of the cytokine cascade and tumor development (Fig 3E; NS vs. Y-CON). In the full ANOVA design, 10 out of 17 age-induced markers were AET-responsive, specifically IL-17a (Fig 3B), IL-13 (Fig 3D), TNF-α (Fig 3F), IL-6, GM-CSF, IFN-γ, IL-2, IL-10, IL-12 (p70), and CCL-2/MCP-1 (S3 and S4 Tables). Although a majority of gerokines were significantly attenuated and even normalized by lifelong running (notably IL-13, GM-CSF, IFN-γ, IL-17, TNF-α, and IL-1β), basal levels of IL-10 and CXCL1/KC were moderately enhanced by AET (+25% vs. O-SED).
IL-10 inhibits the activity of TH1 cells, NK cells, and macrophages and the production of wide range of pro-inflammatory cytokines and chemokines, including TNFα, IL-1α/β, IL-6, and MCP-1 [95]. By counteracting the proteolytic effects of IL-1β, the anti-inflammatory actions of IL-10 are integral for muscle repair and growth following strenuous exercise [96]. CXCL1/KC (GRO-α) is a chemoattractant that stimulates neutrophil recruitment and phagocytosis of injured tissue, but the functional significance of elevated serum KC levels following endurance training is unclear. Overexpression of CXCL1/KC in SM promotes its systemic release and induces an oxidative phenotype in high-fat fed mice, which protects against fat gain and glucose intolerance [97]. CXCL1/KC may therefore play an integral role in mediating the whole-body metabolic benefits of aerobic exercise training and be of therapeutic interest as an exercise mimetic. Lastly, circulating SPARC/osteonectin was restored to young control levels by lifelong running (Fig 5), further suggestive of SPARC being a general biomarker of advancing age and/or pathological aging. We did not observe any significant effects of lifelong AET on basal FSTL-1, musclin, or fractalkine; while MSTN (↑), FGF-21 (↓) and IL-15 (↓) were moderately affected in our study (S5 and S6 Tables).
Lifelong AET alters the inflammatory response to acute exercise
Aerobic exercise is a single, hormetic stress stimulus that encompasses transitory spikes in intracellular danger signals (Ca2+, ROS, pH, and hypoxia), metabolic intermediates (NAD+/NADH and AMP/ATP), and a myriad of neurotransmitters, hormones, and organokines, which act synchronously to stimulate mitochondrial biogenesis, antioxidant defense, cellular repair and recycling, and immunity [45–50]. We recently coined the term ‘exerkine’ to describe any biomolecule released into circulation in response to exercise, including non-coding RNAs, mRNAs, metabolites, peptides, and proteins [49]. Approximately 50% of all SM-derived secretory factors are contractile activity-mediated [64], and other cell types likely contribute significantly to the total exerkine pool in vivo, principally liver, fat, and immune cells [77].
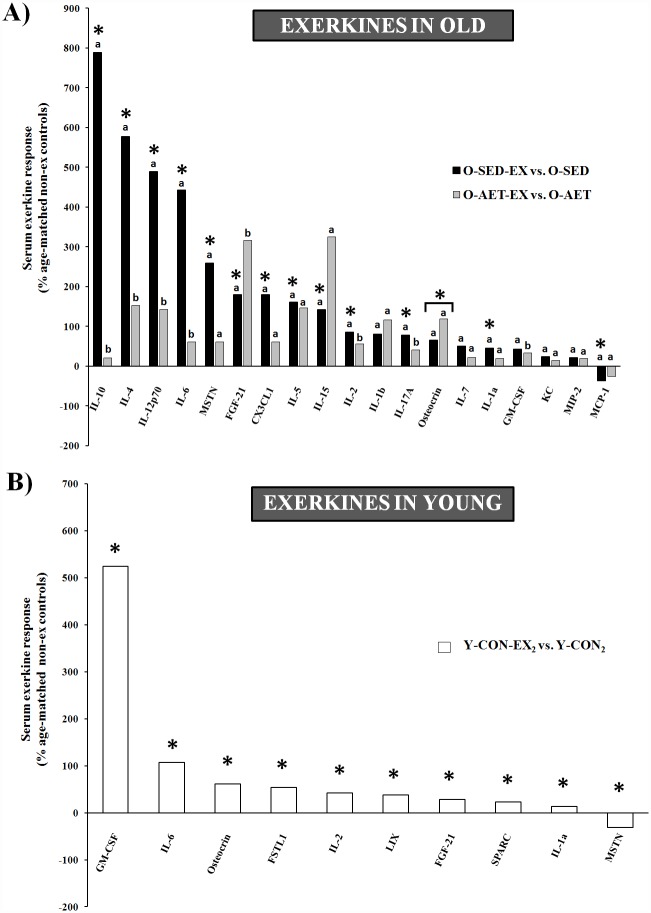
Several basic parameters may affect the systemic response to acute contractile activity, such as exercise variables (e.g., mode, intensity, and duration), blood sampling (time and fraction), training status, gender, and age. As reviewed by Peake at el., the exercise-mediated increase in circulating cytokines is highly variable and typically reflects the relative exercise intensity and degree of intracellular stress/damage [77]. In our study, an acute bout of aerobic exercise elicited a significantly higher inflammatory response in old sedentary C57BL/J6 mice (O-SED-EX) compared to aerobically trained (O-AET-EX) and young counterparts (Y-CON-EX), suggestive of a protective adaptation induced by lifelong AET to intracellular stress (S3 and S4 Tables). Statistical significance was almost exclusively driven by the robust exercise effect in O-SED-EX (AGE x ACUTE EXERCISE x GENDER; Table 1a) and included many classical pro- and anti-inflammatory cytokines previously identified as exercise factors. To illustrate this further, we analyzed the secretory response from old cohorts independently (AET x ACUTE EXERCISE x GENDER; Table 1b), and observed that the vast majority of inflammatory factors (14/18) and myokines (5/7) were contractile activity-regulated at old age (Fig 6A). A second cohort of young animals was vigorously exercised until complete exhaustion (Y-CON-EX2), and this intense physiological stimulus was insufficient to induce the same magnitude and breadth of cytokines as in O-SED (Fig 6B; Table 1c; S7 Table). Other notable age discrepancies in the secretory response were SPARC (↑ young vs. ↔ old), FSTL-1 (↑ young vs. ↔ old), and MSTN (↓ young vs. ↑ old), while musclin and FGF-21 were elevated by acute exercise regardless of age (Table 1b and 1c).
Table 1. Contractile activity-induced cytokines, chemokines, and myokines (‘exerkines’) in serum of young and old C57BL/J6 mice.
| A) ANOVA 1: [age * acute ex * gender] | B) ANOVA 2: [AET* acute ex * gender] | C) Independent T-tests | ||||||
|---|---|---|---|---|---|---|---|---|
| YOUNG AND OLD | OLD | YOUNG | ||||||
| NAME | P-VALUES | DIRECTION | NAME | P-VALUES | DIRECTION | NAME | P-VALUES | DIRECTION |
| Cytokines and chemokines | Cytokines and chemokines | Cytokines and chemokines | ||||||
| IL-10 | PEX = 0.033 | ↑ | IL-10 | PEX = 0.016 | ↑ | IL-6 | P < 0.001 | ↑ |
| IL-4 | PEX = 0.050 | ↑ | IL-4 | PEX = 0.015 | ↑ | IL-2 | P = 0.055 | ↑ |
| IL-12 | PEX = 0.014 | ↑ | IL-12 | PEX = 0.002 | ↑ | IL1α | P = 0.005 | ↑ |
| IL-6 | PEX = 0.024 | ↑ | IL-6 | PEX = 0.007 | ↑ | GM-CSF | P = 0.005 | ↑ |
| IL-2 | PEX = 0.068 | ↑ | IL-2 | PEX < 0.001 | ↑ | LIX | P < 0.001 | ↑ |
| IL1α | PEX = 0.013 | ↑ | IL1α | PEX = 0.003 | ↑ | Myokines | ||
| KC | PEX = 0.009 | ↑ | KC | PEX = 0.030 | ↑ | OSTN | P = 0.038 | ↑ |
| MIP-2 | PEX = 0.019 | ↑ | MIP-2 | PEX = 0.008 | ↑ | FGF-21 | P = 0.006 | ↑ |
| MCP-1 | PEX = 0.001 | ↓ | MCP-1 | PEX = 0.014 | ↓ | Myostatin | P = 0.002 | ↓ |
| Myokines | GM-CSF | PEX = 0.023 | ↑ | FSTL1 | P = 0.00 | ↑ | ||
| OSTN: | PEX = 0.070 | ↑ | IL-1β | PEX = 0.012 | ↑ | SPARC | P < 0.001 | ↑ |
| IL-5 | PEX = 0.011 | ↑ | ||||||
| IL-7 | PEX = 0.004 | ↑ | ||||||
| IL-17 | PEX = 0.033 | ↑ | ||||||
| Myokines | ||||||||
| OSTN | PEX = < 0.001 | ↑ | ||||||
| IL-15 | PEX = < 0.001 | ↑ | ||||||
| FGF-21 | PEX = 0.002 | ↑ | ||||||
| Myostatin | PEX <0.001 | ↑ | ||||||
| Fractalkine | PEX <0.001 | ↑ | ||||||
A) Exerkines according to 2 x 2 x 2 ANOVA across age groups (AGE x ACUTE EXERCISE x GENDER). B) Exerkines according to 2 x 2 x 2 ANOVA in old mice (AET x ACUTE EXERCISE x GENDER) (Fig 6A). C) Exerkines according to an additional cohort of young C57BL/J6 mice exercised until exhaustion and analyzed by independent t-tests (Fig 6B). The arrows depict the directionality of the contractile activity-induced response of each exerkine.
Fig 6. Contractile activity-induced chemokines, cytokines, and myokines (‘exerkines’) in serum of young and old C57BL/J6 mice.
A) Acute exercise response of sedentary (O-SED-EX; black bars) and life-long trained (O-AET-EX; gray bars) C57BL/J6 mice expressed relative to unexercised, age-matched controls (O-SED vs. O-AET, respectively). Main effects of exercise were mainly attributed to old cohorts as shown above and in Table 1a (left column). By removing young animals from the statistical analyses (Table 1b middle column), several additional exerkines were uncovered in old mice. B) Maximal exercise response of young C57BL/J6 mice expressed relative to unexercised controls (Y-CON-EX2 vs. Y-CON2; Table 1c right column). *Significantly different from unexercised, age-matched controls (P ≤ 0.05). Groups that do not share the same letter(s) are significantly different (P ≤ 0.05).
Lifelong AET protects against cancer
Inflammaging predisposes for a wide range of NCDs, including multi-systemic cancers. Chronic inflammation directly underlies ~20% of all cancers and a vast majority of solid malignancies contain inflammatory cells that secrete cytokines and chemokines, which can modulate all stages of tumor development (e.g., initiation, promotion, malignant conversion, invasion, and metastasis) [42, 43]. Macrophages (TAMs) and T-cells (CD8+cytotoxic, CD4+ helper, and NKT) are the most abundant immune cells in the tumor microenvironment, and pro-inflammatory cytokines enable dynamic cross-talk between cell populations [42]. In over 50% of all cancers, apoptotic resistance and high proliferative rates are attributed to aberrant NF-κB, AP-1, and STAT3 signalling caused by the autocrine and paracrine actions of TNF-α and IL-6 [43].
The anti-tumorigenic effects of regular PA may be attributed to attenuating the loss of organ reserves, DAMP removal, and mitigation of the pro-inflammatory, systemic environment. Acutely, aerobic exercise is a stress stimulus that induces a CDR-driven inflammatory response, which is rapidly resolved and favors tumor clearance [98]. Specifically, voluntary wheel running has previously been shown to inhibit cancer development across a range of tumor models in mice, and involves an epinephrine-dependent mobilization of IL-6Rα-positive NK cells that require IL-6 for efficient tumor homing and infiltration [32].
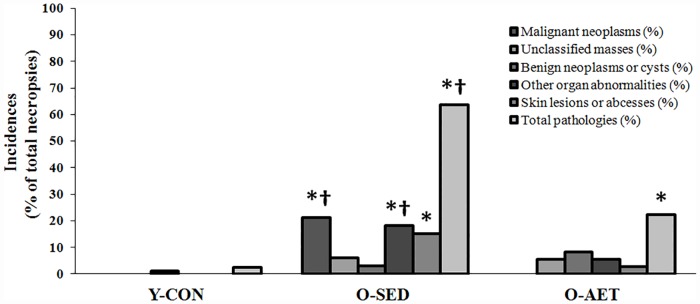
In this study, we found malignant tumors in the liver (N = 3), brain (pituitary; N = 2), intestines (N = 1), and spleen (N = 1) of O-SED C57BL/J6 mice, while lifelong runners were cancer-free (Table 2). In keeping with the Jackson Laboratory Mouse Tumor Biology Database [99], we report that organs most likely to become malignant were the liver, pituitary, and intestines, and that these tumors were mainly hematopoietic malignancies (e.g., lymphomas) and adenocarcinomas (Fig 7A–7C). In contrast to the exclusive finding of malignant tumors in the O-SED cohort, there was a roughly equal distribution of benign tumors in the reproductive (ovary and testicle), digestive (gallbladder and intestine), and urinary (kidney, Fig 7D) systems of non-runners vs. runners. Overall, lifelong running protected against total organ pathology (22% vs. 64% in O-AET vs. O-SED, respectively), multi-systemic cancers (0% vs. 21%), and skin deterioration (3% vs. 15%) (Fig 8).
Table 2. Frequency of cancers, benign cysts, unclassified mass, skin disease, and other organ pathology in young control (Y-CON), old sedentary (O-SED), and lifelong aerobically trained (O-AET) C57BL/J6 mice.
| Cancers, cysts, or other organ pathology* | Necropsies | Brain | Liver | Gallbladder | Kidney | Spleen | Intestine | Ovary | Testicle |
| Y-CON | 40 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| O-SED | 33 | 2 | 4 | 0 | 1 | 3 | 1 | 3 | 2 |
| O-AET | 36 | 0 | 1 | 2 | 0 | 1 | 1 | 2 | 0 |
| Skin pathology | Lesions | Abscesses | Total | ||||||
| Y-CON | 0 | 0 | 0 | ||||||
| O-SED | 4 | 1 | 5 | ||||||
| O-AET | 1 | 0 | 1 | ||||||
| Total pathologies | Cancers | Unclassified mass | Benign cysts | Skin | Other* | Total | |||
| Y-CON | 0 | 0 | 1 | 0 | 0 | 1 | |||
| O-SED | 7 | 2 | 1 | 5 | 6 | 21 | |||
| O-AET | 0 | 2 | 3 | 1 | 2 | 8 |
*Other organ pathology included non-physiological organomegaly and atrophy not associated with neoplasms or cysts.
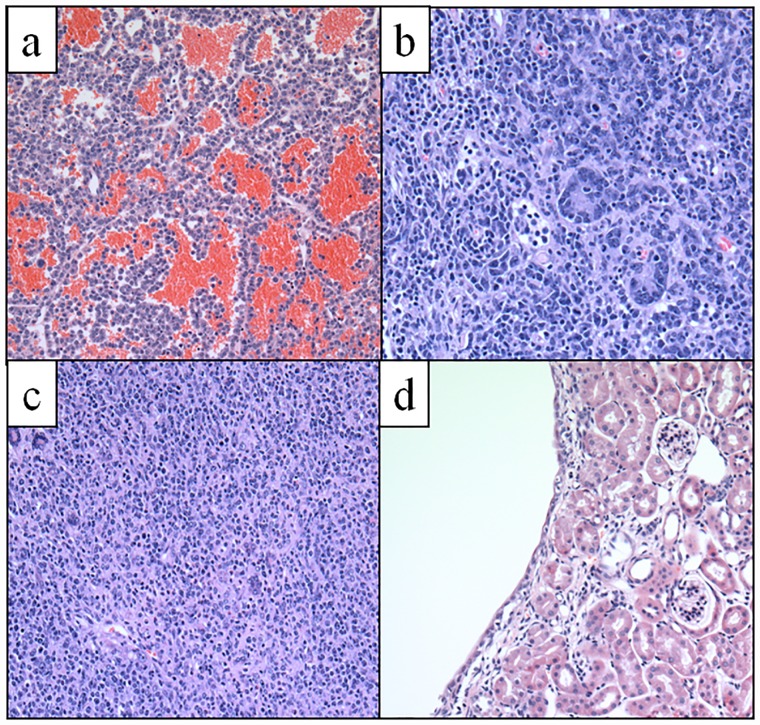
Fig 7. Examples of tumor histopathology.
A) Pituitary neoplasm, B) Intestinal adenocarcinoma, C) Hematopoietic neoplasia, and D) Kidney: Benign renal cysts. H&E stain; 200X.
Fig 8. Incidences of multi-system cancers, benign cysts, unclassified masses, skin pathologies, and other organ abnormalities in young control (Y-CON), old sedentary (O-SED), and lifelong aerobically trained (O-AET) C57BL/J6 mice.
Pathology frequencies in Table 1 were coded as binary data (present vs. non-present), evaluated by either Chi-square or Fisher’s Exact tests, and graphed as % incidences for each group [(total frequency of pathology/number of necropsies)*100)]. Symbols indicate significantly higher (P < 0.05) vs. Y-CON (*) or Y-CON and O-AET (*†).
Lifelong AET extends healthspan
Cell aging is characterized by progressive malfunctioning of pivotal systems that regulate energy production, growth, and quality control (repair, folding, recycling/degradation, and synthesis), progressively depleting organ reserves and eventually causing death. Physical inactivity may accelerate this process by antagonizing a ‘vicious cycle’ of unresolved damage, persistent CDR/DDR activation, and inflammation. In this study, we found that early onset, lifelong AET dampened inflammaging, including master cytokines IL-1, TNF-α, and IL-6 (in circulation and tissue-specific), protected against multi-systemic cancers, and significantly enhanced healthspan of C57BL/J6 mice (cumulative health-index rank; Fig 9). These data support the contention that lifelong running decelerates the ‘vicious cycle’ and protects against the most salient features of the aging process, namely critical organ deterioration/pathology, frailty, and premature death (Fig 10).
Fig 9. Cumulative health index rank in young control (Y-CON), old sedentary (O-SED), and lifelong aerobically trained (O-AET) C57BL/J6 mice.
Common predictors of independence and health- and lifespan, including existing morbidities, aerobic capacity, skeletal muscle mass, muscle strength and endurance, and motor coordination were individually ranked on a 5-grade scale (see Methods), averaged and presented as a cumulative health index, and evaluated by Kruskal Wallis and Dunn’s multiple comparison tests. Group means that are statistically different from each other do not share the same alphabetical letter (P < 0.05).
Fig 10. Integrated hypothesis of aging.
Aging is inevitable and is characterized by progressive accumulation of damage from the 3rd-4th decade and onwards in humans. Physical inactivity accelerates the cellular aging process in a ‘vicious cycle’ and predisposes for a wide spectrum of chronic diseases, including cancers. Aerobic exercise training (AET) mitigates inflammaging, reduces all-cause mortality risk, and extends health- and lifespan.
Conclusions
Cumulative evidence over the last 50 years unequivocally shows that sedentary living accelerates organ deterioration and increases all-cause mortality risk. In contrast, aerobic exercise training promotes healthy aging and extends average life expectancy by 3–10% [20]. Our data confirm that lifelong running provides significant health benefits and protects against cancer, while only modestly improving the median lifespan of naturally-aged mice. For more substantial pro-longevity benefits ‘of mice and men’, a combination of lifestyle modification, rejuvenation biotechnology, and intermittent pharmacotherapy may be necessary (e.g., anti-aging polytherapy). Given that the global burden of chronic disease is steadily rising (population aging and physical inactivity pandemic), the development of innovative strategies to increase daily PA levels at the societal level, and/or alternative means to harness the multi-systemic benefits of exercise (e.g., exercise mimetics), will be of paramount importance in the 21st century.
Supporting information
Voluntary running-wheel distances decreased with aging in C57BL/J6 mice. Groups that do not share the same letter(s) are statistically different (P ≤ 0.05).
(DOC)
Group columns that are significantly different do not share the same letter(s) (P ≤ 0.05).
(TIFF)
Lifelong aerobic exercise training (AET) mitigates age-associated dynapenia and aerobic deconditioning in C57BL/J6 mice. *Significant effects of aging, †lifelong aerobic exercise training, and ‡gender (P ≤ 0.05).
(DOC)
Lifelong aerobic exercise training (AET) mitigates age-associated muscle loss and testicular atrophy in C57BL/J6 mice. Age-associated organ wasting was isolated to fast-twitch muscles and testicles (test.), while slow-twitch muscles (solues; sol.) and other internal organs (heart, brain, liver, kidney, spleen, and lungs) were largely spared or increased in mass. Major thigh (quadriceps complex) and lower leg (anterior and posterior crural) muscles were summed to obtain total hindlimb muscle (HL) mass. *Significant effects of aging, †lifelong aerobic exercise training, and ‡gender (P ≤ 0.05).
(DOC)
Lifelong aerobic exercise training (AET) dampens inflammaging in old C57BL/J6 mice. Serum cytokine and chemokine concentrations are reported in pg/mL and expressed as group means ± SE. Each sample consisted of serum from one or two mice within the same experimental condition. All samples were run in duplicate.
(DOC)
(DOC)
Lifelong aerobic exercise training (AET) mitigates serum SPARC levels in old C57BL/J6 mice. Serum myokine concentrations are in pg/mL and expressed as group means ± SE. Each sample consisted of serum from one or two mice within the same experimental condition. All samples were run in duplicate.
(DOC)
(DOC)
Serum exerkine concentrations are reported in pg/mL and expressed as group means ± SE. Each sample consisted of serum from one or two mice within the same experimental condition. All samples were run in duplicate.
(DOC)
(DOCX)
Acknowledgments
We extend our gratitude to Dr. Mohammad Khan and Max Merilovich, McMaster University, for assisting with necropsies and data collection, respectively. We also thank Drs. Birgit Schilling and Nathan Basisty, Buck Institute for Research on Aging, for defining the term ‘gerokine’.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by CIHR 143325 Dr. Mark A. Tarnopolsky: "Characterization of novel exersomes that mediate the systemic pro-metabolic effects in exercise in aging and neuromuscular disorders", and Exerkine Corporation. Exerkine Corporation provided support in the form of salaries for authors [MIN, BPH, ALB, LM, and DR]. The specific roles of these authors are articulated in the ‘author contributions’ section. The funders of this study did not have additional roles in the study design, data collection and analyses, decision to publish, or preparation of the manuscript.
References
- 1.Oeppen J, Vaupel J. Demography. Broken limits to life expectancy Science. 2002;296(1029–1031). [DOI] [PubMed] [Google Scholar]
- 2.Vaupel J. Biodemography of human ageing Nature. 2010;464:536–42. 10.1038/nature08984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee R. The outlook for population growth. Science. 2011. July 29, 333(6042):569–73. 10.1126/science.1208859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom DE, Chatterji S, Kowal P, Lloyd-Sherlock P, McKee M, Rechel B, et al. Macroeconomic implications of population ageing and selected policy responses. Lancet (London, England). 2015;385(9968):649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. The Lancet. 2014(0). [DOI] [PubMed] [Google Scholar]
- 6.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. The Lancet. 2012. 2017/09/19;380(9838):247–57. [DOI] [PubMed] [Google Scholar]
- 7.Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, van Mechelen W, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. The Lancet. 2016. 2017/09/18;388(10051):1311–24. [DOI] [PubMed] [Google Scholar]
- 8.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet. 2012. 2017/09/19;380(9838):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson M, Rejeski W, Blair S. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–105. 10.1161/CIRCULATIONAHA.107.185650 [DOI] [PubMed] [Google Scholar]
- 10.DHHS. Physical activity guidelines for americans. Rockville (MD): US Department of Health and Human Services. 2008.
- 11.Powell KE, Paluch AE, Blair SN. Physical activity for health: What kind? How Much? How Intense? On top of what? Annual Review of Public Health. 2011;32(1):349–65. [DOI] [PubMed] [Google Scholar]
- 12.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Internal Medicine. 2015;175(6):959–67. 10.1001/jamainternmed.2015.0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stessman J, Hammerman-Rozenberg R, Cohen A, Ein-Mor E, Jacobs JM. Physical activity, function, and longevity among the very old. Arch Intern Med. 2009. September 14;169(16):1476–83. 10.1001/archinternmed.2009.248 [DOI] [PubMed] [Google Scholar]
- 14.Chodzko-Zajko WJ, Proctor DN, Singh F, MA M.D, Minson CT, Nigg CR, et al. Exercise and physical activity for older adults. Medicine & Science in Sports & Exercise:. 2009;41(7):1510–30. [DOI] [PubMed] [Google Scholar]
- 15.Moore SC, Lee I, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Internal Medicine. 2016;176(6):816–25. 10.1001/jamainternmed.2016.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Archives of Internal Medicine. 2005;165(20):2355–60. 10.1001/archinte.165.20.2355 [DOI] [PubMed] [Google Scholar]
- 17.Wen C, Wai J, Tsai M, Yang Y, Cheng T, Lee M, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study Lancet 2011;378:1244–53. 10.1016/S0140-6736(11)60749-6 [DOI] [PubMed] [Google Scholar]
- 18.Swain D, Franklin B. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006. 97:141–47. 10.1016/j.amjcard.2005.07.130 [DOI] [PubMed] [Google Scholar]
- 19.Lee D-c, Brellenthin A, Thompson P, Sui X, Lee I, Lavie C. Running as a Key LIfestyle Medicine for Longevity Prog Cradiovasc Dis. 2017;60(1):45–55. [DOI] [PubMed] [Google Scholar]
- 20.Reimers CD, Knapp G, Reimers AK. Does physical activity increase life expectancy? A review of the literature. Journal of Aging Research. 2012;2012:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakravarty EF, Hubert HB, Lingala VB, Fries JF. Reduced disability and mortality among aging runners: a 21-year longitudinal study. Arch Intern Med. [Research Support, N.I.H., Extramural]. 2008. August 11;168(15):1638–46. 10.1001/archinte.168.15.1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293(20):2479–86. 10.1001/jama.293.20.2479 [DOI] [PubMed] [Google Scholar]
- 23.Siegel R, Miller K, Jemal A. Cancer statistics, 2017. CA: Cancer Journal for Clinicians. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 24.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical Activity and Survival After Prostate Cancer Diagnosis in the Health Professionals Follow-Up Study. Journal of Clinical Oncology. 2011;29(6):726–32. 10.1200/JCO.2010.31.5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and survival in male colorectal cancer survivors. Archives of Internal Medicine. 2009;169(22):2102–8. 10.1001/archinternmed.2009.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. JNCI Journal of the National Cancer Institute. 2012;104(11):815–40. 10.1093/jnci/djs207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk European Journal of Epidemiology. 2015;30(5):397–412. 10.1007/s10654-015-0017-6 [DOI] [PubMed] [Google Scholar]
- 28.Williams PT. Reduced Risk of Brain Cancer Mortality from Walking and Running. Medicine & Science in Sports & Exercise. 2014;46(5):927–32. [DOI] [PubMed] [Google Scholar]
- 29.Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Research. 2016;76(14):4032–50. 10.1158/0008-5472.CAN-16-0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dethlefsen C, Hansen LS, Lillelund C, Andersen C, Gehl J, Christensen JF, et al. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Research. 2017;77(18):4894–904. 10.1158/0008-5472.CAN-16-3125 [DOI] [PubMed] [Google Scholar]
- 31.Pedersen L, Christensen J, Hojman P. Effects of exercise on tumor physiology and metabolism. Cancer J 2015. 21(2):111–6. 10.1097/PPO.0000000000000096 [DOI] [PubMed] [Google Scholar]
- 32.Pedersen L, Idorn M, Olofsson Gitte H, Lauenborg B, Nookaew I, Hansen Rasmus H, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metabolism. 2016. 2017/08/15;23(3):554–62. 10.1016/j.cmet.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 33.Campisi J. Aging, Cellular Senescence, and Cancer. Annual Review of Physiology. 2013;75(1):685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature Reviews Molecular Cell Biology. 2007;8:729 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 35.Coppe J-P, Desprez P-Y, Krtolica A, Campisi J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annual review of pathology. 2010;5:99–118. 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zinger A, Cho WC, Ben-Yehuda A. Cancer and Aging—the Inflammatory Connection. Aging and Disease. 2017;8(5):611–27. 10.14336/AD.2016.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging: An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908(1):244–54. [DOI] [PubMed] [Google Scholar]
- 38.Zong W-X, Rabinowitz JD, White E. Mitochondria and Cancer. Molecular Cell. 2016;61(5):667–76. 10.1016/j.molcel.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and Garb-aging. Trends in Endocrinology & Metabolism. 2017;28(3):199–212. [DOI] [PubMed] [Google Scholar]
- 40.Minciullo PL, Catalano A, Mandraffino G, Casciaro M, Crucitti A, Maltese G, et al. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Archivum Immunologiae et Therapiae Experimentalis. [journal article]. 2016. April 1;64(2):111–26. 10.1007/s00005-015-0377-3 [DOI] [PubMed] [Google Scholar]
- 41.Franceschi C, Valensin S, Bonafe M, Paolisso G, Yashin AI, Monti D, et al. The network and the remodeling theories of aging: historical background and new perspectives. Experimental Gerontology. 2000;35(6):879–96. [DOI] [PubMed] [Google Scholar]
- 42.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010. 2017/08/15;140(6):883–99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Annals of the Rheumatic Diseases. 2011;70(Suppl 1):i104–i8. [DOI] [PubMed] [Google Scholar]
- 44.Laberge R-M, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nature cell biology. 2015;17(8):1049–61. 10.1038/ncb3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Research Reviews. 2008;7(1):34–42. 10.1016/j.arr.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 46.Radak Z, Ishihara K, Tekus E, Varga C, Posa A, Balogh L, et al. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biology. 2017;12:285–90. 10.1016/j.redox.2017.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Experimental Gerontology. 2010;45(6):410–8. 10.1016/j.exger.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 48.Fiuza-Luces C, Garatachea N, Berger N, Lucia A. Exercise is the real polypill. Physiology 2013;28:330–58. 10.1152/physiol.00019.2013 [DOI] [PubMed] [Google Scholar]
- 49.Tarnopolsky M. Exercise Metabolism-Exerkines Are Medicine. Cell Metabolism. 2015;25(5):978–84. [DOI] [PubMed] [Google Scholar]
- 50.Ost M, Coleman V, Kasch J, Klaus S. Regulation of myokine expression: Role of exercise and cellular stress. Free Radical Biology and Medicine. 2016;98(Supplement C):78–89. [DOI] [PubMed] [Google Scholar]
- 51.Booth F, Thomason D. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiological Reviews. 1991;71(2):541–85. 10.1152/physrev.1991.71.2.541 [DOI] [PubMed] [Google Scholar]
- 52.Naviaux RK. Metabolic features of the cell danger response. Mitochondrion. 2014;16(Supplement C):7–17. [DOI] [PubMed] [Google Scholar]
- 53.Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: The Mitochondrial–Lysosomal Axis Theory of Aging. Antioxid Redox Signal. 2010;12(4):503–35. 10.1089/ars.2009.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayflick L. Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both. PLoS Genetics. 2007;3(12):e220 10.1371/journal.pgen.0030220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan R, Tsaih S-W, Petkova SB, De Evsikova CM, Xing S, Marion MA, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging cell. 2009;8(3):277–87. 10.1111/j.1474-9726.2009.00478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lessard-Beaudoin Ml, Laroche M, Demers M-Je, Grenier G, Graham RK. Characterization of age-associated changes in peripheral organ and brain region weights in C57BL/6 mice. Experimental Gerontology. 2015;63:27–34. 10.1016/j.exger.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 57.Cheng A, Morsch M, Murata Y, Ghazanfari N, Reddel SW, Phillips WD. Sequence of Age-Associated Changes to the Mouse Neuromuscular Junction and the Protective Effects of Voluntary Exercise. PloS one. 2013;8(7):e67970 10.1371/journal.pone.0067970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Valles R, Gomez C, Rodriguez-Manaz L, Gracisa-Garcia F, Noguera I, Olaso-Gonzalez G, et al. Life-long spontaneous exercise does not prolong lifespan but improves health span in mice. Longevity & Healthspan. 2013;2(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cahill LS, Bishop J, Gazdzinski LM, Dorr A, Stefanovic B, Sled JG. Altered cerebral blood flow and cerebrovascular function after voluntary exercise in adult mice. Brain Structure and Function. [journal article]. 2017:1–11. [DOI] [PubMed] [Google Scholar]
- 60.Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19(10):937–50. 10.1002/hipo.20543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nilsson M, MacNeil L, Kitaoka Y, Suri R, Young S, Kaczor J, et al. Combined aerobic exercise and enzyme replacement therapy rejuvenates the mitochondrial-lysosomal axis and alleviates autophagic blockage in Pompe disease. Free Radic Biol Med. 2015;87:98–112. 10.1016/j.freeradbiomed.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 62.Nilsson MI, Samjoo IA, Hettinga BP, Koeberl DD, Zhang H, Hawke TJ, et al. Aerobic training as an adjunctive therapy to enzyme replacement in Pompe disease. Molecular genetics and metabolism. 2012;107(3):469–79. 10.1016/j.ymgme.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 63.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, Dynapenia, and the Impact of Advancing Age on Human Skeletal Muscle Size and Strength; a Quantitative Review. Frontiers in Physiology. 2012;3:260 10.3389/fphys.2012.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raschke S, Eckardt K, Bjorklund Holven K, Jensen J, Eckel J. Identification and Validation of Novel Contraction-Regulated Myokines Released from Primary Human Skeletal Muscle Cells. PloS one. 2013;8(4):e62008 10.1371/journal.pone.0062008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Haehling S, Morley J, Anker S. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. Journal of Cachexia, Sarcopenia and Muscle. 2010;1(2):129–33. 10.1007/s13539-010-0014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clinical Cases in Mineral and Bone Metabolism. 2014. Sep-Dec;11(3):177–80. [PMC free article] [PubMed] [Google Scholar]
- 67.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. The lancet Diabetes & endocrinology. 2014;2(10):819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Houtkooper RH, Argmann C, Houten SM, Canto C, Jeninga EH, Andreux PA, et al. The metabolic footprint of aging in mice. Scientific Reports. 2011;1:134 10.1038/srep00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorepiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. Jama. 2009;301(19):2024–35. 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- 70.Konopka AR, Harber M. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. 2014;2:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marsik C, Keazemi-Shirazi L, Joukhadar C, Schickbauer T, Winkler S, Wagner O, et al. C-reactive protein and all cause mortality in large hospital-based cohort. Clinical Chemistry 2008;54(2):3430349. [DOI] [PubMed] [Google Scholar]
- 72.Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. Journal of Applied Physiology. 2005. 2018/05/04;98(4):1154–62. 10.1152/japplphysiol.00164.2004 [DOI] [PubMed] [Google Scholar]
- 73.Franceschi C, Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. The Journals of Gerontology: Series A. 2014;69(Suppl_1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 74.Baylis D, Bartlett DB, Syddall HE, Ntani G, Gale CR, Cooper C, et al. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age. 2013;35(3):963–71. 10.1007/s11357-012-9396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deleidi M, Jaggle M, Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Frontiers in Neuroscience. 2015;9:172 10.3389/fnins.2015.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longevity & Healthspan. 2013;2:8-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peake J, Della Gatta PA, Katsuhiko S, Nieman D. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanism and exercise effects. EIR. 2015;21:8–25. [PubMed] [Google Scholar]
- 78.Dinarello C. Role of Interleukin-1 in Infectious Diseases. Immunological Reviews. 1992. 2018/05/04;127(1):119–46. [DOI] [PubMed] [Google Scholar]
- 79.Dinarello CA, Wolff SM. The Role of Interleukin-1 in Disease. New England Journal of Medicine. 1993. 2018/05/04;328(2):106–13. 10.1056/NEJM199301143280207 [DOI] [PubMed] [Google Scholar]
- 80.Hartwig S, Raschke S, Knebel B, et al. Secretome profiling of primary human skeletal muscle cells. Biochim Biophys Acta. 2014;5:1011–7. [DOI] [PubMed] [Google Scholar]
- 81.Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, et al. Cytokine response of primary human myotubes in an in vitro exercise model. American Journal of Physiology-Cell Physiology. 2013. 2018/05/03;305(8):C877–C86. 10.1152/ajpcell.00043.2013 [DOI] [PubMed] [Google Scholar]
- 82.Le Bihan M-C, Bigot A, Jensen SS, Dennis JL, Rogowska-Wrzesinska A, Laine J, et al. In-depth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts. Journal of Proteomics. 2012;77:344–56. 10.1016/j.jprot.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 83.Frangogiannis NG. Matricellular Proteins in Cardiac Adaptation and Disease. Physiological Reviews. 2012;92(2):635–88. 10.1152/physrev.00008.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tichet M, Prod’Homme V, Fenouille N, Ambrosetti D, Mallavialle A, Cerezo M, et al. Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nature Communications. 2015;6:6993 10.1038/ncomms7993 [DOI] [PubMed] [Google Scholar]
- 85.Jorgensen LH, Jepsen PL, Boysen A, Dalgaard LB, Hvid LG, Ortenblad N, et al. SPARC Interacts with Actin in Skeletal Muscle in Vitro and in Vivo. The American Journal of Pathology. 2017;187(2):457–74. 10.1016/j.ajpath.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 86.Kos K, Wilding JPH. SPARC: a key player in the pathologies associated with obesity and diabetes. Nature Reviews Endocrinology. 2010;6:225 10.1038/nrendo.2010.18 [DOI] [PubMed] [Google Scholar]
- 87.Mazzolini G, Atorrasagasti C, Onorato A, Peixoto E, Schlattjan M, Sowa J-P, et al. SPARC expression is associated with hepatic injury in rodents and humans with non-alcoholic fatty liver disease. Scientific Reports. 2018;8:725 10.1038/s41598-017-18981-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shiba H, Nakanishi K, Sakata M, Fujita T, Uchida Y, Kurihara H. Effects of ageing on proliferative ability, and the expressions of secreted protein, acidic and rich in cysteine (SPARC) and osteoprotegerin (osteoclastogenesis inhibitory factor) in cultures of human periodontal ligament cells. Mechanisms of Ageing and Development. 2000;117(1):69–77. [DOI] [PubMed] [Google Scholar]
- 89.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. American Journal of Physiology—Heart and Circulatory Physiology. 2009;298(2):H614–H22. 10.1152/ajpheart.00474.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trombetta JM, Bradshaw AD. SPARC/Osteonectin Functions to Maintain Homeostasis of the Collagenous Extracellular Matrix in the Periodontal Ligament. Journal of Histochemistry and Cytochemistry. 2010;58(10):871–9. 10.1369/jhc.2010.956144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lecka-Czernik B, Moerman EJ, Jones RA, Goldstein S. Identification of gene sequences overexpressed in senescent and werner syndrome human fibroblasts. Experimental Gerontology. 1996;31(1):159–74. [DOI] [PubMed] [Google Scholar]
- 92.Lee SH, Lee JA, Park HS, Song YS, Jang YJ, Kim JH, et al. Associations among SPARC mRNA expression in adipose tissue, serum SPARC concentration and metabolic parameters in Korean women. Obesity. 2012;21(11):2296–302. [DOI] [PubMed] [Google Scholar]
- 93.Kwak JY, Hwang H, Kim S-K, Choi JY, Lee S-M, Bang H, et al. Prediction of sarcopenia using a combination of multiple serum biomarkers. Scientific Reports. 2018;8(1):8574-. 10.1038/s41598-018-26617-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flynn MG, McFarlin BK, Markofski M. State of the art reviews: The anti-inflammatory effects of exercise training. Am J Lifestyle Med. 2009;1:220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Couper K, Blount D, Riley E. IL-10: The master regulator of immunity to infection. The Journal of Immunology. 2008;180(9):5771–7. [DOI] [PubMed] [Google Scholar]
- 96.Peake J, Della Gatta PA, Cameron-Smith D. Aging and its effects of inflammation in skeletal muscle at rest and following exercise-induced muscle injury American journal of physiology Regulatory, integrative and comparative physiology. 2010;298:R1485–R95. 10.1152/ajpregu.00467.2009 [DOI] [PubMed] [Google Scholar]
- 97.Pedersen L, Olsen CH, Pedersen BK, Hojman P. Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. American Journal of Physiology—Endocrinology and Metabolism. 2012;302(7):E831–E40. 10.1152/ajpendo.00339.2011 [DOI] [PubMed] [Google Scholar]
- 98.Hojman P. Exercise protects from cancer through regulation of immune function and inflammation. Biochemical Society Transactions. 2017;45(4):905–11. 10.1042/BST20160466 [DOI] [PubMed] [Google Scholar]
- 99.Krupke DM, Begley DA, Sundberg JP, Bult CJ, Eppig JT. The Mouse Tumor Biology database. Nature reviews Cancer. 2008;8(6):459–65. 10.1038/nrc2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Voluntary running-wheel distances decreased with aging in C57BL/J6 mice. Groups that do not share the same letter(s) are statistically different (P ≤ 0.05).
(DOC)
Group columns that are significantly different do not share the same letter(s) (P ≤ 0.05).
(TIFF)
Lifelong aerobic exercise training (AET) mitigates age-associated dynapenia and aerobic deconditioning in C57BL/J6 mice. *Significant effects of aging, †lifelong aerobic exercise training, and ‡gender (P ≤ 0.05).
(DOC)
Lifelong aerobic exercise training (AET) mitigates age-associated muscle loss and testicular atrophy in C57BL/J6 mice. Age-associated organ wasting was isolated to fast-twitch muscles and testicles (test.), while slow-twitch muscles (solues; sol.) and other internal organs (heart, brain, liver, kidney, spleen, and lungs) were largely spared or increased in mass. Major thigh (quadriceps complex) and lower leg (anterior and posterior crural) muscles were summed to obtain total hindlimb muscle (HL) mass. *Significant effects of aging, †lifelong aerobic exercise training, and ‡gender (P ≤ 0.05).
(DOC)
Lifelong aerobic exercise training (AET) dampens inflammaging in old C57BL/J6 mice. Serum cytokine and chemokine concentrations are reported in pg/mL and expressed as group means ± SE. Each sample consisted of serum from one or two mice within the same experimental condition. All samples were run in duplicate.
(DOC)
(DOC)
Lifelong aerobic exercise training (AET) mitigates serum SPARC levels in old C57BL/J6 mice. Serum myokine concentrations are in pg/mL and expressed as group means ± SE. Each sample consisted of serum from one or two mice within the same experimental condition. All samples were run in duplicate.
(DOC)
(DOC)
Serum exerkine concentrations are reported in pg/mL and expressed as group means ± SE. Each sample consisted of serum from one or two mice within the same experimental condition. All samples were run in duplicate.
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.