Abstract
Subarachnoid pleural fistula (SPF) is an aberrant communication between the pleural cavity and subarachnoid space, resulting in uncontrolled cerebrospinal fluid drainage. The negative pressure of the pleural cavity creates a continuous suctioning effect, thereby impeding the spontaneous closure of these fistulas. Dural tears or punctures in cardiothoracic procedures, spinal operations, and trauma are known to cause such abnormal communications. Failure to recognize this entity may result in sudden neurological or respiratory complications. Hence, a high index of suspicion is required for early diagnosis and prompt management. Noninvasive positive pressure ventilation has been described to be effective in managing such fistulas, thus mitigating the high morbidity associated with exploratory surgery for primary repair. Herein, we describe the typical presentation of SPF and the clinical course, treatment, and follow-up of a patient who sustained SPF following anterior thoracic spinal surgery.
Keywords: Noninvasive positive pressure ventilation, Subarachnoid pleural fistula, Incidental dural tear
INTRODUCTION
The incidence of iatrogenic dural injury varies from 1%–17%. Noted complications of incidental durotomy are cerebrospinal fluid (CSF) fistulas, pseudomenigocoeles, meningitis, arachnoiditis, epidural abscess, wound and surgical site infections [1]. Subarachnoid pleural fistula (SPF) is a type of CSF fistula resulting in uncontrolled CSF drainage across the channel when a dural tear occurs in close proximity to a rent in pleural cavity. Milloy et al. [2], first reported this entity following anterior thoracic spine surgery. The negative intrapleural pressure (-5 cmH2O) along with the positive subarachnoid pressure (10 cmH2O) creates a high pressure gradient across the fistula, resulting in excess CSF drainage. This vacuum effect causes a massive collection of fluid, as it is much beyond the fluid absorptive capacity of pleura (0.5 mL/kg/hr). This continuous drain of CSF precludes the closure of SPF.
CASE REPORT
A 23-year-old healthy male presented with midthoracic spinal pain with radiation to anterior chest wall since 6 months. Plain radiography revealed mild collapse of D6 vertebra (Fig. 1). Magnetic resonance imaging (MRI) revealed an expansile lytic lesion with multiple fluid levels. Computed topography (CT) revealed thinned out sclerotic margins in anterior aspect of the lesion (Fig. 2). As a first stage procedure, we performed posterior instrumented stabilization from D4 to D9 and biopsy. Histopathological examination was suggestive of aneurysmal bone cyst (Fig. 3).
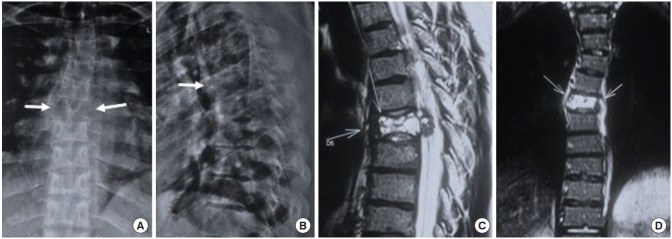
Fig. 1.
(A) Anteroposterior radiograph: arrows point toward loss of pedicle margins on both sides and osteolytic lesion involving the entire D6 vertebra. (B) Lateral radiograph shows mild collapse of the vertebra. (C, D) Sagittal and coronal images of T2-weighted magnetic resonance imaging show an expansile lytic lesion with thinned out sclerotic margin and multiple fluid levels replacing the D6 vertebral body, along with retropulsion and soft tissue expansion of lesion posteriorly into the spinal canal resulting in secondary spinal canal stenosis and cord edema.
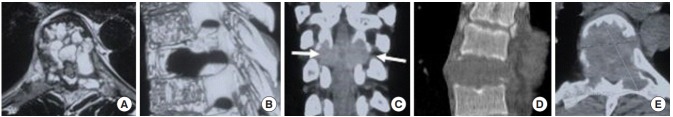
Fig. 2.
(A) Axial T2-weighted magnetic resonance imaging image shows multiple fluid pockets involving the vertebral body, adjacent pedicles and left lamina with posterior extension of lesion causing spinal canal compromise. (B) Sagittal 3-dimensional computed tomography (CT) image shows cortical breech, with posterior and epidural extension of the lesion. (C) Coronal CT arrows marks show distortion of pedicle margins bilaterally. (D) Sagittal CT image showing lytic lesion involving the entire height of vertebral body. (E) Axial CT cut shows thinned out sclerotic margins in anterior aspect of the lesion and grossly thinned out margins in the rest of vertebra.
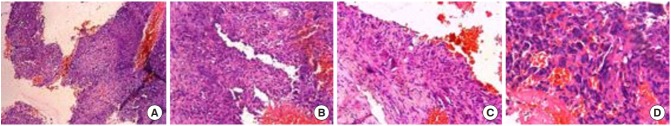
Fig. 3.
Histopathology slides (H&E, × 100). (A) Cystic spaces of varying sizes are filled with blood lined by plump spindle-shaped mononuclear cells and they are separated by cellular stroma. (B) Cystic vascular spaces are lined by fibroblasts and are devoid of endothelium. (C) Vascular space is lined by fibroblasts and the septa shows few osteoclastic giant cells without any evidence of atypia/necrosis/increased mitosis or granulomata. (D) Clusters of multinucleated osteoclastic giant cells seen admixed with spindle stromal cells. The giant cells are small with 3 to 6 nuclei and are lined along the septa. The above images are suggestive of aneurysmal bone cyst.
The second stage tumor excision and anterior reconstruction was done after preoperative embolization. Patient was positioned on right lateral decubitus position and standard left transthoracic approach was employed under general anesthesia. A double lumen endotracheal tube was used for allowing single lung ventilation. Fourth rib was excised, parietal pleura was opened up and lungs were hypo-ventilated to expose D6 vertebra adequately. Corpectomy was performed followed by anterior reconstruction. Ventral surface of dura was visualized and completely decompressed. No CSF leak was noticed during the procedure. Inter costal chest drain tube of size 32F was applied and thoracotomy wound was closed in a water tight fashion.
The chest drain collections were 350, 600, and 850 mL, respectively on the first, second, and third day following surgery. The patient had 3 episodes of vomiting on the third postoperative night .The next day patient started experiencing headache, giddiness and nausea. On auscultation of chest, breath sounds were diminished on left side. Chest radiograph revealed diffuse haziness of left chest cavity suggesting pleural effusion. The volume of fluid drained, started rapidly progressing. The diagnosis of SPF was made after identifying beta 2 transferrin protein in the fluid which is specific to CSF and the patient was started on noninvasive positive pressure ventilation (NPPV). The patient has given a written consent for publishing this material.
He was put on continuous positive airway pressure (CPAP) mode with the FiO2 set at 0.3, pressure support ventilation at 12 cmH2O and positive end-expiratory pressure at 5 cmH2O. Chest drain was clamped intermittently and removed 5 days later. He was continued on NPPV via a full contact face mask for a total of 9 days. Serial chest radiographs (Fig. 4) showed good improvement with clearance of haziness and the patient got symptomatically better gradually, and there was no evidence of recollection at one month following discharge (Fig. 5). On follow-up of 18 months he had recovered completely. His follow-up MRI (Fig. 6) showed clear chest fields and adequate decompression of cord. CT images shows good fusion and cage in good position with no evidence of recurrence. At this point of time patient is employed and has started living a normal lifestyle.
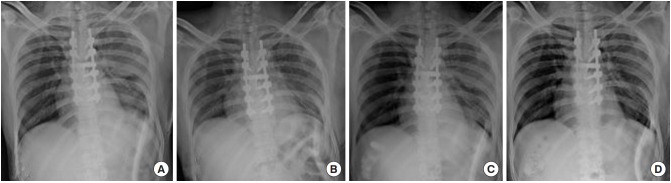
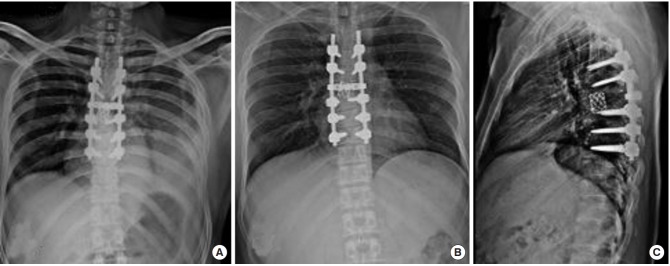
Fig. 4.
Serial chest radiographs with intercostal chest drain (ICD) in situ demonstrating resolution of pleural effusion. (A) Massive pleural effusion on fourth postoperative day (POD). (B) Second day after initiating noninvasive positive pressure ventilation (NPPV) shows reappearance of basal lung air shadows. (C) Lung margins are appreciated better in this image taken 2 days later. (D) Complete resolution of haziness on day 5 of NPPV. ICD was removed on POD 9.
Fig. 5.
(A) Reappearance of minimal basal pleural effusion after removing intercostal chest drain (ICD) on day 6 after noninvasive positive pressure ventilation. (B, C) Complete resolution of pleural effusion with no recurrence after three months with implant cage in good position.
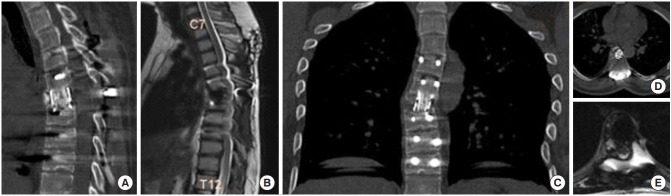
Fig. 6.
(A, B) Sagittal computed tomography (CT) image shows good fusion and bone to bone healing after 6 months with no evidence of recurrence. (C) Coronal magnetic resonance imaging (MRI) image shows clear chest fields with no recurrence of subarachnoid pleural fistula. Axial CT (D) and MRI (E) images show mesh cage in position and adequate decompression of cord.
DISCUSSION
The clinical manifestation of SPF may either be due to excess CSF drainage from subarachnoid space or owing to excess pleural CSF collection [3]. Excess drainage results in Intracranial hypotension, causing headache, nausea, dizziness, and neurological deficits [4]. Massive CSF drainage can also cause rapid deterioration of mental state and level of consciousness due to pneumocephalus [5]. Rapid movement of head in such situations, may make the patient experience a splashing sound and this phenomenon is known as bruit hydroaerique [6]. Rarely Intracranial hypotension may potentiate the downward displacement of cerebrum resulting in subdural hemorrhage [7]. On the other hand, massive pleural fluid collections result in acute respiratory distress, presenting as dyspnea, chest pain or hypoxia. Large CSF collection in pleural cavity due to SPF has been reported and it is recommended to consider SPF as an etiology in pleural effusions, following anterior thoracic spine surgery [8].
Diagnosis of SPF is established based on clinical, radiological and laboratory correlation. Plain chest radiography reveals pleural effusion, but it is not specific. While a simple CT of thorax is not of much value, CT myelography aids in establishing the diagnosis of SPF [9]. MRI of thorax is noninvasive and can demonstrate CSF leaks with high sensitivity. CT of brain is useful in diagnosing pneumocephalus, subdural hemorrhages consequent to SPF. Beta-2 transferrin and beta-trace protein are 2 biomarkers of CSF which can be employed in establishing the diagnosis [10].
Spontaneous closure of SPF is least expected, owing to the high pressure gradient across the fistula. Prolonged bed rest following spine surgery, increases the risk of deep vein thrombosis and pulmonary embolism. Conservative management include bed rest, use of thoracotomy tube or controlled CSF drainage (10 mL/hr) through a lumbar subarachnoid catheter. The usage of divertive CSF drainage by chest tube and/or lumbar sub arachnoid catheters has been recommended. An incidence of 2.7% of SPF following thoracic spine surgery has been observed. In a study in which 8 of 9 patients treated by lumbar CSF drainage alone, required re-explorative surgery.
Mechanical ventilation as a supplementary measure to address SPF has been tried based on the belief that, positive pressure ventilation would provide a tamponade effect and ameliorate the vacuum effect created due to negative pressure in the thorax [11]. This sealing effect would then allow the fistula to close and heal spontaneously. However these were invasive methods, using an endotracheal tube of late, there is growing evidence on the efficacy of NPPV in the treatment of SPF. NPPV can be administered through nasal cushions, nasal masks, full face masks, or total face masks. The ventilator modes can either be biphasic positive airway pressure (BIPAP) or CPAP. CPAP helps in driving the extracellular fluid into the vascular channels.
It has been observed that lumbar drain could not negotiate the suctioning effect of pleura and so NPPV was tried to address the issue. Later BIPAP mode of NPPV was used to treat a SPF following corpectomy in a thoracic spine trauma [12]. Kurata et al. [13] used NPPV in 2 cases of SPF following anterior thoracic spine surgery, when he noted the ineffectiveness of diverted CSF drainage techniques. In 2016, Schlag et al. [14] reported the successful use of NPPV along with a lumbar drain for a 72-yearold female who sustained a SPF following thoracic level excision of a calcified disc which was adherent to the overlying dura.
The unintended durotomy probably a dural puncture with no obvious intraoperative CSF leak in our case, would have occurred while removing the tumor and its soft tissue extension, lying adjacent to dural sheath. The massive pleural effusion and presence of beta 2 transferrin in the fluid helped us establish the diagnosis. We did not use lumbar drain and were able to wean the patient out of NPPV by 9 days and followed him up to 18 months with no evidence of reappearance of the fistula.
SPF associated with low intracranial hypotension may get complicated with the use of thoracic and lumbar drain, as excess CSF drainage would cause further intracranial bleeding and thus are best contraindicated. NPPV seems to be the only rescue, apart from surgical exploration in such cases. The duration of NPPV is tailored according to individual response to treatment. In general NPPV usage for a period of 7 to 10 days would suffice. ICD must first be removed following signs of improvement. NPPV is to be continued for a period of around 1 to 2 weeks. NPPV masks are sleek, weightless, and comfortable and are available in different shapes and sizes. They are completely noninvasive, easily applicable and wearable by awake patients. Patients can feed and carry on routine activities as and when required as muscle relaxants are not necessary. NPPV essentially avoids high morbidity associated with surgical exploration for dural or fistula repair.
CONCLUSION
SPF is a rare entity following surgical intervention in thoracic spine. If untreated it may lead to massive pleural effusion or intracranial hemorrhage and pneumocephalus. NPPV is a noninvasive cost effective intervention which effectively applies a sealant effect to the negative intrapleural pressure, yielding a tamponade effect and helps in spontaneous healing of SPF. NPPV can be a safe alternative in SPF, before embarking on a highly morbid surgical exploration for dural or fistula repair.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Fountas KN, Kapsalaki EZ, Johnston KW. Cerebrospinal fluid fistula secondary to dural tear in anterior cervical discectomy and fusion: case report. Spine (Phila Pa 1976) 2005;30:E277–80. doi: 10.1097/01.brs.0000162399.93992.5c. [DOI] [PubMed] [Google Scholar]
- 2.Milloy FJ, Correll NO, Langston HT. Persistent subarachnoid-pleural space fistula; report of a case. J Am Med Assoc. 1959;169:1467. doi: 10.1001/jama.1959.73000300001012. [DOI] [PubMed] [Google Scholar]
- 3.D'Souza R, Doshi A, Bhojraj S, et al. Massive pleural effusion as the presenting feature of a subarachnoid-pleural fistula. Respiration. 2002;69:96–9. doi: 10.1159/000049379. [DOI] [PubMed] [Google Scholar]
- 4.Khurana A, Brousil J, Russo A, et al. Intracranial hypotension with a sixth cranial nerve palsy subsequent to massive thoracic CSF hygroma: a rare complication of thoracic disc excision. Eur Spine J. 2013;22:2047–54. doi: 10.1007/s00586-013-2818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assietti R, Kibble MB, Bakay RA. Iatrogenic cerebrospinal fluid fistula to the pleural cavity: case report and literature review. Neurosurgery. 1993;33:1104–8. doi: 10.1227/00006123-199312000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Brown WM, 3rd, Symbas PN. Pneumocephalus complicating routine thoracotomy: symptoms, diagnosis and management. Ann Thorac Surg. 1995;59:234–6. doi: 10.1016/0003-4975(94)00434-9. [DOI] [PubMed] [Google Scholar]
- 7.Jung YY, Ju CI, Kim SW. Bilateral subdural hematoma due to an unnoticed dural tear during spine surgery. J Korean Neurosurg Soc. 2010;47:316–8. doi: 10.3340/jkns.2010.47.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monla-Hassan J, Eichenhorn M, Spickler E, et al. Duropleural fistula manifested as a large pleural transudate: an unusual complication of transthoracic diskectomy. Chest. 1998;114:1786–9. doi: 10.1378/chest.114.6.1786. [DOI] [PubMed] [Google Scholar]
- 9.Mantur M, Łukaszewicz-Zając M, Mroczko B, et al. Cerebrospinal fluid leakage--reliable diagnostic methods. Clin Chim Acta. 2011;412:837–40. doi: 10.1016/j.cca.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann-Harildstad G. Diagnostic values of beta-2 transferrin and beta-trace protein as markers for cerebrospinal fluid fistula. Rhinology. 2008;46:82–5. [PubMed] [Google Scholar]
- 11.Samandouras G, Bajtajic V, Cross F, et al. Cranial subdural hygroma complicating thoracic disc surgery. A case report. J Bone Joint Surg Am. 2004;86-A:2033–7. doi: 10.2106/00004623-200409000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Yoshor D, Gentry JB, LeMaire SA, et al. Subarachnoid-pleural fistula treated with noninvasive positive-pressure ventilation. Case report. J Neurosurg. 2001;94(2 Suppl):319–22. doi: 10.3171/spi.2001.94.2.0319. [DOI] [PubMed] [Google Scholar]
- 13.Kurata Y, Yoshimoto M, Takebayashi T, et al. Subarachnoidpleural fistula treated with noninvasive positive pressure ventilation: a two-case report and literature review. Spine (Phila Pa 1976) 2010;35:E908–11. doi: 10.1097/brs.0b013e3181dc57c1. [DOI] [PubMed] [Google Scholar]
- 14.Schlag HR, Muquit S, Hristov TB, et al. Subarachnoidal pleural fistula after resection of intradural thoracic disc herniation and multimodal treatment with noninvasive positive pressure ventilation (NPPV) Eur Spine J. 2016;25:155–9. doi: 10.1007/s00586-015-4137-1. [DOI] [PubMed] [Google Scholar]