Abstract
Background:
Using patient-reported outcomes (PROs) in clinical practice has been shown to enhance detection of health-related quality of life problems and satisfaction with care in children with cancer. This study seeks to identify which PRO information healthcare professionals (HCPs) find useful and what the perceived barriers for routinely assessing PROs are.
Procedure:
A total of 352 pediatric HCPs (43% male) from 52 countries completed a semistructured online 28-item questionnaire. Descriptive statistics (percentages) were used to identify highly important PRO information and perceived barriers. HCPs’ perceived barriers were compared according to gender, years of work experience, and country using a Fishers exact test.
Results:
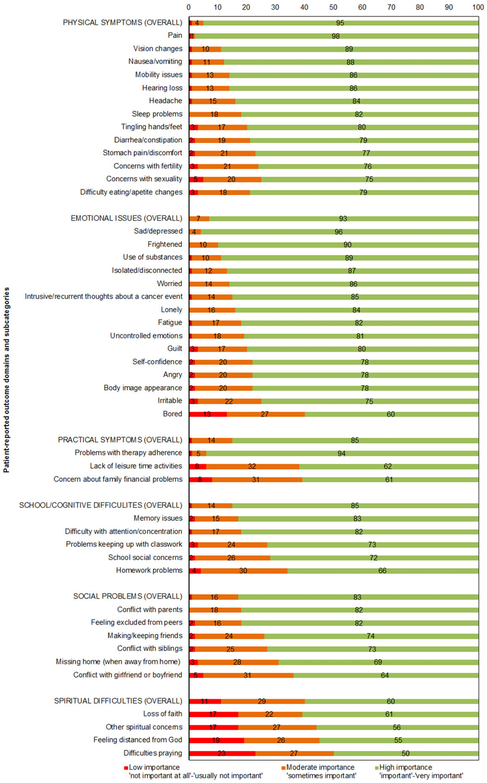
The five highest ranked PRO topics relevant in routine assessment by HCPs were as follows: pain (98%), feeling sad or depressed (96%), overall physical symptoms (95%), problems with therapy adherence (94%), and overall emotional issues (93%). Five lowest ranked topics were as follows: difficulties praying (50%), other spiritual concerns (55, 56, and 60%), and feeling bored (60%). Barriers for assessing PROs included: time (58%), insufficient staff (49%), logistics (32%), and financial resources (26%). Providers from developing countries more often reported barriers concerning insufficient staff, logistics, and financial resources.
Conclusions:
HCPs strongly value the use of physical and psychosocial PROs within pediatric oncology practice, but mainly perceive organizational barriers for routine assessment. To successfully integrate PROs, efforts should be made to address HCP-perceived barriers, such that patient-reported problems can be detected and timely referrals made.
Keywords: patient-reported outcomes, pediatric oncology, psychosocial, quality of life
1 ∣. INTRODUCTION
Children with cancer experience both short- and long-term stressors that can significantly impact their health-related quality of life (HRQoL) and coping ability.1–5 HRQoL is a multidimensional construct and is defined to cover “the subjective perceptions of the positive and. negative aspects of patients’ symptoms, including physical, emotional, social, and cognitive functions and, importantly, disease symptoms and side effects of treatment.”6 Medical healthcare professionals (HCPs; e.g., pediatricians or nurse specialists) play an important role in identifying HRQoL issues with patients and providing information, emotional support, and making appropriate referrals for psychosocial services.7 However, medical HCPs are not always aware of problems, frequently underassess the level of functioning, and underreport symptoms that the patient experiences.6–8 Therefore, it is important to include patient perspectives in the assessment of HRQoL.
HRQoL as reported by the patient is one type of patient-reported outcome (PRO). PROs are a form of patient-centered HRQoL assessment and are defined as a direct report from the patient about its health condition, without the interpretation of the response by a HCP or any other third party.9 Adult10–12 and pediatric13–17 research confirms that the routine use of PROs in clinical practice increases discussion of HRQoL during consultations, helps HCPs to effectively detect HRQoL problems, facilitates good patient–physician communication, and can enhance the satisfaction with care. The current standard of psychosocial care for children with cancer and their families recommends routine and systematic assessment of PROs.18,19
Even though the need for standardized PRO assessment is increasingly being recognized, the actual integration of PROs in clinical practice remains challenging. When trying to implement a change in an organization, individual behavior change and motivation plays an important role.20,21 Greenhalgh et al. indicate the secret behind successful adoption of a new practice or intervention is that it should be compatible with the users’ values and current needs.22 To achieve sustainable PRO implementation, ease of use and focus on clinically relevant items have been described to be of utmost importance.23,24 Therefore, when implementing PROs in clinical practice, it is important to understand the specific information HCPs find useful in the setting in which they work and the obstacles they perceive for the routine assessment of PROs as part of clinical care. Very limited information is available on this topic within the pediatric oncology context. One study that looked at the use of a PRO system in children with advanced cancer reported that HCPs found PRO reports useful, easy to understand, and to contain relevant symptom and HRQoL information.15 Pediatric oncologists and parents recommend that PROs become a part of standard clinical practice with an assessment frequency of every 3 months.25 A study on adult cancer patients showed that HCPs and patients want PRO questionnaires to cover information on physical (e.g., pain, fatigue, and nausea) and nonphysical topics (e.g., emotional distress).8
There are reported barriers to the use of PROs in adult oncology practice, including lack of time, preferences for physiological measures, lack of sufficient clinical relevance for their patients, and uncertainty in interpreting information.24,26–28 Key facilitating factors in the implementation process of PROs seem to lie in the role of a coordinator and sufficient training of staff.29,30 The only pediatric cancer study describing the real-world implementation of a PRO tool as part of standard care found the organization (e.g., not enough time provided, insufficient staffing, not documented in policy plans) and, less frequently, the attitude of HCPs toward PRO assessment or the characteristics of the PRO tool31 to be barriers for routinely discussing PROs in clinical practice. Considering PRO assessment and screening is being internationally conveyed as a standard of care in pediatric cancer,19 little is known about barriers for the use of PROs in pediatric oncology practice across different countries.
The aims of this international survey study were to (i) explore what PRO information medical HCPs find useful when caring for children (8–21 years) with cancer, (ii) identify barriers for the integration of PROs in routine care, and (iii) compare HCPs perceived barriers by gender, practicing duration, and country. Gaining insight into what PRO information HCPs working in pediatric oncology practice would find helpful may make it easier to adapt PRO assessment tools toward the needs of the users in their specific context. Also, when barriers to routine assessment of PROs are known, multifaceted tailored implementation strategies can be developed, so that we can extrapolate the most from existing interventions for the routine assessment and use of PROs in pediatric oncology practice.32
2 ∣. METHODS
2.1 ∣. Participants, design, and procedure
HCPs working in pediatric oncology practice were approached between August 2014 and January 2015 through several major pediatric oncology societies: Children’s Oncology Group (COG), Pediatric Oncology Group Ontario (POGO), Dutch COG, and International Society of Pediatric Oncology (SIOP). Inclusion criteria were as follows: (i) current medical profession in a pediatric oncology care setting (i.e. pediatric oncologist or hematologist, nurse practitioner, physician assistant, radiologist, pediatric surgeon, pediatric neurologist), (ii) working with children with cancer aged 8–21 years, and (iii) ability to speak and read English well enough to complete an online questionnaire in English. After protocol approval at the National Institute of Health Office of Human Subjects Research, HCPs were approached by e-mail through their member list of the different pediatric oncology societies. The e-mail contained an invitational letter in which the study was introduced and a direct secure link to complete a semistructured online questionnaire. If the pediatric oncology society allowed it, a reminder was sent once (COG and POGO) or twice (Dutch COG and SIOP) within 1 month after the invitation was sent. The online questionnaire was completed anonymously and the researchers did not have access to any identifying information of the respondents.
2.2 ∣. Measures
2.2.1 ∣. HCP informational preferences questionnaire
The questionnaire was designed by the investigators to cover relevant physical and psychosocial topics on HRQoL that were addressed by the pediatric cancer PRO literature. These concerned the three major domains of physical, psychological, and social health and 11 accompanying subdomains.33 The instrument was evaluated for face validity by faculty staff (i.e., statistician and pediatric oncologist) at the National Cancer Institute (Bethesda, MD) and the Academic Medical Center (Amsterdam, the Netherlands) and was revised accordingly. The final instrument consisted of 28 items with several sub items divided into five parts: (i) sociodemographic and clinical background information (HCPs’ gender, practicing country, length of work experience in the field of pediatric oncology, and primary profession), (ii) current use of PROs in their organization, (iii) whether they valued the routine use of PROs in clinical practice, and a list of physical and psychosocial PRO content topics (see Fig. 1) with importance ratings on a 5-point Likert scale (1 “not important at all,” 5 “very important”), (iv) HCPs preferences for receiving PRO information (i.e., used for what purposes (“true”/”false”), feedback format (5-point Likert scale: 1 “not useful at all,” 5 “very useful”), preferred informant (self or proxy report) at different age categories (“yes”/”no”), preferred timing on the cancer care continuum (“yes”/”no”), and (v) perceived barriers in the routine assessment of PROs (“yes”/”no”). Item 28 consisted of an open question that inquired whether any important topics were missing from the questionnaire. The questionnaire took approximately 10–15 min to complete.
FIGURE 1.
Patient-reported outcome domains rated by pediatric oncology medical healthcare professionals from highest to lowest importance
2.3 ∣. Statistical analysis
SPSS version 21.0 was used for all analyses. Background characteristics of HCPs and actual PRO use in their organization were analyzed with descriptive statistics (frequencies and percentages). Descriptive statistics (percentages) were also used to identify the five highest and lowest ranked PRO topics (% “important” – “very important”), to identify recommended purpose of use (% “yes”), preferred format (% “useful” –”very useful”), preferred timing and informant (% “yes”), and perceived barriers (% “yes”).
A two-tailed Fisher’s exact test (P< 0.05) was used to analyze possible differences in background characteristics of HCPs (gender, years of work experience, country) regarding perceived barriers. For that purpose, the background variable “work experience” was dichotomized into “less experienced HCPs” (less than 10 years) and “experienced HCPs” (10 years work experience or above). The variable “practicing country” was classified into “low”, “medium”, “high”, and “very high” development, according to the human development index (HDI) classification and was for the purpose of the analysis dichotomized into “developing countries” (low–medium) and “developed countries” (high–very high). The HDI is a “summary measure of average achievement in key dimensions of human development: a long and healthy life, being knowledgeable and have a decent standard of living.”34
Answers on the open-ended question about whether HCPs had missed any topics on the questionnaire were categorized into themes and percentages of missed topics.
3 ∣. RESULTS
3.1 ∣. Participants
Three hundred fifty-nine HCPs completed the survey. Seven HCPs did not meet the inclusion criteria (primary profession: pediatric physical therapist [N = 2], researcher [N = 2], psychologist [N = 2], social worker [N = 1]), resulting in 352 HCPs from 52 countries included in the analyses. The six most represented countries were as follows: United States of America (47%), Canada (9%), the Netherlands (7%), Spain (4%), England (2%), and Australia (2%). Table 1 details sociodemographic and clinical background characteristics of participating HCPs.
TABLE 1.
Demographic characteristics of pediatric oncology medical healthcare professionals (HCP)
| HCP (N = 352) | |
|---|---|
| Frequency (%) | |
| Sex | |
| Male | 151 (42.9) |
| Female | 201(57.1) |
| Primary profession | |
| Pediatric oncologist/hematologist | 307 (87.2) |
| Nurse practitioner | 17(4.8) |
| Pediatric (neuro)surgeon | 7(2.0) |
| Pediatrician | 6(1.7) |
| Radiologist | 4 (1.1) |
| Pediatric neurologist | 6(1.7) |
| Medical oncologist | 2 (0.6) |
| Pediatrician bone marrow transplantation | 1 (0.3) |
| Pediatric urologist | 1 (0.3) |
| Physician assistant | 1 (0.3) |
| Practicing duration | |
| Less than 1 year | 6(1.7) |
| 1–5 years | 62 (17.6) |
| 6–10 years | 77 (21.9) |
| 10–20 years | 95 (27.0) |
| More than 20 years | 112(31.8) |
| Human development index | |
| Low | 8(2.3) |
| Medium | 20 (5.7) |
| High | 32 (9.1) |
| Very high | 291 (82.7) |
| Not applicable | 1 (0.3) |
3.2 ∣. Current use of PROs
The majority of HCPs (61%) indicated that their facility did not obtain any form of standardized PRO information. Twelve percent reported that their organization obtained PROs as part of an outcome measure for research and 27% said the collection of PROs was part of their clinical practice.
3.4 ∣. Preferred PRO information
3.4.1 ∣. Content
HCPs (94%) reported value in the routine use of PROs. As shown in Figure 1, the five highest ranked useful PRO topics reported by HCPs were as follows: pain (98%), feeling sad or depressed (96%), overall physical symptoms (95%), problems with therapy adherence (94%), and overall emotional issues (93%). The five lowest ranked PRO topics by HCPS were as follows: difficulties praying (50%), feeling distanced from God (55%), other spiritual concerns (56%), overall spiritual difficulties (60%), and feeling bored (60%).
3.4.2 ∣. Purpose
HCPs reported PROs to be helpful in making efficient and appropriate referrals as needed (86%), learning more about areas of stress the child may not otherwise raise (83%), being able to address psychosocial concerns (76%), and following a child during his or her developmental trajectory (59%)
3.4.3 ∣. Format
The preferred format for obtaining PROs was the electronic record (84%), printed on paper (58%), or in an online environment, such as a web-based portal (43%). The majority of HCPs (74%) favored a combination of the above-mentioned formats.
3.4.4 ∣. Timing
HCPs reported value in conducting assessments during treatment (87%), at diagnosis (86%), during follow-up (81%), and during end-of-life care (71%). A smaller percentage of HCPs (29%) indicated that this information would be useful at every hospital or clinic visit.
3.4.5 ∣. Informant
When asked about who should report PROs, the majority of HCPs preferred both child and parent report at the age of 8–12 (92%) and 13–17 years (88%). Forages 18–21 years, less than half of HCPs (47%) felt that parent report was necessary.
3.4.6 ∣. Missed topics
Only a minority of HCPs (11%) indicated PRO topics that were missed within the survey options. Missed topics included fear of death/end-of-life concerns (N = 8, 2%), relationship with medical staff (N = 7, 2%), child concerns about the disease/future life (N = 7, 2%), specific leisure time activities (e.g., sports activities, hobbies, pets, play time for young child [N = 5,1%]), specific needs of adolescents and young adults (e.g., school, work, job opportunity [N = 2,1%]), and cultural and ethnic issues (N = 2,1%). Five HCPs (1%) addressed the question of who should address results obtained from the PRO measure (e.g. physician, nurse, social worker, or psychologist).
3.5 ∣. Perceived barriers for routinely assessing PROs
The majority of HCPs (86%) reported barriers at their worksite in routinely assessing PROs. Identified barriers were time (58%), insufficient staff to address issues raised by the assessment (49%), logistical problems (32%), financial resources (26%), and PROs not fitting within the clinical workflow (18%).
Perceived barriers did not significantly differ by gender or length of work experience, but did differ by country development level. HCPs in developing countries more often perceived barriers for obtaining PROs on a routine basis than HCPs in developed countries (P = 0.021, φ = 0.12). HCPs in developing countries more often reported financial resources (P = 0.039, φ = −0.12), logistics (P = 0.011, φ = −0.14), and insufficient staffing (P = 0.005, φ = −0.16) as a barrier than HCPs in developed countries.
4. ∣. DISCUSSION
This is the first study that sought to internationally identify medical HCPs’ preferences and perceived barriers for routine assessment of PRO measures in pediatric oncology practice. The present study illustrates that medical HCPs practicing in the field of pediatric oncology strongly value the routine use of PROs in clinical practice; nonetheless, the actual integration of PROs in their respective organization is limited (about 25%), with specific barriers noted.
4.1 ∣. PRO domains
HCPs indicate that useful PRO measures should cover information on physical (e.g., pain), psychosocial (e.g., sadness, depression, and anxiety), and practical issues (e.g., therapy adherence). This is in line with the pediatric cancer PRO literature, in which it is advocated that PRO instruments used to measure HRQoL should cover physical, psychological, and social functioning as the three primary domains of health.33 One of the topics that was deemed relatively less important by the HCPs in our study was that of spiritual concerns. This may have to do with the fact that the topic of spirituality can be perceived of different importance by HCPs than by patients and their families. In a study that assessed religious perspectives among HCPs, patients, and their families, a large proportion of patients and families saw spirituality as important to be able to cope with their disease, while in the same study, spirituality wasonly considered important by a minority of the HCPs.35
All of the PRO domains and subcategories were considered relevant by at least 50% of the HCPs in our sample, which makes it hard to make a priority list with most essential PRO topics for clinical practice. Still, the most highly ranked topics by HCPs (pain, depression, overall physical issues, adherence, and emotional issues) could be used as a starting point for fundamental elements of PRO assessment in pediatric oncology practice. Also, using computerized adaptive testing to shorten the list of items, the short Patient-Reported Outcomes Information System (PROMIS) pediatric forms, or other feasible and validated pediatric forms covering these domains,41,42 could offer a solution.
4.2 ∣. Formats
HCPs report that PROs should be used to detect HRQoL in an efficient and timely manner so that they can appropriately respond to issues raised by the PRO measure. As a feedback format for PROs, most HCPs prefer the electronic record, but a combination of online, paper, and electronic record is also highly favored. Patients generally also favor electronic PRO measures.36 It is important to take into account that electronic PROs seem to yield similar results as identical paper-pencil questionnaires.37 Using electronic PROs instead of paper-pencil versions has several potential advantages: it may lead to integration of self-report data into the patient’s medical record, efficiency in data management, and the Internet is often easily accessible for both patients and HCPs.38 Finally, PROs could be obtained online prior to a visit. This would limit the possibility of HCPs bias and allow time for the HCP to review the results and arrange for appropriate referrals prior to patients’ visit.31,39
4.3 ∣. Timing of PROs
HCPs recommend assessment of PROs throughout the whole cancer care continuum including at end-of-life. This is in line with other PRO studies performed in pediatric oncology populations, in which PROs were valued by HCPs and parents during the treatment phase,25,31 follow-up, and during end-of-life care.15 HCPs in our study did not want to have this information at every visit. This feedback is consistent with the above-mentioned pediatric oncology study,25 in which HCPs and parents suggested a frequency of every 3 months.
4.4 ∣. Informants
HCPs indicated that both child and parent report is important for youth under the age of 18. For youth ages 18–21 years, more than half of the HCPs felt that child report was sufficient. This corresponds with the existing pediatric literature, where inconsistencies between patient and proxy report are stressed and where parent proxy report is recommended when pediatric patients are too young, too cognitively impaired, too ill, or fatigued to complete a HRQOL instrument, but not as a substitute for child self-report when the child is willing and able to provide his/her perspective.10
4.5 ∣. Benefits and barriers of the use of PROs in clinical practice
There is a large body of evidence on the benefits of using PROs in clinical practice and they are considered important in the provision of quality care.19,32 Yet, from our international cohort, we found that there remains variability in assessment practices and services offered as a result of PRO findings. Moreover, most HCPs do not use any form of standardized PROs, and only a minority use them in clinical practice or for scientific purposes. The barriers for PRO assessment found in our sample were mainly based on practical issues in the organization (i.e., time, insufficient staff, and logistics). These are comparable to those reported in PRO studies performed in adult samples27,28,40 and in one pediatric oncology sample.31 Of concern, we found that HCPs from developing countries indicate routine assessment of PROs to be useful, but do not seem to have the resources (i.e., financial, logistics, and availability of staff) to be able to integrate such assessments in their clinical care. While ways to incorporate assessments into clinical care are now available within the recently published evidence-based standards of care,18 creative programming and infrastructures, particularly for HCPs low in resources, are needed.
Overall, barriers seem to arise when there is no adequate infrastructure provided by the organization to implement PRO measures in clinical practice.24 It is therefore important that professional societies recognize their influencing role in the failure or success of the integration of PROs in clinical practice. This may entail looking for ways to allocate funds for health systems change. In addition, when assessing PRO measures in clinical practice, it stays challenging to deal with methodological issues such as the possible influence of the introduction given prior to the questions and the order of the questions posed.41,42 Future research could therefore look at the possible influence of focusing illusion on response patterns and PRO outcomes in the pediatric oncology setting. Furthermore, as medical HCPs indicate PRO information to be useful, there can be a difference between intentions and actual behavioral change (e.g., when the user lacks control over the behavior, or when psychosocial support is not always readily available).43 Practical issues could be addressed and barriers ameliorated with sufficient staffing and training of staff29,30 and by providing ways to integrate PRO monitoring in clinical routines (e.g., investing in efficient technology systems that can integrate PRO data into the electronic file, and appointing a coordinator/facilitator for the implementation process).24,29,31,32 The PROMIS with its validated items banks44 and innovative electronic PRO systems13–15 could provide an efficient way to collect PRO data in pediatric practice. Regardless of the specific data collected, future studies are needed within and outside the pediatric oncology setting that investigate factors that explain both successful and unsuccessful implementation of PROs in clinical practice.
4.6 ∣. Limitations
This study has some limitations to note. First, as participants were recruited from multiple professional groups where pediatric oncology HCPs are connected, and there is known overlap of members between the groups, we cannot report on an accurate response rate. This can impact external generalizability of the findings. Second, even though HCPs from 52 different countries participated in our survey study, there still appeared to be an overrepresentation of highly developed countries in our sample. Partly, this might be related to the fact that the survey was only offered in English. Also, it might be that HCPs from developing countries have a harder time accessing the Internet. Third, when interpreting the results, it should be recognized that the participating HCPs primarily consisted of pediatric oncologists and hematologists. Caution is therefore warranted regarding the generalizability of the results to all HCPs practicing in the field of pediatric oncology. Finally, this study lacked the perspective of patients and their parents regarding what they want from PRO assessments in clinical practice. A recommendation by the Institute of Medicine is that efforts should be made to work together with patients to build on a common set of data elements that, among others, capture PROs. It should be recognized that the preferences regarding PRO assessment can essentially differ between patients and HCPs8,15,23 and future research should therefore also focus on patients’ and parents’ preferences.13–15,44,45 In addition, while patients (aged 7–21 years) and parents generally report finding completing a number of PRO questionnaires beneficial,46 continuing to assess burden is critically important for good clinical care.
To conclude, medical HCPs practicing in the field of pediatric oncology strongly value the routine use of PROs in clinical practice. However, for future PRO integration initiatives to sustainably succeed in clinical practice, PRO topics should cover information that is perceived as clinically relevant by the user (i.e., patients, parents, and HCPs) with practical barriers acknowledged and implementation challenges creatively tackled.
ACKNOWLEDGMENTS
The authors would like to thank Haven Battles, PhD, and Raphaele van Litsenburg, PhD, for their thorough evaluation of the survey. Furthermore, we would like to thank the Children’s Oncology Group (COG), the International Society for Pediatric Oncology, the Pediatric Oncology Group Ontario, and the Dutch COG that allowed us to distribute the survey. We thank all the healthcare professionals that participated in this study.
ABBREVIATIONS:
- HCP
healthcare professional
- HDI
human development index
- HRQoL
health-related quality of life
- PRO
patient-reported outcome
Footnotes
Grant sponsor: Dutch Cancer Society/Alpe d’Huzes; Grantsponsor: Intramural Programof the National Cancer Institute.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Maurice-Stam H, Oort FJ, Last BF, Brons PP, Caron HN, Grootenhuis MA. Longitudinal assessment of health-related quality of life in preschool children with non-CNS cancer after the end of successful treatment. Pediatr Blood Cancer. 2008;50(5):1047–1051. [DOI] [PubMed] [Google Scholar]
- 2.Price J, Kassam-Adams N, Alderfer MA, Christofferson J, Kazak AE. Systematic review: A reevaluation and update of the integrative (trajectory) model of pediatric medical traumatic stress. J Pediatr Psychol. 2016;41(1):86–97. [DOI] [PubMed] [Google Scholar]
- 3.Eiser C, Penn A, Katz E, Barr R. Psychosocial issues and quality of life. Semin Oncol. 2009;36(3):275–280. [DOI] [PubMed] [Google Scholar]
- 4.Stuber ML, Kazak AE, Meeske K, et al. Predictors of posttraumatic stress symptoms in childhood cancer survivors. Pediatrics. 1997;100(6):958–964. [DOI] [PubMed] [Google Scholar]
- 5.Eiser C, Eiser JR, Stride CB. Quality of life in children newly diagnosed with cancer and their mothers. Health Qual Life Outcomes. 2005;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottomley A The cancer patient and quality of life. Oncologist. 2002;7(2):120–125. [DOI] [PubMed] [Google Scholar]
- 7.Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counselling? Br J Cancer. 2001;84(2):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velikova G, Awad N, Coles-Gale R, Wright EP, Brown JM, Selby PJ. The clinical value of quality of life assessment in oncology practice a qualitative study of patient and physician views. Psychooncology. 2008;17(7):690–698. [DOI] [PubMed] [Google Scholar]
- 9.FDA. Guidance for industry patient-reported outcome measures: Use in medical product development to support labeling claims US Department of Health and Human Services Food and Drug Administration, 2009. [Google Scholar]
- 10.Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014;32(14):1480–1501. [DOI] [PubMed] [Google Scholar]
- 11.Velikova G, Booth L, Smith AB,et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. J Clin Oncol. 2004;22(4):714–724. [DOI] [PubMed] [Google Scholar]
- 12.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. JAMA. 2002;288(23):3027–3034. [DOI] [PubMed] [Google Scholar]
- 13.Engelen V, Detmar S, Koopman H, et al. Reporting health-related quality of life scores to physicians during routine follow-up visits of pediatric oncology patients: Is it effective? Pediatr Blood Cancer. 2012;58(5):766–774 [DOI] [PubMed] [Google Scholar]
- 14.Haverman L, van Rossum MA, van VM, et al. Effectiveness of a web-based application to monitor health-related quality of life. Pediatrics. 2013;131(2):e533–e543. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe J, Orellana L, Cook EF, et al. Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: Results from the PediQUEST randomized controlled trial. J Clin Oncol 2014;32(11):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Wit M, Delemarre-van de Waal HA, Bokma JA, et al. Follow-up results on monitoring and discussing health-related quality of life in adolescent diabetes care: Benefits do not sustain in routine practice. Pediatr Diabetes. 2010;11(3):175–181. [DOI] [PubMed] [Google Scholar]
- 17.De Wit M, Delemarre-van de Waal HA, Bokma JA,et al. Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: A randomized controlled trial. Diabetes Care. 2008;31(8):1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiener L, Kazak AE, Noll RB, Patenaude AF, Kupst MJ. Standards for the psychosocial care of children with cancer and their families: An introduction to the special issue. Pediatr Blood Cancer. 2015;62(Suppl 5):S419–S424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazak AE, Abrams AN, Banks J,et al. Psychosocial assessment as a standard of care in pediatric cancer. Pediatr Blood Cancer. 2015;62(Suppl 5):S426–S459. [DOI] [PubMed] [Google Scholar]
- 20.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grol RP, Bosch MC, Hulscher ME, Eccles MP, Wensing M. Planning and studying improvement in patient care: The use of theoretical perspectives. Milbank Q. 2007;85(1):93–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: Systematic review and recommendations. Milbank Q. 2004;82(4):581–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen RE, Snyder CF, Abernethy AP, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. JOncol Pract 2014;10(4):e215–e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyce MB, Browne JP, Greenhalgh J. The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: A systematic review of qualitative research. BMJ Qual Saf. 2014;23(6):508–518. [DOI] [PubMed] [Google Scholar]
- 25.Schepers SA Engelen VE, Haverman L,et al. Patient reported outcomes in pediatric oncology practice: Suggestions for future usage by parents and pediatric oncologists. Pediatr Blood Cancer. 2014;61(9):1707–1710. [DOI] [PubMed] [Google Scholar]
- 26.Snyder CF, Blackford AL, Brahmer JR, et al. Needs assessments can identify scores on HRQOL questionnaires that represent problems for patients: An illustration with the Supportive Care Needs Survey and the QLQ-C30. Qual Life Res. 2010;19(6):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouette J, Blazeby J, King M, et al. Integrating health-related quality of life findings from randomized clinical trials into practice: An international study of oncologists’ perspectives. Qual Life Res. 2015;24(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 28.Kettis-Lindblad A, Ring L, Widmark E, Bendtsen P, Glimelius B. Patients’and doctors’ views of using the schedule for individual quality of life in clinical practice. J Support Oncol. 2007;5(6):281–287. [PubMed] [Google Scholar]
- 29.Antunes B, Harding R, Higginson IJ, EUROIMPACT. Implementing patient-reported outcome measures in palliative care clinical practice: A systematic review of facilitators and barriers. Palliat Med. 2014;28(2):158–175. [DOI] [PubMed] [Google Scholar]
- 30.Santana MJ, Haverman L, Absolom K, et al. Training clinicians in how to use patient-reported outcome measures in routine clinical practice. Qual Life Res 2015;24(7):1707–1718. [DOI] [PubMed] [Google Scholar]
- 31.Schepers SA, Sint Nicolaas SM, Haverman L, et al. Implementation of electronic patient-reported outcomes in outpatient pediatric cancer care. Psychooncology In press. [DOI] [PubMed] [Google Scholar]
- 32.Sung L Priorities for quality care in pediatric oncology supportive care. J Oncol Pract. 2015;11(3):187–189. [DOI] [PubMed] [Google Scholar]
- 33.Anthony SJ, Selkirk E, Sung L, et al. Considering quality of life for children with cancer: A systematic review of patient-reported outcome measures and the development of a conceptual model. Qual Life Res. 2014;23(3):771–789. [DOI] [PubMed] [Google Scholar]
- 34.Malik K Human Development Report 2014, Sustaining Human Progress: Reducing Vulnerabilities and Building Resilience; United Nations Development Programme: New York, NY, USA, 2014. [Google Scholar]
- 35.Koenig HG, Bearon LB, Hover M, Travis JL, 3rd. Religious perspectives of doctors, nurses, patients, and families. J Pastoral Care. 1991;45(3):254–267. [DOI] [PubMed] [Google Scholar]
- 36.Campbell N, Ali F, Finlay AY, Salek SS. Equivalence of electronic and paper-based patient-reported outcome measures. Qual Life Res. 2015;24(8):1949–1961. [DOI] [PubMed] [Google Scholar]
- 37.Mangunkusumo RT, Duisterhout JS,deGraaff N, Maarsingh EJ,de Koning HJ, Raat H. Internet versus paper mode of health and health behavior questionnaires in elementary schools: Asthma and fruit as examples. J Sch Health. 2006;76(2):80–86. [DOI] [PubMed] [Google Scholar]
- 38.O’Sullivan C, Dupuis LL, Sung L. A review of symptom screening tools in pediatric cancer patients. Curr Opin Oncol. 2015;27(4):285–290. [DOI] [PubMed] [Google Scholar]
- 39.Haverman L, van Oers HA, Limperg PF, et al. Implementation of electronic patient reported outcomes in pediatric daily clinical practice: The KLIK experience. Clin Pract Pediatr Psychol. 2014;2(1): 50–67. [Google Scholar]
- 40.Snyder CF, Jensen RE, Geller G, Carducci MA, Wu AW. Relevant content for a patient-reported outcomes questionnaire for use in oncology clinical practice: Putting doctors and patients on the same page. Qual Life Res. 2010;19(7):1045–1055. [DOI] [PubMed] [Google Scholar]
- 41.Smith DM, Schwarz N, Roberts TR, Ubel PA. Why are you calling me? How study introductions change response patterns. Qual Life Res 2006;15(4):621–630. [DOI] [PubMed] [Google Scholar]
- 42.Kahneman D, Krueger AB, Schkade D, Schwarz N, Stone AA. Would you be happier if you were richer? A focusing illusion. Science. 2006;312(5782):1908–1910. [DOI] [PubMed] [Google Scholar]
- 43.Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol Bull. 2006;132(2):249–268. [DOI] [PubMed] [Google Scholar]
- 44.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007:45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sung L, Regier DA. Decision making in pediatric oncology: Evaluation and incorporation of patient and parent preferences. Pediatr Blood Cancer. 2013;60(4):558–563. [DOI] [PubMed] [Google Scholar]
- 46.Wiener L, Battles H, Zadeh S, Pao M. Is participating in psychological research a benefit, burden, or both for medically ill youth and their caregivers? IRB. 2015;37(6):1–8. [PMC free article] [PubMed] [Google Scholar]