Abstract
Plasmodium vivax is the main cause of malarial disease in Asia and South America. Plasmodium vivax infection was thought to be absent in African populations who are Duffy blood group antigen negative (Duffy-negative). However, many cases of P. vivax infection have recently been observed in Duffy-negative Africans. This raises the question: were P. vivax infections in Duffy-negative populations previously missed or has P. vivax adapted to infect Duffy-negative populations? This review focuses on recent P. vivax findings in Africa and reports views on the parasite ligands that may play a role in Duffy-negative P. vivax infections. In addition, clues gained from studying P. vivax infection of reticulocytes are presented, which may provide possible avenues for establishing P. vivax culture in vitro.
Keywords: Plasmodium vivax, Duffy blood group antigen, Duffy-negative, ligands
Introduction
Plasmodium falciparum is the leading cause of death due to malaria around the world; Plasmodium vivax also causes severe disease in humans, predominantly in Asia and South America [1-3]. The high death rate around the Thames estuary centuries ago was presumed to be caused by P. vivax [4]. Plasmodium vivax was thought to be absent in African populations who were Duffy blood group antigen (Duffy antigen) (see Glossary) negative (Duffy-negative). Recently, however, many cases of P. vivax infection in Duffy-negative populations have been reported in Angola, Benin, Botswana, Cameroon, Ethiopia, Equatorial Guinea, Kenya, Madagascar, Mali, Mauritania, Senegal, Sudan and Uganda [5-20]. Among these new cases, infection has been observed to be less severe in Duffy-negative Africans than in Duffy blood group antigen positive (Duffy-positive) Africans. The concern is that mutations/evolution in P. vivax may put Duffy-negative Africans at risk of severe disease in the future.
The potential importance of P. vivax in Africa may be underestimated by leaders and policy makers. One reason for this could be the reporting of P. vivax infections, usually done on a case-by-case basis, rather than as a holistic map across Africa. In addition, because P. vivax may be asymptomatic, there is a need for PCR diagnostic tools to determine the true frequency of P. vivax throughout Africa. If P. vivax infections are not considered, P. vivax may hamper malaria control efforts and elimination in the future. Here, we summarize the existing evidence for P. vivax malaria in Duffy-negative Africans, highlight the P. vivax ligands and erythrocyte receptors involved in parasite-host interactions, and discuss possible avenues for culturing P. vivax, which will help in understanding parasite biology and support P. vivax elimination efforts.
Evidence for the inability of P. vivax to invade Duffy-negative erythrocytes
Malaria therapy for neurosyphilis was developed in 1919 by Julius Wagner-Jauregg. However, in 1932 P. vivax was found to be ineffective in treating African-Americans, who were thus deemed to be P. vivax-resistant [21]. Although a majority was resistant to P. vivax infection, resistance was not universal. Young et al. demonstrated that refractoriness was present after inoculation of P. vivax-infected blood, indicating that resistance was at the erythrocyte level [22]. Garnham described the absence of P. vivax in West Africa; however, 11 Europeans returning from West Africa were infected with P. vivax [23].
In 1975, to identify the erythrocyte receptors for P. knowlesi (a parasite that causes malaria in primates), P. knowlesi was tested for invasion using erythrocytes that lacked various blood group determinants. Of the tested erythrocytes, it was discovered that the erythrocytes that lacked the Duffy antigen were refractory to invasion by P. knowlesi [24]. It was known at the time that Duffy-negative was common in the parts of Africa where P. vivax did not occur, suggesting that the Duffy antigen was the receptor for P. vivax. Subsequently, this speculation was tested in P. vivax in humans, by demonstrating the resistance of Duffy-negative African Americans to P. vivax infection, induced through infected-mosquito bites [25]. All Duffy-positive African Americans were susceptible to P. vivax infection [25]. Further studies on naturally occurring P. vivax infections demonstrated that Duffy-negative African Americans in Honduras [26] and in the US Army in Vietnam [27] were resistant to infection with P. vivax.
Plasmodium vivax in Duffy-negative African populations: A recent change?
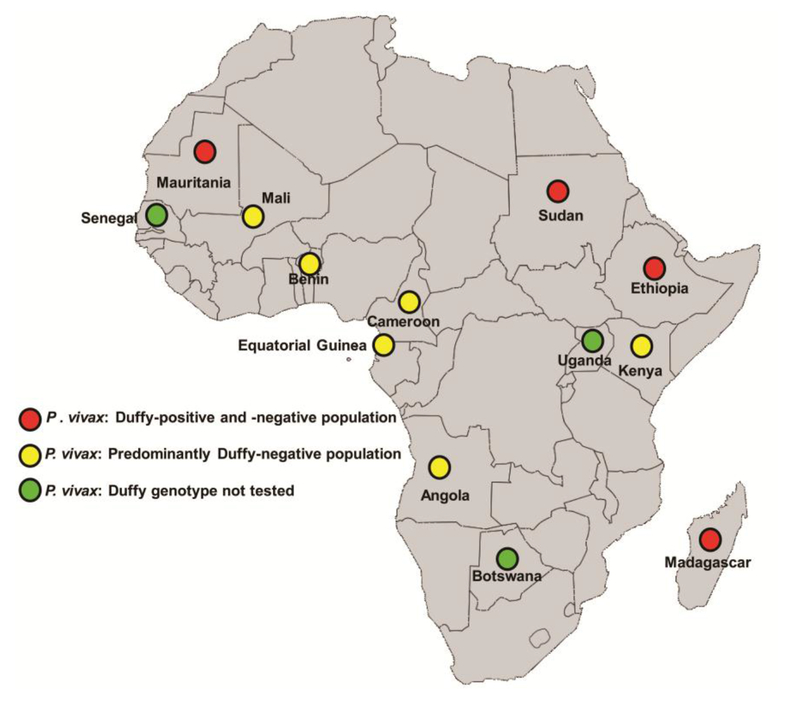
Recently, P. vivax infection of Duffy-negative Africans has been observed in many parts of Africa (Figure 1 and Table 1) and in South America where Duffy-negative people of African origin live [5-11, 15, 19, 28-30]. P. vivax infections have also recently been observed in Senegal, Botswana and Uganda, but the Duffy antigen status in these cases was not determined [12, 16, 18, 31]. The first report of P. vivax infection in Duffy-negative Africans was from Kenya [5]. It is of significance that these infections were detected when the molecular tools became available to identify extremely low levels of P. vivax infection [5-13, 16, 17, 19, 28-30, 32] (Table 1). The work by Didier Menard and colleagues in Madagascar identified multiple cases of P. vivax in Duffy-negative Africans [6] (Figure 1 and Table 1). In addition, P. vivax infection in Duffy-negatives were found in Ethiopia [10, 14] and Sudan [17], where a high proportion of Duffy-positive people live [33]. Infections in Duffy-negative people in Madagascar and Ethiopia were less severe and had a lower parasitemia than in Duffy-positive people [6, 10, 14]. Since both Duffy-positive and -negative people live side-by-side in Madagascar, Ethiopia and Sudan (Figure 1) [33], it was suggested that infections were being passed back and forth between Duffy-positive and -negative people. It was presumed that selection would occur for Duffy-negative erythrocytes under circumstances where P. vivax-infected mosquitoes are constantly feeding on Duffy-positive people. This was found not to be the case, as P. vivax was reported in Duffy-negative populations in multiple places in Africa (Kenya, Mali and Cameroon) (Figure 1 and Table 1), where the predominant population is Duffy-negative [5, 11, 19, 20, 33].
Figure 1. Plasmodium vivax infection in Duffy-negative populations in Africa.
Map showing the regions on the African continent in which P. vivax infection in Duffy-negative people occurs. Red circles represent P. vivax infection in countries where both Duffy-positive and Duffy-negative people live side-by-side. Yellow circles represent molecular evidence of P. vivax infection in Duffy-negative individuals living in predominantly Duffy-negative populations in Sub-Saharan Africa. Green circles represent populations with P. vivax infection, but in which the Duffy status has not been checked. The map has been adapted from Wikimedia.
Table 1.
Latest findings on Duffy-negative Plasmodium vivax infections in Africa and the molecular tools used.
| Countries with P. vivax infection in Africa | Number of Duffy-negative infections | Diagnostic tools | Year | References | |
|---|---|---|---|---|---|
| P. vivax | Duffy genotyping | ||||
| Cameroon | 27 | Nested PCR1, Sequencing | PCR – Melting curve analysis | 2017 | [19] |
| Mali | 25 | Microscopy, qPCR2 | Nested PCR, Sequencing | 2017 | [20] |
| Uganda | 4 | Microscopy, RDT3, Nested PCR | Not tested | 2017 | [18] |
| Ethiopia | 2 | Microscopy, Nested PCR, Sequencing | PCR, Sequencing | 2016 | [14] |
| Benin | 13 | Microscopy, Serology, Nested PCR, Sequencing | Nested PCR, Sequencing | 2016 | [15] |
| Sudan | 4 | Microscopy, RDT, PCR | PCR-RFLP4 | 2016 | [17] |
| Botswana | 169 | Nested PCR | Not tested | 2016 | [16] |
| Cameroon | 10 | Nested PCR, Sequencing | PCR, Sequencing | 2016 | [11] |
| Ethiopia | 4 | Nested PCR, qPCR | PCR, Sequencing | 2015 | [10] |
| Senegal | 4 | Nested PCR, Sequencing | Not tested | 2015 | [12] |
| Cameroon | 8 | Nested PCR, Sequencing | PCR, Sequencing | 2014 | [9] |
| Cameroon | 6 | Microscopy, Nested PCR, Sequencing | PCR-RFLP, Sequencing | 2014 | [8] |
| Mauritania | 1 | qPCR | PCR, Sequencing | 2011 | [7] |
| Equatorial Guinea | 8 | Nested PCR, RFLP | PCR-RFLP, Sequencing | 2011 | [13] |
| Angola | 7 | Nested PCR, RFLP | PCR-RFLP, Sequencing | 2011 | [13] |
| Madagascar | 42 | Microscopy, RDT, PCR, qPCR, Sequencing | PCR, LDR-FMA5, Sequencing, Flow cytometry | 2010 | [6] |
| Kenya | 9 | Microscopy, Nested PCR, Sequencinq | Flow cytometry | 2006 | [5] |
PCR, Polymerase chain reaction;
qPCR, Quantitative PCR;
RDT, Rapid diagnostic test;
RFLP, Restriction fragment length polymorphism;
LDR-FMA, Ligase detection reaction-fluorescent microsphere assay.
In Bandiagara, Mali, a longitudinal study found around 2% of children were infected with P. vivax, all of whom were Duffy-negative [20]. All were asymptomatic, although anemia was seen in some children, which may have resulted from the P. vivax infection [20]. Twenty-seven P. vivax-infected Duffy-negative patients presented with fever in Dschang district hospital in Cameroon [19] (Table 1). Dschang is located 1400 meters above sea level with annual temperatures of 20.5 ± 6°C, which may not be the ideal temperature for P. falciparum development in mosquitoes, but appears to be suitable for P. vivax development. In Madagascar [6] and Ethiopia [10] some patients presented with malaria symptoms; however, a greater number of symptomatic cases were seen in Dschang. It is possible that P. vivax is more severe in Dschang because of mutations in the parasite there or is it because the patients were sampled from a large population of asymptomatic P. vivax in the community?
Merozoite-erythrocyte interactions
In 1995, the molecular basis for Duffy negativity was identified as a single point mutation in the binding site for GATA1, an erythroid transcription factor that binds upstream of the Duffy antigen coding region [34]. Because of the mutation, erythrocytes do not express the Duffy antigen on their surface and hence their refractoriness to P. vivax and P. knowlesi infection [24, 25]. The P. vivax ligands responsible for invasion of Duffy-negative erythrocytes are not known. Multiple ligands are found on Plasmodium merozoites, consisting of two families: Duffy binding proteins (DBP) and reticulocyte binding proteins (RBP) (Table 2). Duffy-binding proteins were first discovered in P. falciparum [35], later in P. knowlesi [36], and then in P. vivax [37], Plasmodium knowlesi has three DBP ligands (DBP α, β and γ). Only DBPα localized in the micronemes binds to human erythrocytes and requires the Duffy antigen for binding [36, 38, 39]. Plasmodium knowlesi merozoites attach to Duffy-negative erythrocytes and reorient apically, but cannot invade the erythrocytes [40]. Transmission electron microscopy has revealed that merozoites remain some distance from the Duffy-negative erythrocytes, with strands extending towards the erythrocyte, but junction formation does not occur [40]. The P. vivax DBP1 full length protein, as well as the recombinant Duffy binding domain (region II) of DBP1, binds strongly to Duffy antigen. This particular interaction is crucial for P. vivax merozoite entry and successful invasion into erythrocytes [14, 37, 41] (Table 2). However, DBP1 full length protein or the region II domain does not bind to Duffy-negative erythrocytes [14, 37, 41] and for this reason, P. vivax could not infect Duffy-negative Africans.
Table 2.
Plasmodium vivax invasion ligands and their host receptor specificities.
| P. vivax ligands | PlasmoD B ID | Localization | Binding to reticulocytes/erythrocytes | Host Receptor specificities based on enzyme treatment | Interaction Partner: Host Receptor/Parasite ligand | References |
|---|---|---|---|---|---|---|
| DBP1 | PvX_110810 | Microneme | Erythrocytes, higher affinity for reticulocytes | Sensitive to chymotrypsin | Duffy blood group antigen | [37, 39, 41] |
| DBP2/EBP | PVP01_0102300 | Unknown | Reticulocytes | Unknown | Unknown | [50] |
| RBP1a1 | PVX_098585 | Microneme | Reticulocytes or all aged erythrocytes? | Sensitive to trypsin and chymotrypsin | Unknown | [47, 55, 57] |
| RBP1b1 | PVX_098582 | Microneme | Reticulocytes or all aged erythrocytes? | Partially sensitive to trypsin and chymotrypsin | Unknown | [47, 57] |
| RBP2a | PVX_121920 | Unknown | Erythrocytes | Sensitive to trypsin | Unknown | [56, 57] |
| RBP2b | PVX_094255 | Unknown | Reticulocytes | Sensitive to trypsin and chymotrypsin | Transferri n receptor | [57, 58] |
| RBP2c | PVX_090325 | Merozoite apical end | Reticulocytes | Resistant to trypsin, chymotrypsin and neuraminidase | Unknown | [55] |
| RBP2-P2 | PVX_101590 | Unknown | Erythrocytes | Unknown | Unknown | [57] |
| GAMA | PVX_088910 | Microneme | Reticulocyte | Sensitive to chymotrypsin, partially resistant to trypsin | Unknown | [59, 60] |
| PvTRAg | PVX_090265 | Unknown | Erythrocytes | Resistant to trypsin, chymotrypsin and neuraminidase | Unknown | [62] |
| PvTRAg26.3 | PVX_112660 | Unknown | Erythrocytes | Resistant to trypsin, chymotrypsin and neuraminidase | Unknown | [61] |
| PvTRA g33.5 | PVX_121897 | Unknown | Erythrocytes | Resistant to trypsin, chymotrypsin and neuraminidase | Unknown | [62] |
| PvTRA g34 | PVX_112685 | Unknown | Erythrocytes | Resistant to trypsin, chymotrypsin and neuraminidase | Unknown | [61] |
| PvTRA g35.2 | PVX_109280 | Unknown | Erythrocytes | Resistant to trypsin, chymotrypsin and neuraminidase | Unknown | [62] |
| PvTRA g36 | PVX_112675 | Unknown | Erythrocytes | Sensitive to chymotrypsin | Band 3 | [61] |
| PvTRA G36.6 | PVX_112690 | Merozoite apical end | Erythrocytes | Resistant to trypsin, chymotrypsin and neuraminidase | ETRAMP | [61, 63] |
| PvTRA g38 | PVX_088820 | Unknown | Erythrocytes | Partially sensitive to chymotrypsin | Band 3, Basigin | [61, 62, 64, 66] |
| PvTRA g69.4 | PVX_115465 | Unknown | Erythrocytes | Resistant to trypsin, chymotrypsin and neuraminidase | Unknown | [62] |
| PvATR Ag74 | PVX_101510 | Unknown | Erythrocytes | Partially sensitive to chymotrypsin | Band 3 | [61, 62] |
Several mutations have been identified in P. vivax DBP1 region II binding domain from Duffy-positive erythrocytes in Madagascar and from Duffy-negative erythrocytes in Ethiopia [14, 29]. None of the mutated DBP1 sequences bound to Duffy-negative erythrocytes [14]. In the Madagascar study, the DBP1 duplication in P. vivax was identified in Duffy-positive erythrocytes for the first time and the duplications were identical and in tandem [29]. Later, the DBP1 duplication was also identified in western Thailand, western Cambodia and Papua, Indonesia [42]. Another DBP1 variant duplication was identified in Cambodia, Ethiopia and Brazil [43]. Similarly, DBPα duplication, the ligand for human erythrocytes, was observed in P. knowlesi when the parasites were adapted to grow in human erythrocytes [44]. Interestingly, the DBP1 copy number expansion was more pronounced in P. vivax samples (three and eight copies) from Duffy-negative erythrocytes from Ethiopia. However, the significance of this expansion in P. vivax for invasion of Duffy-negative erythrocytes has not yet been determined [14]. Based on this result, we speculated that there could be leaky expression of Duffy antigen in Duffy-negative erythrocytes that could have selected for more copies of DBP1. More data are needed to determine if P. vivax infection in Duffy-negative erythrocytes from various regions have more DBP1 copies than in Duffypositive erythrocyte infections in the same region.
Duffy antigen exposure to P. vivax on erythrocytes
Interestingly, DBP1 Region II, expressed on COS cell surfaces, has been shown to bind to all aged erythrocytes [41]. Significantly, Choe et al. found that tyrosine 41 sulfation in the N-terminal region of Duffy antigen is critical for the binding of DBP1 to erythrocytes [45]. The binding of COS cells expressing DBP-1 to erythrocytes was blocked by the wild type Duffy antigen sequence, but not by a mutated sequence (tyrosine 41 to phenylalanine) that lacked sulfation of tyrosine 41 [45]. Subsequently, higher binding affinity of recombinant soluble DBP1 region II to reticulocytes compared to erythrocytes was shown in at least three independent studies [46-48]. However, COS cells expressing DBP1 region II bind to all aged erythrocytes, suggesting that multimeric cell surface expression of DBP1 overcame the low affinity binding of the monomer to all aged erythrocytes. Thus, the ligand avidity would be stronger on reticulocytes than erythrocytes [46-48]? The binding of soluble DBP1 region II is monomeric, whereas DBP1 region II has multiple ligands on the COS cells and at the apical tip of the merozoites, and multiple receptors on the erythrocytes that can compensate for low affinity binding. The greater binding of DBP1 region II soluble proteins to reticulocytes [46-48] suggests that single copy ligands bind more strongly to reticulocytes. Another explanation may be the loss of expression (e.g., clipping by a sulfatase) or masking of sulfation at the tyrosine domain on mature erythrocytes by binding a positively charged molecule. In addition, it was found that the binding of monoclonal antibodies specific for the N-terminal portion of the Duffy antigen decreases as the erythrocytes mature, including Fya- and Fyb- specific antibodies [48]. This may reflect protease clipping or masking of the N-terminus portion of Duffy antigen. Furthermore, there was a drop in binding to Duffy antigen by Fy3-specific antibodies that recognize the loop between the transmembrane domains 6 and 7 [48]. It is possible that some Duffy antigens are lost in the maturation of reticulocytes to mature erythrocytes or masked by other erythrocyte molecules. Yet, Duffy antigens can be detected on mature erythrocytes using Fya- and Fyb- specific antibodies. Perhaps Duffy antigen exposure to the parasite at the immature stage contributes to the inability to culture P. vivax in vitro in mature erythrocytes.
Another ligand of the Duffy family
The de novo genome assembly of a P. vivax isolate from Cambodia led to the identification of erythrocyte binding protein (EBP/DBP2), which has a conserved Duffy binding-like domain and C-terminal cysteine-rich domain before the transmembrane domain [49]. The Duffy binding domain of EBP binds to both Duffy-positive and Duffy-negative erythrocytes at a very low level [14]. However, a study by Ntumngia et al. showed that EBP/DBP2 binds preferentially to young/immature CD71 high reticulocytes (Table 2). Sequence alignment shows EBP region II has 50% similarity with P. vivax DBP1 region II [50]. The antibodies against DBP1 region II, however, did not inhibit EBP binding to immature reticulocytes [50], indicating that immunity to DBP1 does not block binding of DBP2.
Reticulocyte binding proteins of P. vivax
A second family of proteins involved in P. vivax erythrocyte invasion consists of the reticulocyte binding proteins (RBP) (Table 2), that were first discovered in P. yoelii [51] Two proteins (PvRBP1a and PvRBP2c) were identified in a λgt11 P. vivax cDNA expression library that bound to reticulocytes [52]. The RBP family contains five reticulocyte homology (RH) genes in P. falciparum [53]. With the completed P. vivax genome sequences, 11 P. vivax RH genes have been identified [49, 54]. Unlike the DBP genes localized in the micronemes, RH genes in P. falciparum are primarily found in rhoptries [53]. Recently, Gupta et al. identified the binding domains of PvRBP1a and PvRBP2c that bind to reticulocytes to be in the N-terminal region and mapped the regions for both PvRBP1a and PvRBP2c, showing them to be a 749 residue rRBP1.1 region and a 413 residue rRBP2.2 region, respectively [55]. Both bind to different receptors based on the host receptor enzyme specificity; PvRBP1a binds to a receptor that is sensitive to trypsin and chymotrypsin and the PvRBP2c receptor is neuraminidase, trypsin and chymotrypsin resistant [55] (Table 2). Naturally acquired antibodies specific for PvRBP1a or PvRBP2c inhibited the binding of PvRBP1a and PvRBP 2c, respectively, to reticulocytes, suggesting they would make promising vaccine candidates [55]. A 30 kDa region of PfRH4 binding domain that shares homology with PvRBP1a and PvRBP1b was recombinantly synthesized and found to bind to reticulocytes [47]. Antibodies against the PvRBP1a and PvRBP1b binding domain localized the protein in the micronemes [47], unlike the P. falciparum RHs, which are localized to rhoptries [53]. Gruszczyk et al. identified that the PvRBP2a160-1000 domain binds all aged erythrocytes and the erythrocyte binding domain was further narrowed to PvRBP2a160-455 [56]. Furthermore, the critical amino acid residues required for binding were determined to be within the PvRBP2a160-455 region [56]. However, a binding study by Franca et al. identified that PvRBP1a, PvRBP1b, PvRBP2a and PvRBP2-P2 bind to mature erythrocytes and only PvRBP2b binds to reticulocytes [57] (Table 2). The difference in binding of PvRBP1a and PvRBP1b to reticulocytes or all aged erythrocytes needs to be resolved [47, 55, 57]. Recently, the crucial interaction between the erythrocyte receptor transferrin receptor 1 (CD71) and the parasite ligand PvRBP2b was identified, which might be critical for initial reticulocyte recognition [58] (Table 2).
Other potential ligands of P. vivax
Other genes such as glycophosphatidylinositol anchored micronemal antigen (GAMA) and tryptophan-rich antigens may be responsible for the low-density P. vivax invasion of Duffy-negative erythrocytes (Table 2). Like P. falciparum, GAMA is also localized in the micronemes of the P. vivax merozoites and has been shown to bind to Duffy-negative erythrocytes, and more recently has been shown to bind more efficiently to CD71hi immature reticulocytes than to mature erythrocytes [59, 60]. The GAMA-binding receptor is chymotrypsin sensitive (Fig. 2) and the antibodies against GAMA inhibit binding to the receptor [60].
Thirty-six tryptophan-rich antigens are present in P. vivax [54], of which ten proteins (PvTRAg, PvTRAg26.3, PvTRAg33.5, PvTRAg34, PvTRAg35.2, PvTRAg36, PvTRAg36.6, PvTRAg38, PvTRAg69.4 and PvATRAg74) bind to receptors on erythrocytes [61,62] (Table 2). One member of this family, PvTRAg36.6, which is localized on the apical tip of the merozoites, has been shown to interact with P. vivax early transcribed membrane protein (PvETRAMP) [63]. Another member, PvTRAg56.2, which is expressed on the surface of the merozoites but does not bind to erythrocytes, has been shown to interact with the PvMSP7 protein [61,63]. The tryptophan-rich protein PvTRAg38 has two erythrocyte-binding regions, of which one interacts with erythrocyte receptor band 3 [64, 65] and the second binding domain interacts with basigin [66] (Table 2), which is the receptor for RH5 [67, 68] and rhoptry-associated protein 2 [69] in P. falciparum. Interestingly, some tryptophan-rich proteins are highly expressed in clinical isolates, suggesting that these proteins are important in P. vivax infection [70].
Concluding Remarks and Future Perspectives
Technical advancements in diagnostics have improved markedly in the past few decades. Such advancements may account for the recent detection of low-density P. vivax in Africans, which appears to have been originally missed. Although, P. vivax infection in Africa is low density, P. vivax can cause malarial disease in Duffy-negative populations. Some of the reported cases of P. vivax in Africa were symptomatic [6, 10], with some possibly inducing anemia [5, 20]. However, we cannot rule out the possibility that P. vivax evolved to infect Duffy-negative individuals and could evolve further to cause severe disease in these individuals. This could be a disaster for low-income countries where P. falciparum is already a huge public health problem. Molecular tools enable the detection of submicroscopic P. vivax infection. It is critical that the accessibility of diagnostic capacities in malaria endemic areas is increased, to identify the rising incidence of P. vivax infection. Going forward, current inadequate malaria control and elimination strategies will be affected by an increase in P. vivax infections. Adequate investments, development of research capacities and political commitment are required to learn more about P. vivax infection in Duffy-negative individuals and to gain insights into the ligands responsible for the invasion of erythrocytes, transmission and epidemiology.
The leading vaccine candidate for P. vivax is DBP1 region II. It will be interesting to test the DBP1 vaccine in Duffy-negative populations. If the vaccine is effective, it suggests that there may be leaky expression of Duffy antigen on Duffy-negative erythrocytes. At the same time, the role of other invasion pathways is unclear. Hence, more focus should be on other ligands involved in the invasion of Duffy-positive and negative erythrocytes. Are RBPs and EBP/DBP2 involved in Duffy-negative invasion? If so, then can the RBP and EBP/DBP2 ligands replace DBP1 for close apposition to allow junction formation? Furthermore, what ligands lead to apical membrane antigen 1 (AMA1) binding to erythrocytes and junction formation?
At present, research is hampered by the limited availability of P. vivax parasites. While collaborations with teams in different field sites and monkey-based studies have helped to achieve what is currently known about P. vivax, it is important to have a functioning P. vivax culture system, as in P. falciparum, to enable more research on P. vivax. For the past 100 years, studies have been conducted on culturing P. vivax in vitro, with little success. But significant improvements have been made in understanding P. vivax culturing in vitro. It is now known that P. vivax prefers reticulocytes over mature erythrocytes and grows better in reticulocytes from cord blood and maybe from bone marrow [71-74]. However, it is still unclear why P. vivax prefers reticulocytes. However, recently, P. vivax isolates from Madagascar were maintained for more than 200 days in in vitro conditions in Saimiri monkey erythrocytes with AIM V medium at 10% CO2 concentration. The parasites were maintained with blood that was not enriched with reticulocytes [75]. This phenomenon may be restricted only to Saimiri monkey erythrocytes. If P. vivax growth to elevated levels were possible in human erythrocytes, such a culture would be useful for research.
Is it possible that P. vivax requires metabolites that are lost as the erythrocytes mature (e.g. glucose-6-phosphate dehydrogenease to maintain reduced glutathione)? Metabolomics has been used to identify several differences between the reticulocytes and mature erythrocytes in both rats and humans [76]. Metabolomics is one way forward in studying the metabolites of reticulocytes, erythrocytes and the serum components that may provide clues to understand P. vivax biology and for culturing P. vivax.
Highlights.
Plasmodium vivax infection is usually seen in Asia and South America but now is also observed in most parts of Africa.
P. vivax is observed in Africa in places where Duffy-positive and Duffy-negatives live side-by-side and in places where Duffy-negativity is highly prevalent.
Most P. vivax infections observed in Duffy-negatives have a low intensity parasitemia and are asymptomatic.
Several invasion ligands other than DBP1 have been identified to bind to reticulocytes or mature erythrocytes. It will be interesting to identify the parasite ligands involved in P. vivax invasion of Duffy-negative erythrocytes.
Unlike Plasmodium falciparum, P. vivax preferentially invades immature reticulocytes that are mostly present in the bone marrow or cord blood. It is still unclear why P. vivax prefers young reticulocytes.
Outstanding Questions.
When did P. vivax begin occurring in Duffy-negative Africans?
Has P. vivax evolved to infect Duffy-negative Africans, or has technical advances in diagnostics helped identify P. vivax that previously was undetectable in blood films?
If P. vivax parasites were missed in earlier studies in Africans, it is imperative to identify the parasite ligands that are involved in invasion of Duffy-negative erythrocytes. However, what if the parasites were not missed earlier but have evolved to infect Duffy-negative erythrocytes? This could be a major economic burden for countries that are still struggling to cope with P. falciparum infections. Is there a treatment regime for P. vivax in African countries?
How does P. vivax infect Duffy-negative erythrocytes? What is the significance of the increase in the copy number of Duffy binding protein 1 (DBP1) in Duffy-negative infections? Is there leaky expression of Duffy blood group antigen in Duffy negatives or can the Duffy binding protein bind to different receptors with low affinity on Duffy-negative erythrocytes?
Will the vaccine against DBP1 be effective when tested in Duffy-negative populations?
Duffy binding protein 1 from Salvador I strain does not bind to Saimiri monkey erythrocytes but binds efficiently to Aotus monkeys and other P. vivax isolates bind to both. Can Salvador I strain in Saimiri monkeys serve as the model system to study Duffy-negative infections?
Do P. vivax-like parasites infecting apes cause disease in humans?
One major hurdle in P. vivax research is that there is no culture system to grow these parasites in vitro. Is the Duffy blood group antigen blocked or masked by other surface receptors or conformationally changed in mature erythrocytes compared to reticulocytes? If this is the case, is there a way to improve exposure of the Duffy blood group antigen in mature erythrocytes to improve in vitro culture?
Acknowledgements
This work was supported by the Intramural Research Program of the Division of Intramural Research and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. We thank DELTAS Africa Initiative Grant # DEL-15-010 for the support of training through the Malaria Research Capacity Development in West and Central Africa and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, USA, for Award Number R01AI099628 supporting the team in Bandiagara. We thank Wikimedia for the image of the African map (in public domain). We thank Dr. Susan K. Pierce (NIH) for critical reading and inputs for the manuscript.
Glossary
- COS cell
A mammalian fibroblast-like cell line which express the parasite ligand on its surface for binding erythrocytes.
- DBP1 copy number expansion
Duffy binding protein 1 (DBP1) copy number expansion may play a role in P. vivax invasion of Duffy-negative erythrocytes, although duplication also occurs in countries (e.g., South East Asia) where Duffy negativity does not occur.
- Duffy blood group antigen (Duffy-antigen)
Duffy blood group antigen is a protein that is expressed on the surface of the erythrocytes in Duffy-positive populations. It is the erythrocyte receptor for P. vivax invasion.
- Duffy-negative
The GATA 1 transcription factor binding site on Duffy-negative erythrocytes upstream of the start codon of Duffy antigen gene has a point mutation that resists GATA 1 binding. Hence, expression of the Duffy antigen is absent on the surface of the erythrocytes. This mutation occurs in Africa and in Papua New Guinea.
- Junction formation
Tight binding of the parasite to the erythrocyte that depends on the AMA1 and the RON 2 proteins and leads to erythrocyte entrance into a vacuole formed by the erythrocyte membrane.
- Leaky expression
The GATA1 transcription factor could not bind to the Duffy antigen gene due to a mutation at the promotor region and hence the Duffy antigen at the protein level is not detectable by antibodies against it. However, low expression of Duffy antigen could still be possible.
- Ligands
Parasite molecules that bind to erythrocyte receptors. DBP1 is an important ligand for P. vivax. DBP2 (EBP) is structurally similar to DPB1 but binds to a different erythrocyte receptor.
- Merozoite
In blood-stage infections, merozoites are the form of the parasite that invade erythrocytes. After invasion, merozoite develop into rings, trophozoite and schizont stage parasite. DBP is a ligand expressed on the apical tip of merozoites and interact with Duffy antigens on the erythrocyte during invasion.
- Receptors
Erythrocyte molecules to which parasite ligands bind for invasion. The Duffy blood group is the receptor for DBP1.
- Reticulocytes
Reticulocytes are the immature stage of erythrocytes. Reticulocytes contain residual RNA but not the nucleus. P. vivax preferentially invades reticulocytes compared to mature erythrocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Douglas NM et al. (2014) Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua, Indonesia. BMC Med 12, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochar DK et al. (2009) Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg 80 (2), 194–8. [PubMed] [Google Scholar]
- 3.Barber BE et al. (2015) Parasite biomass-related inflammation, endothelial activation, microvascular dysfunction and disease severity in vivax malaria. PLoS Pathog 11 (1), e1004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobson MJ (1994) Malaria in England: a geographical and historical perspective. Parassitologia 36 (1-2), 35–60. [PubMed] [Google Scholar]
- 5.Ryan JR et al. (2006) Evidence for transmission of Plasmodium vivax among a duffy antigen negative population in Western Kenya. Am J Trop Med Hyg 75 (4), 575–81. [PubMed] [Google Scholar]
- 6.Menard D et al. (2010) Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A 107 (13), 5967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wurtz N et al. (2011) Vivax malaria in Mauritania includes infection of a Duffy-negative individual. Malar J 10, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fru-Cho J et al. (2014) Molecular typing reveals substantial Plasmodium vivax infection in asymptomatic adults in a rural area of Cameroon. Malar J 13, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ngassa Mbenda HG and Das A (2014) Molecular evidence of Plasmodium vivax mono and mixed malaria parasite infections in Duffy-negative native Cameroonians. PLoS One 9 (8), e103262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo E et al. (2015) Molecular epidemiology of Plasmodium vivax and Plasmodium falciparum malaria among Duffy-positive and Duffy-negative populations in Ethiopia. Malar J 14, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngassa Mbenda HG et al. (2016) An additional observation of Plasmodium vivax malaria infection in Duffy-negative individuals from Cameroon. J Infect Dev Ctries 10 (6), 682–6. [DOI] [PubMed] [Google Scholar]
- 12.Niang M et al. (2015) A molecular survey of acute febrile illnesses reveals Plasmodium vivax infections in Kedougou, southeastern Senegal. Malar J 14, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendes C et al. (2011) Duffy negative antigen is no longer a barrier to Plasmodium vivax--molecular evidences from the African West Coast (Angola and Equatorial Guinea). PLoS Negl Trop Dis 5 (6), e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunalan K et al. (2016) Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proc Natl Acad Sci U S A 113 (22), 6271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poirier P et al. (2016) The hide and seek of Plasmodium vivax in West Africa: report from a large-scale study in Beninese asymptomatic subjects. Malar J 15 (1), 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motshoge T et al. (2016) Molecular evidence of high rates of asymptomatic P. vivax infection and very low P. falciparum malaria in Botswana. BMC Infect Dis 16 (1), 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelraheem MH et al. (2016) Transmission of Plasmodium vivax in Duffy-negative individuals in central Sudan. Trans R Soc Trop Med Hyg 110 (4), 258–60. [DOI] [PubMed] [Google Scholar]
- 18.Asua V et al. (2017) Plasmodium Species Infecting Children Presenting with Malaria in Uganda. Am J Trop Med Hyg 97 (3), 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo G et al. (2017) Molecular evidence of Plasmodium vivax infection in Duffy negative symptomatic individuals from Dschang, West Cameroon. Malar J 16 (1), 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niangaly A et al. (2017) Plasmodium vivax Infections over 3 Years in Duffy Blood Group Negative Malians in Bandiagara, Mali. Am J Trop Med Hyg 97 (3), 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayne B (1932) Note on experimental infection of Anopheles punctipennis with quartan malaria. Pub H Rep 47, 1771–1774. [Google Scholar]
- 22.Young Martin D., J.M.E.a.T.H.S. (1946) Studies on imported malaria: 5. Transmission of Plasmodium vivax by Anopheles quadrimaculatus. Am J Trop Med Hyg s126 (4), 477–482. [PubMed] [Google Scholar]
- 23.Garnham PCC (1966) Malaria parasites and other Haemosporidia. Bla Sci Pub Oxf, 1114. [Google Scholar]
- 24.Miller LH et al. (1975) Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science 189 (4202), 561–3. [DOI] [PubMed] [Google Scholar]
- 25.Miller LH et al. (1976) The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N. Engl. J. Med. 295 (6), 302–4. [DOI] [PubMed] [Google Scholar]
- 26.Spencer HC et al. (1978) The Duffy blood group and resistance to Plasmodium vivax in Honduras. Am J Trop Med Hyg 27 (4), 664–70. [DOI] [PubMed] [Google Scholar]
- 27.Miller LH et al. (1978) The Duffy blood group phenotype in American blacks infected with Plasmodium vivax in Vietnam. Am J Trop Med Hyg 27 (6), 1069–72. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho TA et al. (2012) Plasmodium vivax infection in Anajas, State of Para: no differential resistance profile among Duffy-negative and Duffy-positive individuals. Malar J 11, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menard D et al. (2013) Whole genome sequencing of field isolates reveals a common duplication of the Duffy binding protein gene in Malagasy Plasmodium vivax strains. PLoS Negl Trop Dis 7 (11), e2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavasini CE et al. (2007) Plasmodium vivax infection among Duffy antigennegative individuals from the Brazilian Amazon region: an exception? Trans R Soc Trop Med Hyg 101 (10), 1042–4. [DOI] [PubMed] [Google Scholar]
- 31.Niang M et al. (2017) Unexpected high circulation of Plasmodium vivax in asymptomatic children from Kedougou, southeastern Senegal. Malar J 16 (1), 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira CM et al. (2015) A systematic review of sub-microscopic Plasmodium vivax infection. Malar J 14, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howes RE et al. (2011) The global distribution of the Duffy blood group. Nat Commun 2, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tournamille C et al. (1995) Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet 10 (2), 224–8. [DOI] [PubMed] [Google Scholar]
- 35.Camus D and Hadley TJ (1985) A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230 (4725), 553–6. [DOI] [PubMed] [Google Scholar]
- 36.Haynes JD et al. (1988) Receptor-like specificity of a Plasmodium knowlesi malarial protein that binds to Duffy antigen ligands on erythrocytes. J Exp Med 167 (6), 1873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wertheimer SP and Barnwell JW (1989) Plasmodium vivax interaction with the human Duffy blood group glycoprotein: identification of a parasite receptor-like protein. Exp Parasitol 69 (4), 340–50. [DOI] [PubMed] [Google Scholar]
- 38.Adams JH et al. (1992) A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci U S A 89 (15), 7085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams JH et al. (1990) The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell 63 (1), 141–53. [DOI] [PubMed] [Google Scholar]
- 40.Miller LH et al. (1979) Interaction between cytochalasin B-treated malarial parasites and erythrocytes. Attachment and junction formation. J Exp Med 149 (1), 172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chitnis CE and Miller LH (1994) Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med 180 (2), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson RD et al. (2016) Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nat Genet 48 (8), 959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hostetler JB et al. (2016) Independent Origin and Global Distribution of Distinct Plasmodium vivax Duffy Binding Protein Gene Duplications. PLoS Negl Trop Dis 10 (10), e0005091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dankwa S et al. (2016) Ancient human sialic acid variant restricts an emerging zoonotic malaria parasite. Nat Commun 7, 11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choe H et al. (2005) Sulphated tyrosines mediate association of chemokines and Plasmodium vivax Duffy binding protein with the Duffy antigen/receptor for chemokines (DARC). Mol Microbiol 55 (5), 1413–22. [DOI] [PubMed] [Google Scholar]
- 46.Tran TM et al. (2005) Detection of a Plasmodium vivax erythrocyte binding protein by flow cytometry. Cytometry A 63 (1), 59–66. [DOI] [PubMed] [Google Scholar]
- 47.Han JH et al. (2016) Identification of a reticulocyte-specific binding domain of Plasmodium vivax reticulocyte-binding protein 1 that is homologous to the PfRh4 erythrocyte-binding domain. Sci Rep 6, 26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ovchynnikova E et al. (2017) DARC extracellular domain remodeling in maturating reticulocytes explains Plasmodium vivax tropism. Blood 130(12), 1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hester J et al. (2013) De novo assembly of a field isolate genome reveals novel Plasmodium vivax erythrocyte invasion genes. PLoS Negl Trop Dis 7 (12), e2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ntumngia FB et al. (2016) A novel erythrocyte binding protein of Plasmodium vivax suggests an alternate invasion pathway into Duffy-positive reticulocytes. MBio 7 (4), e01261–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holder AA and Freeman RR (1981) Immunization against blood-stage rodent malaria using purified parasite antigens. Nature 294 (5839), 361–4. [DOI] [PubMed] [Google Scholar]
- 52.Galinski MR et al. (1992) A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell 69 (7), 1213–26. [DOI] [PubMed] [Google Scholar]
- 53.Gunalan K et al. (2013) The role of the reticulocyte-binding-like protein homologues of Plasmodium in erythrocyte sensing and invasion. Cell Microbiol 15 (1), 35–44. [DOI] [PubMed] [Google Scholar]
- 54.Carlton JM et al. (2008) Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature 455 (7214), 757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta ED et al. (2017) Naturally Acquired Human Antibodies Against Reticulocyte-Binding Domains of Plasmodium vivax Proteins, PvRBP2c and PvRBP1a, Exhibit Binding-Inhibitory Activity. J Infect Dis 215 (10), 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gruszczyk J et al. (2016) Structurally conserved erythrocyte-binding domain in Plasmodium provides a versatile scaffold for alternate receptor engagement. Proc Natl Acad Sci U S A 113 (2), E191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franca CT et al. (2016) Plasmodium vivax Reticulocyte Binding Proteins Are Key Targets of Naturally Acquired Immunity in Young Papua New Guinean Children. PLoS Negl Trop Dis 10 (9), e0005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gruszczyk J et al. (2018) Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax. Science 359 (6371), 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baquero LA et al. (2017) PvGAMA reticulocyte binding activity: predicting conserved functional regions by natural selection analysis. Parasit Vectors 10 (1), 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng Y et al. (2016) Plasmodium vivax GPI-anchored micronemal antigen (PvGAMA) binds human erythrocytes independent of Duffy antigen status. Sci Rep 6, 35581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeeshan M et al. (2015) Host-parasite interaction: selective Pv-fam-a family proteins of Plasmodium vivax bind to a restricted number of human erythrocyte receptors. J Infect Dis 211 (7), 1111–20. [DOI] [PubMed] [Google Scholar]
- 62.Tyagi RK and Sharma YD (2012) Erythrocyte Binding Activity Displayed by a Selective Group of Plasmodium vivax Tryptophan Rich Antigens Is Inhibited by Patients’ Antibodies. PLoS One 7 (12), e50754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyagi K et al. (2016) Plasmodium vivax Tryptophan Rich Antigen PvTRAg36.6 Interacts with PvETRAMP and PvTRAg56.6 Interacts with PvMSP7 during Erythrocytic Stages of the Parasite. PLoS One 11 (3), e0151065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alam MS et al. (2015) Interaction of Plasmodium vivax tryptophan-rich antigen PvTRAg38 with band 3 on human erythrocyte surface facilitates parasite growth. J Biol Chem 290 (33), 20257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alam MS et al. (2016) Host-parasite interaction: multiple sites in the Plasmodium vivax tryptophan-rich antigen PvTRAg38 interact with the erythrocyte receptor band 3. FEBS Lett 590 (2), 232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rathore S et al. (2017) Basigin interacts with Plasmodium vivax tryptophan-rich antigen PvTRAg38 as a second erythrocyte receptor to promote parasite growth. J Biol Chem 292 (2), 462–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crosnier C et al. (2011) Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480(7378) 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aniweh Y et al. (2017) P. falciparum RH5-Basigin interaction induces changes in the cytoskeleton of the host RBC. Cell Microbiol 19 (9). [DOI] [PubMed] [Google Scholar]
- 69.Zhang MY et al. (2018) Disrupting CD147-RAP2 interaction abrogates erythrocyte invasion by Plasmodium falciparum. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venkatesh A et al. (2017) Identification of highly expressed Plasmodium vivax proteins from clinical isolates using proteomics. Prot Clin Appl. [DOI] [PubMed] [Google Scholar]
- 71.Udomsangpetch R et al. (2007) Short-term in vitro culture of field isolates of Plasmodium vivax using umbilical cord blood. Parasitol Int 56 (1), 65–9. [DOI] [PubMed] [Google Scholar]
- 72.Udomsangpetch R et al. (2008) Cultivation of Plasmodium vivax. Trends Parasitol 24 (2), 85–8. [DOI] [PubMed] [Google Scholar]
- 73.Malleret B et al. (2015) Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood 125 (8), 1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell B et al. (2011) A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood 118 (13), e74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mehlotra RK et al. (2017) Long-term in vitro culture of Plasmodium vivax isolates from Madagascar maintained in Saimiri boliviensis blood. Malar J 16 (1), 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srivastava A et al. (2015) Host reticulocytes provide metabolic reservoirs that can be exploited by malaria parasites. PLoS Pathog 11 (6), e1004882. [DOI] [PMC free article] [PubMed] [Google Scholar]