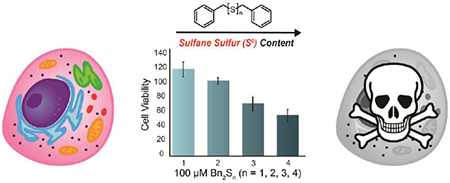
Abstract
Investigations into hydrogen sulfide (H2S) signaling pathways have demonstrated both the generation and importance of persulfides, which are reactive sulfur species that contain both reduced and oxidized sulfur. These observations have led researchers to suggest that oxidized sulfur species, including sulfane sulfur (S0), are responsible for many of the physiological phenomena initially attributed to H2S. A common method of introducing S0 to biological systems is the administration of organic polysulfides, such as diallyl trisulfide (DATS). However, prior reports have demonstrated that commercially-available DATS often contains a mixture of polysulfides, and furthermore a lack of structure-activity relationships for organic polysulfides has limited our overall understanding of different polysulfides and their function in biological systems. Advancing our interests in the chemical biology of reactive sulfur species including H2S and S0, we report our investigations into the rates and quantities of H2S release from a series of synthetic, pure benzyl polysulfides, ranging from monosulfide to tetrasulfide. We demonstrate that H2S is only released from the trisulfide and tetrasulfide, and that this release requires thiol-mediated reduction in the presence of cysteine or reduced glutathione. Additionally, we demonstrate the different effects of trisulfides and tetrasulfides on cell proliferation in murine epithelial bEnd.3 cells.
Graphical Abstract
1. Introduction
Sulfane sulfur (S0) is formally defined as a sulfur atom that bears six valence electrons, no formal charge, can exist in a thiosulfoxide tautomer, and is bonded to two or more sulfur atoms or to a sulfur atom and an ionizable hydrogen.1 This sulfur oxidation state is found in various sulfur-containing species including elemental sulfur, persulfides, and polysulfides.2 Under physiological conditions, S0 can be reduced in the presence of biological thiols, such as reduced glutathione (GSH) or cysteine (Cys), to generate the important signaling molecule hydrogen sulfide (H2S) (Figure 1).3
Figure 1.
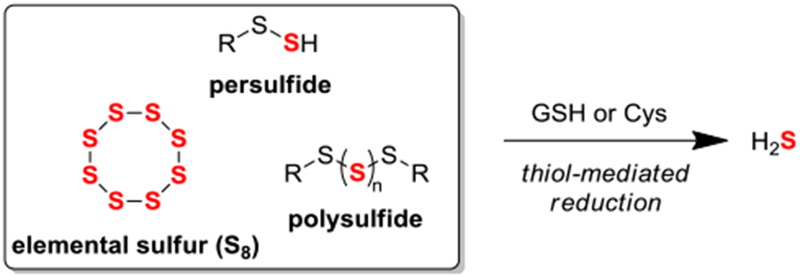
Common S0-containing small molecule species release H2S upon reaction with biological thiols.
H2S is now commonly associated with the gasotransmitter family, which includes nitric oxide and carbon monoxide, because it is produced endogenously, can freely permeate cell membranes, and can act on specific cellular and/or molecular targets to exert physiological effects.4 Recent work has demonstrated H2S-mediated signaling in processes including neurotransmission,5 vasodilation,6 and anti-inflammation.7 Towards efforts to provide exogenous H2S as either a pharmacological tool or as a potential therapeutic,8 a number of groups have prepared small molecules capable of releasing H2S under different conditions.9 Notably, a number of these developed donor motifs generate persulfides en route to H2S release, which complicates whether the observed biological effects from such donors is due to H2S release or S0. Recently, evaluation of H2S-signaling mechanisms has revealed the formation of persulfides as a common intermediate leading to questions of whether persulfides and related S0-containing species are key signaling intermediates.10
When considering sulfur oxidation states, persulfides possess reduced sulfur and S0, implying potential significance of S0 in sulfur biology.11 Under physiological conditions, S0 readily oxidizes free thiol residues to generate persulfides via a reaction mechanism known as persulfidation,12 and this process has been shown to upregulate the activity of key enzymes such as xanthine oxidase.13 Additionally, the formation of persulfides under physiological conditions has been proposed to occur via the reaction of oxidized reactive sulfur species including disulfides, sulfenic acids, and S-nitrosothiols with H2S.14 Interestingly, persulfidation of thiol-based antioxidants such as GSH leads to enhanced antioxidant activity when compared to corresponding thiols.15 Within the active sites of specific enzymes, persulfides can be stabilized and the H2S release from an enzyme-bound persulfide has been shown in 3-mercaptopyruvate sulfurtransferase via thioredoxin-mediated reduction.16 Toward directly studying small molecule persulfide reactivity, recent work has demonstrated the ability to generate persulfides under physiological conditions17 for applications in protein persulfidation,18 reactive oxygen species scavenging,19 and H-atom transfer agents.20 Previous work by our group demonstrated the ability to access discrete persulfides21 and study their reactivity under various conditions.22 A common, yet understudied pathway for accessing persulfides, hydropolysulfides, and other closely related reactive sulfur species is the thiol-mediated reduction of organic polysulfides.
Polysulfides are a class of organosulfur species commonly found in nature,23 that have unique biological properties.24 Readily isolated from alliums such as garlic,25 diallyl trisulfide (DATS) is a simple, organic polysulfide which has been studied heavily in the field of biological sulfur chemistry. The commercial availability of DATS has led to its broad use as an H2S donor and source of S0. For example, the cytotoxicity of DATS has been reported in a wide array of human cancer cell lines,26 although we note the cytotoxicity of DATS appears to be directly correlated to S0 content as the analogous monosulfide and disulfide demonstrate minimal cytotoxicity.27 Upon examining the mechanism of action, DATS was found to suppress cell proliferation in human colon cancer cells (HCT-15) via S-allylation of Cys-12β and Cys-354β in β-tubulin rather than direct H2S or persulfide release.28 By contrast, the thiol-dependent release of H2S from DATS in human red blood cells and the vasoactivity of garlic extract administration was found to be directly related to H2S production.29 In the presence of thiols, DATS releases H2S upon nucleophilic attack at the α-sulfur to generate allyl persulfide, which further reacts with a second equivalent of thiol to generate H2S.30 Despite the accessibility of other organic polysulfides, studies have been primarily limited to DATS. Alternatively, researchers have relied heavily on the use of inorganic polysulfides,31 which spontaneously disproportionate under mild, aqueous conditions to yield complex mixtures.32 Recently, our group and others33 have demonstrated the ability to access34 and utilize organic polysulfides beyond DATS for thiol-triggered H2S delivery.35 Aligned with our interests in studying S0-containing reactive sulfur species, we sought to expand the toolbox of available organic polysulfides and study the effect of varying S0 content in a single series of polysulfides. Herein, we demonstrate the thiol-mediated release of H2S from benzyl trisulfide and benzyl tetrasulfide respectively. Additionally, we demonstrate the ability of S0-containing benzyl polysulfides to suppress cell proliferation in bEnd.3 cells in a S0 content-dependent manner.
2. Materials and Methods.
Reagents were purchased from Sigma-Aldrich, Tokyo Chemical Industry (TCI), and/or Cayman Chemical Company and used directly as received. Deuterated solvents were purchased from Cambridge Isotope Laboratories and used as received. 1H and 13C{1H} NMR spectra were recorded on a Bruker 500 or 600 MHz instrument. Chemical shifts are reported relative to residual protic solvent resonances. Silica gel (SiliaFlash F60, Silicycle, 230-400 mesh) was used for column chromatography. All air-free manipulations were performed under an inert atmosphere using standard Schlenk technique or an Innovative Atmospheres N2-filled glove box. UV-Vis spectra were acquired on an Agilent Cary 60 UV-Vis spectrophotometer equipped with a Quantum Northwest TC-1 temperature controller at 25 °C ± 0.05 °C.
2.1. Synthesis
2.1.1. SS-benzyl O-methyl carbono(dithioperoxoate).
Methoxycarbonylsulfenyl chloride (2.6 mmol, 1.1 equiv.) was added to anhydrous CH2Cl2 (20 mL) and cooled to 0 °C in an ice bath under N2. Once cooled, benzyl mercaptan (2.4 mmol, 1.0 equiv.) was added dropwise, and the reaction mixture was stirred for 1 h at 0 °C under N2. After 1 h, the reaction was quenched with the addition of deionized H2O (30 mL), and the organic layer was separated. The remaining aqueous layer was extracted with CH2Cl2 (2 × 30 mL), and the combined organic layers were washed with brine (×1) and dried over MgSO4. After filtration, the solvent was removed under reduced pressure, and the desired product purified by column chromatography (10% EtOAc in hexanes, Rf = 0.46). The product was isolated as a clear liquid. Mass: 430 mg (83%) 1H NMR (500 MHz, DMSO-d6) δ: 7.38 – 7.26 (m, 5 H), 4.07 (s, 2 H), 3.79 (s, 3 H). 13C{1H} NMR (126 MHz, DMSO-d6) δ: 168.35, 136.13, 129.44, 128.41, 127.60, 55.77, 42.07. TOF MS EI+ (m/z) [M + H]+ calc’d for C9H10O2S2 214.0122; found, 214.0120.
2.1.2. Benzyl trisulfide.
SS-benzyl O-methyl carbono(dithioperoxoate) (0.93 mmol, 1.0 equiv.) was added to THF (5 mL) and stirred briefly. Potassium tert-butoxide (0.46 mmol, 0.5 equiv.) was dissolved in 0.5 mL of deionized H2O in an Eppendorf tube, and then added dropwise to the stirred reaction mixture at room temperature. After 15 h, the solvent was removed under reduced pressure, and the desired product purified by column chromatography (5% EtOAc in hexanes, Rf = 0.50). Bn2S3 was isolated as a white solid. Mass: 47 mg (36%). 1H NMR (500 MHz, CDC13) δ: 7.35 – 7.27 (m, 10 H), 4.03 (s, 4 H). 13C{1H} NMR (126 MHz, CDCl3) δ: 136.69, 129.59, 128.76, 127.73, 43.32. HRMS-EI+ (m/z): [M + H]+ calc’d for C14H14S3, 278.02576; found, 278.02464.
2.1.3. Benzyl tetrasulfide.
Benzyl mercaptan (2.0 mmol, 1.0 equiv.) and pyridine (2.0 mmol, 1.0 equiv.) were added to anhydrous Et2O (20 mL) and cooled to −78 °C over 1 h under N2. Sulfur monochloride (1.2 mmol, 0.6 equiv.) was added dropwise, and the reaction mixture was stirred at −78 °C for 2 h. The reaction was quenched with the addition of dH2O (30 mL) and diluted with CH2Cl2 (30 mL). The organic layer was removed, and the aqueous layer was extracted with CH2Cl2 (20 mL × 2). The combined organic layers were washed organic layers with brine (20 mL × 1), dried over MgSO4, filtered, and evaporated under reduced pressure to yield the product as a yellow solid. Mass: 280 mg (89%). 1H NMR (500 MHz, CDCl3) δ 7.37 – 7.27 (m, 10 H), 4.16 (s, 4 H). 13C{1H} NMR (126 MHz, CDC13) δ 136.39, 129.64, 128.81, 127.87, 43.81. (HRMS experiments did not show the parent ion peak due to the weak internal S-S bond.)
2.1.4. S-Benzyl ethanethioate.
Benzyl mercaptan (1.6 mmol, 1.1 equiv.) and triethylamine (1.6 mmol, 1.1 equiv.) were added to anhydrous CH2Cl2 (30 mL) and cooled to 0 °C in an ice bath under N2. Once cooled, acetyl chloride (1.4 mmol, 1.0 equiv.) was added dropwise, and the reaction was stirred at 0 °C for 2 h under N2. The reaction was quenched with the addition of deionized H2O (20 mL), and the organic layer was separated. The remaining aqueous layer was extracted with CH2Cl2 (2 × 20 mL) and the combined organic extractions were washed with brine (1 × 20 mL) and dried over MgSO4. After filtration, the solvent was removed under reduced pressure, and the desired product purified by column chromatography (20% CH2Cl2 in Hexanes, Rf = 0.25). The product was isolated as a clear liquid. Mass: 126 mg (52%). 1H NMR (600 MHz, CDCl3) δ: 7.31 – 7.27 (m, 4H), 7.27 – 7.23 (m, 1H), 4.13 (s, 2H), 2.35 (s, 3H). 13C{1H} NMR (151 MHz, CDC13) δ: 195.09, 137.56, 128.77, 128.60, 127.24, 33.42, 30.29. TOF MS (EI+) (m/z): [M + H]+ calc’d for C9H10OS 166.0452; found 166.0454.
2.2. H2S Measurement Materials and Methods.
Phosphate buffered saline (PBS) tablets (1×, CalBioChem) were used to prepare buffered solutions (140 mM NaCl, 3 mM KC1, 10 mM phosphate, pH 7.4) in deionized water. Buffer solutions were sparged with N2 to remove dissolved oxygen and stored in an N2-filled glovebox. Donor stock solutions (in DMSO) were freshly prepared inside an N2-filled glovebox prior to an experiment. Thiol (cysteine or GSH) stock solutions (in PBS) were freshly prepared in an N2-filled glovebox immediately before use.
2.3. General Procedure for Measuring H2S Release via Methylene Blue Assay (MBA).
Scintillation vials containing 20 mL of PBS were prepared in an N2-filled glovebox. To these solutions, 20 μL of 500 mM analyte stock solution (in PBS) was added for a final concentration of 500 μM. The solutions were allowed to thermally equilibrate while stirring in a heating block at the desired temperature for approximately 20-30 min. Immediately prior to donor addition, 0.5 mL solution of the methylene blue cocktail were prepared in disposable 1.5 mL cuvettes. The methylene blue cocktail solution contained: 200 μL of 30 mM FeCl3 in 1.2 M HCl, 200 μL of 20 mM N,N-dimethyl-p-phenylene diamine in 7.2 M HCl, and 100 μL of 1% (w/v) Zn(OAc)2. To begin an experiment, 20 μL of 25 mM donor stock solution (in DMSO) was added for a final concentration of 25 μM. At set time points after the addition of donor, 500 μL reaction aliquots were added to the methylene blue cocktail solutions and incubated for 1 h at room temperature shielded from light. Absorbance values were measured at 670 nm 1 h after addition of reaction aliquot. Each experiment was performed in quadruplicate unless stated otherwise.
2.4. MBA Calibration Curve.
Solutions containing 0.5 mL of the methylene blue cocktail and 0.5 mL PBS containing 500 μM thiol (cysteine or GSH) were freshly prepared in disposable cuvettes (1.5 mL). Under inert conditions, a 10 mM stock solution of NaSH (Strem Chemicals) in PBS was prepared and diluted to 1 mM. Immediately after dilution, 1 mM NaSH was added to 1.0 mL solutions for final concentrations of 10, 20, 30, 40, and 50 μM. Solutions were mixed thoroughly, incubated at room temperature for 1 h, and shielded from light. Absorbance values at 670 nm were measured after 1 h.
2.5. Cell Culture.
The murine brain endothelial cell line bEnd.3 was obtained by ATCC (CRL-2299) and cultured in phenol red DMEM containing 10% premium grade fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS) (10,000 units/mL penicillin and 10,000 μg/mL streptomycin). Cells were incubated at 37°C under 5% CO2.
2.6. Cell Proliferation Assay.
bEnd.3 cells were seeded in Nunc® 96 well plates at 20,000 cells/well in 10% FBS, 1% PS DMEM and grown overnight. The next day, media was aspirated off and cells were rinsed in 1% PS-containing DMEM containing no FBS or phenol red. Media was replaced with the FBS-free DMEM containing either 0.5% DMSO (vehicle) or the tested compounds and cells were incubated under these conditions for 24 hours before treatment with Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies). No turbidity of the tested compounds was observed in DMEM up to the concentrations tested.
2.7 Statistical Analysis.
Cell proliferation data is represented as a percentage viability compared to the vehicle (Veh). Data for each treatment condition were averaged from several trials using a pooled average. Error was pooled from the standard deviations for each treatment condition found in each of these trials. A two-tailed Student’s t-test was then performed comparing each treatment condition to the vehicle.
3. Results and Discussion
To investigate the impacts of sulfur content in polysulfides, we chose to study benzyl polysulfides ranging from benzyl sulfide (Bn2S) up to and including benzyl tetrasulfide (Bn2S4). Using previously reported conditions,36 we synthesized Bn2S4 in 89% yield via treatment of benzyl mercaptan with sulfur monochloride (S2Cl2) in the presence of pyridine at −78 °C (Figure 2a). To access benzyl trisulfide (Bn2S3), we evaluated prior reports of symmetrical trisulfide synthesis. Most reports cite the use of sulfur dichloride (SCl2), an unstable sulfur chloride reagent that exists in equilibrium with S2Cl2. We reasoned that using this reagent would provide an inseparable mixture of tri- and tetra-sulfides due to the SCl2/S2Cl2 equilibrium. To avoid the use of SCl2, we were drawn to a report by Harpp and co-workers that used alkoxide-mediated decomposition of sulfenylthiocarbonates to access trisulfides.37 Although treatment of methoxycarbonylsulfenyl chloride with benzyl mercaptan readily afforded the desired precursor in 83% yield, we found that further treatment with potassium tert-butoxide (KOtBu) in methanol provided an inseparable mixture of benzyl disulfide (Bn2S2) and Bn2S3. The proposed reaction mechanism by Harpp and co-workers involves an initial nucleophilic attack by the tert-butoxide anion at the carbonyl to form a carbonate and release benzyl persulfide. Based on previous work from our group, treatment of BnSSH with different bases resulted in disproportionation to yield various benzyl polysulfides, which supports the formation of benzyl persulfide under the current reaction conditions.21 Due to steric congestion and poor nucleophilicity, we viewed that nucleophilic attack by KOtBu was unlikely and hypothesized that the active nucleophile in this reaction was hydroxide, which can be generated upon deprotonation of residual water in methanol by the tert-butoxide anion.
Figure 2.
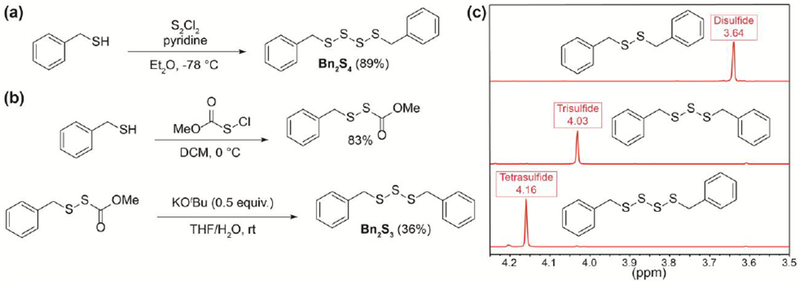
(a) Synthesis of Bn2S4. (b) Synthesis of Bn2S3. (c) Comparison of benzylic 1H NMR (500 MHz) signals between Bn2S2, Bn2S3, and Bn2S4 in CDCl3.
To test this hypothesis, we treated the benzyl sulfenylthiocarbonate precursor with tBuOK in a mixture of tetrahydrofuran and water. Consistent with our hypothesis, we were able to isolate Bn2S3 in 36% yield (Figure 2b). Considering hydroxide as the active nucleophile, we have proposed a new reaction mechanism for the synthesis of symmetrical trisulfides via hydroxide-mediated decomposition of sulfenylthiocarbonates (see Supporting Information). In comparison to current methods of mild trisulfide synthesis, we note the use of hydroxide likely prevents the formation of symmetrical trisulfides containing base-sensitive functional groups. Using 1H NMR spectroscopy, benzyl polysulfides can be identified by their respective peaks corresponding to benzylic protons which are unique to each polysulfide ranging from Bn2S2 to benzyl pentasulfide in deuterated chloroform allowing for ease of characterization (Figure 2c).38
With a series of benzyl polysulfides in hand, we next investigated the release of H2S in the presence of cysteine and GSH. We anticipated that only Bn2S3 and Bn2S4 would release H2S and that Bn2S4 would release twice as much H2S relative to Bn2S3. To test our hypothesis, dibenzyl polysulfides (25 μM) were treated with an excess of cysteine or GSH (500 μM, 20 equiv.) in PBS (10 mM, pH 7.4) at 25 °C and H2S release was measured via the spectrophotometric methylene blue assay (Figure 3).
Figure 3.
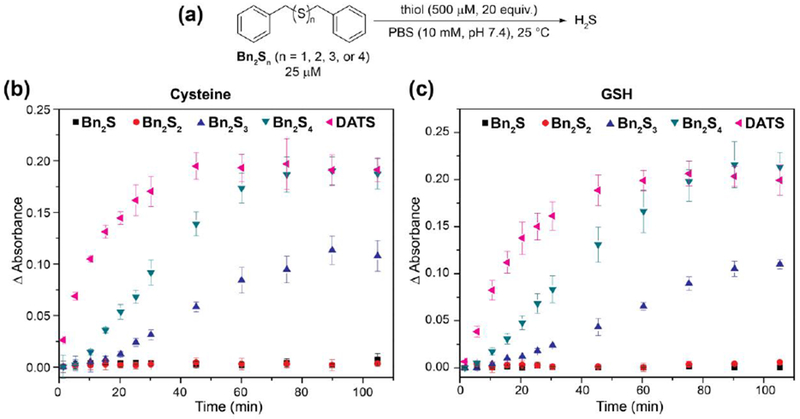
(a) Reaction conditions for thiol-triggered release of H2S from Bn2Sn (n = 1, 2, 3, or 4). (b) Release of H2S in the presence of cysteine. (c) Release of H2S in the presence of GSH.
As expected, we did not observe H2S release from Bn2S and Bn2S2 in the presence of cysteine or GSH, consistent with a lack of S0 content. Additionally, we note that the lack of H2S release from Bn2S2 suggests that nucleophilic attack by Cys or GSH at the benzylic carbon to generate a H2S-releasing persulfide intermediate does not occur.39 For both GSH and Cys, we observed approximately twice as much H2S released from Bn2S4 than from Bn2S3, which is consistent with the higher S0 content of tetrasulfides when compared to trisulfides. In the presence of cysteine (500 μM, 20 equiv.), Bn2S3 and Bn2S4 released 8.6 (34% releasing efficiency) and 17.5 μM (35% releasing efficiency) H2S respectively after 90 min. Similarly, we observed 4.3 μM (17% releasing efficiency) and 16 μM (32% releasing efficiency) H2S released from Bn2S3 and Bn2S4 respectively in the presence of GSH (500 μM, 20 equiv.) after 90 min. To compare the release of H2S from benzyl polysulfides to other commonly-used organic polysulfides, we measured the H2S release from DATS under the reported conditions. By comparison, DATS is a faster H2S donor than the benzyl polysulfides tested and yields approximately the same quantity of H2S after 110 minutes as Bn2S4. To rationalize this observation, we compared the stability of the intermediate persulfides for each donor system (i.e. benzyl persulfide vs. allyl persulfide). To the best of our knowledge, allyl persulfide has never been isolated and is simply observed as a fleeting intermediate, which suggests that is may be significantly less stable than benzyl persulfide, which has been isolated, characterized, and studied extensively.22 Additionally, a comparison of the H2S releasing curves demonstrates a brief induction period for Bn2S3 and Bn2S4 which we attribute to a buildup of benzyl persulfide in solution. Taken together, these results demonstrate the significance of pendant alkyl groups in organic polysulfides and their overall effect on the chemical reactivity of downstream reactive sulfur species including persulfides. Relative to other synthetic H2S donors, we note that organic polysulfides provide lower H2S releasing efficiencies, which is likely a result of higher lipophilicity. We anticipate the design of water-soluble polysulfides should provide more efficient H2S donors.
Further advancing our studies, we sought to examine the effect of benzyl polysulfides on cell proliferation in murine epithelial bEnd.3 cells to determine whether the different releasing efficiencies for tri- and tetra-sulfides in solution translated into cellular environments. Previously, studies have shown administration of sodium hydrosulfide (NaSH) led a pro-proliferative effect in bEnd.3 cells.40 The reported organic polysulfide library provides us a unique opportunity to directly examine the effects of S0 within a single series of polysulfides. With these compounds in hand, we sought to determine the impact of increasing S0 delivery on cell proliferation following 24 h treatments (Figure 4).
Figure 4.
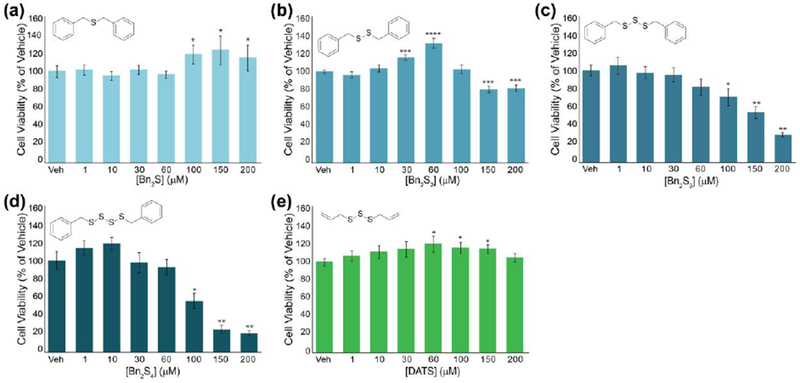
bEnd3 cell viability after 24-hour treatments of a series of organic polysulfides: (a) Bn2S, (b) Bn2S2, (c) Bn2S3, (d) Bn2S4, and (e) DATS. *p < 0.05, **p < 0.01, ***p < 0.001
Use of synthetic H2S donors such as AP3941 and direct administration of H2S via NaSH42 has been shown to have cytoprotective effects on bEnd.3 cells. To the best of our knowledge, the effect of polysulfide administration has not been studied in this specific cell line. Consistent with a lack of S0 content, 24 h incubation with Bn2S and Bn2S2 (up to 200 μM) did not reduce cell viability. We note the slight cytotoxicity of Bn2S2 at concentrations over 100 μM is likely due to perturbation of redox homeostasis arising from thiol-disulfide exchange between biological thiols and Bn2S2. Interestingly, S0-containing Bn2S3 and Bn2S4 at concentrations of 100 μM and higher demonstrated considerable cytotoxicity. Notably, Bn2S4 appears to be significantly more cytotoxic than Bn2S3, which is consistent with the increased S0 content in Bn2S4 relative to Bn2S3.
Because biological thiols react with the tri- and tetrasulfides to generate two equivalents of benzyl mercaptan as a byproduct, we also tested the cytotoxicity of benzyl mercaptan to confirm that the observed cytotoxicity was due to the S0 content rather than the organic byproducts of the reaction. We elected to use S-benzyl ethanethioate (BnSAc) as a source of benzyl mercaptan to enhance cell permeability. We anticipated hydrolysis of this ester under physiological conditions would generate benzyl mercaptan and allow us to directly probe the effect of this thiol on cytotoxicity. Using treatment up to 400 μM, we did not observe significant cytotoxicity from BnSAc suggesting the observed cytotoxicity for benzyl polysulfides is a direct reflection of S0 content, rather than organic byproducts of the reaction (Figure S8). To compare our results with a well-known organic polysulfide, we examined the effect of DATS administration on cell proliferation in bEnd.3 cells under the same conditions. Interestingly, treatment of bEnd.3 cells with increasing concentrations of DATS did not demonstrate significant cytotoxicity and suggests pendant alkyl groups in organic polysulfides can directly alter biological activities. Taken together, these results demonstrate the effect of increasing S0 content on cell proliferation in bEnd.3 cells and suggests organic polysulfides can afford varying biological activities independent of S0 content.
4. Conclusion
The identification of key intermediates in sulfur biology responsible for physiological changes such as vasodilation and anti-inflammation is of utmost importance. Recent work has begun to suggest the importance of S0 over H2S in biology and modem synthetic techniques readily allow for access to simple organic polysulfides beyond DATS. Towards advancing our knowledge of reactive sulfur species in biology, we report our studies on examining S0 content in a single series of organic polysulfides ranging from monosulfide up to and including tetrasulfide. In the presence of biological thiols including cysteine and GSH, we demonstrate the release of H2S from Bn2S3 and Bn2S4 respectively under physiological conditions. Additionally, we demonstrate the unique ability of Bn2S3 and Bn2S4 to suppress cell proliferation, whereas DATS has no effect in bEnd.3 cells. The results of this study warrant future investigations into the effects of various organic polysulfides and supports the significance of S0 in sulfur biology.
Supplementary Material
Highlights.
A series of benzyl (poly)sulfides with 1-4 sulfur atoms is prepared
Rates of H2S release after treatment with Cys and GSH are reported
H2S is only released from tri- and tetra-sulfides, but not mono- or di-sulfides
The effects of trisulfides and tetrasulfides on cell proliferation in bEnd.3 cells are reported
5. Acknowledgements
This work was supported by the NSF (CHE-1454747), the Dreyfus Foundation, and an NIH training grant (T32 GM007759, to SGB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toohey JI, Sulfur signaling: is the agent sulfide or sulfane? Anal Biochem 2011, 413 (1), 1–7. [DOI] [PubMed] [Google Scholar]
- 2.Toohey JI, Sulphane sulphur in biological systems: a possible regulatory role. Biochemical Journal 1989, 264 (3), 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R, Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 2012, 92 (2), 791–896. [DOI] [PubMed] [Google Scholar]
- 4.Wang R, Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 2002, 16 (13), 1792–1798. [DOI] [PubMed] [Google Scholar]
- 5.Kimura H; Nagai Y; Umemura K; Kimura Y, Physiological roles of hydrogen sulfide: synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid Redox Signal 2005, 7 (5–6), 795–803. [DOI] [PubMed] [Google Scholar]
- 6.Yang G; Wu L; Jiang B; Yang W; Qi J; Cao K; Meng Q; Mustafa AK; Mu W; Zhang S; Snyder SH; Wang R, H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322 (5901), 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiteman M; Li L; Rose P; Tan CH; Parkinson DB; Moore PK, The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal 2010, 12 (10), 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace JL; Wang R, Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov 2015, 14 (5), 329–345. [DOI] [PubMed] [Google Scholar]
- 9.(a) Szabo C; Papapetropoulos A, International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H2S Levels: H2S Donors and H2S Biosynthesis Inhibitors. Pharmacol Rev 2017, 69 (4), 497–564 [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Powell CR; Dillon KM; Matson JB, A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem Pharmacol 2018, 149, 110–123 [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhao Y; Biggs TD; Xian M, Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem Commun 2014, 50 (80), 11788–11805 [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bora P; Chauhan P; Pardeshi KA; Chakrapani H, Small molecule generators of biologically reactive sulfur species. RSC Advances 2018, 8 (48), 27359–27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipovic MR; Zivanovic J; Alvarez B; Banerjee R, Chemical Biology of H2S Signaling through Persulfidation. Chem Rev 2018, 118 (3), 1253–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau N; Pluth MD, Reactive sulfur species (RSS): persulfides, polysulfides, potential, and problems. Curr Opin Chem Biol 2018, 49, 1–8. [DOI] [PubMed] [Google Scholar]
- 12.Kimura H, Hydrogen sulfide and polysulfides as biological mediators. Molecules 2014, 19 (10), 16146–16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iciek M; Wlodek L, Biosynthesis and biological properties of compounds containing highly reactive, reduced sulfane sulfur. Polish Journal of Pharmacology 2001, 53 (3), 215–225. [PubMed] [Google Scholar]
- 14.Mishanina TV; Libiad M; Banerjee R, Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat Chem Biol 2015, 11 (7), 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ida T; Sawa T; Ihara H; Tsuchiya Y; Watanabe Y; Kumagai Y; Suematsu M; Motohashi H; Fujii S; Matsunaga T; Yamamoto M; Ono K; Devarie-Baez NO; Xian M; Fukuto JM; Akaike T, Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci U S A 2014, 111 (21), 7606–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikami Y; Shibuya N; Kimura Y; Nagahara N; Ogasawara Y; Kimura H, Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J 2011, 439 (3), 479–485. [DOI] [PubMed] [Google Scholar]
- 17.Artaud I; Galardon E, A persulfide analogue of the nitrosothiol SNAP: formation, characterization and reactivity. Chembiochem 2014, 15 (16), 2361–2364. [DOI] [PubMed] [Google Scholar]
- 18.Yu B; Zheng Y; Yuan Z; Li S; Zhu H; De La Cruz LK; Zhang J; Ji K; Wang S; Wang B, Toward Direct Protein S-Persulfidation: A Prodrug Approach That Directly Delivers Hydrogen Persulfide. J Am Chem Soc 2018, 140 (1), 30–33. [DOI] [PubMed] [Google Scholar]
- 19.Powell CR; Dillon KM; Wang Y; Carrazzone RJ; Matson JB, A Persulfide Donor Responsive to Reactive Oxygen Species: Insights into Reactivity and Therapeutic Potential. Angew Chem Int Ed Engl 2018, 57 (21), 6324–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauvin JR; Griesser M; Pratt DA, Hydropersulfides: Η-Atom Transfer Agents Par Excellence. J Am Chem Soc 2017,139 (18), 6484–6493. [DOI] [PubMed] [Google Scholar]
- 21.Bailey TS; Pluth MD, Reactions of isolated persulfides provide insights into the interplay between H2S and persulfide reactivity. Free Radic Biol Med 2015, 89, 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey TS; Zakharov LN; Pluth MD, Understanding hydrogen sulfide storage: probing conditions for sulfide release from hydrodisulfides. J Am Chem Soc 2014,136 (30), 10573–10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Pluth M; Bailey T; Hammers M; Hartle M; Henthorn H; Steiger A, Natural Products Containing Hydrogen Sulfide Releasing Moieties. Synlett 2015, 26 (19), 2633–2643 [Google Scholar]; (b) Jiang CS; Muller WE; Schroder HC; Guo YW, Disulfide- and multisulfide-containing metabolites from marine organisms. Chem Rev 2012,112 (4), 2179–2207. [DOI] [PubMed] [Google Scholar]
- 24.Steudel R, The Chemistry of Organic Polysulfanes R–Sn–R (n> 2). Chemical Reviews 2002,102 (11), 3905–3946. [DOI] [PubMed] [Google Scholar]
- 25.Block E, The Organosulfur Chemistry of the GenusAllium - Implications for the Organic Chemistry of Sulfur. Angew. Chem. Int. Ed 1992, 31 (9), 1135–1178. [Google Scholar]
- 26.(a) Powolny AA; Singh SV, Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett 2008, 269 (2), 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li Y; Zhang J; Zhang L; Si M; Yin H; Li J, Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of osteosarcoma cells by switching on suppressor microRNAs and inactivating of Notch-1 signaling. Carcinogenesis 2013, 34 (7), 1601–1610 [DOI] [PubMed] [Google Scholar]; (c) Xiao D; Lew KL; Kim YA; Zeng Y; Hahm ER; Dhir R; Singh SV, Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res 2006, 12 (22), 6836–6843 [DOI] [PubMed] [Google Scholar]; (d) Li W; Tian H; Li L; Li S; Yue W; Chen Z; Qi L; Hu W; Zhu Y; Hao B; Gao C; Si L; Gao F, Diallyl trisulfide induces apoptosis and inhibits proliferation of A549 cells in vitro and in vivo. Acta Biochim Biophys Sin 2012, 44 (7), 577–583 [DOI] [PubMed] [Google Scholar]; (e) Wang YB; Qin J; Zheng XY; Bai Y; Yang K; Xie LP, Diallyl trisulfide induces Bcl-2 and caspase-3-dependent apoptosis via downregulation of Akt phosphorylation in human T24 bladder cancer cells. Phytomedicine 2010, 17 (5), 363–368. [DOI] [PubMed] [Google Scholar]
- 27.Antosiewicz J; Herman-Antosiewicz A; Marynowski SW; Singh SV, c-Jun NH(2)-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res 2006, 66 (10), 5379–5386. [DOI] [PubMed] [Google Scholar]
- 28.Hosono T; Fukao T; Ogihara J; Ito Y; Shiba H; Seki T; Ariga T, Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of beta-tubulin. J Biol Chem 2005, 280 (50), 41487–41493. [DOI] [PubMed] [Google Scholar]
- 29.Benavides GA; Squadrito GL; Mills RW; Patel HD; Isbell TS; Patel RP; Darley-Usmar VM; Doeller JE; Kraus DW, Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A 2007, 104 (46), 17977–17982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang D; Wu H; Wong MW; Huang D, Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org Lett 2015, 17 (17), 4196–4199. [DOI] [PubMed] [Google Scholar]
- 31.Liu H; Radford MN; Yang CT; Chen W; Xian M, Inorganic hydrogen polysulfides: chemistry, chemical biology and detection. Br J Pharmacol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licht S; Davis J, Disproportionation of Aqueous Sulfur and Sulfide: Kinetics of Polysulfide Decomposition. J. Phys. Chem. B 1997, 101 (14), 2540–2545. [Google Scholar]
- 33.Ercole F; Whittaker MR; Halls ML; Boyd BJ; Davis TP; Quinn JF, Garlic-inspired trisulfide linkers for thiol-stimulated H2S release. Chem Commun 2017, 53 (57), 8030–8033. [DOI] [PubMed] [Google Scholar]
- 34.Xu S; Wang Y; Radford MN; Ferrell AJ; Xian M, Synthesis of Unsymmetric Trisulfides from 9-Fluorenylmethyl Disulfides. OrgLett 2018, 20 (2), 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerda MM; Hammers MD; Earp MS; Zakharov LN; Pluth MD, Applications of Synthetic Organic Tetrasulfides as H2S Donors. Org Lett 2017, 19 (9), 2314–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zysman-Colman E; Harpp DN, Optimization of the synthesis of symmetric aromatic tri- and tetrasulfides. J Org Chem 2003, 68 (6), 2487–2489. [DOI] [PubMed] [Google Scholar]
- 37.Harpp DN; Granata A, Organic sulfur chemistry. XXI. Trisulfide formation by alkoxide decomposition of sulfenylthiocarbonates. Tetrahedron Letters 1976, 17 (35), 3001–3004. [Google Scholar]
- 38.Ahrika A; Robert J; Anouti M; Paris J; Jiang Z-H; Yan S-P; Wang G-L; Yao XK; Wang H-G; Tuchagues JP; Ögren M, Nucleophilic Substitution of Alkyl Halides by Electrogenerated Polysulfide Ions in N,N-dimethylacetamide. Acta Chemica Scandinavica 1999, 53, 513–520. [Google Scholar]
- 39.Cai YR; Hu CH, Computational Study of H2S Release in Reactions of Diallyl Polysulfides with Thiols. J Phys Chem B 2017, 121 (26), 6359–6366. [DOI] [PubMed] [Google Scholar]
- 40.Coletta C; Papapetropoulos A; Erdelyi K; Olah G; Modis K; Panopoulos P; Asimakopoulou A; Gero D; Sharina I; Martin E; Szabo C, Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA 2012,109 (23), 9161–9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szczesny B; Modis K; Yanagi K; Coletta C; Le Trionnaire S; Perry A; Wood ΜE; Whiteman M; Szabo C, AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 2014, 41, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan S; Shen X; Kevil CG, Beyond a Gasotransmitter: Hydrogen Sulfide and Polysulfide in Cardiovascular Health and Immune Response. Antioxid Redox Signal 2017, 27(10), 634–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


