Abstract
Background
Trehalose is a natural, non-reducing disaccharide synthesized by lower organisms. Trehalose exhibits an extraordinary ability to protect cells against different kinds of stresses through activation of autophagy. However, the effect of trehalose on the heart during stress has never been tested.
Objectives
We evaluated the effect of trehalose administration in a mouse model of chronic ischemic remodeling.
Methods
Wild type (WT) or beclin 1+/− mice were subjected to permanent ligation of the left anterior descending artery (LAD) and then treated with either placebo or trehalose (1 mg/g/day i.p. for 48 hours, then 2% in the drinking water). After 4 weeks, echocardiographic, hemodynamic, gravimetric, histological and biochemical analyses were conducted.
Results
Trehalose reduced left ventricular (LV) dilation and increased ventricular function in mice with LAD ligation, as compared to placebo. Sucrose, another non-reducing disaccharide, did not exert protective effects during post-infarction LV remodeling. Trehalose administration to mice overexpressing GFP-tagged LC3 significantly increased the number of GFP-LC3 dots, both in the presence and absence of chloroquine administration. Trehalose also increased cardiac LC3-II levels after 4 weeks of myocardial infarction (MI), indicating that it induces autophagy in the heart in vivo. In order to evaluate whether trehalose exerts beneficial effects through the activation of autophagy, trehalose was administered to beclin 1+/− mice. The improvement of LV function, lung congestion, cardiac remodeling, apoptosis and fibrosis following trehalose treatment observed in WT mice were all significantly blunted in beclin 1+/− mice.
Conclusions
Trehalose reduces MI-induced cardiac remodeling and dysfunction through activation of autophagy.
Keywords: trehalose, cardiac remodeling, autophagy, heart failure, ventricular function
Introduction
Cardiovascular diseases remain the first cause of death in Western countries (1). The final common pathway of chronic cardiovascular disorders, including coronary artery disease, is heart failure, a highly morbid and disabling condition that leads to death after recurrent hospitalizations (1,2). It is therefore important to develop pharmacological therapies targeting chronic cardiac remodeling following myocardial injury, such as acute myocardial infarction (MI), in order to reduce the incidence of heart failure and death.
We hypothesized that trehalose might be a potentially beneficial compound for the treatment of chronic ischemic remodeling. Trehalose is a natural, non-reducing α-linked disaccharide composed of two molecules of glucose that is synthesized by lower organisms, such as yeasts, bacteria, insects and plants, but not by mammals (3–6). Accumulating lines of evidence indicate that trehalose has an extraordinary ability to protect cells in response to different kinds of stresses (3–6) Trehalose rapidly accumulates in lower organisms such as yeasts and tardigrades, enabling them to survive conditions of dehydration, thermal shock, oxidative stress and protein aggregation (3–6). Trehalose can also protect mammalian cells against stress. Trehalose confers a high water retention capability to cells, thereby protecting intracellular organelles from disruption by hydration/dehydration cycles during stress (7,8). In addition, trehalose elicits antioxidant functions (5,9,10) and dramatically reduces intracellular protein aggregates and misfolded protein accumulation (11). It exerts salutary effects in mouse models of neurodegenerative disorders by promoting the clearance of β-amyloids and huntingtin aggregates (12–14). In addition, trehalose can reduce hepatic steatosis by promoting the clearance of intracellular lipid droplets (15). However, the effect of trehalose administration during cardiac stress is currently unknown.
The clearance of protein aggregates by trehalose is accompanied by induction of autophagy in COS-7 and PC12 cells (11). Autophagy is an evolutionarily-conserved intracellular mechanism that mediates degradation of misfolded proteins, lipid aggregates and damaged organelles (16). Autophagy is activated in response to cellular stresses, including starvation, endoplasmic reticulum (ER) stress and oxidative stress, thereby limiting cell death (16). Genetic or pharmacological inhibition of autophagy exacerbates myocardial ischemic injury and chronic cardiac remodeling in mouse models of myocardial ischemia and MI (17–20). Conversely, activation of autophagy limits myocardial damage in response to ischemia and reduces chronic ischemic remodeling and heart failure (18,21,22). The salutary effects of autophagy are mediated by the preservation of energy content, the clearance of misfolded proteins/damaged intracellular organelles, and the improvement of mitochondrial function. However, clinical application of interventions to stimulate autophagy is challenging due to issues of specificity and the side effects inherent to each intervention. Thus, the pharmacological compound most suitable for activating autophagy has yet to be identified (23).
The aim of this study was to test whether trehalose administration reduces cardiac remodeling and dysfunction in a mouse model of chronic MI and, if so, whether these effects are mediated by autophagy activation.
Methods
Animal models and trehalose administration
Heterozygous GFP-LC3 transgenic (Tg-GFP-LC3) mice (C57BL/6J background, strain GFP-LC3#53, RIKEN BioResource Center) containing a rat LC3-EGFP fusion under control of the chicken β-actin promoter and heterozygous Beclin1 knock-out (beclin 1+/−) mice (C57BL/6J background) were bred in-house, as previously described (21).
Wild-type (WT) C57BL/6J mice were subjected to chronic MI for 4 weeks by permanent left anterior descending coronary artery (LAD) ligation. After LAD ligation, mice were divided into 3 treatment groups: one group was treated with trehalose (1 mg/g/day i.p. for 48 hours, then 2% in the drinking water until the end of the 4-week period), while the other 2 groups were control groups that received either placebo (saline for the initial 48 hours and then regular water) or sucrose (same dosage as for trehalose). Sham mouse groups that were not subjected to LAD ligation also received placebo, sucrose or trehalose. After 4 weeks, echocardiographic, hemodynamic, gravimetric, histological and biochemical analyses were conducted as previously described (21,24).
In a different set of experiments, WT and beclin 1+/− mice were subjected to LAD ligation and received either placebo or trehalose for 4 weeks. Finally, in order to evaluate the acute effects of trehalose on myocardial autophagy and autophagic flux, Tg-GFP-LC3 mice were administered either trehalose (1 mg/g/day i.p.) or placebo (saline) for 48 hours, with or without chloroquine administration (10 mg/kg i.p. 4 hours before sacrifice), as previously described (21,25). Trehalose, chloroquine and sucrose were all purchased from Sigma-Aldrich. All animal protocols were approved by the local Institutional Animal Care and Use Committee of Rutgers New Jersey Medical School.
LAD ligation procedures
Mice were anesthetized by intraperitoneal administration of pentobarbital sodium (60 mg/kg), and then ventilated for the entire procedure through an endotracheal tube connected to a mouse ventilator. The LAD was visualized through a left thoracotomy across the third intercostal space, and then ligated 1–2 mm distal to the left atrial appendage with an 8–0 nylon suture placed around the artery. After closure of the chest wall and extubation, mice were placed in a recovery cage with the temperature maintained at 37°C overnight and then housed normally.
Echocardiographic analysis
Mice were anesthetized by intraperitoneal injection of 2, 2, 2- tribromoethanol (Sigma-Aldrich, 300 mg/kg). Mouse chests were shaved and the animals were positioned on a warm cushion. All left ventricular (LV) measurements were taken in M-mode short-axis at the level of papillary muscles. LV end-diastolic diameter (LVEDD) and diastolic wall measurements were obtained at the time of the apparent maximal diastolic diameter, while LV end-systolic diameter (LVESD) and systolic wall measurements were obtained at the time of the most anterior systolic excursion of the posterior wall. LV fractional shortening (FS) was calculated as follows: FS= (LVEDD-LVESD)/LVEDD × 100.
Hemodynamic analysis
Pressure-volume analysis was performed using the Millar PV system MPVS-300/400. After anesthesia with 2, 2, 2- tribromoethanol (Sigma-Aldrich, 300 mg/kg), the right carotid artery was cannulated with a high-fidelity Mikro-Tip catheter transducer (1.0 Fr, Model PVR-1030; Millar Instruments, Houston, Texas). LV diastolic and systolic pressures and performance were measured as previously described (26,27).
Histological analyses
De-paraffinized tissue sections were antigen-unmasked and wheat germ agglutinin staining and Masson’s trichrome staining were performed as previously described (21,24). For GFP-LC3 dot visualization in Tg-GFP-LC3 mice, myocardial samples were embedded in Tissue-Tek OCT compound (Sakura Finetechnical Co. Ltd) and stored at −80°C. The samples were sectioned at 5–7 μm thickness with a cryostat (CM3050 S, Leica). GFP-LC3 dots were observed under a fluorescence microscope as previously described (17,21).
For immunofluorescent staining, fixed cardiomyocytes were incubated overnight with anti-TFEB antibody (MyBioSource) and then with Alexa Fluor 568 dye-conjugated secondary antibody (Life Technologies). Samples were washed and mounted on glass slides with DAPI (Vectashield; Vector Laboratories).
Cell cultures
Neonatal rat cardiomyocytes were isolated and cultured as previously reported (21). Cell viability was assessed by MTT assay. In order to knock down TFEB, cardiomyocytes were infected with adenovirus expressing a short hairpin sequence targeting TFEB for 72 hours. In order to evaluate the effects of trehalose on mitophagy, cardiomyocytes were transduced with an adenovirus harboring mitochondria-targeted Keima, a protein that emits different fluorescent signals depending on the microenvironment pH. In this way, it is possible to track the localization of mitochondria inside lysosomes (acidic microenvironment). Mitophagy was assessed as previously described (28).
Immunoblot analysis and antibody
Myocardial and cardiomyocyte samples were lysed in RIPA buffer and immunoblot analyses were performed as previously described (24). Nuclear and cytosolic fractions were isolated from LV samples as previously described (29). Anti-LC3 and GAPDH antibodies were purchased from MBL and Cell Signaling Technology, respectively. Antibodies detecting cleaved Caspase-3, Histone H3, p-ERK1/2 (Thr202/Tyr204) and total ERK1/2 were purchased from Cell Signaling Technology. Ubiquitin antibody was purchased from Santa Cruz. Anti-SERCA2 and anti-Cathepsin D antibodies were purchased from Novus Biologicals and Abcam, respectively.
Statistical analysis
Continuous variables were expressed as mean ± SD. The Student’s t test was used to compare means of two groups. When ≥3 groups were specifically intercompared, the one-way ANOVA test followed by the Bonferroni post-hoc test was used. A p-value <0.05 was considered statistically significant. Analyses were performed using Graphpad 7 and SPSS statistical software (SPSS, Chicago, Illinois, version 20).
Results
Trehalose attenuates chronic ischemic remodeling
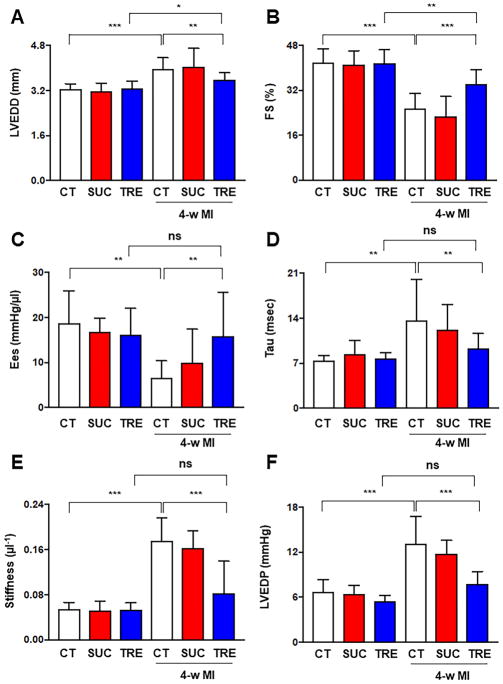
In order to evaluate the effects of trehalose on cardiac remodeling after MI, WT C57BL/6J mice were subjected to LAD ligation and treated with either placebo, trehalose or sucrose, as depicted in Online Figure 1. After 4 weeks, echocardiographic analyses showed that mice with LAD ligation treated with placebo displayed significant LV dilation and a reduction in systolic LV function compared to control mice that underwent a sham operation and placebo treatment. On the other hand, mice with LAD ligation treated with trehalose exhibited attenuated LV remodeling compared to those with LAD ligation treated with placebo and to sham-operated groups (Figure 1A–B, Online Figure 2A). Interestingly, sucrose treatment did not exert any protective effect with respect to LV remodeling after LAD ligation and its effects were comparable to those of placebo (Figure 1A–B, Online Figure 2A). Hemodynamic studies also showed that trehalose treatment significantly ameliorated systolic and diastolic dysfunction after 4 weeks of MI, as indicated by the significantly increased slope of the end-systolic pressure volume relationship, lower LV relaxation constant (Tau) and lower index of myocardial stiffness in the trehalose-treated group as compared to the placebo group (Figure 1C–E). LV end-diastolic pressure was significantly lower in the trehalose-treated group than in the placebo group (Figure 1F). Trehalose-treated groups with MI also did not exhibit a significant alteration of these hemodynamic parameters with respect to sham-operated groups. Taken together, these results indicate that trehalose reduces LV remodeling and improves both systolic and diastolic function in mice with chronic MI. These effects are specific to trehalose and not disaccharides in general, since sucrose did not exhibit similar beneficial effects on ischemic remodeling.
Figure 1. Trehalose treatment ameliorates chronic ischemic remodeling.
A–F. Mice with and without LAD ligation received placebo (CT), sucrose (SUC) or trehalose (TRE). After 4 weeks, they were subjected to echocardiographic analysis to assess left ventricular end-diastolic diameter (LVEDD, B) and fractional shortening (FS, C). N=9 in placebo sham group; N=9 in sucrose sham group; N=10 in trehalose sham group; N=19 in placebo MI group; N=14 in sucrose MI group; N=21 in trehalose MI group. Mice were also subjected to hemodynamic analysis in order to assess the slope of the end-systolic pressure volume relationship (ESPVR), the relaxation constant Tau (D), the left ventricular stiffness (E) and the left ventricular end-diastolic pressure (LVEDP, F). N=7 in each sham group; N=18 in placebo MI group; N=14 in sucrose MI group; N=19 in trehalose MI group. *p<0.05; **p<0.01; ***p<0.001.
Trehalose induces autophagy in the heart
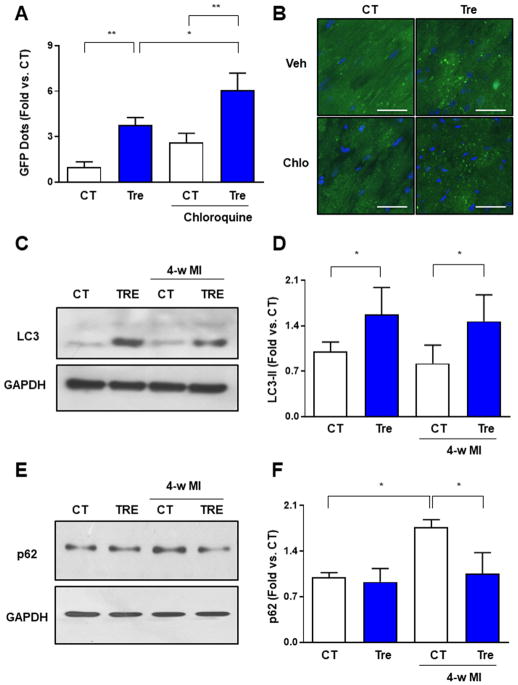
Previous studies demonstrated that trehalose exerts beneficial effects in other organs, in part through activation of autophagy (11,14,15,30). To test whether trehalose induces autophagy in the heart, we administered trehalose to Tg-GFP-LC3 mice and evaluated the extent of autophagosome formation. We found that 48 hours of trehalose treatment significantly increased the number of LC3 puncta in both the absence and presence of chloroquine, a lysosome inhibitor (Figure 2A–B) (25). This result indicates that trehalose promotes both autophagosome formation and autophagic flux in the heart. Chronic treatment with trehalose also increased autophagy both in control mice without MI and in mice subjected to 4 weeks of LAD ligation, as indicated by an increase in LC3-II, a biochemical indicator of autophagosome accumulation (Figure 2C–D). Trehalose treatment also significantly attenuated the accumulation of p62, a protein that is degraded by autophagy, in the hearts of mice with chronic MI, further demonstrating that this molecule induces cardiac autophagy (Figure 2E–F). In addition, chronic trehalose treatment enhanced autophagosome formation in the hearts of mice with LAD ligation, as indicated by the increased number of LC3 puncta in Tg-GFP-LC3 mice treated with trehalose compared to in those treated with placebo, either with or without chloroquine treatment (Online Figure 3). Furthermore, we observed an increase in the number of LC3 dots in the hearts of Tg-GFP-LC3 mice treated with trehalose in response to chloroquine administration, but not in placebo-treated mice. This suggests a reduction of autophagic flux in the heart during the chronic phase of cardiac remodeling that can be recovered by trehalose administration, which is consistent with the observed p62 accumulation (Figure 2E–F) and with previously published evidence (31). Finally, we found that myocardial levels of cathepsin D are increased by trehalose treatment, suggesting enhanced lysosomal biogenesis, which may contribute to the observed increase in autophagic flux (Online Figure 4).
Figure 2. Trehalose promotes myocardial autophagy.
A–B. Tg-GFP-LC3 mice received placebo (saline) or trehalose (1 mg/g/day i.p.) for 48 hours. In some animals, chloroquine was also administered (10 mg/kg i.p., 4 hours before sacrifice). Representative pictures of myocardial GFP-LC3 dots are shown (A), together with quantification of the number of dots/field (B). Data are expressed as fold vs. placebo without chroloquine (CT). N=4 per group. Scale bar: 50 μm. C–F. Mice with LAD ligation received placebo (CT) or trehalose (TRE). After 4 weeks, cardiac levels of LC3, p62 and GAPDH were analysed by immunoblot. A representative immunoblot is shown (C, E) together with the densitometric analysis of normalized LC3-II (D) and p62 levels. Data are expressed as fold vs. CT. N=4–5 per group. *p<0.05; **p<0.01.
Previous work demonstrated that autophagy activation limits cardiomyocyte apoptosis, misfolded protein accumulation and mitochondrial dysfunction in response to stress (17–22). In line with this evidence, we found reductions in cleaved caspase 3 levels and protein ubiquitination in mice with chronic MI treated with trehalose (Online Figure 5A–B). Trehalose treatment also increased SERCA2 levels in mice with LAD ligation (Online Figure 5A), in accordance with the increased cardiac contractility observed in these animals.
Then, we tested whether trehalose promotes mitophagy in cardiomyocytes. Mitophagy is a critical mechanism for degradation of damaged mitochondria and preservation of mitochondrial function. Trehalose promoted mitophagy in cardiomyocytes, at baseline and in response to energy deprivation, as indicated by an increased number of Keima dots with a high 560/440 fluorescent signal ratio, corresponding to mitochondria localized in lysosomes (Online Figure 6).
Trehalose induces autophagy through upregulation of TFEB
It is known that trehalose induces autophagy independently of the mTOR pathway in COS-7 cells (11). Previous studies showed that trehalose promotes activation of Transcription Factor EB (TFEB) (32–34), which is involved in upregulation of autophagy genes and activation of autophagy (35,36). We found that trehalose promotes nuclear localization of TFEB in cardiomyocytes in vitro and in the mouse heart in vivo, confirming that TFEB is activated (Online Figure 7A–B). Importantly, we found that TFEB knockdown significantly attenuates trehalose-induced cardiomyocyte autophagy and survival in response to H2O2 (Online Figure 7C–E), suggesting that TFEB mediates the beneficial effects of trehalose in cardiomyocytes. Bafilomycin treatment also attenuated the antiapoptotic effect of trehalose in cardiomyocytes exposed to H2O2 (Online Figure 7F), indicating that preserved lysosomal function is required for the beneficial effect of trehalose in response to stress in cardiomyocytes, likely to maintain autophagic flux. Of note, it was previously demonstrated that TFEB nuclear localization may be mediated by inhibition of the ERK signaling pathway (35). However, trehalose administration did not significantly modulate ERK activity, as evaluated by its phosphorylation, in the mouse heart either at baseline or during chronic MI (Online Figure 8A–B), suggesting that this pathway may not be important for trehalose-induced TFEB nuclear localization.
Trehalose attenuates chronic ischemic remodeling through the activation of autophagy
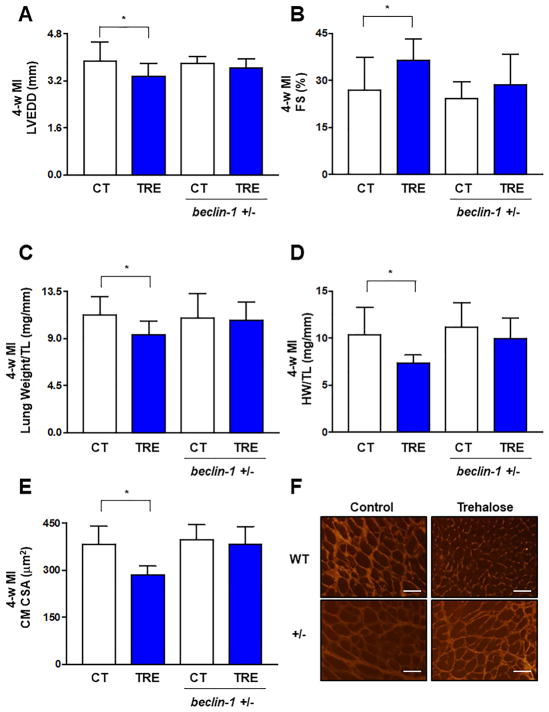
In order to evaluate whether trehalose attenuates ischemic remodeling through the activation of autophagy, WT and beclin 1+/− mice were subjected to LAD ligation and received either placebo or trehalose treatment for 4 weeks. Autophagy is genetically disrupted in beclin 1+/− mice and these mice are insensitive to autophagy inducers (21,28). Although trehalose significantly attenuated LAD ligation-induced LV dilation and systolic dysfunction in WT mice, these effects were blunted in beclin 1+/− mice (Figure 3A–B). Similarly, trehalose significantly reduced lung weight, a sign of lung congestion and heart failure, in WT mice but not in beclin 1+/− mice subjected to chronic MI (Figure 3C). Heart weight/tibial length and cardiomyocyte size after 4 weeks of MI were also reduced by trehalose in WT mice, but these effects were blunted in beclin 1+/− mice (Figure 3D–F).
Figure 3. The beneficial effects of trehalose on cardiac remodeling and function in response to LAD ligation are attenuated in beclin 1+/− mice.
A–F. WT and beclin 1+/− mice with LAD ligation received placebo (CT) or trehalose (TRE). After 4 weeks, they were subjected to echocardiographic analysis to assess left ventricular end-diastolic diameter (LVEDD, A) and fractional shortening (FS, B). Lung (C) and heart weight (D) were also measured. N=9 in placebo group; N=11 in trehalose group; N=8 in beclin-1 +/− placebo group; N=9 in beclin-1 +/− trehalose group. Cell size was measured in left ventricular myocardial tissue sections. Quantification of cross-sectional area (CSA, E) together with representative pictures of wheat germ agglutinin staining are shown (F). Data are presented as fold vs. WT CT. N=4 per group. *p<0.05. Scale bar: 50 μm.
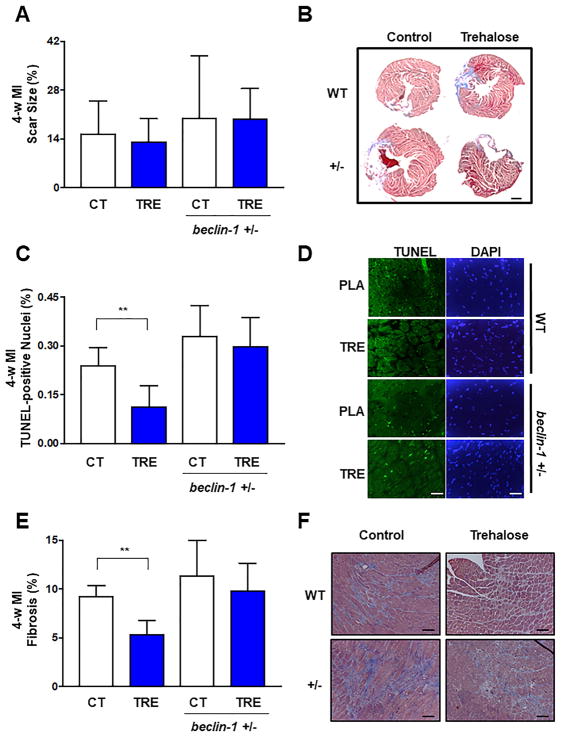
Trehalose treatment did not significantly affect the MI scar size 4 weeks after LAD ligation in either WT or beclin 1+/− mice, as evaluated with Masson’s trichrome staining (Figure 4A–B). However, cardiomyocyte apoptosis in the remote area was significantly reduced by trehalose treatment in WT mice but not in beclin 1+/− mice (Figure 4C–D). Trehalose treatment also significantly reduced myocardial fibrosis in WT mice after 4 weeks of MI compared to placebo treatment, whereas it failed to reduce myocardial fibrosis in beclin 1+/− mice (Figure 4E–F). These results demonstrate that trehalose treatment reduces cardiac remodeling and dysfunction, cardiac hypertrophy, apoptosis and fibrosis in response to chronic MI, at least partially through activation of autophagy (Online Figure 9).
Figure 4. The beneficial effects of trehalose on cardiac apoptosis and fibrosis in response to LAD ligation are attenuated in beclin 1+/− mice.
A–F. WT and beclin 1+/− mice with LAD ligation received placebo (CT) or trehalose (TRE). After 4 weeks, infarct size was measured (A). Representative pictures of Masson’s trichrome staining of the whole left ventricular transverse section are also shown (B). Scale bar: 1 mm. Apoptosis was evaluated by quantification of TUNEL positive cells (C–D). Data are expressed as fold vs. CT. N=4–7 per group. The percentage of cardiac fibrosis was also quantified (E) and representative pictures of Masson’s trichrome staining are shown (F). N=4 per group. **p<0.01. Scale bar: 50 μm.
Discussion
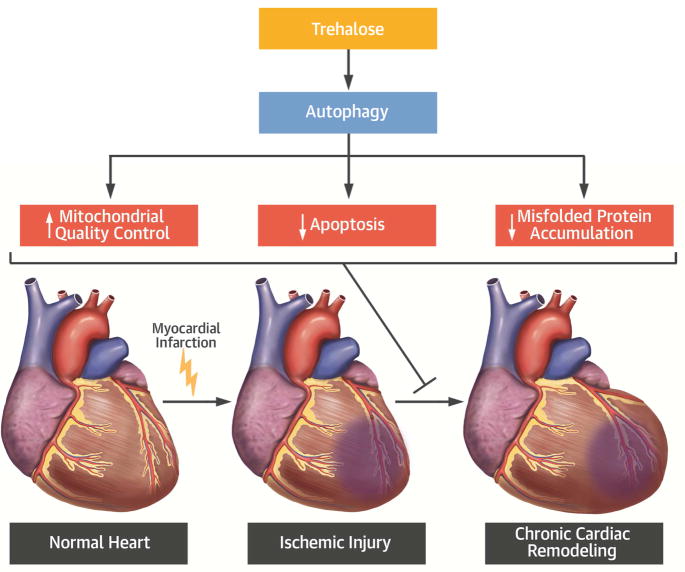
In the present study, we investigated the effect of trehalose administration on LV remodeling after chronic MI. Chronic trehalose treatment significantly attenuated MI-induced cardiac remodeling and improved both systolic and diastolic LV function (Central Illustration). These beneficial effects were associated with a reduction in cardiac hypertrophy, apoptosis and fibrosis. Trehalose significantly activated autophagy in the heart and the cardio-protective effect of trehalose during cardiac remodeling was blunted in mice with genetic disruption of autophagy, suggesting that autophagy activation mediates the salutary effects of trehalose. Since sucrose, another non-reducing disaccharide, did not exhibit the same beneficial effects on cardiac remodeling that trehalose did, the protective effects of trehalose would appear to be specific.
Central Illustration. Trehalose administration hampers the detrimental cellular effects induced by chronic myocardial infarction and reduces cardiac remodeling by activating autophagy.
Autophagy activation by trehalose increases mitochondrial quality control and attenuates misfolded protein accumulation and apoptosis induced by chronic myocardial infarction.
Our work significantly extends previous evidence demonstrating that trehalose protects cells in response to stress. In lower organisms, trehalose is rapidly synthesized in response to stress, thereby preserving cell viability and functions (3–6). On the other hand, trehalose is not synthesized in mammals. However, several studies have demonstrated that exogenously applied trehalose exerts beneficial effects on mammalian cells in response to oxidative stress, DNA damage, and misfolded protein accumulation (9,11,37). Trehalose has also been shown to ameliorate pathological conditions in animal models of human diseases in vivo. For example, chronic oral administration of trehalose to a mouse model of Huntington disease dramatically ameliorated motor dysfunction, extended survival, and reduced polyglutamine aggregates (12). Chronic treatment with trehalose, but not sucrose, also preserved motor neuron survival and mitochondrial function in a mouse model of amyotrophic lateral sclerosis, thereby delaying the onset of the disease and extending the lifespan (14). Trehalose reduced intracellular protein aggregates and reduced neuron death in a model of Alzheimer’s disease (13). Recently, trehalose administration in drinking water was found to reduce high fructose-induced hepatic steatosis through increased clearance of intracellular lipid droplets (15).
Previous studies have suggested that the beneficial effects of trehalose may be mediated by autophagy activation (11,13–15). In this study, we, for the first time, provide in vivo genetic evidence that the protective effects of trehalose on cardiac remodeling are mediated through stimulation of autophagy, since they are lost when trehalose is administered to beclin 1+/− mice.
The mechanisms through which trehalose induces autophagy remain to be clarified. It has been suggested that this process is independent of the mTOR pathway (11). We found that trehalose induces dramatic nuclear localization of TFEB and that TFEB knockdown attenuates the pro-autophagic and pro-survival effects exerted by trehalose. These data suggest that trehalose promotes autophagy in part through stimulation of TFEB, in line with previous evidence (32–34). TFEB promotes not only lysosomal biogenesis but also autophagosome formation, by directly controlling the expression of autophagy-related genes (35,36). In addition, like trehalose, TFEB activation dramatically reduces protein aggregates (38). Future studies are warranted to understand how trehalose regulates TFEB. The Akt and AMPK signaling pathways were previously shown to be involved in these mechanisms (15,34). In addition, it will be important to conduct studies to evaluate whether TFEB is required for trehalose-induced autophagy in vivo and to clarify the specific role of lysosomal biogenesis in the beneficial effects of trehalose.
We have shown previously that autophagy is negatively regulated by Mst1, a pro-apoptotic kinase, through Thr108 phosphorylation of Beclin1, during cardiac remodeling after MI (21). Cardiac remodeling and LV dysfunction are often accompanied by oxidative stress and Ca2+ overload, and consequent development of mitochondrial dysfunction and ER stress (39). In the failing heart, accumulation of protein aggregates and dysfunctional mitochondria and ER is often observed (39). Since autophagy protects the heart not only through recycling of ATP but also by eliminating protein aggregates and damaged intracellular organelles such as mitochondria, an intervention that stimulates autophagy should be beneficial. In fact, previous studies have shown that interventions to stimulate autophagy, including rapamycin (40), inhibition of Mst1 (21), and TAT-Beclin 1 (28), improve the function of the heart in the presence of hemodynamic overload. Considering the specificity and potential toxicity of these available interventions in experimental animals, the development of safe and effective interventions to stimulate autophagy is needed.
Our study suggests that trehalose might represent a potentially useful molecule to reduce cardiac remodeling and heart failure in human patients and to activate autophagy in a physiological manner. Trehalose is a natural compound with apparently no side effects in human subjects. It can be found in certain foods and it is currently used as a sweetener or supplement (41). Furthermore, prolonged trehalose treatment was not found to have adverse metabolic effects (12). In addition, trehalose could be administered orally. Although the human and mouse intestine expresses trehalase, an enzyme that degrades trehalose, a small percentage of trehalose can pass the intestinal barrier and reach the bloodstream and different organs (12,15).
Finally, we did not apply a correction for multiple testing to our statistical analyses. All the analyses were performed to test specific hypotheses and the results had biological explanations. In several cases, multiple analyses were performed to test the same hypothesis in different ways and the results of these multiple analyses were consistent. For this reason, we believe we can exclude the possibility that our results may be affected by a multiple testing bias.
Conclusions
Our study demonstrated that oral administration of trehalose reduces MI-induced cardiac remodeling and dysfunction through the activation of autophagy (Supplemental Figure 9). Our results suggest that trehalose may be a potentially useful pharmacological agent to activate autophagy and reduce cardiac remodeling and heart failure. Additional studies are encouraged to further validate this possibility, including in other models of heart diseases. In addition, our results indicated that trehalose significantly increases mitophagy. It will be interesting to study in the future whether trehalose administration limits the development of mitochondrial dysfunction during stress in the heart.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Incidence of heart failure is still very high in Western countries. It is important to discover new pharmacological therapies that can delay or even treat chronic cardiac remodeling following myocardial injury, such as an acute MI, in order to reduce the incidence of heart failure and death. We found that oral administration of trehalose, a natural non-reducing disaccharide, reduces MI-induced cardiac remodeling and dysfunction in mice through activation of autophagy.
Translational Outlook 1
Trehalose may be a potentially useful pharmacological agent to reduce cardiac remodeling and heart failure.
Translational Outlook 2
Trehalose represents a suitable compound to activate autophagy in a physiological manner during cardiac stress.
Acknowledgments
Funding: This work was supported in part by U.S. Public Health Service Grants HL67724, HL91469, HL102738, HL112330 and AG23039 (J.S.), and by the Leducq Foundation Transatlantic Network of Excellence (J.S.). This work was also partially supported by a grant from the Italian Ministry of Health (GR-2013-02355401) to S.S.
We thank Daniela Zablocki (Rutgers New Jersey Medical School) for assistance with the manuscript.
Abbreviations
- beclin 1+/−
heterozygous Beclin1 knock-out mice
- FS
fractional shortening
- LAD
left anterior descending coronary artery
- LVEDD
LV end-diastolic diameter
- LVESD
LV end-systolic diameter
- SUC
sucrose
- TFEB
Transcription Factor EB
- Tg-GFP-LC3 mice
transgenic mice expressing LC3 conjugated with a green fluorescent protein
- TRE
trehalose
- WT
wild type
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 3.Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13:17R–27R. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Haddad GG. Role of trehalose phosphate synthase and trehalose during hypoxia: from flies to mammals. J Exp Biol. 2004;207:3125–9. doi: 10.1242/jeb.01133. [DOI] [PubMed] [Google Scholar]
- 5.Benaroudj N, Lee DH, Goldberg AL. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J Biol Chem. 2001;276:24261–7. doi: 10.1074/jbc.M101487200. [DOI] [PubMed] [Google Scholar]
- 6.Tapia H, Young L, Fox D, Bertozzi CR, Koshland D. Increasing intracellular trehalose is sufficient to confer desiccation tolerance to Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2015;112:6122–7. doi: 10.1073/pnas.1506415112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iturriaga G, Suarez R, Nova-Franco B. Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci. 2009;10:3793–810. doi: 10.3390/ijms10093793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tapia H, Koshland DE. Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Curr Biol. 2014;24:2758–66. doi: 10.1016/j.cub.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Echigo R, Shimohata N, Karatsu K, et al. Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage. J Transl Med. 2012;10:80. doi: 10.1186/1479-5876-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez-Peral FJ, Zaragoza O, Pedreno Y, Arguelles JC. Protective role of trehalose during severe oxidative stress caused by hydrogen peroxide and the adaptive oxidative stress response in Candida albicans. Microbiology. 2002;148:2599–606. doi: 10.1099/00221287-148-8-2599. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–52. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Machida Y, Niu S, et al. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med. 2004;10:148–54. doi: 10.1038/nm985. [DOI] [PubMed] [Google Scholar]
- 13.Schaeffer V, Goedert M. Stimulation of autophagy is neuroprotective in a mouse model of human tauopathy. Autophagy. 2012;8:1686–7. doi: 10.4161/auto.21488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Chen S, Song L, et al. MTOR-independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy. 2014;10:588–602. doi: 10.4161/auto.27710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBosch BJ, Heitmeier MR, Mayer AL, et al. Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci Signal. 2016;9:ra21. doi: 10.1126/scisignal.aac5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 18.Sciarretta S, Zhai P, Shao D, et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–46. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanamori H, Takemura G, Goto K, et al. Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion. Am J Physiol Heart Circ Physiol. 2011;300:H2261–71. doi: 10.1152/ajpheart.01056.2010. [DOI] [PubMed] [Google Scholar]
- 20.Kanamori H, Takemura G, Goto K, et al. The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovasc Res. 2011;91:330–9. doi: 10.1093/cvr/cvr073. [DOI] [PubMed] [Google Scholar]
- 21.Maejima Y, Kyoi S, Zhai P, et al. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med. 2013;19:1478–88. doi: 10.1038/nm.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buss SJ, Muenz S, Riffel JH, et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–46. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Sciarretta S, Zhai P, Volpe M, Sadoshima J. Pharmacological modulation of autophagy during cardiac stress. J Cardiovasc Pharmacol. 2012;60:235–41. doi: 10.1097/FJC.0b013e3182575f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sciarretta S, Zhai P, Maejima Y, et al. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep. 2015;11:125–36. doi: 10.1016/j.celrep.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–34. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YC, Park HW, Sciarretta S, et al. Rag GTPases are cardioprotective by regulating lysosomal function. Nat Commun. 2014;5:4241. doi: 10.1038/ncomms5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirakabe A, Zhai P, Ikeda Y, et al. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation. 2016;133:1249–63. doi: 10.1161/CIRCULATIONAHA.115.020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushima S, Kuroda J, Ago T, et al. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res. 2013;112:651–63. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang YL, Saleem MA, Chan KW, Yung BY, Law HK. Trehalose, an mTOR independent autophagy inducer, alleviates human podocyte injury after puromycin aminonucleoside treatment. PLoS One. 2014;9:e113520. doi: 10.1371/journal.pone.0113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, He L, Chen F, et al. Impaired autophagy contributes to adverse cardiac remodeling in acute myocardial infarction. PLoS One. 2014;9:e112891. doi: 10.1371/journal.pone.0112891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter K, Nallathambi J, Lin Y, Liton PB. Lysosomal basification and decreased autophagic flux in oxidatively stressed trabecular meshwork cells: implications for glaucoma pathogenesis. Autophagy. 2013;9:581–94. doi: 10.4161/auto.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqui A, Bhaumik D, Chinta SJ, et al. Mitochondrial Quality Control via the PGC1alpha-TFEB Signaling Pathway Is Compromised by Parkin Q311X Mutation But Independently Restored by Rapamycin. J Neurosci. 2015;35:12833–44. doi: 10.1523/JNEUROSCI.0109-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmieri M, Pal R, Nelvagal HR, et al. mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat Commun. 2017;8:14338. doi: 10.1038/ncomms14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Settembre C, Di Malta C, Polito VA, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–33. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–14. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emanuele E, Bertona M, Sanchis-Gomar F, Pareja-Galeano H, Lucia A. Protective effect of trehalose-loaded liposomes against UVB-induced photodamage in human keratinocytes. Biomed Rep. 2014;2:755–759. doi: 10.3892/br.2014.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci U S A. 2013;110:E1817–26. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMullen JR, Sherwood MC, Tarnavski O, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–5. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 41.Richards AB, Krakowka S, Dexter LB, et al. Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem Toxicol. 2002;40:871–98. doi: 10.1016/s0278-6915(02)00011-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.