Abstract
Early-life stress modulates the development of cortico-limbic circuits and increases vulnerability to adult psychopathology. Given the important stressbuffering role of endocannabinoid (eCB) signaling, we performed a comprehensive investigation of the developmental trajectory of the eCB system and the impact of exposure to early life stress induced by repeated maternal separation (MS; 3 hours/day) from postnatal day 2 (PND2) to PND12. Tissue levels of the eCB molecules anandamide (AEA) and 2-arachidonoylglycerol (2-AG) were measured after MS exposures, as well under basal conditions at juvenile (PND14), adolescent (PND40) and adult (PND70) timepoints in the prefrontal cortex (PFC), amygdala and hippocampus. We also examined the effects of MS on CB1 receptor binding in these three brain regions at PND40 and PND70. AEA content was found to increase from PND2 into adulthood in a linear manner across all brain regions, while 2-AG was found to exhibit a transient spike during the juvenile period (PND12-14) within the amygdala and PFC, but increased in a linear manner across development in the hippocampus. Exposure to MS resulted in bidirectional changes in AEA and 2-AG tissue levels within the amygdala and hippocampus and produced a sustained reduction in eCB function in the hippocampus at adulthood. CB1 receptor densities across all brain regions were generally found to be downregulated later in life following exposure to MS. Collectively, these data demonstrate that early life stress can alter the normative ontogeny of the eCB system, resulting in a sustained deficit in eCB function, particularly within the hippocampus, in adulthood.
Introduction
It is widely accepted that early life stress exposure can have long-term ramifications for the development and maturation of limbic circuits that subserve the regulation of stress, mood and anxiety (Andersen, 2015; Callaghan et al., 2014; Gee et al., 2013; Malter Cohen et al., 2013). In fact, several clinical studies have identified that exposure to early life stress is one of the biggest predictors of vulnerability to the development of psychiatric illnesses, such as major depression and post-traumatic stress disorder (PTSD), in adulthood (Davidson & McEwen, 2012; Heim & Binder, 2012; Pratchett & Yehuda, 2011). Interestingly, while exposure to stress during the early life period clearly has immediate effects on the activation of stress responsive systems in the brain and body, many of these detrimental effects do not emerge until later in life, such as during adolescence or even adulthood (Andersen, 2015; Gee & Casey, 2015). A large body of evidence has focused on the potential role of epigenetic programming in these delayed effects, with a particular focus on the effects of early life stress on the methylation and silencing of glucocorticoid receptor expression (Klengel & Binder, 2015; Turecki & Meaney, 2016). This stream of research suggests that early life stress produces a long-lasting suppression of glucocorticoid receptor expression, particularly in the hippocampus, which results in a state of glucocorticoid resistance and impaired regulation of the neurobiological cascades that are evoked by stress exposure in adulthood (Turecki & Meaney, 2016; Zhang et al., 2013). While these findings are quite promising and provide a logical mechanism for the transfer of stress in early life to behavioral alterations as the animal matures, the investigation of other target systems that could result in altered stress regulation in adulthood are warranted at this early stage.
The endocannabinoid (eCB) system is a neuromodulatory lipid signaling system in the brain that primarily acts as a retrograde signaling system that gates the synaptic release of many neurotransmitters in the brain (Katona & Freund, 2012; Ohno-Shosaku & Kano, 2014). Cannabinoid type 1 (CB1) receptors are abundantly expressed across glutamatergic, GABAergic, monoaminergic and neuropeptidergic neurons throughout the brain (Katona & Freund, 2012; Ohno-Shosaku & Kano, 2014). Activation of this receptor suppresses neurotransmitter release and contributes to multiple forms of synaptic plasticity that appear to be important for learning, adaptation, and other physiological processes including pain perception and feeding (Mechoulam & Parker, 2013; Melis et al., 2014). N- rachidonylethanolamine (anandamide; AEA) and 2-arachidonoylglycerol (2-AG) represent the two most characterized eCB molecules to date and are believed to be primarily formed in post-synaptic cells where they act in a retrograde fashion to modulate neurotransmitter release in response to a variety of intracellular signals, including increased calcium and activation of metabotropic receptors (Katona & Freund, 2012; Ohno-Shosaku & Kano, 2014). With respect to the regulation of stress, the eCB system is widely distributed throughout cortico-limbic circuits such as the prefrontal cortex (PFC), amygdala and hippocampus (Herkenham et al., 1991), that are important for the processing of emotionally salient information and the generation of neurobehavioral responses to stress (Davidson & McEwen, 2012).
In general, eCB signaling buffers against the effects of stress as disruption of this system results in exaggerated neurobehavioral and hormonal responses to stress, impairs appropriate termination of stress responses, compromises adaptation to stress and promotes structural changes in the brain associated with mood and anxiety disorders (Hill & Patel, 2013; Hillard, 2014; Morena et al., 2015). More so, there is a rapidly increasing body of clinical literature indicating that alterations in eCB signaling are associated with the development of psychiatric illnesses, such as depression and PTSD (see Hill et al., 2018; Hill & Patel, 2013; Hillard et al., 2012). Building on these findings, it has been well established that exposure to chronic stress results in a weathering of eCB signaling that compromises the stress-inhibitory role this system normally exerts, which in turn may contribute to the development of the pathological effects of chronic stress, also often referred to as allostatic load (Gorzalka et al., 2008; Hillard, 2014; Morena et al., 2016).
It is known that the eCB system undergoes dramatic reorganization during development, playing a prominent role in axon guidance and circuit formation in the perinatal period (Alpár et al., 2014; Berghuis et al., 2007; Mulder et al., 2008), followed by a progressive increase in the ability of eCBs to regulate synaptic transmission through early development (Liang et al., 2014; Zhu & Lovinger, 2010). The transition of adolescence to adulthood is also associated with alterations in the ability of eCB signaling to regulate excitatory and inhibitory transmission in regions such as the PFC and hippocampus (Heng et al., 2011; Kang-Park et al., 2007). Interestingly, several reports have indicated that the eCB system is responsive to stress exposure in early life (D’Asti et al., 2010; Llorente et al., 2008; Marco et al., 2013), including potential long-lasting effects that continue into adulthood (Atsak et al., 2018; Llorente-Berzal et al., 2013; López-Gallardo et al., 2012; Naudon et al., 2013). However, no studies to date have examined how the normal developmental trajectory of eCB signaling is influenced by exposure to stress during the early postnatal period. Given the importance of eCB signaling in the regulation of stress responses (Hill & Tasker, 2012; Morena et al., 2016), and the enhanced vulnerability to stress-related conditions that emerges in individuals following early life stress exposure, the aim of the current study was to characterize the immediate and sustained effects of neonatal stress exposure on the developmental trajectory of the eCB system.
Methods
Animals
Timed-pregnant Sprague-Dawley rats obtained from Charles River Laboratories (Kingston, NY) arrived at our animal facility on gestational day 13. The dams were singly housed. Two days after delivery (postnatal day 2; PND2), litters were culled to 12 pups with equal number of males and females where possible. Litters were randomly assigned to a control (CONT) or maternal separation (MS) group. Animals were maintained on a 12h light-dark schedule (lights on from 0600h to 1800h) and the ambient temperature was maintained at 21 ± 2°C. Food and water were available ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee of Rockefeller University.
Maternal Separation
MS dams were removed from the home cage and their litters were transported in the home cage to a separate room and placed in a clean cage inside of a temperature-controlled isolette for a period of three hours a day on PND2-PND12. The temperature of the isolette was set at 32°C from PND2-PND6 and at 30°C from PND7-PND12. Additionally, pups were permitted to huddle with their littermates during the separation period. Previous studies demonstrate that under similar conditions, the core temperature of pups is maintained at 36-37°C (Jans & Woodside, 1990). Dams remained in the home cage undisturbed during the separation period. At the end of the three-hour separation period, litters were returned to the housing room and their respective dams were returned to the home cage. Non-maternally separated litters (CON) were left undisturbed with their dams from PND2-PND12. Twice weekly cage cleaning commenced on PND13 for all litters. All litters were weaned on PND20 and housed in same-sex groups of 2-3 rats per cage.
Tissue Harvest
All biochemical analyses were completed using male pups and only 2 pups per litter were used for any assay to prevent litter specific effects. Animals (n = 7-8 / treatment condition) were sacrificed by decapitation at the following developmental time points to assess eCB function: PND2 -immediately after the 1st MS session; PND12- immediately after the final MS session; PND14- juvenile (basal juvenile state, 2 days after the final MS session); PND40 adolescent basal state and PND70 adult basal state. All tissues from all treatment conditions at all age points were collected during the first 3 hours of the light cycle. Tissue was harvested and analyzed from cortico-limbic brain regions known to modulate the stress response: 1. the prefrontal cortex (PFC)- composed of medial prefrontal cortex and anterior cingulate; 2. the hippocampus - containing all subregions of the hippocampus, as well as both dorsal and ventral regions; and 3. the amygdala- composed of central, basolateral, and medial nuclei. The regions were anatomically identified as described previously (Hill et al., 2010). The PFC, hippocampus, and amygdala were dissected out on ice, immediately frozen in liquid nitrogen, and stored at −80°C degrees until analysis. Samples from all time points were used for analysis of eCB content, while only PND40 and PND70 (n = 4 / treatment condition) samples were used for CB1 receptor binding density assays due to limitations from protein quantity and tissue size that are required for accurate assessment of CB1 receptor binding site densities using this assay.
Analysis of Endocannabinoid Ligands
For analysis of eCB content, brain regions were subjected to a lipid extraction process as described previously (Patel et al., 2003). The contents of the two primary eCBs, AEA and 2-AG, within lipid extracts were determined using isotope-dilution liquid chromatography–mass spectrometry as described previously (Patel et al., 2005).
CB1 Receptor Binding Assay
CB1 receptor binding assays were performed using a Multiscreen Filtration System with Durapore 1.2-μM filters (Millipore, Bedford, MA) as described previously (Hillard et al., 1995). Incubations (total volume = 0.2 mL) were carried out using TME buffer containing 1 mg/mL bovine serum albumin (TME/BSA). Membranes (10 μg protein per incubate) were added to the wells containing 0.25, 0.5, 1.0, or 2.5 nM 3H-CP 55,940. Ten μM Δ9-tetrahydrocannabinol was used to determine non-specific binding. KD and Bmax values were determined by nonlinear curve fitting to the single site binding equation using GraphPad Prism (San Diego, CA, USA).
Data Analysis
The effects of developmental age and exposure to MS on eCB ligand levels (AEA and 2-AG) and CB1 receptor binding site densities were analyzed using a 2 factor analysis of variance, with age and stress exposure acting as fixed factors. Post hoc analysis was performed using a Tukeys test. Significance was established against an alpha level equal to 0.05. For all eCB measures an n = 8, and for CB1 receptor binding an n = 4, was used per treatment condition at each age epoch.
Results
Developmental Trajectory of Endocannabinoid Content in the Prefrontal Cortex and its Modulation by Early Life Stress
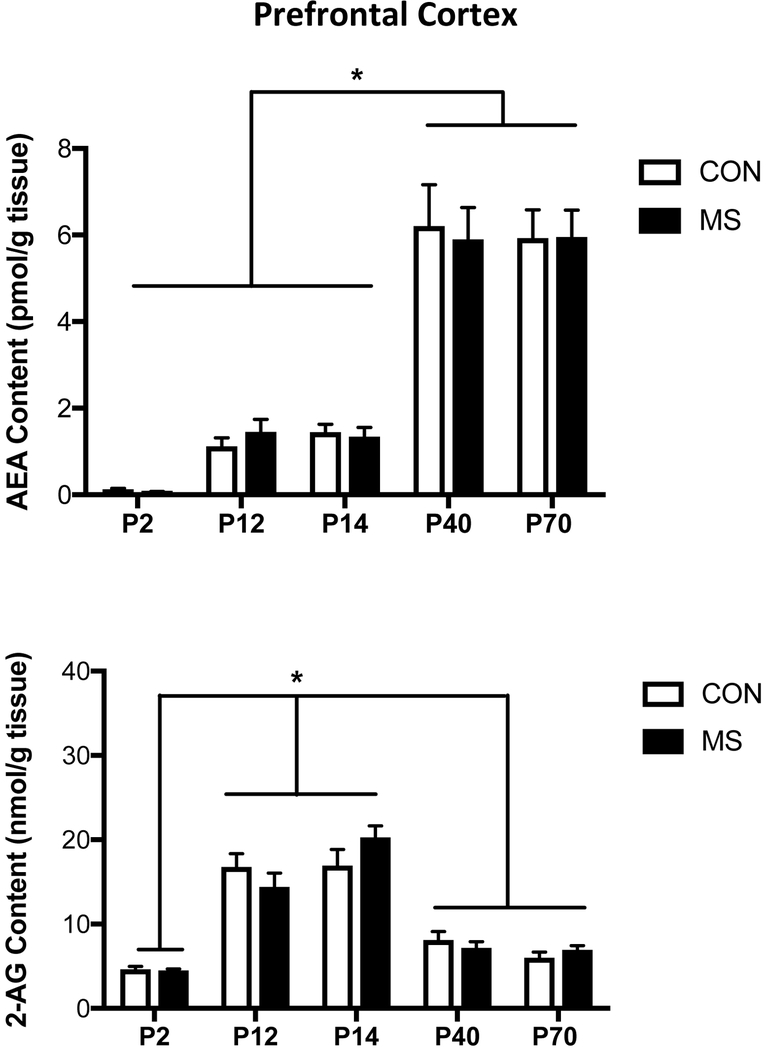
Within the PFC, there was no interaction between age and MS on AEA content [F (4, 68) = 0.11, p > 0.05; Fig. 1], nor a main effect of MS [F (1, 68) = 0.01, p > 0.05], but there was a main effect of age [F (4, 68) = 61.99, p < 0.001], such that AEA levels at P40 and P70 were higher than at P2, P12 and P14 (p < 0.001 for all comparisons). With respect to 2-AG, there was no interaction between age and MS [F (4, 69) = 1.76, p > 0.05; Fig. 1], nor an effect of MS [F (1, 68) = 0.06, p > 0.05], but there was a main effect of age [F (4, 68) = 56.90, p < 0.001]. Unlike AEA, however, this was not a linear change, as 2-AG tissue levels in the PFC were significantly higher at P12 and P14, relative to P2, P40 and P70 (p < 0.01 for all comparisons).
Figure 1. The Effect of Maternal Separation Stress on the Developmental Trajectory of the Endocannabinoid Contents in the Prefrontal Cortex.
Tissue content of the endocannabinoid anandamide (AEA; upper panel) is found to elevate in the prefrontal cortex (PFC) at postnatal day (PND) 40 and 70 relative to PND 2, 12 and 14. This developmental change was uninfluenced by exposure to 3 hours of maternal separation (MS) stress per day from PND 2-11. 2-arachidonoylglycerol (2-AG; lower panel), the other primary endocannabinoid molecule, exhibited a different developmental trajectory where it was found to be significantly higher at PND 12 and 14 relative to both PND 2 as well as PND 40 and 70. Again, there was no influence of MS stress on PFC levels of 2-AG at any age, relative to control (CON) animals. Data are presented as means +/− SEM. * denotes significant differences (p < .05) between identified age windows. All n = 7-8 / treatment condition.
Developmental Trajectory of Endocannabinoid Content in the Amygdala and its Modulation by Early Life Stress
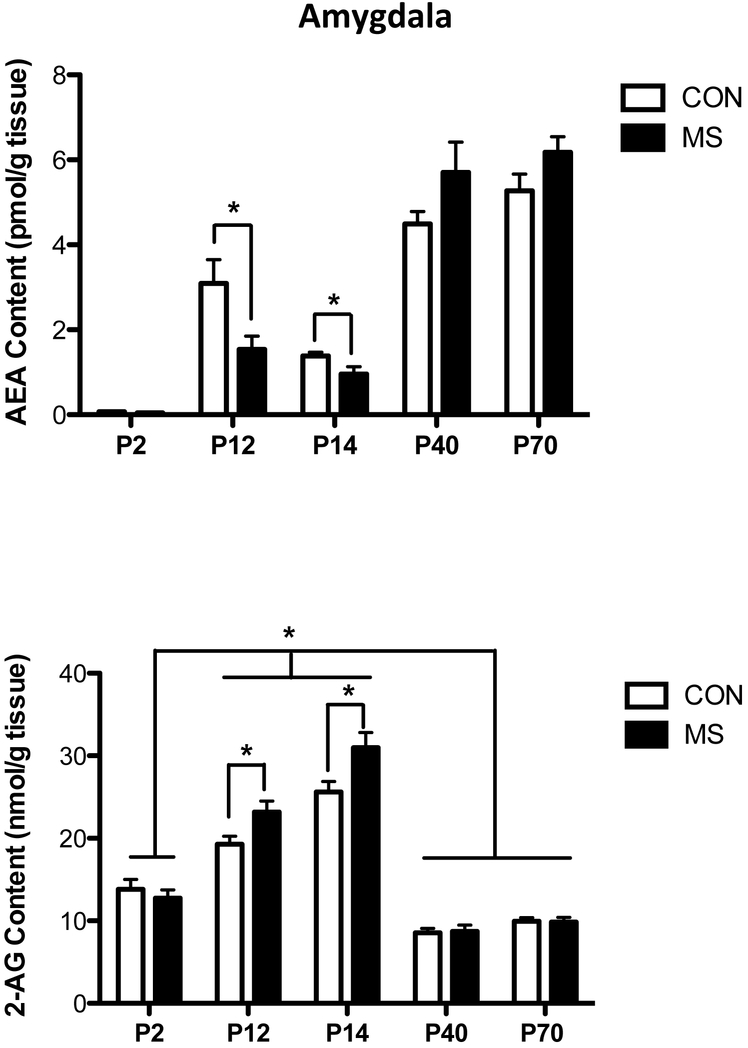
Within the amygdala, there was a significant interaction between age and MS exposure [F (4, 66) = 4.39, p < 0.01; Fig. 2]; post-hoc analysis revealed both age and MS dependent changes in AEA content within the amygdala. With respect to age, AEA levels in the amygdala at P2 were significantly lower than at P12 (p < 0.01), P14 (p < 0.01), P40 (p < 0.001) and P70 (p < 0.01). Similarly, AEA levels in the amygdala at P12 and P14, while not different from each other (p > 0.05), were significantly lower than at P40 (P < 0.001) and P70 (p < 0.001). With respect to stress, however, there was no difference in AEA levels between control rats and those exposed to MS at P2 (p > 0.05), or at adolescence at P40 (p > 0.05) or adulthood at P70 (p > 0.05). However, immediately following the final session of MS stress (P12), and, 2 days following the final bout of MS (P14), AEA content within the amygdala in rats exposed to early life stress were significantly reduced compared to control rats (P12: p < 0.01; P14: p < 0.05).
Figure 2. The Effect of Maternal Separation Stress on the Developmental Trajectory of the Endocannabinoid Contents in the Amygdala.
Tissue content of the endocannabinoid anandamide (AEA; upper panel) was found to elevate in the amygdala in an age-dependent manner, with AEA content being higher at PND 12 and 14, relative to PND2, while AEA content at P40 and P70 was higher than at the earlier time points. Exposure to 3 hours of maternal separation (MS) stress per day from PND 2-11 resulted in significant reductions in AEA content in the amygdala both at PND 12 and PND 14, relative to control (CON) animals. These effects had normalized by PND40 and 70. 2-arachidonoylglycerol (2-AG; lower panel), the other primary endocannabinoid molecule, exhibited a different developmental trajectory where it was found to be significantly higher at PND 12 and 14 relative to both PND 2 as well as PND 40 and 70. Similar to AEA, but in the opposite direction, exposure to MS stress from PND2-11 resulted in an elevation in 2-AG content in the amygdala at both PND12 and PND14. Data are presented as means +/− SEM. * denotes significant differences (p < .05) between identified age windows or between identified CON and MS groups. All n = 7-8 / treatment condition.
There was a significant interaction between age and MS on amygdalar 2-AG content [F (4, 67) = 3.25, p < 0.02; Fig. 2]. Specifically, as was seen in the PFC, there was an age effect on 2-AG content, regardless of MS exposure, such that 2-AG contents in the amygdala at P12 and P14 were significantly higher than at P2, P40 and P70 (all comparisons p < 0.01). With respect to MS, it was the P12 and P14 ages when the impact of MS was apparent. Specifically, at P2, P40 and P70, there were no differences in 2-AG content in rats that had been exposed to early like stress versus control rats, but at both P12 (p < 0.01) and P14 (p < 0.05), animals that had been exposed to MS exhibited higher levels of 2-AG than control rats.
Developmental Trajectory of Endocannabinoid Content in the Hippocampus and its Modulation by Early Life Stress
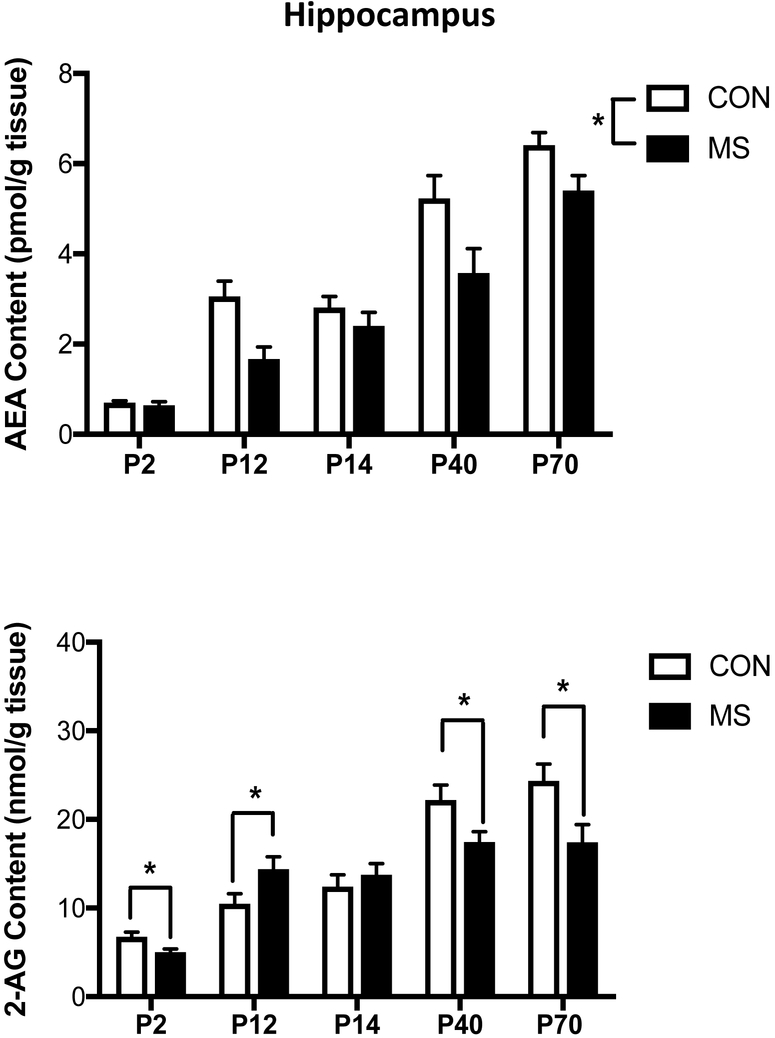
Within the hippocampus, there was no significant interaction between exposure to MS and age on AEA content [F (4, 67) = 2.10, p > 0.05; Fig. 3]. There was a main effect of MS [F (1, 67) = 19.91, p < 0.001], such that exposure to early life stress caused a general reduction in AEA content in the hippocampus at all ages. There was also a main effect of age [F (4, 67) = 79.22, p < 0.001], similar to what was seen in other structures, such that AEA content in the hippocampus was higher at P40 and P70 relative to all younger ages (p < 0.05 for all comparisons). There was a significant interaction between MS exposure and age on 2-AG content in the hippocampus [F (4, 68) = 5.20, p < 0.01; Fig. 3]. Unlike the PFC and amygdala, 2-AG changed in a more linear manner across ages; 2-AG content was significantly higher at P40 and P70 relative to all younger ages (p < 0.05 for all comparisons). However, when post-hoc analysis examined the impact of MS exposure, the effects were very age dependent. Specifically, at P2, immediately after the first exposure to MS stress, there was a significant reduction in 2-AG content in the hippocampus in the stressed rats relative to the control rats (p < 0.05). Immediately after the final MS exposure (P12), 2-AG levels in the hippocampus were significantly higher in the stressed animals relative to the control animals (p < 0.05). At P14, there were no differences in 2-AG content between the two groups in the hippocampus. By P40 and continuing to P70, 2-AG levels within the hippocampus were significantly lower in the animals that had experienced MS, relative to the control animals (p < 0.05 and p<0.01, respectively).
Figure 3. The Effect of Maternal Separation Stress on the Developmental Trajectory of the Endocannabinoid Contents in the Hippocampus.
Tissue content of the endocannabinoid anandamide (AEA; upper panel) was found to elevate in the hippocampus in an age-dependent manner, with AEA content being higher at postnatal day (PND) 40 and 70 relative to PND 2, 12 and 14. Exposure to 3 hours of maternal separation (MS) stress per day from PND 2-11 resulted in a main effect of reduced in AEA content in the hippocampus across all age points. 2-arachidonoylglycerol (2-AG; lower panel), the other primary endocannabinoid molecule, was also found to be higher at postnatal day (PND) 40 and 70 relative to PND 2, 12 and 14 in the hippocampus. Exposure to MS stress had age specific effects on 2-AG content in the hippocampus, with 2-AG levels significantly reducing on PND2 after the first exposure to MS, but then elevating on PND12 after the last MS stress, relative to control (CON) animals. These effects had normalized by PND 14, but then again at both PND40 and PND70 tissue levels of 2-AG in the hippocampus were found to be significantly reduced in rats which had been exposed to MS stress relative to CON animals. Data are presented as means +/− SEM. * denotes significant differences (p < .05) between identified CON and MS groups. All n = 7-8 / treatment condition.
The impact of early life stress on CB1 receptor binding site densities in adolescence and adulthood.
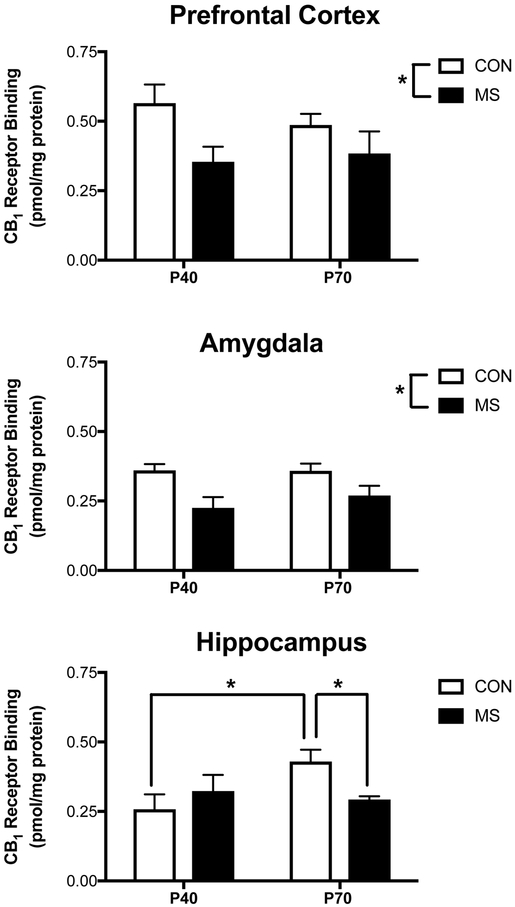
Within the PFC, there was no interaction between MS exposure and age on the Bmax of the CB1 [F (1, 12) = 0.78, p > 0.05; Fig. 4], nor an effect of age [F (1, 12) = 0.16, p > 0.05], but there was a significant main effect of MS exposure [F (1, 12) = 6.46, p < 0.03], such that exposure to MS was related to reductions in CB1 receptor binding site density in the PFC, at both ages, relative to control rats. A similar effect was seen with CB1 receptor binding affinity, where there was no interaction between MS and age [F (1, 12) = 1.50, p > 0.05], nor a main effect of age [F (1, 12) = 0.44], but there was a main effect of MS exposure [F (1, 12) = 5.26, p < 0.05], such that regardless of age, exposure to MS reduced the Km for the CB1 receptor (P40 CON: 0.63 nM +/− 0.10; P40 MS: 0.26 nM +/− 0.04; P70: CON 0.43 nM +/− 0.1; P70 MS: 0.32 nM +/− 0.12).
Figure 4. Maternal Separation Impacts CB1 Receptor Densities in the Adolescent and Adult Brain.
The maximal binding site density (Bmax) of the cannabinoid CB1 receptor, as determined by specific binding of the 3H-CP55,940, was examined in the prefrontal cortex, amygdala and hippocampus of the adolescent brain at PND40 and the adult brain at PND70. Within the prefrontal cortex (upper panel) and amygdala (middle panel), maternal separation (MS) stress was found to result in a main effect of reduced CB1 receptor binding site densities at both PND40 and PND70. Within the hippocampus, there was an age dependent effect, such that CB1 receptor binding site densities were found to be higher at PND70 relative to PND40. Exposure to MS stress had no effect on CB1 density within the hippocampus at PND40, but at PND70 animals exposed to MS stress had reduced CB1 receptor binding site densities relative to control (CON) animals. Data are presented as means +/− SEM. * denotes significant differences (p < .05) between CON and MS groups or identified age groups. All n = 4 / treatment condition.
A similar pattern emerged within the amygdala, such that there was no interaction between age and MS exposure on the Bmax of the CB1 receptor [F (1, 12) = 0.53, p > 0.05; Fig. 4], or a main effect of age [F (1, 12) = 0.47, p > 0.05]; but there was a significant main effect of MS exposure [F (1, 12) = 12.65, p < 0.005], such that regardless of age, exposure to MS reduced the Bmax of the CB1 receptor within the amygdala. However, there was no significant interaction [F (1, 12) = 0.01, p > 0.05], no main effect of age [f (1, 12) = 0.13, p > 0.05] nor a significant main effect of MS exposure [F (1, 12) = 3.73, p > 0.05] on the binding affinity of the CB1 receptor in the amygdala (P40 CON: 0.47 nM +/− 0.15; P40 MS: 0.20 nM +/− 0.12; P70 CON: 0.51 nM +/− 0.21; P70 MS: 0.26 nM +/− 0.04).
Analysis of the binding parameters of the CB1 receptor within the hippocampus revealed a main effect between age and MS exposure [F (1, 12) = 4.97, p < 0.05; Fig 4], with post hoc analysis demonstrating effects of both age and MS. Specifically, there was an increase in the Bmax of the CB1 receptor between P40 and P70 (p < 0.05) in control animals. While there was no difference between the Bmax of the CB1 receptor at P40 in control rats versus those that were exposed to MS (p > 0.05), at P70, rats exposed to MS exhibited a significant reduction in CB1 receptor binding sites relative to control rats (p < 0.05). With respect to the binding affinity of the CB1 receptor, there was no interaction between age and MS exposure [F (1, 12) = 3.16, p > 0.05], nor an effect of age [F (1, 12) = 0.70, p > 0.05], there was a main effect of stress [F (1, 12) = 9.25, p < 0.02], such that, regardless of age, there was a reduction in the binding affinity of the CB1 receptor in rats that had been exposed to MS (P40 CON: 0.62 nM +/− 0.10; P40 MS: 0.52 nM +/− 0.06; P70 CON: 0.84 nM +/− 0.05; P70 MS: 0.44 nM +/− 0.10).
Discussion
This study demonstrated that exposure of neonatal rats to MS stress from PND2-12 resulted in both immediate and sustained effects on the corticolimbic eCB system. Generally, dynamic stress-induced changes in neonatal eCB levels are consistent with changes documented in adult rats (Morena et al., 2016), suggesting that the eCB system is responsive to stress, even during the early life period. This is consistent with the limited data available from previous reports indicating that eCB signaling is modulated by stress during early development (D’Asti et al., 2010; Llorente et al., 2008; Marco et al., 2013). More so, it is likely that these eCB changes are functionally relevant to the effects of stress since previous work has demonstrated that eCB signaling can regulate both behavioral and neuroendocrine responses to stress at this early developmental window (Buwembo et al., 2013; D’Asti et al., 2010; Fride et al., 2005). It should be noted that these changes were only seen after the final stress exposure, however, due to low tissue concentrations of AEA at PND2, and the possibility that rapid changes could have occurred which may have habituated by the conclusion of the 3h separation period, we cannot rule out that the first exposure to separation stress also had an impact on eCB signaling in the neonates.
Impact of Early Life Stress on the Endocannabinoid System within the PFC
Tracking changes in the eCB system across development, following exposure to MS, revealed that stress exposure in early life modulates the normative ontogeny of the eCB system in a region-specific manner. Quite surprisingly, the PFC appeared entirely resistant to any impact of early life stress on eCB content at any of the time points measured, but did exhibit a persistent downregulation of CB1 receptors in adolescence and adulthood in animals exposed to MS. This reduction in prefrontal CB1 receptor binding sites after developmental exposure to stress mirrors data from our group that exposure to stress during adolescence also resulted in a sustained downregulation of prefrontal cortical CB1 receptors in adulthood (Lee & Hill, 2013). The functional relevance of these changes is not yet known, however, given the importance of CB1 receptor signaling within the PFC to regulate dendritic plasticity (Hill et al., 2011a), termination of the stress response (Hill et al., 2011b) and emotional behavior (McLaughlin et al., 2014; McLaughlin et al., 2012; Rubino et al., 2008), all of which are influenced by developmental exposure to stress, we hypothesize that these changes are functionally related.
Impact of Early Life Stress on the Endocannabinoid System within the Amygdala
Repeated exposure to MS stress in neonates produced similar changes in eCB content as to what is seen in adults exposed to chronic stress (Morena et al., 2016), whereby repeated exposure to MS stress resulted in a reduction in AEA content throughout the juvenile period (PND12-14), coupled to an increase in 2-AG content at the same time points. This suggests that the effect of stress on eCB signaling within the amygdala is preserved in early life, which is interesting given the importance of amygdalar eCB signaling to various aspects of the stress response, including activation of the HPA axis and the generation of an anxious state. Similar to the PFC, CB1 receptor binding site densities within the amygdala were downregulated both at adolescence and adulthood in animals exposed to MS, despite normalization of eCB content. While not examined in this study, an impairment in CB1 receptor signaling within the amygdala in adulthood would likely render an organism more sensitive to the adverse effects of stress. Future work will have to examine a “double hit” approach to establish if the downregulation of amygdalar CB1 receptors contributes to the altered stress sensitivity animals exposed to early stress exhibit in adulthood.
Impact of Early Life Stress on the Endocannabinoid System within the Hippocampus
Within the hippocampus, there was a general effect of MS stress such that AEA levels were reduced at every single developmental time point following MS stress, indicating an exquisite sensitivity of AEA signaling in the hippocampus to early life adversity. 2-AG content, on the other hand, was reduced in response to the first bout of neonatal stress, but was then transiently elevated after the final exposure to MS stress, only to return to baseline levels by PND14. By adolescence, and into adulthood, however, 2-AG content exhibited a prominent reduction in tissue levels in animals exposed to MS indicating that early life stress resulted in a profound, and sustained, reduction in both eCB ligands within the hippocampus at later developmental windows. Unlike what was seen in the PFC and amygdala, however, there was no impact of MS stress on CB1 receptor binding site densities at adolescence. By adulthood, however, when control animals exhibited a significant elevation in CB1 receptor density relative to adolescence, rats exposed to early life stress exhibited no such developmental change, and accordingly, there was a significant downregulation of CB1 receptors in the hippocampus of these animals. Together, these data indicate that the most profound effects of early life stress on the eCB system appeared to be within the hippocampus where every aspect of the system (the primary receptor and both ligands) exhibited a significant downregulation, relative to animals reared under standard conditions.
Previous work has demonstrated that the hippocampus is particularly sensitive to the effects of early life stress (Andersen & Teicher, 2008; Brunson et al., 2003), such that basal changes in the hippocampus of adult rodents exposed to early life stress appear to mirror dynamic changes that are induced by exposure to chronic stress as adults, such as reductions in neurogenesis, neuronal complexity, dendritic arborization and spine densities (Andersen & Teicher, 2004; Brunson et al., 2005; Eiland & McEwen, 2012; Ivy et al., 2010; Karten et al., 2005; Leslie et al., 2011; Wang et al., 2011, 2013). These changes in hippocampal plasticity and architecture following early life stress are met by alterations in hippocampalmediated cognitive function (Brunson et al., 2005, 2003; Wang et al., 2011), including findings from our group using this model where we have demonstrated persistent impairments in object recognition memory in adults (Eiland & McEwen, 2012). Consistent with these findings, our data demonstrate that exposure to early life stress causes a robust impairment in the adult hippocampal eCB system (downregulation of CB1 receptors as well as reduced AEA and 2-AG contents), which parallels data indicating that exposure to chronic stress in adulthood causes an impairment in eCB signaling within the hippocampus (Hill et al., 2005, 2008; Hu et al., 2011; Lee & Hill, 2013; Reich et al., 2009; Wang et al., 2014). One interpretation of these data is that the adult hippocampus of rodents exposed to early life stress appears to exist in a persistent state of allostatic load. As eCB signaling is a mediator of both synaptic and structural plasticity in the hippocampus (Chevaleyre & Castillo, 2004; Monory et al., 2015), these findings could suggest that exposure to early life stress compromises plasticity-related mechanisms within the hippocampus rendering this structure highly inflexible to experiential change in adulthood, which is consistent with a state of allostatic load (McEwen, 2007). The particular sensitivity of the hippocampus to these effects could relate to the timing of stress exposure. Specifically, several studies have demonstrated that the hippocampus is undergoing significant development during this early life window, in both animals and humans, and is accordingly, highly susceptible to the long-term effects of stress exposure (Andersen & Teicher, 2008; Loi et al., 2014; McClelland et al., 2011; Qiu et al., 2013).
It is interesting to note, that for measures of CB1 receptor densities, almost all of the reductions in CB1 receptor binding site density were coupled to an enhancement of the binding affinity for ligand binding to the CB1 receptor. The relevance of these differential effects on CB1 receptor density and affinity is not clear at this time, but the increased affinity could be a compensatory mechanism attempting to counter the reduced level of receptor. Future studies employing electrophysiology will be required to determine the impact of early life stress on CB1 receptor functionality and the neuronal populations on which this occurs. Regardless, these findings are generally consistent with data generated using a similar model of maternal deprivation where early life stress produced reductions in CB1 receptor expression (using immunohistochemistry) in the adult hippocampus (López-Gallardo et al., 2012) and reductions in CB1 receptor expression in the adolescent frontal cortex (Romano-López et al., 2012).
Normative Developmental Trajectory of the Endocannabinoid System
Examination of eCB content across multiple developmental epochs allowed us to determine a general trajectory for the normative ontogeny of this system (also see Lee and Gorzalka, 2012 for review of previous work on development of the eCB system, particularly during discrete periods of adolescence which was not assessed to the same degree herein). Both the amygdala and PFC appeared to exhibit a comparable trajectory whereby AEA levels were almost undetectable at PND2, increase slightly at PND12-14 and then dramatically increase by PND40 and 70. 2-AG, on the other hand, was comparable to adult levels at PND2, but then exhibited a dramatic elevation in tissue levels on PND12-14, declining to adult levels at PND40 and 70. The comparable levels of 2-AG in neonates and adults, but progressive increase in AEA levels is consistent with a previous report (Berrendero et al., 1999), however, the current study extends these investigations into the juvenile period where a robust increase in 2-AG content is observed. The functional nature of these evolving eCB levels remains elusive, but it is interesting to note that the developmental window in which 2-AG levels appear to be higher in corticoamygdala circuits is during a period of significant development in this circuit and approximately corresponds to the period when the amygdala develops the ability to assign negative valence to stimuli and recognize aversive cues (Barr et al., 2009; Moriceau & Sullivan, 2006; Sullivan & Holman, 2010). Given that 2-AG signaling during development has been identified to influence axon guidance and circuit formation (Keimpema et al., 2010, 2013; Oudin et al., 2011; Wu et al., 2010), the possibility exists that this signal could be involved in the development of the frontocortical-amygdala circuit that is essential for the top down control of emotional behavior and valence detection. Stress exposure early in life has been shown to accelerate the maturation and ability of the amygdala to respond to aversive cues (Gee et al., 2013; Moriceau et al., 2009), and in the current study, stress exposure significantly increased 2-AG content within the amygdala during this window, suggesting that future work should examine whether there is any role for 2-AG in the maturation of the amygdala, its connections with the frontal cortex and whether stress-induced changes in eCB signaling are relevant to stress exposure’s known ability to modulate maturational processes within this circuit (Gee et al., 2013; Malter Cohen et al., 2013). Of note, the ontogeny of hippocampal eCB signaling did not parallel the developmental trajectories of eCB signaling in the amygdala and PFC in that hippocampal AEA and 2-AG levels appeared to increase progressively in a linear manner from birth into adulthood. While the relevance of the ontogenetic dissociation between these structures is currently unknown, it does suggest that there are differential trajectories of eCB signaling across the brain and that future work should examine this profile in more depth to understand both the mechanisms and relevance of these differences.
Limitations and Considerations
A limitation of this study was that all of the analysis was only performed in male, and not female rats. To date, there is almost no information available regarding how stress, even in adulthood, modulates eCB content in females relative to males. Ongoing research in our group is beginning to investigate sex-specific changes in eCB content and CB1 receptors in response to stress. Once a more comprehensive understanding of the sex-specific nature by which stress modulates eCB signaling is established, the developmental effects of stress will also have to be investigated. This is particularly relevant given that sex differences in eCB signaling have been widely established, both in a developmental context as well as in adulthood (Huang & Woolley, 2012; Krebs-Kraft et al., 2010; Tabatadze et al., 2015). Similarly, another issue of consideration in the interpretation of these data is that all of the pregnant dams were exposed to shipping during gestational day 10-12 of pregnancy. The stress of shipment during this period of gestation has been found to impact neural adaptations to additional stimuli (Ogawa et al., 2007; Moriyama et al., 2013), and thus could have an impact on the generalization of the data generated herein.
Conclusions
Collectively this study highlights a previously undetermined non-linear and region-specific developmental trajectory of the eCB system within corticolimbic structures that is influenced by exposure to stress during the postnatal period. The hippocampus appears to be particularly sensitive to these sustained effects into adulthood, which could be due to its stage of development during the stress exposure. This is consistent with other studies identifying the PND2-12 window as being particularly sensitive to manipulations, such as elevated 5-HT signaling (Rebello et al., 2014), which produce sustained alterations in neurodevelopment that continue into adulthood. These data also indicate that the eCB system in adulthood is sensitive to programming by early life experience. Given the important stress-inhibitory role of eCB signaling, the ability of early life stress to compromise this system could represent one mechanism by which early life stress increases vulnerability to stress-related psychiatric illnesses in adulthood. In this light, it is interesting to note that gene variants in the CB1 receptor, and enzymes which metabolizes AEA and 2-AG, have been shown to influence the effects of early life stress on the development of anhedonia, stress sensitivity and depression in adulthood (Agrawal et al., 2012; Carey et al., 2015; Lazary et al., 2016).
Highlights.
Maternal separation stress modulates endocannabinoid levels in neonates
Endocannabinoid signaling in the adult hippocampus is impaired by early life stress
Early life stress does not impact cortical and amygdala endocannabinoid levels in adulthood
Acknowledgements
The authors would like to thank Praveen Chatani for his technical assistance. This research was supported operating funds from the National Institutes of Health to BSM (R01 MH041256) and CJH (R01 DA026996), with additional support to CJH from the Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin. MNH was the recipient of a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR) and TTL was the recipient of a doctoral fellowship from CIHR. Funding for this study was also provided to LE by a Weill Cornell Medical College Friedman Clinical Scholarship in Newborn Medicine and by a National Institute of Mental Health (NIMH) Diversity Supplement 3R01MH041256-22S1. MNH currently receives salary support in the form of a Tier II Canada Research Chair supported by the CIHR. All funding bodies had no role in the design, acquisition or interpretation of this data or in the writing of, or decision to submit, this manuscript.
Footnotes
Conflict of Interest Statement
BSM has received unrestricted operating funds from Johnson and Johnson Pharmaceuticals Ltd that is unrelated to the current project. All other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Nelson EC, Littlefield AK, Bucholz KK, Degenhardt L, Henders AK, … Lynskey MT. (2012). Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Archives of General Psychiatry, 69(7), 732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpár A, Tortoriello G, Calvigioni D, Niphakis MJ, Milenkovic I, Bakker J, … Harkany T. (2014). Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling. Nature Communications, 5, 4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL (2015). Exposure to early adversity: Points of cross-species translation that can lead to improved understanding of depression. Development and Psychopathology, 27(2), 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, & Teicher MH (2004). Delayed effects of early stress on hippocampal development. Neuropsychopharmacology, 29(11), 1988–93. [DOI] [PubMed] [Google Scholar]
- Andersen SL, & Teicher MH (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences, 31(4), 183–191. [DOI] [PubMed] [Google Scholar]
- Atsak P, Morena M, Schoenmaker C, Tabak E, Oomen CA, Jamil S, Hill MN, & Roozendaal B (2018). Glucocorticoid-endocannabinoid uncoupling mediates fear suppression deficits after early-life stress. Psychoneuroendocrinology, 91, 41–49. [DOI] [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, & Sullivan RM (2009). Transitions in infant learning are modulated by dopamine in the amygdala. Nature Neuroscience, 12(11), 1367–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, … Harkany T. (2007). Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science, 316(5828), 1212–6. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, & Fernández-Ruiz JJ (1999). Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse, 33(3), 181–91. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Chen Y, Avishai-Eliner S, & Baram TZ (2003). Stress and the developing hippocampus: a double-edged sword? Molecular Neurobiology, 27(2), 121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, … Baram TZ. (2005). Mechanisms of late-onset cognitive decline after early-life stress. Journal of Neuroscience, 25(41), 9328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwembo A, Long H, & Walker C-D (2013). Participation of endocannabinoids in rapid suppression of stress responses by glucocorticoids in neonates. Neuroscience, 249, 154–61. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Sullivan RM, Howell B, & Tottenham N (2014). The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits--a cross-species analysis. Developmental Psychobiology, 56(8), 1635–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CE, Agrawal A, Zhang B, Conley ED, Degenhardt L, Heath AC, Li D, Lynskey MT, Martin NG, Montgomery GW, Wang T, Bierut LJ, Hariri AR, Nelson EC, & Bogdan R (2015). Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: Evidence from an endocannabinoid system-level analysis. Journal of Abnormal Psychology, 124(4), 860–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, & Castillo PE (2004). Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron, 43(6), 871–81. [DOI] [PubMed] [Google Scholar]
- D’Asti E, Long H, Tremblay-Mercier J, Grajzer M, Cunnane SC, Di Marzo V, & Walker C-D (2010). Maternal dietary fat determines metabolic profile and the magnitude of endocannabinoid inhibition of the stress response in neonatal rat offspring. Endocrinology, 151(4), 1685–94. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, & McEwen BS (2012). Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuroscience, 15(5), 689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, & McEwen BS (2012). Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus, 22(1), 82–91. [DOI] [PubMed] [Google Scholar]
- Fride E, Suris R, Weidenfeld J, & Mechoulam R (2005). Differential response to acute and repeated stress in cannabinoid CB1 receptor knockout newborn and adult mice. Behav Pharmacol, 16(5–6), 431–440. [DOI] [PubMed] [Google Scholar]
- Gee DG, & Casey BJ (2015). The impact of developmental timing for stress and recovery. Neurobiology of Stress, 1, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N. (2013). Early developmental emergence of human amygdalaprefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America, 110(39), 15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, & Hillard CJ (2008). Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neuroscience and Biobehavioral Reviews, 32(6), 1152–60. [DOI] [PubMed] [Google Scholar]
- Heim C, & Binder EB (2012). Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology, 233(1), 102–11. [DOI] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, & Tseng KY (2011). Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse, 65(4), 278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, & Rice KC (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience, 11(2), 563–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Campolongo P, Yehuda R, & Patel S (2018). Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology, 43(1), 80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, & Gorzalka BB (2008). Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem, 106(6), 2322–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, & McEwen BS (2011a). Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptordeficient mice parallel the effects of chronic stress. Cereb Cortex, 21(9), 2056–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Karatsoreos IN, Hillard CJ, & McEwen BS (2010). Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology, 35(9), 1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT-Y, … Hillard CJ. (2011b). Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. Journal of Neuroscience, 31(29), 10506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, & Patel S (2013). Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biology of Mood & Anxiety Disorders, 3(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, & Gorzalka BB (2005). Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology, 30(3), 508–515. [DOI] [PubMed] [Google Scholar]
- Hill MN, & Tasker JG (2012). Endocannabinoid signaling, glucocorticoid- mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience, 204, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ (2014). Stress regulates endocannabinoid-CB1 receptor signaling. Seminars in Immunology, 26(5), 380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, & Campbell WB (1995). Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. Journal of Neurochemistry, 64(2), 677–83. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Weinlander KM, & Stuhr KL (2012). Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience, 204, 207–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czéh B, Zhang W, & Flügge G (2011). Chronic restraint stress impairs endocannabinoid mediated suppression of GABAergic signaling in the hippocampus of adult male rats. Brain Research Bulletin, 85(6), 374–9. [DOI] [PubMed] [Google Scholar]
- Huang GZ, & Woolley CS (2012). Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron, 74(5), 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, … Baram TZ. (2010). Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. Journal of Neuroscience, 30(39), 13005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans JE, & Woodside BC (1990). Nest temperature: effects on maternal behavior, pup development, and interactions with handling. Developmental Psychobiology, 23(6), 519–34. [DOI] [PubMed] [Google Scholar]
- Kang-Park M-H, Wilson WA, Kuhn CM, Moore SD, & Swartzwelder HS (2007). Differential sensitivity of GABA A receptor-mediated IPSCs to cannabinoids in hippocampal slices from adolescent and adult rats. Journal of Neurophysiology, 98(3), 1223–30. [DOI] [PubMed] [Google Scholar]
- Karten YJG, Olariu A, & Cameron HA (2005). Stress in early life inhibits neurogenesis in adulthood. Trends in Neurosciences, 28(4), 171–2. [DOI] [PubMed] [Google Scholar]
- Katona I, & Freund TF (2012). Multiple functions of endocannabinoid signaling in the brain. Annual Review of Neuroscience, 35, 529–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keimpema E, Alpár A, Howell F, Malenczyk K, Hobbs C, Hurd YL, … Harkany T. (2013). Diacylglycerol lipase α manipulation reveals developmental roles for intercellular endocannabinoid signaling. Scientific Reports, 3, 2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keimpema E, Barabas K, Morozov YM, Tortoriello G, Torii M, Cameron G, … Harkany T. (2010). Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding. Journal of Neuroscience, 30(42), 13992–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, & Binder EB (2015). Epigenetics of Stress-Related Psychiatric Disorders and Gene × Environment Interactions. Neuron, 86(6), 1343–1357. [DOI] [PubMed] [Google Scholar]
- Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM (2010). Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proceedings of the National Academy of Sciences, 107(47), 20535–20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazary J, Eszlari N, Juhasz G, Bagdy G (2016). Genetically reduced FAAH activity may be a risk for the development of anxiety and depression in persons with repetitive childhood trauma. European Neuropsychopharmacology, 26(6), 1020–1028. [DOI] [PubMed] [Google Scholar]
- Lee TT-Y, & Gorzalka BB (2012). Timing is everything: evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic–pituitary–adrenal axis activity across developmental periods. Neuroscience, 204, 17–30. [DOI] [PubMed] [Google Scholar]
- Lee TTY, & Hill MN (2013). Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB1 receptor binding in male rats. Neuroscience, 249, 106–14. [DOI] [PubMed] [Google Scholar]
- Leslie AT, Akers KG, Krakowski AD, Stone SSD, Sakaguchi M, Arruda-Carvalho M, & Frankland PW (2011). Impact of early adverse experience on complexity of adult-generated neurons. Translational Psychiatry, 1, e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S-L, Alger BE, & McCarthy MM (2014). Developmental increase in hippocampal endocannabinoid mobilization: role of metabotropic glutamate receptor subtype 5 and phospholipase C. Journal of Neurophysiology, 112(10), 2605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Berzal A, Assis MA, Rubino T, Zamberletti E, Marco EM, Parolaro D, … Viveros M-P. (2013). Sex-dependent changes in brain CB1R expression and functionality and immune CB2R expression as a consequence of maternal deprivation and adolescent cocaine exposure. Pharmacological Research, 74, 23–33. [DOI] [PubMed] [Google Scholar]
- Llorente R, Llorente-Berzal A, Petrosino S, Marco E-M, Guaza C, Prada C, … Viveros M-P. (2008). Gender-dependent cellular and biochemical effects of maternal deprivation on the hippocampus of neonatal rats: A possible role for the endocannabinoid system. Developmental Neurobiology, 68(11), 1334–1347. [DOI] [PubMed] [Google Scholar]
- Loi M, Koricka S, Lucassen PJ, & Joels M (2014). Age- and sex-dependent effects of early life stress on hippocampal neurogenesis. Frontiers in Endocrinology, 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüpez-Gallardo M, Lüpez-RodrÍguez AB, Llorente-Berzal Á, Rotllant D, Mackie K, Armario A, … Viveros M-P. (2012). Maternal deprivation and adolescent cannabinoid exposure impact hippocampal astrocytes, CB1 receptors and brain-derived neurotrophic factor in a sexually dimorphic fashion. Neuroscience, 204, 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, & Casey BJ (2013). Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences, 110(45), 18274–18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Scattoni ML, Rapino C, Ceci C, Chaves N, MacrÍ S, … Laviola G (2013). Emotional, endocrine and brain anandamide response to social challenge in infant male rats. Psychoneuroendocrinology, 38(10), 2152–62. [DOI] [PubMed] [Google Scholar]
- McClelland S, Korosi A, Cope J, Ivy A, & Baram TZ (2011). Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiology of Learning and Memory, 96(1), 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews, 87(3), 873–904. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, & Gorzalka BB (2014). A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neuroscience & Biobehavioral Reviews, 42, 116–131 [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Bambico FR, Stuhr KL, Gobbi G, Hillard CJ, & Gorzalka BB (2012). Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway. Eur Neuropsychopharmacol, 22(9), 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, & Parker LA (2013). The endocannabinoid system and the brain. Annual Review of Psychology, 64, 21–47. [DOI] [PubMed] [Google Scholar]
- Melis M, Greco B, & Tonini R (2014). Interplay between synaptic endocannabinoid signaling and metaplasticity in neuronal circuit function and dysfunction. The European Journal of Neuroscience, 39(7), 1189–201. [DOI] [PubMed] [Google Scholar]
- Monory K, Polack M, Remus A, Lutz B, & Korte M (2015). Cannabinoid CB1 Receptor Calibrates Excitatory Synaptic Balance in the Mouse Hippocampus. Journal of Neuroscience, 35(9), 3842–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, & Hill MN (2016). Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology, 41(1), 80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, & Sullivan RM (2009). Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. Journal of Neuroscience, 29(50), 15745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, & Sullivan RM (2006). Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience, 9(8), 1004–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama C, Galic MA, Mychasiuk R, Pittman QJ, Perrot TS, Currie W & Esser MJ (2013). Prenatal transport stress, postnatal maternal behavior and offspring sex differentially affect seizure suceptibility in young rats. Epilepsy and Behavior, 29(1), 19–27. [DOI] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabás K, Ballester Rosado CJ, Nguyen L, … Harkany T. (2008). Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proceedings of the National Academy of Sciences of the United States of America, 105(25), 8760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudon L, Piscitelli F, Giros B, Di Marzo V, & Daugé V (2013). Possible involvement of endocannabinoids in the increase of morphine consumption in maternally deprived rat. Neuropharmacology, 65, 193–9. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Kuwagata M, Hori Y & Shioda S (2007). Valproate-induced developmental neurotoxicity is affected by maternal conditions including shipping stress and environmental change during pregnancy. Toxicol Letters 174(1-3), 18–24. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, & Kano M (2014). Endocannabinoid-mediated retrograde modulation of synaptic transmission. Current Opinion in Neurobiology, 29, 1–8. [DOI] [PubMed] [Google Scholar]
- Oudin MJ, Hobbs C, & Doherty P (2011). DAGL-dependent endocannabinoid signalling: roles in axonal pathfinding, synaptic plasticity and adult neurogenesis. European Journal of Neuroscience, 34(10), 1634–46. [DOI] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho W-SV, Rademacher DJ, Cunningham S, Reddy DS, … Hillard CJ (2005). The postmortal accumulation of brain Narachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. Journal of Lipid Research, 46(2), 342–9. [DOI] [PubMed] [Google Scholar]
- Patel S, Rademacher DJ, & Hillard CJ (2003). Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. The Journal of Pharmacology and Experimental Therapeutics, 306(3), 880–8. [DOI] [PubMed] [Google Scholar]
- Pratchett LC, & Yehuda R (2011). Foundations of posttraumatic stress disorder: Does early life trauma lead to adult posttraumatic stress disorder? Development and Psychopathology, 23(2), 477–491. [DOI] [PubMed] [Google Scholar]
- Qiu A, Rifkin-Graboi A, Chen H, Chong Y-S, Kwek K, Gluckman PD, … Meaney MJ. (2013). Maternal anxiety and infants’ hippocampal development: timing matters. Translational Psychiatry, 3(9), e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello TJ, Yu Q, Goodfellow NM, Caffrey Cagliostro MK, Teissier A, Morelli E, … Ansorge MS. (2014). Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(37), 12379–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, & McCarthy MM (2009). Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behavioural Brain Research, 203(2), 264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-López A, Méndez-DÍaz M, Ruiz-Contreras AE, Carrisoza R, & Prospéro-GarcÍa O (2012). Maternal separation and proclivity for ethanol intake: a potential role of the endocannabinoid system in rats. Neuroscience, 223, 296–304. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, … Parolaro D. (2008). Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex, 18(6), 1292–1301. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, & Holman PJ (2010). Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neuroscience and Biobehavioral Reviews, 34(6), 835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Huang G, May RM, Jain A, Woolley CS (2015). Sex Differences in Molecular Signaling at Inhibitory Synapses in the Hippocampus. Journal of Neuroscience, 35(32), 11252–11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G, & Meaney M (2016). Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biological Psychiatry 79(2), 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang L, Zhang R, Chen Y, Liu L, Gao F, … Tan Q. (2014). Antidepressive mechanism of repetitive transcranial magnetic stimulation in rat: The role of the endocannabinoid system. Journal of Psychiatric Research, 51, 79–87. [DOI] [PubMed] [Google Scholar]
- Wang X-D, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, … Schmidt MV. (2011). Forebrain CRF1 modulates early-life stress-programmed cognitive deficits. Journal of Neuroscience, 31(38), 13625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-D, Su Y-A, Wagner KV, Avrabos C, Scharf SH, Hartmann J, … Schmidt MV. (2013). Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nature Neuroscience, 16(6), 706–13. [DOI] [PubMed] [Google Scholar]
- Wu C-S, Zhu J, Wager-Miller J, Wang S, O’Leary D, Monory K, … Lu H-C. (2010). Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. The European Journal of Neuroscience, 32(5), 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Labonté B, Wen XL, Turecki G, & Meaney MJ (2013). Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology, 38(1), 111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, & Lovinger DM (2010). Developmental alteration of endocannabinoid retrograde signaling in the hippocampus. Journal of Neurophysiology, 103(2), 1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]