Abstract
Background:
Phthalates are common plasticizer chemicals that have been linked to glucose intolerance in the general population, but there is only limited research on their association with gestational diabetes (GDM).
Objective:
We evaluated the association between 11 urinary phthalate metabolites and GDM, impaired glucose tolerance (IGT), and continuous blood glucose concentration during pregnancy in The Infant Development and Environment Study (TIDES). Based on prior study results, our primary analyses focused on monoethyl phthalate (MEP) in relation to our outcomes of interest.
Study Design:
We used multi-variable logistic regression to examine the odds of GDM and IGT in relation to an interquartile-range (IQR) increase in natural log (ln)-transformed, specific gravity (SG)-adjusted first trimester (T1) and average of T1 and third trimester (T3) (“T1T3avg”) phthalate metabolite concentrations. We fit linear regression models to examine the percent change in blood glucose per IQR increase in ln-transformed, SG-adjusted T1 and T1T3avg phthalates. In sensitivity analyses, we examined interactions between exposure and race. We adjusted for maternal age, maternal body mass index, study center, race/ethnicity, parity, and gestational age at glucose testing.
Results:
In our sample of 705 pregnant women, we observed 60 cases of GDM, 90 cases of IGT, and an average GLT blood glucose of 113.6 ±27.7 mg/dL. In our primary analysis, T1T3avg MEP was positively associated with GDM ([OR (95% CI) per IQR increase] T1T3avg MEP: 1.61 (1.10, 2.36)). In secondary analyses, most other phthalates were not found to be associated with study outcomes, though some associations were noted. Sensitivity analyses indicated strong race-specific associations in Asians, though these results are based on a small sample size (n=35).
Conclusion:
In alignment with our a priori selection, we documented an association between T1T3avg MEP and GDM. Additional phthalate metabolites were also found to be linked to glucose intolerance, with possible stronger associations in certain racial/ethnic subgroups. Given the prevalence of phthalate exposures and the growing evidence of associations with metabolic outcomes, future studies should continue to examine this question in diverse cohorts of pregnant women, particularly in those who may be at higher risk for GDM and IGT.
Keywords: gestational diabetes, impaired glucose intolerance, blood glucose, phthalates, endocrine disruptors, pregnancy
1. Introduction
Gestational diabetes mellitus (GDM) is glucose intolerance that develops during pregnancy (American Diabetes Association 2012). In GDM, there is insufficient insulin released from the beta cells and decreased skeletal muscle glucose uptake, resulting in maternal hyperglycemia. Incidence of GDM in the United States has increased dramatically in the past twenty years, (Albrecht et al. 2010; Bardenheier et al. 2015; Thorpe et al. 2005) partially attributed to improved detection. GDM is now detected in 8–9% percent of pregnancies in the U.S., though estimates vary based on ethnicity and diagnostic criteria (Caughey et al. 2010; Coustan et al. 2010; DeSisto et al. 2014; Ehrlich et al. 2016; Reece 2010). Risk factors include prior GDM (Holmes et al. 2010; Kim et al. 2007), previous large for gestational age (LGA) infant (Simmons et al. 2009), family history of GDM or type 2 diabetes mellitus (T2DM) (Ben‐ Haroush et al. 2003), pre-pregnancy obesity (Chu et al. 2007; Torloni et al. 2009), nonwhite race/ethnicity (Berkowitz et al. 1992), high gestational weight gain in early pregnancy (Morisset et al. 2011), and increased maternal age (Lao et al. 2006; Reece 2010). GDM is associated with health consequences for the mother and child, but recent research suggests that elevated maternal blood glucose even without overt GDM diagnosis, such as in impaired glucose tolerance (IGT), is also associated with adverse fetal and maternal outcomes (The HAPO Study Cooperative Research Group 2008).
The etiology of beta cell dysfunction and related pathologies in GDM is not well understood. Possible contributing factors and mediators include: alterations in inflammatory signaling, which can affect the insulin receptor and glucose transporters (Winkler et al. 2002); changes in expression of peroxisome proliferator activated receptors (PPARs) (Catalano et al. 2002); and oxidative stress (Chen and Scholl 2005; Coughlan et al. 2004; Lappas et al. 2011). Some women (<10% of GDM cases) have circulating antibodies to islet cells or key cellular enzymes, similar to those in type 1 diabetes (T1DM) (Catalano et al. 1990). Increasing evidence suggests that chemical exposure may also play a role in metabolic dysregulation in pregnancy, perhaps through the pathways described above (Ehrlich et al. 2016).
Phthalates are chemicals utilized in numerous consumer applications, including plastics, personal care products, food packaging, and medical devices (Sathyanarayana 2008; Schettler 2006) Because they are not bound to products, phthalates can easily migrate into air, dust, and food. Exposure occurs through ingestion, inhalation, and/or dermal contact (Schettler 2006). Phthalates are known endocrine disruptors (National Research Council 2008), and growing evidence indicates a link to metabolic dysfunction, including obesity and diabetes (Hatch et al. 2008; James-Todd et al. 2012; Stahlhut et al. 2007).
Published studies on the relationship between phthalates and gestational glucose intolerance are limited. The only cohort study (n= 1274) to examine GDM diagnosis in relation to phthalate exposure found no evidence of increased risk (Shapiro et al. 2015). However, other studies have documented associations between phthalate exposure and both increases and decreases in blood glucose concentration and/or risk factors for GDM (Fisher et al. 2018; James-Todd et al. 2018; James-Todd et al. 2016; Robledo et al. 2015).
To further address this question, we utilized data from The Infant Development and Environment Study (TIDES) to investigate the associations of urinary phthalate metabolite concentrations with GDM, IGT, and continuous blood glucose. Based on results from prior studies (James-Todd et al. 2018; James-Todd et al. 2016), our primary analytical hypothesis was that concentrations of monoethyl phthalate (MEP) would be higher among mothers with GDM compared to those who did not develop GDM. Secondary analyses considered additional individual phthalate metabolites and one weighted summary score of di-2-ethylhexyl phthalate (DEHP) metabolites. In sensitivity analyses, we evaluated race-specific associations and non-linear associations. Our study improves and expands upon previous related analyses by examining a large population from four distinct geographic regions, utilizing more than one urinary measure of phthalate exposure, and including subclinical measures (such as continuous glucose and IGT) based on medical records, in addition to clinical diagnosis of GDM.
2. MATERIALS AND METHODS
2.1. Study design
From 2010–2012, TIDES recruited first trimester (T1) pregnant women at the University of California San Francisco (UCSF), University of Minnesota (UMN), University of Rochester Medical Center (URMC), and Seattle Children’s Hospital/University of Washington (SCH/UW). Eligibility criteria included: <13 weeks pregnant, English speaking, ≥18 years of age, no severe threat to pregnancy, and intention to deliver at a study hospital. After providing informed consent, participants completed questionnaires and provided urine samples during each trimester of pregnancy. Glucose tolerance test results and other data were abstracted by staff at each site from birth and medical records, with one in ten records undergoing double abstraction for quality control. This study was approved by the UW Institutional Review Board (IRB) (Study ID: #00002643).
The overall TIDES cohort includes 753 women, but this analysis is limited to 705 women who completed a T1 questionnaire, had T1 phthalate data, and underwent a glucose load test (GLT). Because a GLT is only administered to pregnant women without prior diabetes, our study is limited to individuals without previous diabetes diagnoses.
2.2. Exposure Assessment
Data from T1 and third trimester (T3) urine samples were available for this analysis. T3 collection occurred concurrent with or after GDM screening. Samples were collected in sterile, phthalate-free specimen cups, transferred to cryovials, and stored at −80°C until analysis. Specific gravity was measured with a handheld refractometer at the time of urine collection. Phthalate metabolite analyses were carried out at two laboratories. Most samples were analyzed at the National Center for Environmental Health, Centers for Disease Control and Prevention (CDC). This process involved enzymatic deconjugation of phthalate metabolites from glucuronidated form, automated online solid phase extraction, separation with high performance liquid chromatography (HPLC), and detection by isotope-dilution tandem mass spectrometry (Silva et al. 2007). A subset of samples were analyzed at the University of Washington (UW) with a modified version of CDC method 6306.03, which included HPLC with electrospray ionization-tandem mass spectrometry (Calafat A.M. 2010). Process and instrument blanks were included for quality assurance. The limit of detection (LOD) was between 0.2–2.0 ng/mL for UW and 0.2–0.6 ng/mL for CDC.
Eleven individual phthalate metabolites (mono-isobutyl phthalate (MIBP), MEP, mono-n-butyl phthalate (MNBP), mono-benzyl phthalate (MBZP), mono-carboxy-isononyl phthalate (MCNP), mono-carboxy-iso-octyl phthalate (MCOP), mono-(3-carboxypropyl) phthalate (MCPP), mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP)) and the molar sum of DEHP metabolites(Ferguson et al. 2014b) (∑DEHP) were included. T1 MCOP and MCNP were only available for mothers of male infants, while T3 MCOP and MCNP were available for mothers of male and female infants. All urinary phthalates were corrected for dilution using specific gravity (SG) measurements with the following formula: Pc = P[(SGmedian-1)/SG-1] (Pc = SG-corrected concentration; P = measured urinary concentration; SG = specific gravity for the individual sample; SGmedian = median SG over all samples).(Ferguson et al. 2014b) The metabolites were also natural log (ln)- transformed to approximate normal distributions. To calculate ∑DEHP, we divided each metabolite by its molecular weight and summed these totals.(Hauser et al. 2016) Correlation between SG-adjusted T1 and T3 phthalates were assessed through Spearman correlation coefficients.
We utilized two exposure metrics: 1) arithmetic mean of ln-transformed, SG-adjusted T1 and T3 urinary phthalate metabolite concentrations (“T1T3avg”), and 2) ln-transformed, SG-adjusted T1 urinary phthalate metabolite concentrations. The subject-specific T1T3avg, our primary exposure metric, was used to reduce exposure misclassification and obtain a more representative measure, given that phthalates are nonpersistent chemicals that exhibit variability over time (Fisher et al. 2015). T1 concentrations were utilized because this exposure assessment preceded glucose tolerance testing.
2.3. Outcome Assessment
GDM screening occurred during weeks 24–28 of pregnancy. The most common GDM screening approach, and the one utilized by all TIDES clinics, is a two-step test, comprised of a 1-hour 50-g glucose load test (GLT) followed by a 3-hour 100-g oral glucose tolerance test (OGTT) for those who screen positive in the initial GLT (American Diabetes 2014; Vandorsten et al. 2012). GDM is diagnosed in women with two or more abnormal values in the OGTT. Because of varying diagnostic thresholds (American College of Obstetricians and Gynecologists (ACOG) 2018; Carpenter and Coustan 1982; National Diabetes Data 1979), we standardized across the TIDES clinics with respect to GLT and OGTT test results. We classified a GLT result of ≥ 135 mg/dL as GLT exceedance/failure, since this was the midpoint of the threshold used across TIDES clinics. We then utilized results from the OGTT to classify women using the Carpenter-Coustan (CC) thresholds for exceedance (fasting: 95 mg/dL; 1 hour: 180 mg/dL; 2 hour: 155 mg/dL; and 3 hours: 140 mg/dL) (Carpenter and Coustan 1982). IGT was defined as a failed GLT but less than two exceedances on the OGTT. Therefore, by definition, GDM and IGT are mutually exclusive. Blood glucose concentrations from the GLT were utilized in our continuous outcome analysis. Two GLT values (x=1 & x=38 mg/dL) deemed implausible by study clinicians were dropped.
2.4. Statistical Analysis
Based on published literature and a priori model conceptualization, we considered the following covariates and precision variables in the analyses: maternal age (Hatch et al. 2008; Lao et al. 2006), maternal pre-pregnancy body mass index (BMI) (Torloni et al. 2009), gestational weight gain (GWG) (Hedderson et al. 2010), study center, race/ethnicity (Berkowitz et al. 1992; Caughey et al. 2010; Ferrara 2007; Savitz et al. 2008; Thorpe et al. 2005), maternal education (Bouthoorn et al. 2015), smoking (Wendland et al. 2008), alcohol use (Bouthoorn et al. 2015), infant sex, parity, and gestational age at glucose testing (Di Cianni et al. 2003). Our final model, based on statistical significance of variables in stepwise model building, adjusted for maternal age, maternal pre-pregnancy BMI, study center, race/ethnicity, parity, and gestational age at glucose testing. Ultimately, we included BMI instead of GWG, because adjustment for GWG may have led to bias because of its possible role as a mediator.
We first conducted univariate analyses of covariates and then assessed the relation between covariates and outcomes. For the primary inferential analyses, we used multivariable regression models to estimate associations between T1T3avg urinary MEP concentrations and outcomes of interest (logistic regression: odds ratio (OR) of GDM or IGT; linear regression: difference in blood glucose concentrations from the GLT). In secondary analyses, we estimated associations between: 1) T1T3avg urinary phthalate concentrations (for all other individual phthalates separately and ∑DEHP metabolites) and the outcomes; and 2) T1 urinary phthalate concentrations (for all individual phthalates separately and ∑DEHP metabolites) and the outcomes. To enhance comparability and interpretation, we present results in terms of an interquartile range (IQR) difference in phthalate metabolite concentrations.
Since most studies suggest that women with polycystic ovary syndrome (PCOS) are at increased risk for GDM (Ben‐ Haroush et al. 2003; Toulis et al. 2009), we conducted sensitivity analyses excluding individuals with PCOS (n=45). Additionally, because of the strong associations of race/ethnicity with phthalate exposure (Huang et al. 2014; Mitro et al. 2018) and glucose intolerance (Berkowitz et al. 1992; Caughey et al. 2010) as well as the differing associations between BMI and glucose tolerance by race (Hsu et al. 2015), we used interaction and stratification by group to assess potential racial/ethnic-specific effects. We also created phthalate exposure quartiles to evaluate possible non-linear associations.
To address potential concerns with reverse causality and/or changes in metabolism due to disease status, we conducted a sensitivity analysis of our primary aim after dropping individuals whose T3 urine samples were obtained after their prenatal visit with GLT screening (n=47). To evaluate the potential influence of between-lab differences in LODs, we included laboratory as a covariate in the models.
All analyses were performed using STATA (version 14.1, StataCorp, College Station, TX, USA) with complete case analysis.
3. RESULTS
Table 1 describes our study population for this analysis (n=705). Our cohort was predominantly non-Hispanic white (66.4%) and well educated (74.2% had college/postgraduate education). Average maternal age was 31 years (standard deviation (SD) = 5.6 years), and average first trimester BMI was 26.1 kg/m2 (SD = 6.2 kg/m2).
Table 1:
Demographic characteristics of mothers included in analyses1
| Characteristic | Total (n=705) n (%) | GDM (n=60) n (%) | IGT (n=90) n (%) |
|---|---|---|---|
| Study Center | |||
| University of California, San Francisco (UCSF) | 165 (23.4) | 20 (33.3) | 21 (23.3) |
| University of Minnesota (UMN) | 192 (27.2) | 8 (13.3) | 31 (34.4) |
| University of Rochester Medical Center (URMC) | 201 (28.5) | 15 (25) | 25 (27.8) |
| University of Washington (UW) | 131 (18.6) | 17 (28.3) | 11 (12.2) |
| Maternal Age (years) | |||
| <=20 | 21 (2.9) | 1 (1.7) | 2 (2.2) |
| 21–30 | 262 (37.2) | 20 (33.3) | 31 (34.4) |
| 31–40 | 393 (55.7) | 31 (51.7) | 54 (60.0) |
| >40 | 29 (4.1) | 8 (13.3) | 3 (3.3) |
| Pre-pregnancy Body Mass Index (BMI) (kg/m2) | |||
| <=24.9 | 379 (53.8) | 23 (38.3) | 42 (46.7) |
| 25–29.9 | 155 (22.0) | 11 (18.3) | 27 (30.0) |
| >=30 | 159 (22.6) | 24 (40.0) | 21 (23.3) |
| Race/Ethnicity Category | |||
| Non-Hispanic White | 468 (66.4) | 31 (51.7) | 62 (68.9) |
| Non-Hispanic Black | 81 (11.5) | 9 (15.0) | 8 (8.9) |
| Non-Hispanic Asian | 38 (5.4) | 6 (10.0) | 6 (6.7) |
| Hispanic | 55 (7.8) | 9 (15.0) | 5 (5.6) |
| Other/Mixed | 49 (6.9) | 5 (8.3) | 6 (6.7) |
| Highest Education Attended | |||
| High School or less | 97 (13.8) | 8 (13.3) | 11 (12.2) |
| Some College | 85 (12.1) | 7 (11.7) | 13 (14.4) |
| College/post-graduate | 523 (74.2) | 45 (75.0) | 66 (73.3) |
| Any Smoking During Pregnancy | |||
| Yes | 47 (6.7) | 4 (6.7) | 7 (7.8) |
| No | 591 (83.4) | 50 (83.3) | 75 (83.3) |
| Any Alcohol During Pregnancy | |||
| Yes | 91 (12.9) | 7 (11.7) | 10 (11.1) |
| No | 546 (77.4) | 48 (80.0) | 71 (78.9) |
| Infant Sex | |||
| Boy | 323 (45.8) | 33 (55.0) | 44 (48.9) |
| Girl | 345 (48.9) | 27 (45.0) | 41 (45.6) |
| Previous Live Birth | |||
| Yes | 296 (42.0) | 22 (36.7) | 44 (48.9) |
| No | 367 (52.0) | 35 (58.3) | 43 (47.8) |
Summaries provided for entire cohort (total) and also stratified by diagnosis (gestational diabetes (GDM) or impaired glucose tolerance (IGT))
We observed 60 cases of GDM and 90 cases of IGT in the cohort, for overall frequencies of 8.5% and 12.8% respectively. As noted above, these outcomes are mutually exclusive. The prevalence of these conditions varied by race/ethnicity (p = 0.04) (Table A1). GDM was observed in 6.6% of the non-Hispanic white population but in 16.4% of Hispanics, 15.6% of Asians, and 11.1% of Blacks. IGT was observed in 15.6% of the Asian population, compared to 13.2% in the non-Hispanic white population. The mean (SD) glucose concentration in the GLT test was 113.6 (27.7) mg/dL across the cohort; Asians had the highest mean glucose concentrations among race/ethnic subgroups (121.9 (24.8) mg/dL).
Summary statistics on phthalate metabolites and Spearman correlation coefficients are found in Table 2. Within-woman, between-trimester correlations were low, ranging from 0.11 for ∑DEHP to 0.46 for mBzP.
Table 2:
Descriptive data for phthalate metabolites
| Phthalate Metabolite | T11 | T32 | T1 T3 Spearman Correlation7 | ||||
|---|---|---|---|---|---|---|---|
| N | %>LOD3 | GM4 (GSD)5,6 | N | %>LOD | GM (GSD) | ||
| Mono-isobutyl phthalate (MIBP) | 668 | 99 | 5.2 (2.4) | 679 | 95 | 7.2 (2.6) | 0.40 |
| Monoethy 1 phthalate (MEP) | 668 | 100 | 37.6 (3.8) | 679 | 98 | 42.1 (4.5) | 0.40 |
| Mono-n-butyl phthalate (MNBP) | 668 | 95 | 8.3 (2.4) | 679 | 98 | 9.7 (2.7) | 0.27 |
| Mono-benzyl phthalate (MBZP) | 668 | 95 | 4.3 (2.9) | 679 | 95 | 4.8 (3.2) | 0.46 |
| Mono-carboxy-iso-nonyl phthalate (MCNP) | 406 | 96 | 2.7 (2.8) | 679 | 98 | 2.8 (2.7) | 0.15 |
| Mono-carboxy-iso-octyl phthalate (MCOP) | 406 | 100 | 19.3 (3.5) | 679 | 100 | 16.0 (3.3) | 0.27 |
| Mono-(3-carboxypr opyl) phthalate (MCPP) | 668 | 94 | 2.5 (3.6) | 679 | 87 | 2.4 (3.7) | 0.20 |
| Mono-(2-ethylhexyl) phthalate (MEHP) | 668 | 70 | 2.5 (2.5) | 679 | 76 | 2.1 (2.4) | 0.14 |
| Mono-(2-ethyl-5-hydroxyh exyl) phthalate (MEHHP) | 668 | 100 | 7.9 (2.6) | 679 | 99 | 6.7 (2.5) | 0.12 |
| Mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) | 668 | 99 | 5.5 (2.5) | 679 | 100 | 5.3 (2.5) | 0.13 |
| Mono-(2-ethyl-5-carboxype ntyl) phthalate (MECPP) | 668 | 100 | 10.6 (2.4) | 679 | 100 | 12.2 (2.3) | 0.20 |
| ∑Di-2-ethylhexyl phthalate (DEHP) | 668 | N/A | 93.4 (2.3) | 679 | N/A | 47.7 (2.4) | 0.11 |
T1 = First trimester
T3 = Third trimester
LOD = Limit of detection
GM = Geometric mean
GSD = Geometric standard deviation
Units are μg/Lfor all individual phthalates; nmol/mL for ∑DEHP
Within-woman, between-trimester correlations on SG-adjusted phthalates
3.1. Primary & secondary analyses
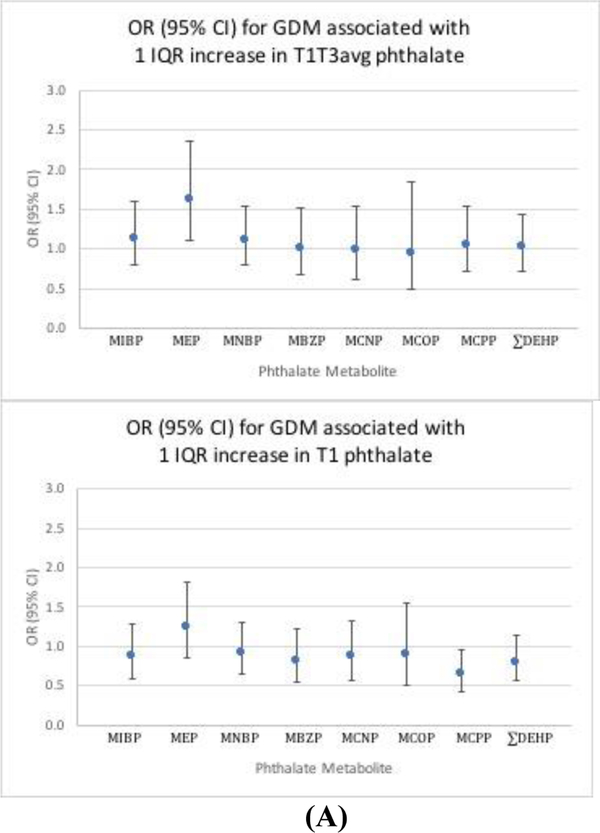
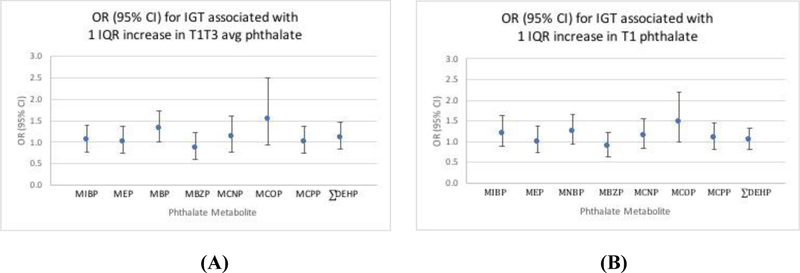
Regression results are presented Figures 1–2 and Table 3, and IQRs are presented in Table A2. Because regression results for individual DEHP metabolites were similar, results are presented for ∑DEHP only. Our primary analysis indicated that T1T3avg MEP was associated with increased odds of GDM (OR (95% CI) per IQR increase: 1.61 (1.10, 2.36)). In secondary analyses, other T1T3avg phthalate metabolites were generally found to have slight positive associations; however, the confidence intervals were wide and overlapped the null, suggesting no overall effect. Most T1 metabolites were estimated to have negative associations; yet with the exception of MCPP ([OR (95% CI) per IQR increase]: (0.64 (0.43, 0.96)), these confidence intervals were also consistent with no effect. Analyses of both exposure metrics of phthalate metabolites with IGT generally suggested slight positive associations; most of these confidence intervals overlapped the null, indicating no effect, with the exception of T1T3avg MNBP ([OR (95% CI) per IQR increase]: 1.32 (1.00, 1.75)). Finally, our analyses suggested positive associations between phthalate metabolites and blood glucose difference for both exposure metrics. However, only the confidence intervals for MCOP excluded the null ((blood glucose difference (95%CI) per IQR increase) T1: 1.91 (0.25, 3.55), T1T3avg: 1.50 (0.02, 2.98)).
Figure 1: Phthalates in association with GDM.
Adjusted odds ratio (OR) and 95% confidence interval (95% CI) for the association between one interquartile range (IQR) increase in urinary phthalate metabolite and gestational diabetes mellitus (GDM).1 A) T1T3avg phthalate metabolite; B) T1 phthalate metabolite
1 Model adjusted for maternal age, maternal BMI, study center, race/ethnicity, parity, and gestational age at glucose testing.
Figure 2: Phthalates in association with IGT.
Adjusted odds ratio (OR) and 95% confidence interval (95% CI) for the association between one interquartile range (IQR) increase in phthalate and IGT.1 A) T1T3avg phthalate metabolite; B) T1 phthalate metabolite
1 Model adjusted for maternal age, maternal BMI, study center, race/ethnicity, parity, and gestational age at glucose testing
Table 3:
Adjusted mean difference in glucose concentration (mg/dL) and 95% CI measured during glucose load test associated with one IQR increase in urinary phthalate metabolites.1
| Phthalate | T1 Estimates | T1 T3avg Estimates |
|---|---|---|
| MIBP | −0.03 (−1.84, 1.79) | 0.19 (−1.26, 1.63) |
| MEP | 0.11 (−1.05, 1.28) | 0.60 (−0.25, 1.44) |
| MNBP | 0.60 (−1.17, 2.37) | 0.88 (−0.62, 2.36) |
| MBZP | −0.19 (−1.74, 1.35) | 0.01 (−1.20, 1.21) |
| MCNP | −0.52 (−2.47, 1.44) | −0.70 (−2.30, 0.91) |
| MCOP | 1.91 (0.25, 3.55) | 1.50 (0.02, 2.98) |
| MCPP | −0.13 (−1.31, 1.05) | 0.09 (−0.85, 1.01) |
| ∑DEHP | 0.29 (−1.51, 2.08) | 0.56 (−0.91, 2.02) |
Model adjusted for maternal age, maternal BMI, study center, race/ethnicity, parity, and gestational age at glucose testing. IQR = interquartile range.
3.2. Sensitivity analyses
Sensitivity analyses excluding individuals with PCOS did not alter results. Race/ethnicity-stratified analyses adjusting for maternal age, maternal BMI, and parity suggested associations between several phthalates on blood glucose among Asians only (Table A3). Quartile analyses did not indicate any non-linear associations (results not shown). Sensitivity analyses excluding women whose T3 urine samples occurred after GLT (n=47) and adjusting for laboratory did not change our estimates (results not shown).
4. DISCUSSION
Our primary finding that T1T3avg MEP is significantly associated with increased odds of GDM (OR (95%CI): 1.61 (1.10, 2.36)) supports our a priori primary analytical hypothesis and previous studies suggesting associations with GDM risk factors and blood glucose.(James-Todd et al. 2018; James-Todd et al. 2016) In secondary analyses, most other phthalates were not found to be associated with study outcomes; however, we did detect a positive association between T1T3avg MNBP and IGT, and between both T1T3avg and T1 MCOP and blood glucose. By contrast, T1 MCPP was found to be inversely associated with GDM.
Previously, five studies had evaluated the association between phthalate exposure and outcomes related to gestational glucose intolerance. It is challenging to draw overall conclusions from this body of work, given their disparate results. Yet, of relevance, MEP has been positively associated with both IGT (James-Todd et al. 2016) and blood glucose (James-Todd et al. 2018), though two other studies observed no association (Fisher et al. 2018; Robledo et al. 2015). Other phthalate metabolites have been estimated to have both negative and positive associations with gestational glucose tolerance outcomes, though many of these estimates had wide confidence intervals overlapping the null (Fisher et al. 2018; James-Todd et al. 2018; James-Todd et al. 2016; Robledo et al. 2015; Shapiro et al. 2015). In the only previous study to assess GDM in relation to phthalates (Shapiro et al. 2015), wide confidence intervals for all metabolites precluded any meaningful conclusions; though some metabolites were associated with increased risk while others were associated with decreased risk (Shapiro et al. 2015). Shapiro et al. evaluated phthalates using only T1 urine samples, which may not capture the relevant exposure period given their short half-life (Fromme et al. 2007). Furthermore, they utilized different criteria for OGTT results, based on Canadian guidelines (Berger et al. 2002) that are less conservative than the classifications we used. These less stringent diagnostic criteria may partially account for their null findings.
The overall prevalence of GDM in our population was 8.5%, consistent with national estimates of 8–9% (DeSisto et al. 2014). The prevalence of GDM was highest in Asians and Hispanic subgroups (15.8% and 16.4%, respectively) (Table A1); national estimates and previous studies have also documented high burdens of metabolic conditions in these subgroups (Ferrara 2007; Thorpe et al. 2005). Concentrations of phthalate metabolites detected in our cohort of pregnant women (Table 2) were similar to those found in nationally representative data from the general population in the 2011–2012 National Health and Nutrition Examination Survey (NHANES) (Centers for Disease Control and Prevention 2018) and for the full TIDES cohort (Swan et al. 2015). We found that within woman, between trimester correlations of SG-adjusted phthalate metabolite concentrations were moderately low, ranging from 0.11 for ∑DEHP metabolites to 0.46 for MBzP (Table 2). Our findings are consistent with previous studies of pregnant populations that have documented low to moderate correlations between phthalates at different timepoints (Adibi et al. 2008; Ferguson et al. 2014a; Valvi et al. 2015), which helped to inform our decision to use T1T3avg phthalates as the primary exposure variable.
Phthalates may alter glucose metabolism through several mechanisms. They can selectively modulate PPARs, altering lipid processing and glucose homeostasis (Desvergne et al. 2009; Grun and Blumberg 2007; Liu and Sun 2016; Sarath Josh et al. 2014; Tordjman et al. 2002). Phthalates are also known endocrine disruptors, linked to changes in sex steroid hormones and related outcomes (Diamanti-Kandarakis et al. 2009), and several phthalates have been found to have estrogenic activity specifically (Harris et al. 1997; Jobling et al. 1995; Sathyanarayana et al. 2017). Substantial evidence indicates that alterations in estrogens are linked to insulin resistance, changes in adipocytes, and related metabolic disruptions in females (Chen et al. 2009; Ding et al. 2007; Liu and Sun 2016; Livingstone and Collison 2002; Louet et al. 2004; Pallottini et al. 2008). In the short term, phthalate-driven elevations in estrogen could lead to increased insulin signaling through an estrogen receptor alpha (ERalpha)-mediated pathway. However, over time, prolonged activation could result in excess insulin release, beta cell exhaustion, and peripheral insulin resistance (Aston-Mourney et al. 2008; Nadal et al. 2009). Some but not all experimental studies support a link between phthalates and abnormal glucose metabolism, including changes in insulin signaling molecules, glucose transporters, glucose uptake, and blood glucose concentrations after exposure (Rajesh et al. 2013; Rengarajan et al. 2007; Srinivasan et al. 2011; Viswanathan et al. 2017). MEP, the a priori focus of our study, may act by estrogenic and/or PPAR-related pathways (Guven et al. 2016).
Certain racial/ethnic groups, particularly Asians, have higher risk of GDM and other metabolic disorders (Ferrara 2007; Thorpe et al. 2005). Sensitivity analyses to evaluate our study question using race-stratification suggested elevated risk among Asians from numerous phthalate metabolites (Table A3). This exploratory finding is interesting given that, while Asians are at highest risk for GDM and other metabolic diseases, they have low rates of obesity, one of the strongest risk factors (Hedderson et al. 2012). Genetic factors may play a role in the observed susceptibility among Asians. The PPARgamma2 polymorphism Pro12Ala is associated with decreased risk of diabetes (Stumvoll and Haring 2002). Due to reduced binding of this variant to PPARgamma-responsive DNA elements, there is altered production and release of adipose factors, including reductions in free fatty acids, TNF-alpha, and resistin –all of which reduce insulin sensitivity –and increases in adiponectin – which improves insulin sensitivity (Stumvoll and Haring 2002). Given that PPARgamma is one possible mode of action of phthalates on insulin resistance, individuals with this variant would be less susceptible to phthalate-mediated insulin resistance. The prevalence of this polymorphism varies by race, with the highest frequencies among Caucasians (~12%) and the lowest among certain Asian groups [e.g. Japanese (~4%), Chinese (%1)] and African Americans (~3%)] (Mori et al. 2001; Stumvoll and Haring 2002; Vigouroux et al. 1998). The low frequency of this protective variant among Asians may confer greater vulnerability to the effects of exposure. Ethnic and cultural differences in diet, particularly with respect to high glycemic foods, also likely play a role (Hiza et al. 2013; Zhang and Ning 2011).
Our research has several limitations. Phthalate metabolites were only measured in two samples per woman, but phthalate concentrations during pregnancy can change considerably from day-to-day (Fisher et al. 2015). Furthermore, MCOP and MCNP were only available on a subset of the population, which reduced our sample size for these analyses. We were unable to adjust for several confounders, including prior GDM in pregnancy, prior T2DM, or family history of T2DM (Kim et al. 2007); diet (Serrano et al. 2014; Zhang and Ning 2011); and/or other exogenous compounds with similar exposure sources also linked to diabetes and metabolic dysfunction, such as bisphenol-A (Song et al. 2015). Additionally, we were underpowered to thoroughly evaluate racial/ethnic subpopulations and did not have detailed information on the different sub-groups within this category, suggesting that a very cautious interpretation of our intriguing findings from sensitivity analyses is warranted. Finally given the uncertainty regarding the critical windows of exposure for GDM, we cannot be certain that exposure assessment preceded initiation of the disease process. Future research to elucidate disease progression can inform the design of epidemiological studies that can more accurately assess exposure during critical windows.
Despite these limitations, our study had important strengths. The TIDES cohort is derived from four centers across the country, providing geographic diversity not present in some prior studies. Furthermore, our study is unique in utilizing phthalate measures from two timepoints during pregnancy. The low within-woman correlation between T1 and T3 phthalates underscores the importance of utilizing multiple exposure measurements. Finally, we also had access to continuous measures of glucose intolerance. These data allowed us to investigate not only the clinical outcome of GDM but also subclinical measures of glucose intolerance linked to adverse pregnancy outcomes (The HAPO Study Cooperative Research Group 2008), but not as thoroughly investigated.
5. CONCLUSION
Overall, this study adds to growing literature on the association between phthalates and gestational glucose intolerance, and in particular provides additional data to support the link between MEP and GDM. There are several mechanisms by which phthalates may affect metabolic function and, given the significant maternal and fetal consequences of hyperglycemia during pregnancy, research should continue to address this subject – especially in susceptible populations.
Highlights.
Growing evidence suggests an association between certain phthalates and diabetes, but few studies have focused on gestational diabetes or impaired glucose tolerance
In agreement with prior studies, we observed an association between elevated mono-ethyl phthalate (MEP) and gestational diabetes
Secondary and sensitivity analyses indicated that some other common phthalates were linked to glucose intolerance, with possible stronger associations in certain racial/ethnic groups
Acknowledgements:
We thank Dr. Sarah Lowry (Seattle Children’s Research Institute) for her statistical programming support during the analysis and Dr. Antonia Calafat (Centers for Disease Control and Prevention) for the coordination and processing of urine sample analyses.
We would also like to thank the TIDES study team: Garry Alcedo (UW), Sabrina Bedell (UMN), Ashley Carter (UW), Sarah Caveglia (URMC), Alana Cordeiro (UCSF), Sarah Evans (Mt. Sinai School of Medicine), Andrea Hart (URMC), Savannah King (UCSF), Ellen Laschansky (UW), Stacey Moe (UMN), Ashley Santilli (UMN), and Simar Singh (UCSF). The TIDES study team was funded by NIEHS R01ES0125169–01.
Finally, we also acknowledge and thank the TIDES study participants who made this study possible.
Funding: RMS is supported by the Seattle Chapter of the ARCS Foundation and NIEHS T32ES015459. TIDES is funded by NIEHS R01ES0125169–01. This research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (ZIA103313), and NIH-NIEHS P30 ES005022. The funding sources had no role in the design, collection, analysis, or interpretation of the data, nor the writing of the publication.
APPENDIX:
A1: Race-specific and overall frequencies of glucose intolerance outcomes in study population1
| Race/Ethnicity | n | GDM (n (%)) | IGT (n (%)) | GLT (mean (SD)(mg/dL)) |
|---|---|---|---|---|
| Non-Hispanic White | 468 | 31 (6.6) | 62 (13.2) | 112.9 (27.1) |
| Non-Hispanic Black | 81 | 9 (11.1) | 8 (9.9) | 108.3 (31.0) |
| Non-Hispanic Asian | 38 | 6 (15.8) | 6 (15.8) | 121.9 (24.8) |
| Hispanic | 55 | 9 (16.4) | 5 (9.1) | 114.6 (29.9) |
| Other/Mixed | 49 | 5 (10.2) | 6 (12.2) | 119.0 (25.7) |
| Missing | 14 | 0 (0) | 3 (21.4) | 118.9 (25.0) |
| Total | 705 | 60 (8.5) | 90 (12.8) | 113.6 (27.7) |
GDM = Gestational diabetes; IGT = impaired glucose tolerance; GLT = glucose load test; SD = standard deviation
A2: Interquartile range (IQR) for ln-transformed phthalate metabolites
| Phthalate | T1 IQR | T1 T3avg IQR |
|---|---|---|
| MIBP | 1.1 | 0.9 |
| MEP | 1.8 | 1.8 |
| MNBP | 1.1 | 0.9 |
| MBZP | 1.4 | 1.3 |
| MCNP | 1.3 | 1.2 |
| MCOP | 1.7 | 1.6 |
| MCPP | 1.6 | 1.5 |
| MEHP | 1.1 | 0.8 |
| MEHHP | 1.0 | 0.9 |
| MEOHP | 1.0 | 0.8 |
| MECPP | 1.0 | 0.8 |
| ∑DEHP | 0.9 | 0.9 |
A3: Race-stratified adjusted mean difference in glucose concentration (mg/dL) in the glucose load test (GLT) associated with one IQR increase in T1T3avg phthalate1
| Phthalate | White (n=413; MCOP/MCNP n=249) |
Black (n=68; MCOP/MCNP n=38) |
Asian (n=35; MCOP/MCNP n=21) |
Hispanic (n=45; MCOP/MCNP n=33) |
|---|---|---|---|---|
| MIBP | −0.78 (−2.49, 0.93) | 0.56 (−3.77, 7.03) | 10.73* (5.29, 14.59) | 0.41 (−5.42, 6.33) |
| MEP | 0.38 (−.62, 1.38) | −0.14 (−2.70, 2.41) | 4.80* (1.39, 8.21) | 0.25 (−2.75, 3.26) |
| MNBP | 0.14 (−1.29, 1.58) | 0.46 (−3.81, 4.73) | 4.60* (0.85, 8.36) | −0.10 (−5.10, 4.90) |
| MBZP | −0.23 (−2.74, 2.28) | −1.79 (−5.37, 1.78) | 10.65 (−0.46, 21.78) | 0.34 (−7.53, 8.22) |
| MCNP | −2.66 (−7.33, 1.99) | .4.41 (−24.54, 15.72) | 1.49 (−11.34, 14.33) | −5.85 (−17.92, 6.21) |
| MCOP | 1.36 (−0.53, 3.24) | 3.96 (−0.80, 8.72) | 0.98 (−5.41, 7.36) | −2.36 (−7.72, 3.00) |
| MCPP | −0.27 (−4.11, 3.56) | 0.17 (−8.34, 8.67) | 11.53 (−9.40, 32.43) | 0.18 (−9.70, 10.05) |
| MEHP | −0.25 (−4.07, 3.56) | 0.35 (−8.45, 9.16) | 7.74 (−6.24, 21.71) | 2.69 (−11.02, 16.42) |
| MEHHP | −0.30 (−1.65, 1.04) | 1.07 (−2.29, 4.43) | 4.24* (0.81, 7.67) | −1.48 (−6.95, 3.98) |
| MEOHP | −0.29 (−1.82, 1.25) | 0.32 (−3.75, 4.39) | 5.26* (0.79, 9.74) | −0.96 (−6.86, 4.95) |
| MECPP | −0.29 (−1.48, 0.90) | 0.73 (−2.48, 3.94) | 3.74* (0.81, 6.67) | −1.51 (−6.87, 3.86) |
| ∑DEHP | −0.004 (−0.69, 0.68) | 0.14 (−2.03, 2.31) | 2.59* (0.41, 4.76) | −0.41 (−2.77, 1.95) |
Model adjusted for maternal age, maternal BMI, and parity. Star denotes statistical significance at the 0.05 alpha level.
IQR = interquartile range.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The views expressed in this publication do not necessarily reflect the views of the funding agencies or the federal government.
Declarations of interest: none
References
- Adibi JJ; Whyatt RM; Williams PL; Calafat AM; Camann D; Herrick R; Nelson H; Bhat HK; Perera FP; Silva MJ; Hauser R Characterization of Phthalate Exposure among Pregnant Women Assessed by Repeat Air and Urine Samples. Environmental Health Perspectives 2008;116:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht SS; Kuklina EV; Bansil P; Jamieson DJ; Whiteman MK; Kourtis AP; Posner SF; Callaghan WM Diabetes trends among delivery hospitalizations in the United States, 1994–2004. Diabetes care 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists (ACOG). ACOG Practice Bulletin No. 190 Summary: Gestational Diabetes Mellitus. Obstet Gynecol 2018;131:406–408 [DOI] [PubMed] [Google Scholar]
- American Diabetes A Standards of medical care in diabetes—2014. Diabetes care 2014;37:S14–S80 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes—2013. Diabetes Care 2012;36:S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Mourney K; Proietto J; Morahan G; Andrikopoulos S Too much of a good thing: why it is bad to stimulate the beta cell to secrete insulin. Diabetologia 2008;51:540–545 [DOI] [PubMed] [Google Scholar]
- Bardenheier BH; Imperatore G; Gilboa SM; Geiss LS; Saydah SH; Devlin HM; Kim SY; Gregg EW Trends in Gestational Diabetes Among Hospital Deliveries in 19 U.S. States, 2000–2010. Am J Prev Med 2015;49:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Haroush A; Yogev Y; Hod M Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabetic Medicine 2003;21:103–113 [DOI] [PubMed] [Google Scholar]
- Berger H; Crane J; Farine D; Armson A; De SLR; Keenan-Lindsay L; Leduc L; Reid G; Van JA Screening for gestational diabetes mellitus. Journal of obstetrics and gynaecology Canada: JOGC= Journal d’obstetrique et gynecologie du Canada: JOGC 2002;24:894–912 [DOI] [PubMed] [Google Scholar]
- Berkowitz GS; Lapinski RH; Wein R; Lee D Race/Ethnicity and Other Risk Factors for Gestational Diabetes. American Journal of Epidemiology 1992;135:965–973 [DOI] [PubMed] [Google Scholar]
- Bouthoorn SH; Silva LM; Murray SE; Steegers EA; Jaddoe VW; Moll H; Hofman A; Mackenbach JP; Raat H Low-educated women have an increased risk of gestational diabetes mellitus: the Generation R Study. Acta Diabetol 2015;52:445–452 [DOI] [PubMed] [Google Scholar]
- Calafat AM Phthalate Metabolites Method 6306.03. Centers for Disease Control and Prevention; Atlanta, GA; 2010 [Google Scholar]
- Carpenter MW; Coustan DR Criteria for screening tests for gestational diabetes. American Journal of Obstetrics & Gynecology 1982;144:768–773 [DOI] [PubMed] [Google Scholar]
- Catalano PM; Nizielski SE; Shao J; Preston L; Qiao L; Friedman JE Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab 2002;282:E522–533 [DOI] [PubMed] [Google Scholar]
- Catalano PM; Tyzbir ED; Sims EAH Incidence and significance of islet cell antibodies in women with previous gestational diabetes. Diabetes care 1990;13:478–482 [DOI] [PubMed] [Google Scholar]
- Caughey AB; Cheng YW; Stotland NE; Washington AE; Escobar GJ Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol 2010;202:616.e611–615 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2018 [Google Scholar]
- Chen JQ; Brown TR; Russo J Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim Biophys Acta 2009;1793:1128–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X; Scholl TO Oxidative stress: changes in pregnancy and with gestational diabetes mellitus. Current diabetes reports 2005;5:282–288 [DOI] [PubMed] [Google Scholar]
- Chu SY; Callaghan WM; Kim SY; Schmid CH; Lau J; England LJ; Dietz PM Maternal Obesity and Risk of Gestational Diabetes Mellitus. Diabetes Care 2007;30:2070. [DOI] [PubMed] [Google Scholar]
- Coughlan MT; Vervaart PP; Permezel M; Georgiou HM; Rice GE Altered placental oxidative stress status in gestational diabetes mellitus. Placenta 2004;25:78–84 [DOI] [PubMed] [Google Scholar]
- Coustan DR; Lowe LP; Metzger BE; Dyer AR The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol 2010;202:654.e651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSisto CL; Kim SY; Sharma AJ Prevalence Estimates of Gestational Diabetes Mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Preventing Chronic Disease 2014;11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B; Feige JN; Casals-Casas C PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol Cell Endocrinol 2009;304:43–48 [DOI] [PubMed] [Google Scholar]
- Di Cianni G; Miccoli R; Volpe L; Lencioni C; Del Prato S Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes/Metabolism Research and Reviews 2003;19:259–270 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E; Bourguignon J-P; Giudice LC; Hauser R; Prins GS; Soto AM; Zoeller RT; Gore AC Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocrine Reviews 2009;30:293–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding EL; Song Y; Manson JE; Rifai N; Buring JE; Liu S Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia 2007;50:2076–2084 [DOI] [PubMed] [Google Scholar]
- Ehrlich S; Lambers D; Baccarelli A; Khoury J; Macaluso M; Ho SM Endocrine Disruptors: A Potential Risk Factor for Gestational Diabetes Mellitus. Am J Perinatol 2016;33:1313–1318 [DOI] [PubMed] [Google Scholar]
- Ferguson KK; McElrath TF; Ko YA; Mukherjee B; Meeker JD Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int 2014a;70:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK; McElrath TF; Meeker JD Environmental phthalate exposure and preterm birth. JAMA Pediatr 2014b;168:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara A Increasing Prevalence of Gestational Diabetes Mellitus. Diabetes Care 2007;30:S141. [DOI] [PubMed] [Google Scholar]
- Fisher BG; Frederiksen H; Andersson A-M; Juul A; Thankamony A; Ong KK; Dunger DB; Hughes IA; Acerini CL Serum Phthalate and Triclosan Levels Have Opposing Associations With Risk Factors for Gestational Diabetes Mellitus. Frontiers in Endocrinology 2018;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M; Arbuckle TE; Mallick R; LeBlanc A; Hauser R; Feeley M; Koniecki D; Ramsay T; Provencher G; Bérubé R Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. Journal of Exposure Science and Environmental Epidemiology 2015;25:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H; Bolte G; Koch HM; Angerer J; Boehmer S; Drexler H; Mayer R; Liebl B Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hyg Environ Health 2007;210:21–33 [DOI] [PubMed] [Google Scholar]
- Grun F; Blumberg B Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord 2007;8:161–171 [DOI] [PubMed] [Google Scholar]
- Guven C; Dal F; Aydogan Ahbab M; Taskin E; Ahbab S; Adin Cinar S; Sirma Ekmekci S; Gulec C; Abaci N; Akcakaya H Low dose monoethyl phthalate (MEP) exposure triggers proliferation by activating PDX-1 at 1.1B4 human pancreatic beta cells. Food Chem Toxicol 2016;93:41–50 [DOI] [PubMed] [Google Scholar]
- Harris CA; Henttu P; Parker MG; Sumpter JP The estrogenic activity of phthalate esters in vitro. Environmental health perspectives 1997;105:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE; Nelson JW; Qureshi MM; Weinberg J; Moore LL; Singer M; Webster TF Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health 2008;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R; Gaskins AJ; Souter I; Smith KW; Dodge LE; Ehrlich S; Meeker JD; Calafat AM; Williams PL Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing in Vitro Fertilization: Results from the EARTH Study. Environ Health Perspect 2016;124:831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedderson M; Ehrlich S; Sridhar S; Darbinian J; Moore S; Ferrara A Racial/Ethnic Disparities in the Prevalence of Gestational Diabetes Mellitus by BMI. Diabetes Care 2012;35:1492–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedderson MM; Gunderson EP; Ferrara A Gestational Weight Gain and Risk of Gestational Diabetes Mellitus. Obstetrics and gynecology 2010;115:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiza HAB; Casavale KO; Guenther PM; Davis CA Diet Quality of Americans Differs by Age, Sex, Race/Ethnicity, Income, and Education Level. Journal of the Academy of Nutrition and Dietetics 2013;113:297–306 [DOI] [PubMed] [Google Scholar]
- Holmes HJ; Lo JY; McIntire DD; Casey BM Prediction of diabetes recurrence in women with class A1 (diet-treated) gestational diabetes. American journal of perinatology 2010;27:047–052 [DOI] [PubMed] [Google Scholar]
- Hsu WC; Araneta MRG; Kanaya AM; Chiang JL; Fujimoto W BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes care 2015;38:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T; Saxena AR; Isganaitis E; James-Todd T Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001–2008. Environ Health 2014;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd T; Stahlhut R; Meeker JD; Powell SG; Hauser R; Huang T; Rich-Edwards J Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect 2012;120:1307–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM; Chiu Y-H; Messerlian C; Mínguez-Alarcón L; Ford JB; Keller M; Petrozza J; Williams PL; Ye X; Calafat AM; Hauser R Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environmental Health 2018;17:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM; Meeker JD; Huang T; Hauser R; Ferguson KK; Rich-Edwards JW; McElrath TF; Seely EW Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ Int 2016;96:118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S; Reynolds T; White R; Parker MG; Sumpter JP A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environmental health perspectives 1995;103:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C; Berger DK; Chamany S Recurrence of Gestational Diabetes Mellitus. Diabetes Care 2007;30:1314. [DOI] [PubMed] [Google Scholar]
- Lao TT; Ho L-F; Chan BCP; Leung W-C Maternal Age and Prevalence of Gestational Diabetes Mellitus. Diabetes Care 2006;29:948. [DOI] [PubMed] [Google Scholar]
- Lappas M; Hiden U; Desoye G; Froehlich J; Hauguel-de Mouzon S; Jawerbaum A The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal 2011;15:3061–3100 [DOI] [PubMed] [Google Scholar]
- Liu S; Sun Q Sex differences, endogenous sex-hormone hormones, sex-hormone binding globulin, and exogenous disruptors in diabetes and related metabolic outcomes. J Diabetes 2016; [DOI] [PubMed] [Google Scholar]
- Livingstone C; Collison M Sex steroids and insulin resistance. Clin Sci (Lond) 2002;102:151–166 [DOI] [PubMed] [Google Scholar]
- Louet J-F; LeMay C; Mauvais-Jarvis F Antidiabetic actions of estrogen: Insight from human and genetic mouse models. Current Atherosclerosis Reports 2004;6:180–185 [DOI] [PubMed] [Google Scholar]
- Mitro SD; Chu MT; Dodson RE; Adamkiewicz G; Chie L; Brown FM; James-Todd TM Phthalate metabolite exposures among immigrants living in the United States: findings from NHANES, 1999–2014. Journal of exposure science & environmental epidemiology 2018:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H; Ikegami H; Kawaguchi Y; Seino S; Yokoi N; Takeda J; Inoue I; Seino Y; Yasuda K; Hanafusa T The Pro12→ Ala substitution in PPAR-γ is associated with resistance to development of diabetes in the general population: possible involvement in impairment of insulin secretion in individuals with type 2 diabetes. Diabetes 2001;50:891–894 [DOI] [PubMed] [Google Scholar]
- Morisset AS; Tchernof A; Dube MC; Veillette J; Weisnagel SJ; Robitaille J Weight gain measures in women with gestational diabetes mellitus. J Womens Health (Larchmt) 2011;20:375–380 [DOI] [PubMed] [Google Scholar]
- Nadal A; Alonso-Magdalena P; Soriano S; Quesada I; Ropero AB The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol 2009;304:63–68 [DOI] [PubMed] [Google Scholar]
- National Diabetes Data G Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- National Research Council. Phthalates and Cumulative Risk Assessment The Task Ahead ed^eds. Washington, DC: The National Academies Press; 2008 [PubMed] [Google Scholar]
- Pallottini V; Bulzomi P; Galluzzo P; Martini C; Marino M Estrogen regulation of adipose tissue functions: involvement of estrogen receptor isoforms. Infectious Disorders-Drug Targets (Formerly Current Drug Targets-Infectious Disorders) 2008;8:52–60 [DOI] [PubMed] [Google Scholar]
- Rajesh P; Sathish S; Srinivasan C; Selvaraj J; Balasubramanian K Phthalate is associated with insulin resistance in adipose tissue of male rat: role of antioxidant vitamins. J Cell Biochem 2013;114:558–569 [DOI] [PubMed] [Google Scholar]
- Reece EA The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med 2010;23:199–203 [DOI] [PubMed] [Google Scholar]
- Rengarajan S; Parthasarathy C; Anitha M; Balasubramanian K Diethylhexyl phthalate impairs insulin binding and glucose oxidation in Chang liver cells. Toxicology in Vitro 2007;21:99–102 [DOI] [PubMed] [Google Scholar]
- Robledo CA; Peck JD; Stoner J; Calafat AM; Carabin H; Cowan L; Goodman JR Urinary phthalate metabolite concentrations and blood glucose levels during pregnancy. International Journal of Hygiene and Environmental Health 2015;218:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarath Josh MK; Pradeep S; Vijayalekshmi Amma KS; Balachandran S; Abdul Jaleel UC; Doble M; Spener F; Benjamin S Phthalates efficiently bind to human peroxisome proliferator activated receptor and retinoid X receptor alpha, beta, gamma subtypes: an in silico approach. J Appl Toxicol 2014;34:754–765 [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S Phthalates and children’s health. Curr Probl Pediatr Adolesc Health Care 2008;38:34–49 [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S; Butts S; Wang C; Barrett E; Nguyen R; Schwartz SM; Haaland W; Swan SH Early Prenatal Phthalate Exposure, Sex Steroid Hormones, and Birth Outcomes. J Clin Endocrinol Metab 2017;102:1870–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA; Janevic TM; Engel SM; Kaufman JS; Herring AH Ethnicity and gestational diabetes in New York City, 1995–2003. Bjog 2008;115:969–978 [DOI] [PubMed] [Google Scholar]
- Schettler T Human exposure to phthalates via consumer products. Int J Androl 2006;29:134–139; [DOI] [PubMed] [Google Scholar]
- Serrano SE; Braun J; Trasande L; Dills R; Sathyanarayana S Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 2014;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GD; Dodds L; Arbuckle TE; Ashley-Martin J; Fraser W; Fisher M; Taback S; Keely E; Bouchard MF; Monnier P; Dallaire R; Morisset A; Ettinger AS Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ Int 2015;83:63–71 [DOI] [PubMed] [Google Scholar]
- Silva MJ; Samandar E; Preau JL Jr.; Reidy JA; Needham LL; Calafat AM Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 2007;860:106–112 [DOI] [PubMed] [Google Scholar]
- Simmons D; Devers MC; Wolmarans L; Johnson E Difficulties in the use of risk factors to screen for gestational diabetes mellitus. Diabetes Care 2009;32:e8–e8 [DOI] [PubMed] [Google Scholar]
- Song Y; Chou Elizabeth L; Baecker A; You Nai‐Chieh Y; Song Y; Sun Q; Liu S Endocrine‐disrupting chemicals, risk of type 2 diabetes, and diabetes‐related metabolic traits: A systematic review and meta‐analysis. Journal of Diabetes 2015;8:516–532 [DOI] [PubMed] [Google Scholar]
- Srinivasan C; Khan AI; Balaji V; Selvaraj J; Balasubramanian K Diethyl hexyl phthalate-induced changes in insulin signaling molecules and the protective role of antioxidant vitamins in gastrocnemius muscle of adult male rat. Toxicol Appl Pharmacol 2011;257:155–164 [DOI] [PubMed] [Google Scholar]
- Stahlhut RW; van Wijngaarden E; Dye TD; Cook S; Swan SH Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult US males. Environmental health perspectives 2007:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumvoll M; Haring H The peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism. Diabetes 2002;51:2341–2347 [DOI] [PubMed] [Google Scholar]
- Swan SH; the TST; Sathyanarayana S; Barrett ES; Janssen S; Liu F; Nguyen RHN; Redmon JB First trimester phthalate exposure and anogenital distance in newborns. Human Reproduction 2015;30:963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcomes. New England Journal of Medicine 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- Thorpe LE; Berger D; Ellis JA; Bettegowda VR; Brown G; Matte T; Bassett M; Frieden TR Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990–2001. American journal of public health 2005;95:1536–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman K; Standley KN; Bernal-Mizrachi C; Leone TC; Coleman T; Kelly DP; Semenkovich CF PPARalpha suppresses insulin secretion and induces UCP2 in insulinoma cells. J Lipid Res 2002;43:936–943 [PubMed] [Google Scholar]
- Torloni MR; Betrán AP; Horta BL; Nakamura MU; Atallah AN; Moron AF; Valente O Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obesity Reviews 2009;10:194–203 [DOI] [PubMed] [Google Scholar]
- Toulis KA; Goulis DG; Kolibianakis EM; Venetis CA; Tarlatzis BC; Papadimas I Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertil Steril 2009;92:667–677 [DOI] [PubMed] [Google Scholar]
- Valvi D; Monfort N; Ventura R; Casas M; Casas L; Sunyer J; Vrijheid M Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int J Hyg Environ Health 2015;218:220–231 [DOI] [PubMed] [Google Scholar]
- Vandorsten JP; Dodson WC; Espeland MA; Grobman WA; Guise JM; Mercer BM; Minkoff HL; Poindexter B; Prosser LA; Sawaya GF NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH consensus and state-of-the-science statements 2012;29:1–31 [PubMed] [Google Scholar]
- Vigouroux C; Fajas L; Khallouf E; Meier M Human peroxisome proliferator-activated receptor-gamma2: genetic mapping, identification of a variant in the coding sequence, and exclusion as the gene responsible for lipoatrophic diabetes. Diabetes 1998;47:490. [DOI] [PubMed] [Google Scholar]
- Viswanathan MP; Mullainadhan V; Chinnaiyan M; Karundevi B Effects of DEHP and its metabolite MEHP on insulin signalling and proteins involved in GLUT4 translocation in cultured L6 myotubes. Toxicology 2017;386:60–71 [DOI] [PubMed] [Google Scholar]
- Wendland EM; Pinto ME; Duncan BB; Belizán JM; Schmidt MI Cigarette smoking and risk of gestational diabetes: a systematic review of observational studies. BMC pregnancy and childbirth 2008;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler G; Cseh K; Baranyi É; Melczer Z; Speer G; Hajós P; Salamon F; Turi Z; Kovács M; Vargha P; Karádi I Tumor necrosis factor system in insulin resistance in gestational diabetes. Diabetes Research and Clinical Practice 2002;56:93–99 [DOI] [PubMed] [Google Scholar]
- Zhang C; Ning Y Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. The American Journal of Clinical Nutrition 2011;94:1975S–1979S [DOI] [PMC free article] [PubMed] [Google Scholar]