Abstract
The persistence of measurable residual disease (MRD) following induction chemotherapy is the single most powerful prognostic factor available to clinicians treating patients with acute myeloid leukemia (AML). How to use this information to guide subsequent therapy is complex, and influenced by the category of AML being treated, the assays used to measure MRD, MRD levels and kinetics, and the spectrum of therapies available to the patient. In this literature-based review, each of these issues will be discussed, with a particular emphasis on the role of hematopoietic cell transplantation in the treatment of MRD-positive patients.
Keywords: acute myeloid leukemia, AML, core binding factor, hematopoietic cell transplantation, HCT, measurable residual disease, MFC, MRD, multi-parameter flow cytometry, next generation sequencing, NGS, NPM1, reverse transcriptase polymerase chain reaction, RT-PCR
Introduction
The persistence of measureable residual disease (MRD) in patients with acute myeloid leukemia (AML) who have entered an initial complete remission is the single most powerful prognostic factor available to clinicians. Yet, exactly how to use the information provided by tests measuring MRD remains unclear. This uncertainty is a product of the complexity of the problem, but a number of answers are starting to come into focus.
The central questions at hand are whether specific patients with MRD after consolidation therapy benefit from allogeneic hematopoietic cell transplant (HCT), and if so, how can that benefit best be achieved. Major factors that enter into the complexity surrounding these questions include: (1) disease and patient specific characteristics, (2) assays used to measure MRD, (3) MRD levels and kinetics, (4) method of transplantation, and (5) alternative approaches available.
Concerning first disease and patient characteristics, the European Leukemia Net (ELN) criteria, which combine cytogenetic and mutational data, readily categorize AML patients into favorable, intermediate, and unfavorable prognostic groups [1]. It’s hardly surprising that the relative benefit of HCT in patients with and without MRD might differ among prognostic groups. Similarly, it would be expected that the relative benefit of an aggressive intervention like HCT might differ depending on the expected tolerance of the individual patient to the intervention [2].
In addition to disease and patient heterogeneity, there is considerable variability among assays used to measure MRD. The two most common methods employed have been reverse transcriptase polymerase-chain reaction (RT-PCR)-based assays and multi-dimensional flow cytometry (MFC) [3]. Both have individual strengths, and both have added to our prognostic prowess, but the variety of assays used has added to the difficulty of reaching firm recommendations about application to therapeutic decision making. Further complexity comes from recent studies demonstrating that next generation sequencing (NGS)-based assays provide yet additional prognostic information [4]. In addition to the assay employed, both the level of MRD and the kinetics of its rise, fall, or persistence are of prognostic importance.
The impact of HCT in the care of MRD+ patients may vary depending on the choice of donor and perhaps the preparative regimen used. Several studies suggest that the impact of the presence of MRD prior to HCT is mitigated if cord blood is used as the source of stem cells [5]. In contrast, it is as yet unclear how other transplant approaches, including intensity of the preparative regimen and form of graft-versus-host disease prophylaxis might differentially impact patients with and without MRD.
Finally, whether patients with MRD should proceed directly to transplant depends on the alternative possibilities. These alternatives range from only considering HCT if and when a patient relapses, to attempting interventions to eradicate the MRD prior to proceeding to transplantation. In the following discussion, each of these issues will be briefly considered.
Impact of MRD by disease category
RT-PCR for core binding factor (CBF) AML
The impact of the presence of MRD after consolidation in patients with CBF AML treated on the Medical Research Council (MRC) AML-15 trial was investigated by Liu Yin et al [6]. Using a quantitative RT-PCR assay, they were able to identify levels of MRD in the peripheral blood (100 copies of the transcript for t(8;21) and 10 copies for inv(16), both normalized to 105 ABL copies ) that were associated with a 100% incidence of subsequent relapse if patients were not transplanted in first remission. Similarly, Jourdan writing for the French AML Intergroup, identified that a less than 3 log reduction in MRD was associated with a far higher relapse rate (54%) than seen in those with greater degrees of reduction (22%) [7].
While both of these studies identify patients with a very high probability of subsequent relapse, neither formally tested whether transplanting such patients in first complete remission (CR) would necessarily improve outcome. The only study to prospectively test this approach comes from the Chinese AML-05 study, which was a prospective risk-directed trial for patients with t(8;21) AML and reported improved survival with HCT compared to chemotherapy (71.6% vs 26.7% at 3 years) in patients deemed high risk by virtue of MRD status [8]. While there were many limitations to the AML-05 study, it does seem reasonable that if one can identify AML patients with a close to 100% probability of disease recurrence, transplantation while in first remission should be considered.
RT-PCR for NPM1-mutated AML
Using an RT-PCR assay for NPM1 mutations, Ivey et al showed that NPM1-mutated transcripts persisted in the blood in 15% of patients in CR after the second chemotherapy cycle on the British AML-17 trial, and that such persistence was associated with a far greater risk of subsequent relapse (82% vs 30%) and a lower survival at 3 years (24% vs 75%) [9]. Balsat et al have published similar data from the Acute Leukemia French Association (ALFA) 0702 study that show that failure to achieve a 4-log reduction in NPM1 transcripts predicts a higher incidence of subsequent relapse and shorter overall survival [10]. They further report that among patients with NPM1-mutated AML who had <4-log reduction in peripheral blood MRD, disease-free survival and overall survival were significantly improved if patients underwent allogeneic HCT in first remission (overall survival 55% with HCT versus 10% without, P= 0.047).
Multi-parameter flow cytometry (MFC) for all AML categories
RT-PCR assays are currently restricted to AML cases with certain translocations and NPM1-mutations, which combined make up perhaps 50% of patients less than age 60, and a substantially smaller proportion of older AML patients [3]. In contrast, MFC-based methods, although perhaps less sensitive and more difficult to standardize, are applicable to almost all cases of AML. A number of studies have documented the prognostic power of MFC-based MRD determination in adult AML. For example, we analyzed data from 245 adults with AML treated with contemporary high-dose induction regimens [11]. Among those with MRD following induction, the relapse rates approached 100% if patients were not taken to allogeneic HCT (Figure 1).
Figure 1.
Impact of response to induction (CR versus CRi) and persistence of MRD as determined by MFC on outcome of therapy in 109 patients with AML treated without transplantation [11]. As shown, the relapse rates ranged from 90-100% among those with persistence MRD and/or incomplete count recovery.
Unfortunately, presence of MRD in patients taken to allogeneic transplant is also highly predictive of outcome [12]. While the relapse for such patients is not 100%, it does appear to approach 60%, although as will be explained, it may be possible to reduce the impact of pre-transplant MRD. Similar findings about the impact of MFC detection of MRD has been published by others, including a recent report from Freeman et al concerning the British AML-17 trial [13]. In their study, they found that presence of MFC-detected MRD was highly predictive of overall survival, and that allogeneic transplant benefit was more apparent in MRD+ than in MRD− patients.
Impact of next generation sequencing
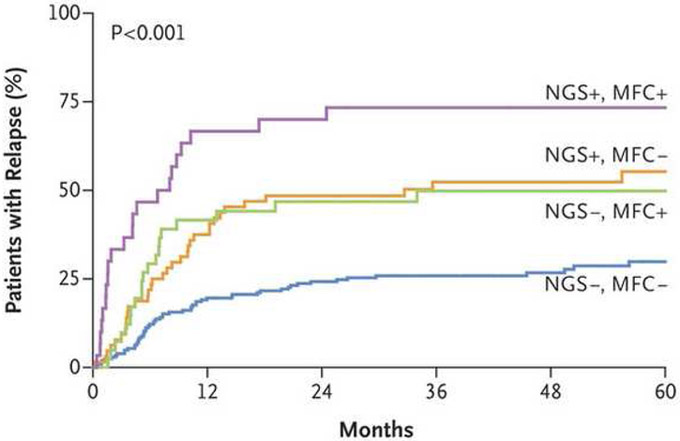
The Dutch group recently presented data showing that next generation sequencing (NGS) using a 54 gene panel could detect mutations in about 90% of AML cases, and that in about half of these, mutations persisted once patients entered CR at allelic frequencies ranging from 0.02% to 47% [4]. Persistent DNMT3A, TET2 or ASXL1 mutations were of no significance, but persistence of all other mutations was associated with an increased risk of disease recurrence. The information provided by NGS was complementary to that provided by MFC (Figure 2). Approximately 50% of the patients described in this study underwent allogeneic HCT in first remission, with a significant decrease in relapse rates and increase in relapse-free survival in the transplanted group.
Figure 2.
Rates of relapse among 340 patients with AML based on persistence of MRD as detected by NGS and/or MFC [4].
From The New England Journal of Medicine, Jongen-Lavrencic M, Grob T, Hanekamp D, et al. Molecular minimal residual disease in acute myeloid leukemia, Volume No. 378, Page Nos 1189-99. Copyright © 2018 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Similar results were published by Morita et al showing that relapse rates were significantly higher among those who failed to clear somatic mutations once in remission, and that this impact was restricted to non- DNMT3A, TET2, ASXL1 mutations [14]. In their report, Morita include an analysis showing that allogeneic HCT was of significant benefit among those who failed to clear somatic mutations after initial induction/consolidation.
Approach to the MRD+ patient
Although differences may exist depending on disease population and specific assay employed, the studies reviewed above are consistent in that they show that presence of MRD following consolidation predicts for a poor outcome, and although definitive prospective trials are lacking, retrospective analyses suggest that allogeneic HCT may be of benefit for such patients. This then leads to a series of questions: (1) Should patients go directly to HCT or should further efforts be made to clear MRD before proceeding to HCT? (2) Is one donor source preferable to others? (3) Does the presence of MRD affect the choice of preparative regimen? (4) Do methods exist to prevent post-transplant relapse?
Conversion of MRD+ to MRD−
Although there are few detailed studies sequentially measuring MRD levels in AML after each cycle of therapy, those that do exist suggest that the major impact of standard chemotherapy on disease burden occurs within the first two cycles of post-remission therapy, and there appears to be little further reduction in disease burden with subsequent cycles of conventional chemotherapy [9, 15]. Studies are now exploring the concept of taking patients with MRD+ CRs and treating them with therapies specifically selected to eliminate MRD prior to transplant, but whether this approach will be successful in eliminating MRD, and whether such elimination will impact subsequent transplant outcomes is unknown. The setting where an AML patient has known residual disease, a transplant is planned, and a donor has already been identified, may provide a unique situation in which therapies likely associated with profound and prolonged myelosuppression could be tested, with the idea that once MRD was eliminated the patient could proceed directly to transplant.
Donor selection for MRD+ patients
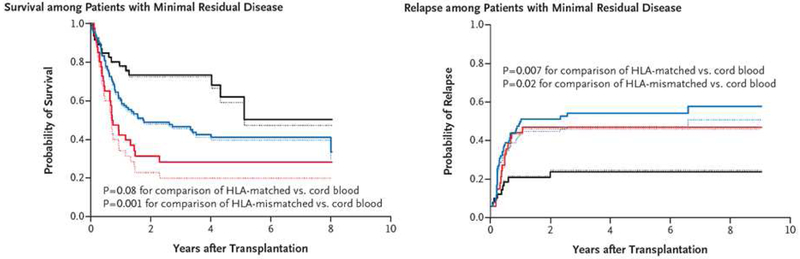
In those cases where it has been studied, the graft-versus-leukemia effect appears to be equally present in MRD-positive and MRD-negative patients [16]. We and others have previously reported that the graft-versus-leukemia effect may be more pronounced after cord blood transplantation compared to use of other donors [17]. More recently, we conducted a retrospective analysis of transplant outcomes in 582 consecutive patients with acute leukemia transplanted in Seattle following a myeloablative preparative regimen from an unrelated cord donor (n=140), an HLA-matched unrelated donor (n=344), or an HLA-mismatched donor (n=98) [5]. Survival was similar following cord blood and matched unrelated donor recipients, but somewhat worse in recipients of mismatched unrelated donors. In patients without MRD, outcomes were again similar in cord blood and matched unrelated donor recipients, but in patients with MRD at the time of transplant, the risk of relapse was significantly higher in the two unrelated-donor groups than in the cord blood group (hazard ratio 3.01, P=0.02) leading to improved overall outcomes with cord blood (Figure 3). In fact, in cord blood recipients, there was no detectable impact of MRD on transplant outcomes.
Figure 3.
Unadjusted and adjusted estimates of overall survival and relapse among 184 patients with MRD who underwent a myeloablative transplant with cord blood, HLA-matched unrelated or HLA-mismatched unrelated stem cell sources [5].
"From The New England Journal of Medicine, Milano F, Gooley T, Wood B, et al. Cord-blood transplantation in patients with minimal residual disease. Volume No. 375, Page No s 944-53. Copyright © 2016 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society."
Preparative regimen choice for MRD+ patients
The recently published results of BMT CTN 0901 suggest that, for patients with AML younger than age 60 with adequate performance status, myeloablative preparative regimens are preferred over reduced intensity regimens [18]. Measurements of MRD were not reported in that study. One might imagine that the importance of dose intensity would, if anything, be greater among those entering transplant with MRD. While prospective randomized trials testing this hypothesis are lacking, retrospective studies are at least consistent with this notion [19].
Prevention of post-transplant relapse
Because patients entering transplant with MRD are at increased risk of relapse, there has been intense interest in developing methods to try and prevent this occurrence. Unfortunately, there are as yet no randomized trials demonstrating a benefit of any post-transplant intervention (reviewed in [20]). While FLT3 inhibitors, histone deacetylase (HDAC) inhibitors, and lenalidomide all have been studied to assess safety and feasibility, randomized trials are lacking. Particularly exciting are newer immunologically based therapies [21].
Conclusion
Although the precise implications vary depending on disease category and method of detection, the persistence of measurable residual disease in patients with AML in otherwise complete remission is the most powerful predictor of the subsequent behavior of the disease available to the clinician. While the prognostic implications of MRD measurement are undisputed, there is less agreement about their use. The multiplicity of disease categories and available assays has been an impediment to the conduct of prospective randomized trials vigorously testing the utility of these assays in directing therapeutic interventions. Nonetheless, the available retrospective data are consistent in showing that patients who are MRD positive after consolidation therapy do very poorly if further treatment is restricted to chemotherapy, and that taking such patients to allogeneic transplantation appears to reduce their risk of subsequent relapse with a resulting improvement in disease-free and possibly overall survival. The preliminary data suggesting that MRD-positive patients specifically benefit from the use of cord blood as a stem cell source and from the receipt of intensive preparative regimens need to be confirmed by additional studies. Effective therapies that eradicate MRD prior to transplant and prevent post-transplant disease recurrence are badly needed.
Footnotes
Disclosure:
Consulting fees: Igenica, Adaptive Biotechnologies; Ownership interest: Igenica, Adaptive Biotechnologies
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sorror ML, Storer BE, Fathi AT, Gerds AT, Medeiros BC, Shami P, et al. Development and validation of a novel acute myeloid leukemia-composite model to estimate risks of mortality. JAMA Oncol 2017;3:1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for "prime time"? Blood 2014;124:3345–55. [DOI] [PubMed] [Google Scholar]
- [4].Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med 2018;378:1189–99. [DOI] [PubMed] [Google Scholar]
- [5].Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med 2016;375:944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yin JA, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood 2012;120:2826–35. [DOI] [PubMed] [Google Scholar]
- [7].Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood 2013;121:2213–23. [DOI] [PubMed] [Google Scholar]
- [8].Zhu HH, Zhang XH, Qin YZ, Liu DH, Jiang H, Chen H, et al. MRD-directed risk stratification treatment may improve outcomes of t(8.21) AML in the first complete remission: results from the AML05 multicenter trial. Blood 2013;121:4056–62. [DOI] [PubMed] [Google Scholar]
- [9].Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med 2016;374:422–33. [DOI] [PubMed] [Google Scholar]
- [10].Balsat M, Renneville A, Thomas X, de Botton S, Caillot D, Marceau A, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: A study by the Acute Leukemia French Association Group. J Cliln Oncol 2017;35:185–93. [DOI] [PubMed] [Google Scholar]
- [11].Chen X, Xie H, Wood BL, Walter RB, Pagel JM, Becker PS, et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol 2015;33:1258–64. [DOI] [PubMed] [Google Scholar]
- [12].Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol 2016;34:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Freeman SD, Hills RK, Virgo P, Khan N, Couzens S, Dillon R, et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol 2018;36:1486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Morita K, Kantarjian HM, Wang F, Yan Y, Bueso-Ramos C, Sasaki K, et al. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J Clin Oncol 2018;36:1788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maurillo L, Buccisano F, Del Principe MI, Del Poeta G, Spagnoli A, Panetta P, et al. Toward optimization of postremission therapy for residual disease-positive patients with acute myeloid leukemia. J Clin Oncol 2008;26:4944–51. [DOI] [PubMed] [Google Scholar]
- [16].Versluis J, Kalin B, Zeijlemaker W, Passweg J, Graux C, Manz MG, et al. Graft-versus-leukemia effect of allogeneic stem-cell transplantation and minimal residual disease in patients with acute myeloid leukemia in first complete remission. JCO Precision Oncology 2017:1–13. [DOI] [PubMed] [Google Scholar]
- [17].Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematological malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010;116:4693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol 2017;35:1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Festuccia M, Deeg HJ, Gooley TA, Baker K, Wood BL, Fang M, et al. Minimal identifiable disease and the role of conditioning intensity in hematopoietic cell transplantation for myelodysplastic syndrome and acute myelogenous leukemia evolving from myelodysplastic syndrome. Biol Blood Marrow Transplant 2016;22:1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rashidi A, Walter RB, Tallman MS, Appelbaum FR, DiPersio JF. Maintenance therapy in acute myeloid leukemia: an evidence-based review of randomized trials. Blood 2016;128:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med 2013;5:174ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]