Abstract
Background:
Mesolimbic dopamine system dysfunction is believed to contribute to major depressive disorder (MDD), but molecular neuroimaging of striatal dopamine neurotransmission has yielded mixed results, possibly due to limited sensitivity of antagonist radioligands used with positron emission tomography (PET) to assess dopamine release capacity. This study used an agonist radioligand with agonist challenge to assess dopamine release capacity and D2/D3 receptor availability in MDD.
Methods:
Twenty-six treatment-naïve adults with MDD, and 26 healthy comparison participants underwent functional magnetic resonance imaging during a probabilistic reinforcement task, and PET with the D3-preferring ligand [11C]-(+)-PHNO, before and after oral dextroamphetamine. MDD participants then received pramipexole treatment for 6 weeks.
Results:
MDD participants had trend-level greater ΔBPND (a measure of dopamine release capacity) in the ventral striatum (−34% vs. −30%, p = .072, d = .58) but no difference in D2/D3 receptor availability (BPND). Striatal and extrastriatal BPND and ΔBPND were not significantly associated with blood oxygen level dependent response to reward prediction error in ventral striatum, severity of depression and anhedonia, or antidepressant response to pramipexole (response rate = 72.7%).
Conclusions:
[11C]-(+)-PHNO demonstrated high sensitivity to displacement by amphetamine-induced dopamine release, but dopamine release capacity and D2/D3 availability were not associated with ventral striatal activation to reward prediction error or clinical features, in this study powered to detect large effects. While a preponderance of indirect evidence implicates dopaminergic dysfunction in MDD, these findings suggest presynaptic dopamine dysregulation may not be a feature of MDD or a prerequisite for treatment response to dopamine agonists.
https://clinicaltrials.gov/ct2/show/NCT02033369 Registration number: NCT02033369
Keywords: positron emission tomography, functional magnetic resonance imaging, major depressive disorder, dopamine, [11C]-(+)-PHNO, pramipexole
Introduction
Major depressive disorder (MDD) is often characterized by anhedonia or low reward motivation (1), features that predict poor treatment response (2), particularly to selective serotonin reuptake inhibitors (SSRIs) (3,4). Better understanding of neurobiological processes underlying motivational symptoms in MDD has the potential to improve personalization of treatment.
Converging evidence implicates dysfunction of the mesolimbic dopamine (DA) system in reward-related deficits that have been associated with MDD. Preclinical studies show that phasic DA learning signals in midbrain and striatum mediate the ability to anticipate, learn from, and integrate reward information (5–9). In healthy volunteers, functional magnetic resonance imaging (fMRI) studies support striatal involvement in reward processing and reinforcement learning (10,11). Positron emission tomography (PET) studies of individual differences in healthy subjects have found associations of DA receptor availability and/or DA release with self-report and behavioral measures of reward learning and motivation (12–18).
In MDD, low motivation has been linked to impairment in integrating reinforcement over time and adapting behavior (19–22). Some fMRI studies have reported diminished striatal reactivity during reward anticipation and reward prediction error in MDD (23–26), though others have not (27). While these fMRI findings indirectly implicate DA dysfunction in MDD, more direct assessment of DA is needed to support this association with MDD and motivational deficits. DA agonist challenge has been shown to enhance striatal response during reward learning in MDD (28) and healthy subjects (29–31). Other evidence comes from animal models of depression (32), and from MDD studies of DA depletion (33) and antidepressant response to DA agonists. The DA agonist that has been most extensively studied for depression treatment is pramipexole (34–40), a D3-preferring agonist that increases dopaminergic transmission (41,42).
Despite evidence for DA dysfunction in MDD, molecular neuroimaging using PET or single photon emission computed tomography (SPECT) – among the most direct approaches for assessing DA function in the living human brain – has yielded mixed findings. Of six PET and seven SPECT studies assessing striatal D2/D3 receptor availability in MDD relative to healthy comparison subjects (HC), four reported receptor availability to be greater in MDD (43–46), one less in MDD (47), and eight reported no difference (48–55). Two studies of amphetamine-induced DA release in MDD (one PET study (49) and one SPECT study (56)) also found no difference. These studies have been variously limited, however, by use of D2/D3 antagonist ligands, limited assessment of anhedonia or reward motivation, and variability in antidepressant exposure, substance use, and in females, menstrual status.
[11C]-(+)-PHNO is a D2/D3 agonist radioligand with potentially advantageous features for studying MDD. Because [11C]-(+)-PHNO is D3-preferring, it permits measurement of D3 availability in regions where D3 receptors predominate, such as substantia nigra (57,58). D3 receptors, which are preferentially distributed in the mesolimbic DA system, are believed to be important for affective processes (59). As an agonist, [11C]-(+)-PHNO binds only to high affinity state receptors and is more sensitive to displacement by endogenous DA relative to antagonists, resulting in greater power to assess amphetamine-induced displacement as a measure of DA release capacity (60–61).
The aim of this study, the first to use [11C]-(+)-PHNO in MDD, was to capitalize on the sensitivity of this radioligand to test whether depression is associated with abnormal striatal DA release. We hypothesized that DA release in the ventral striatum would be decreased in MDD. We also investigated associations of PET measures of D2/D3 availability and DA release capacity with other indicators of DA function, including ratings of motivational anhedonia, fMRI assessment of ventral striatal response to reward prediction error, and symptomatic response to pramipexole treatment.
Methods and Materials
Participants:
Participants were recruited from research clinics at New York State Psychiatric Institute and Icahn School of Medicine at Mount Sinai between April 2014 and August 2016. Diagnoses were assessed by clinical interview and confirmed using the Structured Clinical Interview for DSM-IV (62). Medical screenings included history and physical examination, blood and urine tests including urine toxicology, electrocardiogram, and structural MRI of the brain. Plasma estradiol and progesterone levels were obtained for females on the PET imaging day. MDD participants had a current major depressive episode without psychotic features, a Hamilton Rating Scale for Depression (63) 17-item score of 17–28, <2 weeks of lifetime psychiatric medication (none for past 3 months), and no lifetime psychotic, bipolar, attention deficit, or substance use disorders (including nicotine). HC participants had no lifetime psychiatric disorders and were matched for age, sex, and race/ethnicity (see Table 1). All participants had no tobacco or illicit substance use for 3 months, no family history of schizophrenia, were medically healthy, and were not pregnant, nursing, postmenopausal, or using hormonal contraception. This study was approved by institutional review boards of New York State Psychiatric Institute and Icahn School of Medicine at Mount Sinai, and participants provided written informed consent.
Table 1:
Demographics, Clinical Features, and PET Scan Parameters
| Mean (SD) | ||||
|---|---|---|---|---|
| HC (n=20) | MDD (n=20) | P valuea | ||
| Demographics | ||||
| Age, yrs | 26.9 (5.4) | 26.8 (6.9) | .95 | |
| Sex, N (%) | ||||
| Female | 10 (50) | 10 (50) | .00 | |
| Male | 10 (50) | 10 (50) | ||
| Race, N (%) | ||||
| White | 8 (40) | 8 (40) | .95 | |
| African American | 3 (15) | 4 (20) | ||
| Asian | 3 (15) | 2 (10) | ||
| Other | 6 (30) | 6 (30) J | ||
| Ethnicity, N (%) | ||||
| Hispanic | 9 (45) | 6 (30) | .33 | |
| Non-Hispanic | 11 (55) | 14 (70) | ||
| Education, yrs | 15.3 (1.6) | 14.6 (1.4) | .13 | |
| NAART (estimated verbal IQ) | 111.3 (8.9) | 112.1 (7.5) | .75 | |
| Body Mass Index | 25.2 (5.1) | 24.9 (5.0) | .87 | |
| Edinburgh Handedness LQ | 67.8 (42.5) | 62.1 (39.3) | .67 | |
| Clinical Features | ||||
| MDE specifiers, N (%) | ||||
| With Melancholic Features | NA | 0 (0) | ||
| With Atypical Features | NA | 4 (20) | ||
| Comorbid anxiety disorder, N (%) | NA | 14 (70) | ||
| Age onset MDD, yrs | NA | 16.8 (7.0) | ||
| Hamilton Rating Scale for Depression, 17-item total | 0.2 (0.4) | 20.3 (2.5) | <.001 | |
| MASQ - Anxious Arousal | 19.2 (2.8) | 26.1 (7.3) | .001 | |
| MASQ - Anhedonic Depression | 39.0 (9.8) | 82.4 (10.7) | <.001 | |
| MASQ - General Distress Anxious | 13.2 (2.6) | 24.4 (6.8) | <.001 | |
| MASQ - General Distress Depressive | 14.5 (2.9) | 40.2 (11.2) | <.001 | |
| MASQ - Total | 87.8 (13.4) | 173.0 (27.1) | <.001 | |
| Snaith Hamilton Pleasure Scale | 19.5 (5.0) | 32.0 (7.1) | <.001 | |
| TEPS - Anticipatoryb | 47.7 (4.7) | 37.1 (8.5) | <.001 | |
| TEPS - Consummatoryb | 37.6 (7.7) | 30.3 (7.8) | .005 | |
| Apathy Evaluation Scale | 24.3 (5.3) | 40.9 (9.5) | <.001 | |
| PET Scan Parameters and Amphetamine-Related Measures | ||||
| Baseline Injected radioactivity, MBq | 267.2 (94.2) | 213.3 (85.5) | .07 | |
| Post-amphetamine Injected radioactivity, MBq | 224.0 (79.4) | 197.9 (81.5) | .43 | |
| Baseline Injected mass of radiotracer, ug | 1.9 (0.4) | 1.9 (0.3) | .63 | |
| Post-amphetamine Injected mass of radiotracer, ug | 1.9 (0.2) | 1.9 (0.2) | .86 | |
| Baseline specific activity, MBq/nmol | 34.6 (12.7) | 28.2 (11.8) | .11 | |
| Post-amphetamine specific activity, MBq/nmol | 29.2 (11.7) | 26.2 (11.2) | .43 | |
| Post-amphetamine peak change in AIRS -happiness | 1.4 (1.6) | 2.4 (2.0) | .08 | |
| Post-amphetamine peak change in AIRS - energy | 1.4 (2.4) | 3.3 (2.2) | .009 | |
| Post-amphetamine peak change in AIRS - restlessness | 2.6 (2.6) | 1.9 (3.3) | .46 | |
| Post-amphetamine peak change in AIRS - anxiety | 0.9 (1.5) | 0.3 (2.9) | .43 | |
| Plasma amphetamine (ng/mL, mean of 3 levels at 0, 15, and 30 minutes postinjection of PHNO) | 62.0 (13.2) | 68.4 (16.8) | .19 | |
| Oral dose dextroamphetamine (mg) | 36.8 (8.5) | 36.0 (7.9) | .78 | |
| Serum progesterone (ng/ml) | 2.7 (4.5) in n=8 females | 1.7 (1.7) in n=9 females | .55 | |
| Serum estradiol (pg/ml) | 104.0 (101.2) in n=8 females | 163.6 (274.5) in n=8 females | .57 | |
Abbreviations: HC, healthy comparison subjects; MDD, major depressive disorder; MDE, major depressive episode; MASQ, Mood and Anxiety Symptom Questionnaire; TEPS, Temporal Experience of Pleasure Scale; NAART, North American Adult Reading Test; AIRS, Amphetamine Interview Rating Scale (change from baseline to post-amphetamine maximum); LQ, laterality quotient; NA, not applicable.
Two-group t test for continuous variables, Χ2 or Fisher’s Exact for categorical.
Greater scores represent less anhedonia
Overall Study Design:
Baseline assessments included rating scales and fMRI during a reinforcement learning task. Two PET scans were then performed on a separate day. MDD participants started pramipexole treatment one day after PET, returning weekly for assessments. A separate probabilistic reward task (21) without imaging was conducted pre- and post-treatment. Those results and the full fMRI results will be reported elsewhere.
Clinical Assessments:
Baseline ratings included the North American Adult Reading Test (NAART) (64) and the Edinburgh Handedness Scale (65). The Hamilton Rating Scale for Depression (HRSD) and the Clinical Global Impression -- Change Scale (CGIC) were primary treatment outcome measures, rated weekly (66). As dopaminergic dysfunction has been hypothesized to be associated with anhedonia, and particularly with motivational or anticipatory anhedonia (67,68), we included pre- and post-treatment ratings designed to assess specific forms of anhedonia -- the Temporal Experience of Pleasure Scale (TEPS) (69) assessing anticipatory and consummatory physical pleasure, and the Apathy Evaluation Rating Scale (AES-S) (70) assessing motivational anhedonia, as well as ratings of anhedonia that have been commonly used in MDD --the Mood and Anxiety Symptom Questionnaire (MASQ) Short Form, with Anhedonic Depression subscale (71), and the Snaith-Hamilton Pleasure Scale (SHAPS) (72,73). The Amphetamine Interview Rating Scale (AIRS) (74) assessed mood hourly on the PET day. Treating clinicians administered a Side Effect Checklist devised for this study (see Supplement).
Reinforcement Learning Task:
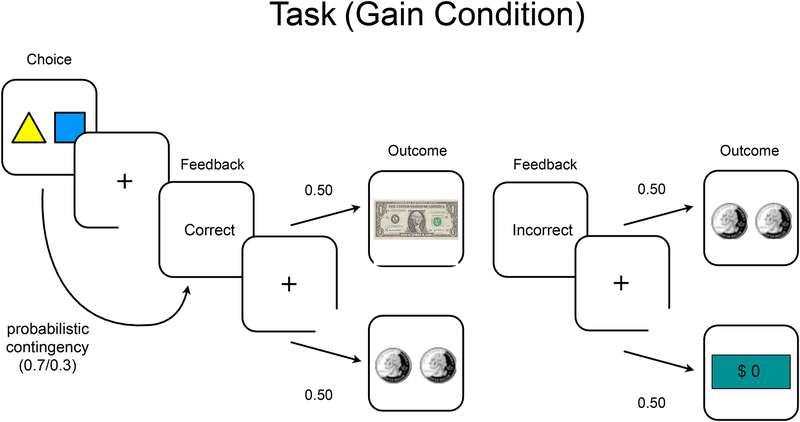
During fMRI, participants performed a probabilistic reinforcement learning task (75, 76) with two counterbalanced phases (60 non-intermixed trials each): gain (winning money), and loss (avoiding loss of money from endowment). The trials were designed to separate motor response (choice), anticipatory reinforcement feedback, and actual reward receipt. In each condition, participants 1) chose one of two stimuli, 2) received stochastically-delivered feedback (correct or incorrect, 70/30 contingency based on choice), and 3) received a monetary outcome. In the gain condition, for example, feedback “correct” triggered a $1 or $0.50 monetary outcome (at 50/50 contingency), whereas “incorrect” triggered a $0.50 or $0 monetary outcome (at 50/50 contingency) (see Figure 1). Conversely, in the loss condition, “correct” triggered losing $0 or $0.50, and “incorrect” triggered losing $0.50 or $1. This yielded reward prediction error responses separately during feedback and monetary outcome. Reinforcement contingencies were learned through trial and error.
Figure 1:
Probabilistic Reinforcement Learning Task
MRI data acquisition:
Scans utilized a GE Signa 3T scanner (Milwaukee, WI) with 32-channel head coil. Participants viewed images on a screen and responded using a trackball. T1-weighted structural images (1mm isotropic voxels, 200 slices, FOV = 25.6) and functional EPI images (TR = 2000ms, TE = 28ms, flip angle = 77 degrees, FOV = 19.2, 3mm isotropic voxels, 40 slices) were acquired in six runs of 20 trials each. Five volumes were discarded for magnetic stabilization.
fMRI analysis:
Functional images were preprocessed with SPM12 and analyzed with NeuroElf (http://neuroelf.net/) software. Images were slice-time corrected and realigned to the first volume of each run for motion correction, then warped to Montreal Neurological Institute template and smoothed with a 6mm Gaussian kernel. Data were forced to single precision to decrease impact of rounding errors. After preprocessing, first-level analyses used a general linear model (GLM), including six stick function regressors convolved with hemodynamic response: choice, feedback, outcome, each with trial-specific parametric regressors (choice value, feedback prediction error, and monetary outcome prediction error). Learning rates and choice value for model-based fMRI analyses were estimated using a reinforcement learning model (77,78). A high-pass temporal filter (Fourier transform, 200s) and motion parameters were incorporated as regressors of no interest.
For parametric regressors, a computational Q-learning model (79) generated behavioral learning parameters for each participant (learning rate/alpha, temperature/beta), and trial-specific learning signal regressors (prediction error) for fMRI GLM-based analyses. Analyses here are limited to the a priori hypothesis of altered ventral striatal BOLD response to reward prediction error in MDD. We extracted beta values reflecting each participant’s response in an a priori defined nucleus accumbens region of interest (ROI) using the Harvard-Oxford Atlas. Group difference analyses were conducted for the nucleus accumbens, small volume corrected at p<0.05. Each set of analyses was performed for each prediction error event (feedback and outcome) and condition (gain and loss).
PET Imaging Procedures:
Participants completed two [11C]-(+)-PHNO PET scans, five hours apart, on one day, following previous methods (80). A molded polyurethane head immobilizer (Soule Medical, Tampa, FL) minimized head motion. Following a 7s CT scan for attenuation correction, a 120-minute baseline scan was acquired, followed by oral amphetamine (0.5mg/kg) administration. Three hours later (5h after first radiotracer injection) another CT and 120-minute scan were administered. Data were acquired in list mode on a Biograph mCT PET-CT (Siemens, Knoxville TN), binned into a frame sequence of increasing duration and reconstructed by filtered back-projection using manufacturer-provided software.
PET Data Analysis:
Preprocessing:
ROIs, drawn on each T1-weighted MRI as previously described (81), included globus pallidus, pre-commissural dorsal caudate, post-commissural caudate, pre-commissural dorsal putamen, post-commissural putamen, ventral striatum, midbrain encompassing substantia nigra and ventral tegmentum, thalamus, and cerebellum. PET data were coregistered to MRI data using normalized maximization of mutual information (SPM8) and ROIs were transferred to coregistered PET using MEDx software (Medical Numerics).
Kinetic Analysis:
Time activity curves were generated as mean activity in each frame for each ROI. Reference tissue-based kinetic modeling (SRTM) (82) using cerebellum as reference tissue yielded binding potential relative to non-displaceable compartment (BPND) (83). Percent change from baseline BPND in each ROI following amphetamine (ΔBPND) was taken as a measure of DA release capacity (84).
Statistics:
Ventrostriatal BPND and ΔBPND were compared between groups by two-group t-tests and correlated with clinical features within the MDD group. In secondary analyses, for other ROIs, groups were compared by t-tests with FDR correction for multiple comparisons. Additionally, all regions were tested simultaneously, both for group mean comparisons and associations with other variables including BOLD response in the nucleus accumbens, in the mixed model framework (SPSS 24) with ROI as repeated measure, and group and ROI as fixed effects. Paired t-tests compared pre-and post-treatment values for clinical outcomes.
Treatment:
Following PET, MDD participants started 6 weeks of pramipexole treatment, with dose (0.5–2.5 mg/day) adjusted at weekly visits, based on clinical response.
Results
Participants:
Twenty-six adults with MDD and 26 HC participated. Twenty MDD and 20 HC participants completed PET with analyzable data, and 23 MDD and 24 HC participants completed fMRI with analyzable data (see Consort Diagram in the Supplement). Demographic and clinical features of PET completer samples are shown in Table 1. Demographic and clinical features of fMRI completers, and intercorrelations among clinical ratings of anhedonia and depression are shown in the Supplement Tables S2 and S3.
PET:
Groups did not differ in mean injected activity, injected mass, regional volumes or plasma amphetamine levels (Table 1). Age was significantly correlated with BPND across both groups (BPND: F1,36 = 17.34, p < .001, decrease = 0.9%/year (95% CI = [−1.3%, −0.5%]), group by age interaction, NS). Baseline BPND and ΔBPND (Table 2 and Figure 2) did not differ significantly between groups for any ROI or across all ROIs after covarying for age (BPND: F1,36.17 = 0.50, p = .48; ΔBPND: F1, 35.70 = 1.78, p = .19). There was trend-level greater DA release in the MDD group in the ventral striatum (−34% vs. −30%, p = .072, Cohen’s d = .58) and the globus pallidus (−27% vs. −22%, p = .096, d = .54) relative to HC.
Table 2.
Binding Potential (BP) and Post-Amphetamine Change in Binding Potential (ΔBPND)
| Mean (SD) | MDD vs. HC | |||||||
|---|---|---|---|---|---|---|---|---|
| HC (n=20) | MDD (n=20) | |||||||
| Region | Baseline | Post-amphetamine | ΔBPND% | Baseline | Post-amphetamine | ΔBPND% | P BPND | P ΔBPND |
| Anterior putamen | 2.7 (0.2) | 2.1 (0.2) | −22.9 (6.0) | 2.6 (0.5) | 2.0 (0.4) | −25.8 (6.4) | .75 | .15 |
| Dorsal caudate | 2.3 (0.2) | 1.8 (0.2) | −18.7 (5.3) | 2.2 (0.5) | 1.7 (0.4) | −19.8 (5.6) | .83 | .53 |
| Midbrain | 0.8 (0.1) | 0.5 (0.1) | −30.5 (14.6) | 0.7 (0.2) | 0.4 (0.1) | −39.2 (24.2) | .19 | .17 |
| Posterior caudate | 1.3 (0.2) | 1.0 (0.2) | −23.3 (6.8) | 1.3 (0.3) | 1.0 (0.3) | −23.3 (6.6) | .76 | .20 |
| Globus pallidus | 4.7 (0.6) | 3.6 (0.6) | −22.0 (8.8) | 4.5 (1.1) | 3.2 (0.8) | −26.9 (9.0) | .46 | .10 |
| Posterior putamen | 2.3 (0.2) | 1.6 (0.1) | −29.5 (6.9) | 2.3 (0.5) | 1.6 (0.4) | −29.6 (5.7) | .80 | .95 |
| Thalamus | 0.6 (0.2) | 0.5 (0.1) | −22.8 (10.3) | 0.6 (0.2) | 0.5 (0.1) | −18.6 (20.5) | .95 | .42 |
| Ventral striatum | 3.9 (0.4) | 2.7 (0.3) | −29.6 (7.6) | 3.9 (0.8) | 2.5 (0.6) | −34.2 (8.3) | .92 | .07 |
Abbreviations:
MDD, Major Depressive Disorder; HC, healthy comparison subjects
Figure 2:
PET scatterplot of post-amphetamine change in binding potential (ΔBPND) as a measure of dopamine release in the ventral striatum (VST): Healthy Comparison subjects, mean 29.6% (SD = 7.6) vs. Major Depressive Disorder subjects, mean 34.2% (SD = 8.3), t = 1.85, P = .07
Baseline clinical features in the MDD group, including severity of depression and severity of anhedonia on all measures, were not significantly associated with BPND and ΔBPND across all ROIs or within any ROI. Six MDD patients who evidenced ventral striatal ΔBPND outside the range of HC participants (i.e., greater DA release) did not differ significantly from the other 14 MDD patients in any clinical features except for greater scores on the MASQ total (t18 = 2.65, p = .02) and on two of its four subscales, anxious arousal (t5.49 = 2.53, p = .05) and general distress anxious (t18 = 2.91, p = .01). These differences did not survive correction for multiple comparisons. MDD patients showed significantly greater post-amphetamine increase in energy relative to HC and trend-level greater increase in happiness (Table 1), but BPND and ΔBPND did not predict changes in mood after amphetamine. PET outcomes across all ROIs for BPND and ΔBPND, respectively, were also not associated with antidepressant response to pramipexole, as assessed by slope of change in HRSD or SHAPS total scores over time (BPND - HRSD F1,17 = 0.008, p = .93; BPND - SHAPS F1,17 = 0.12, p = .73; ΔBPND -HRSD F1,17 = 0.59, p = .452; ΔBPND - SHAPS F1,17 = 0.18, p = 0.68).
fMRI:
Overall, participants performed well on the fMRI learning task, with all but two (controls) performing above chance in the gain condition, and all but one (control) in the loss condition. Similarly, maximum likelihood of the model did not differ between groups (gain: t46 = −0.93, p = 0.36; loss: t46 = −0.59, p = 0.56) and showed near-chance estimates for only one (control) participant; all others were fit better than chance. However, no correlations between PET and behavioral metrics (reaction time, performance, or model-based analyses) in either group survived correction. BOLD responses in nucleus accumbens for prediction error at feedback and outcome in each condition (gain or loss) were not significantly correlated with PET outcomes in ventral striatum or other ROIs (all p > 0.05). The MDD group had decreased prediction error responses relative to HC in the ventral striatum in the gain condition during both feedback and outcome (at feedback PE: 7 voxels, peak at (−12,6,0), tmax = 3.47, p < 0.001; at outcome PE, 6 voxels, peak at (18,18,−3), tmax = 4.06, p = 0.001). No differences were identified in the loss condition during feedback or outcome.
Treatment:
Twenty-two patients with MDD entered treatment. Twenty-one completed 6 weeks and one discontinued treatment at week 4 due to adverse events (nausea, headaches). Mean maximum dose was 1.6 ±0.7mg/d (range 0.75 to 2.5mg/d). Sixteen patients (72.7%) were responders, defined a priori by Clinical Global Improvement – Change score of 1 (very much improved) or 2 (much improved) at endpoint. Depressive symptoms and measures of motivational, anticipatory, and consummatory anhedonia all improved, as shown in Table 3. Treatment-emergent adverse events were generally mild (see the Supplement Table S4).
Table 3:
Treatment Outcomes
| Mean (SD) | Paired t Test P value | Cohen’s d | ||
|---|---|---|---|---|
| Assessment | Baseline | Week 6 | ||
| Hamilton Rating Scale for Depression | 20.2 (2.5) | 8.4 (5.4) | <.001 | 2.2 |
| MASQ - Anxious Arousal | 23.4 (6.1) | 21.7 (6.0) | .163 | 0.3 |
| MASQ - Anhedonic Depression | 82.5 (12.1) | 59.6 (18.5) | <.001 | 1.3 |
| MASQ - General Distress Anxious | 22.7 (6.7) | 18.0 (5.4) | .002 | 0.8 |
| MASQ - General Distress Depressive | 39.3 (10.9) | 23.9 (12.9) | <.001 | 1.4 |
| MASQ - Total | 167.9 (24.5) | 123.1 (36.3) | <.001 | 1.3 |
| Snaith Hamilton Pleasure Scale | 31.8 (6.5) | 26.0 (7.5) | .005 | 0.7 |
| TEPS - Anticipatorya | 35.6 (10.3) | 43.2 (7.9) | <.001 | 0.6 |
| TEPS - Consummatorya | 29.4 (7.4) | 35.6 (6.5) | .012 | 0.9 |
| Apathy Evaluation Scale | 42.7 (8.9) | 31.7 (9.5) | .0001 | 1.1 |
Abbreviations: MASQ, Mood and Anxiety Symptom Questionnaire; TEPS, Temporal Experience of Pleasure Scale.
Greater scores represent less anhedonia
Discussion
This study did not find abnormal D2/D3 receptor availability or DA release capacity in MDD, as measured by [11C]-(+)-PHNO PET before and after amphetamine administration. Nor were PET outcomes associated with clinical features within the MDD group. MDD patients with greatest DA release in the ventral striatum did evidence greater anxiety scores. These did not survive correction for multiple comparisons but suggest the need for further study of the relationship of anxiety to striatal DA. PET outcome measures showed no association with ratings of anticipatory or consummatory anhedonia and did not predict clinical response to pramipexole treatment. PET indices were also uncorrelated with ventrostriatal BOLD response to reward prediction error, which was blunted in MDD in the gain condition.
The MDD sample did have elevated anhedonia on each of several measures, including motivational anhedonia, hypothesized to be a clinical indicator of DA dysfunction. Depressive symptoms were generally responsive to treatment with the DA agonist pramipexole, suggesting DA system mediation of treatment response. Inference of a specific dopaminergic mechanism of response, however, is limited by absence of a placebo treatment group and likelihood of nonspecific influences in an antidepressant-naive sample (85).
The absence of [11C]-(+)-PHNO PET outcome associations with MDD – in a sample with motivational deficits and robust response to treatment with a DA agonist – was unexpected, given the study’s methodological advantages. The sample size of 20 PET completers per group constitutes one of the largest DA receptor imaging studies in MDD. The groups were well-matched for features associated with DA indices in some studies, including age, sex, estradiol and progesterone levels in females, and body mass index. This study was tightly controlled by exclusion of lifetime antidepressant treatment and current and lifetime comorbid DA-associated conditions, including ADHD, substance use disorders, and recent subsyndromal use of psychoactive substances or tobacco. Estrogen and progesterone levels in females also did not differ between groups. Clinical assessments included multiple measures of depressive symptomatology, including motivational anhedonia, that have been indirectly associated with DA function.
Validity of the PET results here is supported by replication of the well-established finding of decreased DA release with increasing age (despite a constrained range in this sample). The mean magnitude of post-amphetamine ΔBPND of 29.6% in the HC group is in a similar range as that reported for a prior PHNO study at our center (80) that utilized the same dose of oral amphetamine (24.9%), suggesting that the lack of group differences here was unlikely due to aberrant HC sample results.
The absence of an association between PET results and BOLD response to reward prediction error in the ventral striatum suggests that D2/D3 receptor availability and DA release capacity may not mediate neural response to reward prediction error both within MDD and more generally. However, the lack of an association here may be due in part to the different probes: an instrumental reinforcement learning task during fMRI and pharmacological amphetamine challenge during PET. Also, PET and fMRI were performed on separate days. Reinforcement learning tasks similar to the one used here during fMRI have been associated with phasic release of striatal DA (5,14). Amphetamine, however, increases synaptic DA via multiple mechanisms, including reversing the DA transporter (86). The absence of differences in amphetamine-induced dopamine release observed here suggests presynaptic dopamine levels may not be dysregulated in MDD. For example, the kinetics of dopaminergic cell firing and the resultant phasic DA release that tracks reward prediction error could be altered in MDD, yet DA storage in vesicles and release upon amphetamine administration may not be substantially affected. Another presynaptic PET measure, assessment of DA synthesis capacity with 6-[(18) F]fluoro-L-DOPA, was found in healthy subjects to be negatively associated with ventral striatal learning signal using a task that isolated model-based learning, whereas our task assessed model-free learning (87). These approaches to assessing presynaptic DA and reward learning warrant further investigation in MDD.
Our observation that PET results in the MDD group were also not associated with antidepressant response to pramipexole suggests that the robust symptomatic response to chronic pramipexole treatment does not represent normalization of a deficit in dopaminergic storage or release capacity in MDD. Similarly, the tendency for the MDD group to report a greater post-amphetamine increase in energy and mood (consistent with a prior report (88) was not significantly associated with BPND and ΔBPND. The group difference in mood response may be related to ceiling effects in the HC or to the effects of amphetamine on neurotransmitters other than DA.
An alternative to using PET with amphetamine to stimulate DA release in MDD might be to utilize a reward motivation task during PET to more directly assess DA release that occurs during reward prediction error, as has been conducted with healthy subjects (12–18). However, the relatively low temporal resolution of PET neuroreceptor imaging and the low magnitude of DA displacement in response to a behavioral reward task (relative to amphetamine or methylphenidate challenge) limit sensitivity of such as an assessment, and prior efforts to assess DA released by a behavioral task in healthy volunteer samples have had mixed results (89). Another study using a behavioral task recorded striatal DA signaling directly, from a sample of Parkinson’s disorder patients who had deep brain electrodes placed for therapeutic stimulation, using fast-scan cyclic voltammetry during an investment task. Comparisons to a healthy sample undergoing fMRI during the same task found only partial correspondence between direct recording signals and BOLD responses (90).
The sample size, although large for a PET study, afforded power to detect only large effects. The largest group difference in PET outcomes was for DA release in the ventral striatum, where a trend significant finding of p = 0.072 represented a medium-sized effect of d = 0.58. A future study designed to detect a group difference of this magnitude with 80% power at a significance level of p < 0.05 would require 47 subjects per group, a sample size of limited feasibility given current costs of PET imaging. The well-known heterogeneity of the diagnostic category of MDD may have limited power to detect group differences in this study. Despite strict exclusion of subjects with hypothesized confounders including comorbid substance use and prior antidepressant treatment, careful matching of comparison subjects, and exploration of dimensional features such as anhedonia and response to DA agonist treatment, unmeasured heterogeneity such as genetic variability may have limited ability to detect associations. Alternative clinical samples, such as nonresponders to first-line serotonergic medications, might be more likely to be enriched for dopaminergic dysfunction.
Other limitations involve the PET assessments. For example, the study was limited by the specificity of the PET amphetamine challenge for assessment of DA storage and release capacity, rather than other physiological aspects of DA function. Additionally, specific contributions of D2 and D3 receptors to [11C]-(+)-PHNO binding potential could not be discriminated in regions known to contain both receptor types, including ventral striatum. Another limitation was administration of the reinforcement learning task in a separate session on separate day from dual PET scans. Future studies should more directly explore the functional relationship between reward motivation tasks that putatively elicit phasic DA signals and PET metrics that track the activity of D2 and D3 receptors.
Additionally, other neural processes both within and outside the ventral striatum could contribute to blunting of the BOLD response to reward prediction error in MDD, in the absence of abnormal DA release capacity or D2/D3 receptor density. D2/D3 receptor interacting partners within the ventral striatum, such as DA transporter, D1 receptors, or components of the signaling pathways downstream of D2/D3 receptors, could impact response to reward in MDD (91–93). The serotonergic system also modulates reward responses (94,95), and abnormalities of glutamate or dopamine in other brain regions, such as in the frontal cortex, could also influence BOLD response in the striatum (96–97). The role of frontostriatal pathways in anhedonic depression might be clarified by PET studies with radioligands allowing assessment of cortical D2/D3 receptors (e.g. [11C]FLB 457) and D1 receptors (e.g. [11C]NNC 112) (97–98).
In conclusion, this multimodal imaging study, incorporating fMRI and [11C]-(+)-PHNO PET with amphetamine in MDD and HC groups, did not identify group differences in D2/D3 receptor binding or DA release capacity, although ventrostriatal BOLD response to reward prediction error was decreased in the MDD group. PET outcomes also did not predict response of MDD symptoms to treatment with pramipexole. While the positive therapeutic response could suggest a D2/D3 dopaminergic dysfunction that was reversed by pramipexole treatment, the normal baseline D2/D3 PET and DA release measures suggest that [11C]-(+)-PHNO PET with amphetamine may not target the specific molecular mechanisms underlying response to pramipexole treatment. Better understanding of the precise molecular and functional abnormalities in the cortico-basal ganglia loops in MDD may help to explain these negative results and should guide future investigations.
Supplementary Material
Acknowledgments
Funding for this study was provided by grant R01MH099322 from the National Institute of Mental Health to FRS. DAP was partially supported by National Institute of Mental Health grant R37 MH068376.
Presented in part at the 56th Annual Meeting of the American College of Neuropsychopharmacology, Palm Springs, CA, December 3–7, 2017.
We thank Roberto Valdovinos and Danielle Moskow for assistance with data collection, and Page van Meter for assistance with data management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Schneier has received research support from Forest Laboratories; Dr. Slifstein has received research support from Forest Laboratories, Pierre-Fabre, CHDI, and Otsuka and has provided consultation for Amgen. Dr. Pizzagalli has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Pfizer and Posit Science, for activities unrelated to the current research. Dr. Iosifescu has received consulting fees from Axsome, MyndAnalytics (CNS Response), Jazz, Lundbeck, Otsuka, Sunovion, and has received research support (through his academic institutions) from Alkermes, Astra Zeneca, Brainsway, LiteCure, Neosync, Roche, Shire. Dr. Abi-Dargham has received research support from Takeda and Forest Pharmaceuticals and has served on advisory boards for Roche, Forum, and Otsuka. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- 2.Spijker J, Bijl RV, de Graaf R, Nolen WA (2001): Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr Scand 103: 122–130. [DOI] [PubMed] [Google Scholar]
- 3.McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, et al. (2012): Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry 51: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. (2012): Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med 42: 967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz W, Dayan P, Montague PR (1997): A neural substrate of prediction and reward. Science 275: 1593–1599. [DOI] [PubMed] [Google Scholar]
- 6.Berridge KC (2007): The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 191: 391–431. [DOI] [PubMed] [Google Scholar]
- 7.Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M (2009): Dopamine, behavioral economics, and effort. Front Behav Neurosci 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott R, Newman JL, Longe OA, William Deakin JF (2004): Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. Neuroimage 21: 984–990. [DOI] [PubMed] [Google Scholar]
- 9.Bayer HM, Glimcher PW (2005): Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 47:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang KS, Smith DV, Delgado MR (2016): Using fMRI to study reward processing in humans: past, present, and future. J Neurophysiol 115:1664–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chase HW, Kumar P, Eickhoff SB, Dombrovski AY (2015): Reinforcement learning models and their neural correlates: An activation likelihood estimation meta-analysis. Cogn Affect Behav Neurosci 15: 435–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. (2012): Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci 32: 6170–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonasson LS, Axelsson J, Riklund K, Braver TS, Ögren M, Bäckman L, Nyberg L (2014): Dopamine release in nucleus accumbens during rewarded task switching measured by [11C]raclopride. Neuroimage 99: 357–364. [DOI] [PubMed] [Google Scholar]
- 14.Kasanova Z, Ceccarini J, Frank MJ, Amelsvoort TV, Booij J, Heinzel A, et al. (2017): I. Striatal dopaminergic modulation of reinforcement learning predicts reward-oriented behavior in daily life. Biol Psychol 127:1–9. [DOI] [PubMed] [Google Scholar]
- 15.Caravaggio F, Fervaha G, Browne CJ, Gerretsen P, Remington G, Graff-Guerrero A (2018): Reward motivation in humans and its relationship to dopamine D(2/3) receptor availability: A pilot study with dual [(11)C]-raclopride and [(11)C]-(+)-PHNO imaging. J Psychopharmacol 32: 357–366. [DOI] [PubMed] [Google Scholar]
- 16.Vrieze E, Ceccarini J, Pizzagalli DA, Bormans G, Vandenbulcke M, Demyttenaere K, et al. (2013): Measuring extrastriatal dopamine release during a reward learning task. Human Brain Mapping 34: 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, et al. (2008): Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci 28: 14311–14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urban NB, Slifstein M, Meda S, Xu X, Ayoub R, Medina O, et al. (2012): Imaging human reward processing with positron emission tomography and functional magnetic resonance imaging. Psychopharmacology (Berl) 221: 67–77. [DOI] [PubMed] [Google Scholar]
- 19.Henriques JB, Glowacki JM, Davidson RJ (1994): Reward fails to alter response bias in depression. J Abnorm Psychol 103: 460–466. [DOI] [PubMed] [Google Scholar]
- 20.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M (2008): Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 43:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pizzagalli DA, Jahn AL, O’Shea JP (2005): Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry 57: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, deBoer P, et al. (2013): Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry 73: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD (2008): Abnormal temporal difference reward-learning signals in major depression. Brain 131(Pt 8): 2084–2093. [DOI] [PubMed] [Google Scholar]
- 24.Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS (2009): fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord 118: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrison J, Erdeniz B, Done J (2013): Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 37: 1297–1310. [DOI] [PubMed] [Google Scholar]
- 26.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. (2009): Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry 166: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutledge RB, Moutoussis M, Smittenaar P, Zeidman P, Taylor T, Hrynkiewicz L, et al. (2017): Association of neural and emotional impacts of reward prediction errors with major depression. JAMA Psychiatry 74:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Admon R, Kaiser RH, Dillon DG, Beltzer M, Goer F, Olson DP et al. (2017): Dopaminergic enhancement of striatal response to reward in major depression. Am J Psychiatry 174: 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury R, Guitart-Masip M, Lambert C, Dayan P, Huys Q, Düzel E, et al. (2013): Dopamine restores reward prediction errors in old age. Nat Neurosci 16:648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jocham G, Klein TA, Ullsperger M (2011): Dopamine-mediated reinforcement learning signals in the striatum and ventromedial prefrontal cortex underlie value-based choices. J Neurosci 31:1606–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD (2006): Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442: 1042–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willner P (2006): Animal models of depression: validity and applications. Adv Biochem Psychopharmacol 49: 19–41. [PubMed] [Google Scholar]
- 33.Ruhe HG, Mason NS, Schene AH (2007): Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry 12: 331–359. [DOI] [PubMed] [Google Scholar]
- 34.Lattanzi L, Dell’Osso L, Cassano P, Pini S, Rucci P, Houck PR, et al. (2002): Pramipexole in treatment-resistant depression: a 16-week naturalistic study. Bipolar Disord 4: 307–314. [DOI] [PubMed] [Google Scholar]
- 35.Cassano P, Lattanzi L, Soldani F, Navari S, Battistini G, Gemignani A, Cassano GB, et al. (2004): Pramipexole in treatment-resistant depression: an extended follow-up. Depress Anxiety 20:131–138. [DOI] [PubMed] [Google Scholar]
- 36.Inoue T, Kitaichi Y, Masui T, Nakagawa S, Boku S, Tanaka T, et al. (2010): Pramipexole for stage 2 treatment-resistant major depression: an open study. Prog Neuropsychopharmacol Biol Psychiatry 34: 1446–1449. [DOI] [PubMed] [Google Scholar]
- 37.Franco-Chaves JA, Mateus CF, Luckenbaugh DA, Martinez PE, Mallinger AG, Zarate CA Jr. (2013): Combining a dopamine agonist and selective serotonin reuptake inhibitor for the treatment of depression: a double-blind, randomized pilot study. J Affect Disord 149: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cusin C, Iovieno N, Iosifescu DV, Nierenberg AA, Fava M, Rush AJ, Perlis RH (2013): A randomized, double-blind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J Clin Psychiatry 74: e636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL (2000): Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety 11: 58–65. [DOI] [PubMed] [Google Scholar]
- 40.Aiken CB (2007): Pramipexole in psychiatry: a systematic review of the literature. J Clin Psychiatry 68:1230–1236. [PubMed] [Google Scholar]
- 41.Piercey MF (1998): Pharmacology of pramipexole, a dopamine D3-preferring agonist useful in treating Parkinson’s disease. Clin Neuropharmacol 21: 141–151. [PubMed] [Google Scholar]
- 42.Chernoloz O, El Mansari M, Blier P (2012): Long-term administration of the dopamine D3/2 receptor agonist pramipexole increases dopamine and serotonin neurotransmission in the male rat forebrain. J Psychiatry Neurosci 37: 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NP, et al. (2006): Elevated putamen D(2) receptor binding potential in major depression with motor retardation: an [11C]raclopride positron emission tomography study. Am J Psychiatry 163: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 44.Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP (1997): Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med 27: 1247–1256. [DOI] [PubMed] [Google Scholar]
- 45.D’Haenen HA, Bossuyt A (1994): Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biol Psychiatry 35: 128–132. [DOI] [PubMed] [Google Scholar]
- 46.Peciña M, Sikora M, Avery ET, Heffernan J, Peciña S, Mickey BJ, Zubieta JK (2017): Striatal dopamine D2/3 receptor-mediated neurotransmission in major depression: Implications for anhedonia, anxiety and treatment response. Eur Neuropsychopharmacol 27: 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montgomery AJ, Stokes P, Kitamura Y, Grasby PM (2007): Extrastriatal D2 and striatal D2 receptors in depressive illness: pilot PET studies using [11C]FLB 457 and [11C]raclopride. J Affect Disord 101: 113–122. [DOI] [PubMed] [Google Scholar]
- 48.Yang YK, Yeh TL, Yao WJ, Lee IH, Chen PS, Chiu NT, Lu RB (2008): Greater availability of dopamine transporters in patients with major depression--a dual-isotope SPECT study. Psychiatry Res 162: 230–235. [DOI] [PubMed] [Google Scholar]
- 49.Parsey RV, Oquendo MA, Zea-Ponce Y, Rodenhiser J, Kegeles LS, Pratap M, et al. (2001): Dopamine D(2) receptor availability and amphetamine-induced dopamine release in unipolar depression. Biol Psychiatry 50: 313–322. [DOI] [PubMed] [Google Scholar]
- 50.Klimke A, Larisch R, Janz A, Vosberg H, Muller-Gartner HW, Gaebel W (1999): Dopamine D2 receptor binding before and after treatment of major depression measured by [123I]IBZM SPECT. Psychiatry Res 90: 91–101. [DOI] [PubMed] [Google Scholar]
- 51.Ebert D, Feistel H, Kaschka W, Barocka A, Pirner A (1994): Single photon emission computerized tomography assessment of cerebral dopamine D2 receptor blockade in depression before and after sleep deprivation--preliminary results. Biol Psychiatry 35:880–885. [DOI] [PubMed] [Google Scholar]
- 52.Hirvonen J, Karlsson H, Kajander J, Markkula J, Rasi-Hakala H, Någren K, et al. (2008): Striatal dopamine D2 receptors in medication-naive patients with major depressive disorder as assessed with [11C]raclopride PET. Psychopharmacology (Berl) 197: 581–590. [DOI] [PubMed] [Google Scholar]
- 53.Messa C, Colombo C, Moresco RM, Gobbo C, Galli L, Lucignani G, et al. (2003): 5-HT(2A) receptor binding is reduced in drug-naive and unchanged in SSRI-responder depressed patients compared to healthy controls: a PET study. Psychopharmacology (Berl) 167: 72–78. [DOI] [PubMed] [Google Scholar]
- 54.Kuroda Y, Motohashi N, Ito H, Ito S, Takano A, Nishikawa T, Suhara T (2006): Effects of repetitive transcranial magnetic stimulation on [11C]raclopride binding and cognitive function in patients with depression. J Affect Disord 95: 35–42. [DOI] [PubMed] [Google Scholar]
- 55.de Kwaasteniet BP, Pinto C, Ruhé HG, van Wingen GA, Booij J, Denys D (2014): Striatal dopamine D2/3 receptor availability in treatment resistant depression. PLoS One 9:e113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Busto UE, Redden L, Mayberg H, Kapur S, Houle S, Zawertailo LA (2009): Dopaminergic activity in depressed smokers: a positron emission tomography study. Synapse 63: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, et al. (2011): Imaging dopamine receptors in humans with[11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 54: 264–277. [DOI] [PubMed] [Google Scholar]
- 58.Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, et al. (2006): Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem 97: 1089–1103. [DOI] [PubMed] [Google Scholar]
- 59.Leggio GM, Salomone S, Bucolo C, Platania C, Micale V, Caraci F, Drago F (2013): Dopamine D(3) receptor as a new pharmacological target for the treatment of depression. Eur J Pharmacol 719: 25–33. [DOI] [PubMed] [Google Scholar]
- 60.Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, et al. (2008): First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology 33: 279–289. [DOI] [PubMed] [Google Scholar]
- 61.Caravaggio F, Kegeles LS, Wilson AA, Remington G, Borlido C, Mamo DC, Graff-Guerrero A, et al. (2016): Estimating the effect of endogenous dopamine on baseline [(11) C]-(+)-PHNO binding in the human brain. Synapse 70: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.First MB, Spitzer RL,Gibbon M, Williams JBW (2002): Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- 63.Hamilton M (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uttl B (2002): North American Adult Reading Test: age norms, reliability, and validity. J Clin Exp Neuropsychol 24: 1123–1137. [DOI] [PubMed] [Google Scholar]
- 65.Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 66.Guy W (1976): ECDEU Assessment Manual for Psychopharmacology—Revised (DHEW Publ No ADM 76–338). Rockville, MD, U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs, pp 218–222. [Google Scholar]
- 67.Schmidt K, Nolte-Zenker B, Patzer J, Bauer M, Schmidt LG, Heinz A (2001): Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry 34:66–72. [DOI] [PubMed] [Google Scholar]
- 68.Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH (2016): Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev 65:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gard DE, Gard MG, Kring AM, John OP (2006): Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality 40: 1086–1102. [Google Scholar]
- 70.Marin RS, Biedrzycki RC, Firinciogullari S (1991): Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 38: 143–162. [DOI] [PubMed] [Google Scholar]
- 71.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA (1995): Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology 104: 3–14. [DOI] [PubMed] [Google Scholar]
- 72.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P (1995): A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 167: 99–103. [DOI] [PubMed] [Google Scholar]
- 73.Franken IH, Rassin E, Muris P (2007): The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS). J Affect Disord 99:83–89. [DOI] [PubMed] [Google Scholar]
- 74.van Kammen DP, Murphy DL (1975): Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia (Berl.) 44: 215–224. [DOI] [PubMed] [Google Scholar]
- 75.Insel C, Reinen J, Weber J, Wager TD, Jarskog LF, Shohamy D, et al. (2014): Antipsychotic dose modulates behavioral and neural responses to feedback during reinforcement learning in schizophrenia. Cogn Affect Behav Neurosci. 14:189–201. [DOI] [PubMed] [Google Scholar]
- 76.Reinen J, Smith EE, Insel C, Kribs R, Shohamy D, Wager TD, et al. (2014): Patients with schizophrenia are impaired when learning in the context of pursuing rewards. Schizophr Res 152: 309–310. [DOI] [PubMed] [Google Scholar]
- 77.Daw ND (2011): Trial-by-trial data analysis using computational models In: Delgado MR, Phelps EA, Robbins TW, eds. Decision Making, Affect, and Learning. Oxford University Press. [Google Scholar]
- 78.Sutton RS, Barto AG (1998): Reinforcement learning: an introduction. Cambridge, MA: MIT. [Google Scholar]
- 79.Reinen JM, Van Snellenberg JX, Horga G, Abi-Dargham A, Daw ND, Shohamy D (2016): Motivational context modulates prediction error response in schizophrenia. Schizophr Bull 42: 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van de Giessen E, Weinstein JJ, Cassidy CM, Haney M, Dong Z, Ghazzaoui R, et al. (2017): Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry 22: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. (2001): Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21: 1034–1057. [DOI] [PubMed] [Google Scholar]
- 82.Lammertsma AA, Hume SP (1996): Simplified reference tissue model for PET receptor studies. NeuroImage 4: 153–158. [DOI] [PubMed] [Google Scholar]
- 83.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. (2007): Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 84.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. (2003): Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23: 285–300. [DOI] [PubMed] [Google Scholar]
- 85.Hunter AM, Cook IA, Tartter M, Sharma SK, Disse GD, Leuchter AF (2015): Antidepressant treatment history and drug-placebo separation in a placebo-controlled trial in major depressive disorder. Psychopharmacology (Berl) 232: 3833–3840. [DOI] [PubMed] [Google Scholar]
- 86.Sulzer D, Sonders MS, Poulsen NW, Galli A (2005): Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75: 406–433. [DOI] [PubMed] [Google Scholar]
- 87.Deserno L, Huys QJ, Boehme R, Buchert R, Heinze HJ, Grace AA, Dolan RJ, Heinz A, Schlagenhauf F (2015): Ventral striatal dopamine reflects behavioral and neural signatures of model-based control during sequential decision making. Proc Natl Acad Sci U S A. 112:1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE (2002): Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Arch Gen Psychiatry 59: 409–416. [DOI] [PubMed] [Google Scholar]
- 89.Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ, et al. (2009): The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neurosci Biobehav Rev 33:1109–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lohrenz T, Kishida KT, Montague PR (2016): BOLD and its connection to dopamine release in human striatum: a cross-cohort comparison. Philos Trans R Soc Lond B Biol Sci 371(1705). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soares-Cunha C, Coimbra B, Sousa N, Rodrigues AJ (2016): Reappraising striatal D1 and D2-neurons in reward and aversion. Neurosci Biobehav Rev 68:370–386. [DOI] [PubMed] [Google Scholar]
- 92.Bamford NS, Wightman RM, Sulzer D (2018): Dopamine’s effects on corticostriatal synapses during reward-based behaviors. Neuron 97:494–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaiser RH, Treadway MT, Wooten DW, Kumar P, Goer F, Murray L, et al. (2017): Frontostriatal and dopamine markers of individual differences in reinforcement learning: A multi-modal investigation. Cereb Cortex 31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kranz GS, Kasper S, Lanzenberger R (2010): Reward and the serotonergic system. Neuroscience 166:1023–1035. [DOI] [PubMed] [Google Scholar]
- 95.Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, et al. (2012): Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol 26:677–688. [DOI] [PubMed] [Google Scholar]
- 96.Jenni NL, Larkin JD, Floresco SB (2017): Prefrontal dopamine D(1) and D(2) receptors regulate dissociable aspects of decision making via distinct ventral striatal and amygdalar circuits. J Neurosci 37:6200–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, et al. (2009): Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse 63:447–461. [DOI] [PubMed] [Google Scholar]
- 98.Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, et al. (2000): Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab 20:225–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.