Abstract
Background:
Tungsten (W) interferes with molybdenum (Mo) binding sites and has been associated with prevalent cardiovascular disease (CVD). We evaluated if (1) W exposure is prospectively associated with incident CVD and (2) the association between urinary W levels and incident CVD is modified by urinary Mo levels.
Methods:
We estimated multi-adjusted hazard ratios (HRs) for incident CVD outcomes by increasing W levels for 2726 American Indian participants in the Strong Heart Study with urinary metal levels measured at baseline (1989–1991) and CVD events ascertained through 2008.
Results:
Increasing levels of baseline urinary W were not associated with incident CVD. Fully-adjusted HRs (95% CIs) of incident CVD comparing a change in the IQR of W levels for those in the lowest and highest tertile of urinary Mo were 1.05 (0.90, 1.22) and 0.80 (0.70, 0.92), respectively (p-interaction = 0.02); for CVD mortality, the corresponding HRs were 1.05 (0.82, 1.33) and 0.73 (0.58, 0.93), respectively (p-interaction = 0.03).
Conclusions:
The association between W and CVD incidence and mortality was positive although non-significant at lower urinary Mo levels and significant and inverse at higher urinary Mo levels. Although prior cross-sectional epidemiologic studies in the general US population found positive associations between urinary tungsten and prevalent cardiovascular disease, our prospective analysis in the Strong Heart Study indicates this association may be modified by molybdenum exposure.
Keywords: Tungsten, Molybdenum, Cardiovascular disease, Strong Heart Study, American Indians
1. Introduction
Tungsten (W) is a toxic metal naturally occurring in rocks and soil and is deposited into the environment from ore processing, municipal waste incineration, and hard metal industrial output (Grimes et al., 1995; Smith et al., 2014; Agency for Toxic Substances and Disease Registry, 2005). In the U.S., W is highest in topsoil in the Southwestern region (Smith et al., 2014), and soluble W compounds readily leach into groundwater (Grimes et al., 1995; Smith et al., 2014; Agency for Toxic Substances and Disease Registry, 2005). The general population is likely exposed to W through food, drinking water, and industrial output, although relevant sources of exposure have not been well characterized (Grimes et al., 1995; Witten et al., 2012). The median (interquartile range) urinary W levels in the National Health and Nutrition Examination Survey 1999–2000 was 0.06 µg/L (0.03–0.13) and 70% had detectable W levels; for molybdenum, an essential element which may interact with W, the median (interquartile range) was 41.2 µg/L (21.2–71.3) and > 99% had detectable levels (Navas-Acien et al., 2004). Urinary W levels reflect mostly recent exposure, with a rapid urinary excretion phase of 5 days (70%) and a longer excretion phase of 100 days (30%) (Agency for Toxic Substances and Disease Registry, 2005; Lemus and Venezia, 2015). The U.S. Environmental Protection Agency (EPA) selected W as an emerging contaminant in 2009, although it has not yet been regulated in drinking water in the U.S. (US Environmental Protection Agency (2009).
Relatively little is known about the health effects of W. In hard metal workers, the inhalation of W-Cobalt (Co) dust particles causes hard metal disease, characterized by asthma and fibrosis (Lison, 1996; Moulin et al., 1998). Sources of high-level tungsten exposure are likely occupational, including manufacturing and metal work. Tungsten has also been detected in air and a municipal water supply in a town with significant tungsten industrial output (Agency for Toxic Substances and Disease Registry, 2005). In the National Health and Nutrition Examination Survey (NHANES), a survey of the general U.S. population, W exposure has been associated with elevated blood pressure levels (Shiue and Hristova, 2014) and with prevalent stroke, peripheral arterial disease, and composite cardiovascular disease (Navas-Acien et al., 2004; Agarwal et al., 2011; Tyrrell et al., 2013). The association between W exposure and CVD has not been evaluated in population studies outside of NHANES, and the prospective association between W exposure and incident CVD remains unknown. Tungsten measured in blood, urine, or feces is an accepted biomarker of recent exposure. Physiologically based pharmacokinetic models indicate that tungsten is rapidly excreted in urine, with a half-life in kidneys of 5 days (70%) and 100 days (30%); one study of an industrially-exposed community found that municipal water concentrations correlated well with urinary tungsten concentrations (Agency for Toxic Substances and Disease Registry, 2005).
Increasing evidence suggests that W exposure augments toxicity from exposure to other metals (Bolt and Mann, 2016). In particular, although experimental evidence is limited, W likely modifies Co toxicity (Agency for Toxic Substances and Disease Registry, 2005; Lasfargues et al., 1992). It is also well established that W may modify the action of molybdenum (Mo), as both are group VI transition metals and share similar chemical properties, including binding site structure (Jelikic-Stankov et al., 2007). Mo is an essential element and cofactor for several molybdozymes including xanthine oxidase, sulfite oxidase, and aldehyde oxidase (Klaassen and Watkins, 2015). Tungsten may render molybdozymes inactive by interfering at active sites (Brondino et al., 2006). In a rat model, W administration in the presence of low dietary Mo induced xanthine oxidase and sulfite oxidase deficiency (Yoshida et al., 2015; Herken et al., 2009). However, no epidemiological study has evaluated if W toxicity is modified by the presence of low Mo levels, or by exposure to other metals that have been causally associated with incident CVD.
The Strong Heart Study is a prospective population-based cohort initiated in 1989 to evaluate CVD and its risk factors in American Indian adults living in rural communities in Arizona, Oklahoma, and North and South Dakota (Lee et al., 1990). Some SHS communities are located in areas with elevated topsoil W (Welty et al., 1995; Smith et al., 2014). Urinary W levels in the SHS participants were higher than in adults from 6 U.S. urban sites who participated in the Multi-Ethnic Study of Atherosclerosis (MESA) (Pang et al., 2016). Exposure to W may thus potentially be of particular importance in these communities. The objective of this study was to evaluate the prospective association of baseline W exposure (1989–1991), as reflected in urinary W levels, with incident fatal and non-fatal CVD through the end of 2008 in the Strong Heart Study. As both inorganic arsenic (iAs) and cadmium (Cd) have been associated with incident CVD in the SHS (Moon et al., 2013; Tellez-Plaza et al., 2013), we also evaluated the possibility of an interaction between those metals and W. Finally, given previous knowledge of a biological interaction between W and Mo, we hypothesized that levels of urinary Mo would modify the association between W and incident CVD. Information on Co exposure is not available in the SHS and the interaction Co-W could not be evaluated. To our knowledge, this is the first prospective study evaluating W exposure and incident CVD, and the first epidemiologic study to explore effect modification of W toxicity by Mo exposure.
2. Materials and methods
2.1. Study population & data collection
The Strong Heart Study recruited 4549 American Indians from thirteen tribes and communities in rural Arizona, Oklahoma, and North and South Dakota (Welty et al., 1995; Lee et al., 1990). All adults aged 45–74 years of age at baseline were invited to participate. The participation rates for Arizona, Oklahoma, and North/South Dakota were 72%, 62%, and 55%, respectively (62% overall) (Stoddart et al., 2000). Demographic and examination data and biological samples were collected at baseline (1989–1991) and follow-up clinical visits in 1993–1995 (88% response rate) and 1997–1999 (89% response rate). Participants were followed until death or until the end of follow-up in 2008.
Data from a total of 3974 individuals from the Strong Heart Study with urinary W measured were available for analysis. We excluded 925 individuals from a community who withdrew consent for further inclusion in research studies in 2016, 213 individuals with prevalent CVD at baseline, 3 individuals missing urinary W measurements, and 107 individuals missing education, smoking, alcohol consumption, urinary creatinine, body mass index (BMI), low-density lipoprotein (LDL), hypertension, estimated glomerular filtration rate (eGFR), diabetes, or systolic blood pressure (SBP), leaving a total analytic sample of 2726 individuals. Diabetes status at baseline was defined as fasting glucose ≥ 126 mg/dl, two-hour plasma glucose ≥ 200 mg/dl, hemoglobin A1C level ≥ 6.5%, self-reported history of diagnosis, or current use of diabetic medication. The Strong Heart Study protocol was approved by institutional review boards, participating tribes, and the Indian Health Service. All participants gave informed consent.
2.2. Urinary tungsten measurement
Urinary W, As, Cd, and Mo concentrations were measured in spot urine samples collected at the baseline visit. Detailed analytical methods and quality control criteria have been described elsewhere (Scheer et al., 2012). Briefly, urine was collected in a polypropylene screw-cap tube, frozen within 1–2 h of collection, shipped on dry ice, and stored at − 80 °C at the Medstar Health Research Institute in Hyattsville, MD. Portions were then shipped on dry ice to Graz University, Austria for trace metal analysis in 2009–2010 via inductively coupled plasma-mass spectrometry (ICP-MS) using a multi-element protocol (Scheer et al., 2012). Reference water provided certified values for As, Cd, and Mo concentrations, but not W concentrations. For iAs, we used the sum of inorganic arsenic (arsenate and arsenite) and methylated species (monomethylarsonate, MMA, and dimethylarsinate, DMA) (ΣAs) as the biomarker of inorganic As exposure to account for interindividual variation in arsenic metabolism. The limit of detection for W was 0.005 µg/L of urine (1.1% undetectable in our study sample). For Mo, Cd, iAs, MMA, and DMA, the LOD in µg/L (% below the limit of detection) was 0.10 (0%), 0.015 (0.03%), 0.10 (6.1%), 0.10 (0.8%), and 0.10 (0%), respectively. Urinary metal concentrations below the limit of detection were replaced with the limit of detection divided by the square root of two.
2.3. Incident cardiovascular disease follow-up
Detailed morbidity and mortality surveillance protocol has been described previously (Strong Heart Study, 2001). Death and cardiovascular disease outcomes were ascertained by annual contact with the Coordinating Center, review of death and hospitalization records, and during both clinical follow-up visits (Strong Heart Study, 2001). All deaths and cardiovascular outcomes were reviewed by the Morbidity and Mortality Review Committee, and all discrepancies were adjudicated (Strong Heart Study, 2001). Follow-up through 2008 for fatal and non-fatal endpoints was 99.8% and 99.2% complete, respectively.
Incident coronary heart disease (CHD) was defined as the first occurrence of definite fatal or non-fatal myocardial infarction, definite sudden death due to CHD, and definite and possible non-fatal CHD. Incident stroke was defined as the first occurrence of definite or possible fatal or non-fatal stroke. Incident CVD was defined as any definite or possible fatal or non-fatal CHD, stroke, or heart failure (Lee et al., 1990; Strong Heart Study, 2001).
2.4. Other variables
Baseline participant sociodemographic and covariate information (age, sex, education, study center, BMI, LDL, hypertension, diabetes, smoking status) was collected from Strong Heart Study baseline questionnaires and physical exams. We calculated eGFR using age, sex, and creatinine via the Chronic Kidney Disease – Epidemiology Collaboration formula (Levey et al., 2009). We defined hypertension as systolic blood pressure ≥ 140, diastolic blood pressure ≥ 90, or reported use of antihypertensive medication.
2.5. Statistical analysis
Urinary metals were skewed and log-transformed for analysis. All analyses were conducted in R version 3.2.4 (R Core Team, 2013). We used Cox proportional hazards models to evaluate progressively adjusted hazard ratios (HRs) of incident CVD by increasing quartile of urinary W concentrations. To allow a more flexible dose-response analysis, we also modeled urinary W continuously using a restricted quadratic spline model with knots at the 10th, 50th, and 90th percentiles with the reference at the 10th percentile. We used age as the time metric, with participants entering as late entries corresponding to age at baseline, and censored participants after age 90. P-values for trend were determined by assigning each participant the median level of W within their quartile. For all outcomes, Model 1 was stratified by study center (Arizona, Oklahoma, and North/South Dakota) and adjusted for sex and urinary creatinine. Model 2 was further adjusted for education (≤ 12 years/ > 12 years), BMI (continuous), LDL (continuous), hypertension (binary), diabetes (binary), eGFR (continuous), and smoking (current/former/never). Model 3 was further adjusted for urinary ΣAs (log-transformed continuous). We conducted sensitivity analyses further adjusting for urinary Cd and Mo. As an additional sensitivity analysis, we expressed urinary metal levels in µg/g creatinine, and did not adjust for urinary creatinine in regression models. All variables were treated as time fixed. Proportional hazards assumptions were evaluated by examining Schoenfeld residuals.
In effect modification analyses, we evaluated whether the HRs for an increase in urinary W corresponding to the interquartile range (IQR) (0.18 µg/L) differed by the following subgroups: age (< 55/≥ 55), sex (male/female), education (< 12 yrs/≥ 12 yrs), smoking status (never/former/current), BMI (< 25/25–29/ ≥ 30), urinary ΣAs tertiles (< 6.78/6.78–14.39/ > 14.39 µg/L), urinary Cd tertiles (< 0.77/0.77–1.52/ < 1.52 µg/L), and urinary Mo tertiles (< 24.89/24.89–45.45/ > 45.45 µg/L). Cox proportional hazards models included interaction terms between the subgroup indicator and an increase in urinary W corresponding to the IQR (0.18 µg/L). We hypothesized a priori that Mo levels would modify the association between W and CVD. We had no hypotheses regarding the direction of effect modification for other subgroups, including ΣAs and Cd.
3. Results
Among all participants, the median (IQR) of baseline urinary W was 0.13 (0.07, 0.25) µg/L. Urinary W concentrations (µg/L) for participants who later experienced incident CVD and no CVD were 0.13 (0.08, 0.24) and 0.13 (0.07, 0.27), respectively. Participants with urinary W measurements in the highest quartile were more likely male, more likely to live in Arizona and less likely to live in Oklahoma, more likely to have diabetes, less likely to have completed high school, had higher BMI and eGFR, and had lower LDL (Table 2). Participants in the highest quartile of urinary Mo levels were younger, more likely male, and from Arizona, to have diabetes, higher BMI and lower eGFR (Supplementary Material Table 1). Participants who later developed incident CVD had higher Mo levels at baseline than those who did not (Table 1).
Table 2.
Participant characteristics by urinary tungsten quartile in µg/L.
| Overall | ≤ 0.072 µg/L (N = 677) |
0.073–0.131 µg/L (N = 696) |
0.132–0.254 µg/L (N = 671) |
> 0.254 µg/L (N = 682) |
P for trenda | |
|---|---|---|---|---|---|---|
| Age - ± SE | 56.12 ± 0.2 | 56.3 ± 0.3 | 56.8 ± 0.3 | 56.2 ± 0.3 | 55.2 ± 0.3 | p = 0.004 |
| Female - % (SE) | 59.2 (0.9) | 62.5 (1.9) | 59.1 (1.9) | 59.6 (1.9) | 55.7 (1.9) | p = 0.019 |
| Center - % (SE) | ||||||
| Arizona | 14.0 (0.7) | 4.6 (0.8) | 9.1 (1.1) | 16.7 (1.4) | 25.8 (1.7) | p < 0.001 |
| North/South Dakota | 42.6 (0.9) | 40.5 (1.9) | 43.2 (1.9) | 45.2 (1.9) | 41.3 (1.9) | p = 0.597 |
| Oklahoma | 43.4 (0.9) | 54.9 (1.9) | 47.7 (1.9) | 38.2 (1.9) | 32.8 (1.8) | p < 0.001 |
| Less than high school - % (SE) | 40.6 (0.9) | 39.7 (1.9) | 40.4 (1.9) | 39.2 (1.9) | 43.0 (1.9) | p = 0.313 |
| MI – mean ± SE | 30.4 ± 0.1 | 30.0 ± 0.2 | 30.3 ± 0.2 | 30.5 ± 0.2 | 30.7 ± 0.2 | p = 0.044 |
| Smoking - % (SE) | ||||||
| Never | 29.3 (0.8) | 27.5 (1.7) | 30.3 (1.7) | 29.7 (1.8) | 29.6 (1.7) | p = 0.458 |
| Former | 32.9 (0.9) | 34.6 (1.8) | 33.3 (1.8) | 30.7 (1.8) | 33.1 (1.8) | p = 0.400 |
| Current | 37.8 (0.9) | 38.0 (1.9) | 36.4 (1.8) | 39.6 (1.9) | 37.2 (1.9) | p = 0.890 |
| SBP - mean ± SE | 126.1 ± 0.4 | 125.6 ± 0.7 | 126.6 ± 0.7 | 126.3 ± 0.7 | 125.7 ± 0.7 | p = 0.92 |
| Hypertensive - % (SE) | 35.5 (0.9) | 34.3 (1.8) | 37.9 (1.8) | 34.6 (1.8) | 35.3 (1.8) | p = 0.979 |
| LDL - mean ± SE | 120.0 ± 0.64 | 122.3 ± 1.3 | 122.0 ± 1.3 | 118.2 ± 1.3 | 117.6 ± 1.3 | p = 0.002 |
| eGFR - mean ± SE | 97.5 ± 0.3 | 96.6 ± 0.7 | 95.8 ± 0.6 | 99.0 ± 0.7 | 98.6 ± 0.7 | p = 0.001 |
| Diabetes - % (SE) | 42.2 (0.9) | 35.6 (1.8) | 41.8 (1.9) | 45.2 (1.9) | 46.3 (1.9) | p < 0.001 |
| Arizona | 67.5 (2.5) | |||||
| North/South Dakota | 36.3 (1.4) | |||||
| Oklahoma | 39.9 (1.4) | |||||
P-for trend obtained by adding quartile of tungsten into model as continuous variable.
Table 1.
Participant characteristics by incident CVD status (N = 2726).
| No CVD (N = 1824) | CVD (N = 902) | |
|---|---|---|
| Age - mean (SE) | 55.1 (0.2) | 58.2 (0.3) |
| Female - % (SE) | 60.9 (1.1) | 55.8 (1.7) |
| Education - % (SE) | ||
| No high school | 14.7 (0.8) | 22.1 (1.4) |
| Some high school | 22.4 (1.0) | 25.5 (1.5) |
| Completed high school | 62.9 (1.1) | 52.4 (1.7) |
| < 12 years | 37.1 (1.1) | 47.6 (1.7) |
| Smoking - % (SE) | ||
| Current | 36.2 (1.1) | 40.9 (1.6) |
| Former | 33.0 (1.1) | 32.8 (1.6) |
| Never | 30.8 (1.1) | 26.3 (1.5) |
| Alcohol - % (SE) | ||
| Current | 45.6 (1.2) | 37.5 (1.6) |
| Former | 39.8 (1.1) | 46.6 (1.7) |
| Never | 14.4 (0.8) | 16.0 (1.2) |
| BMI - mean (SE) | 30.0 (0.1) | 31.1 (0.2) |
| SBP - mean (SE) | 123.7 (0.4) | 130.9 (0.6) |
| Hypertensive - % (SE) | 29.7 (1.1) | 47.5 (1.7) |
| LDL - mean (SE) | 118.4 (0.8) | 123.3 (1.1) |
| eGFR - mean (SE) | 98.6 (0.4) | 95.2 (0.6) |
| Diabetes - % (SE) | 34.3 (1.1) | 58.3 (1.6) |
| aUrinary Cd µg/L | 1.09 (0.62, 1.75) | 1.10 (0.67, 1.87) |
| aUrinarybiAs µg/L | 9.52 (5.35, 17.22) | 10.08 (6.05, 18.35) |
| aUrinary Mo µg/L | 34.01 (19.68, 52.75) | 35.02 (21.32, 55.65) |
| aUrinary W µg/L | 0.13 (0.07, 0.27) | 0.13 (0.08, 0.24) |
Cd = cadmium; iAs = inorganic arsenic; Mo = molybdenum; W = tungsten.
Values are median (interquartile range).
iAs measured as sum of inorganic (arsenate and arsenite) and methylated species (MMA and DMA).
Out of 2726 study participants followed over 40,091 person-years, 317 experienced incident CVD mortality, of which 242 were CHD mortality and 41 were stroke mortality; 902 experienced non-fatal incident CVD, of which 643 were non-fatal CHD and 200 were non-fatal stroke (Tables 3 and 4). At baseline, participants experiencing an incident CVD event were older, more likely male, less likely to complete high school, more likely to have hypertension and diabetes, more likely to be current smokers but less likely to be current drinkers, and had higher BMI, systolic blood pressure, and LDL measurements, and lower eGFR (Table 1).
Table 3.
Hazard ratios for CVD, CHD, and stroke mortality by quartiles of urinary tungsten in µg/L (N = 2726).
| Total | ≤ 0.072 µg/L (N = 677) | 0.073–0.131 µg/L (N = 696) | 0.132–0.254 µg/L (N = 671) | > 0.254 µg/L (N = 682) | P-for trend | |
|---|---|---|---|---|---|---|
| CVD Mortality | ||||||
| No cases/ non-cases | 317 / 2409 | 73 / 604 | 97 / 599 | 76 / 595 | 71 / 611 | |
| Person-years | 40,091 | 10,127 | 10,378 | 9734 | 9852 | |
| HRs (95% CI) | ||||||
| Model 1 d | 1 (reference) | 1.28 (0.94, 1.74) | 1.09 (0.78, 1.53) | 1.05 (0.74, 1.49) | p = 0.67 | |
| Model 2 e | 1 (reference) | 1.16 (0.85, 1.58) | 1.01 (0.72, 1.42) | 0.91 (0.64, 1.30) | p = 0.29 | |
| Model 3 f | 1 (reference) | 1.15 (0.84, 1.57) | 1.00 (0.71, 1.41) | 0.90 (0.63, 1.29) | p = 0.28 | |
| CHD Mortality | ||||||
| No cases/ non-cases | 242 / 2484 | 58 / 619 | 71 / 625 | 56 / 615 | 57 / 625 | |
| Person-years | 40,091 | 10,127 | 10,378 | 9734 | 9852 | |
| HRs (95% CI) | ||||||
| Model 1 | 1 (reference) | 1.12 (0.79, 1.60) | 0.95 (0.65, 1.40) | 0.96 (0.64, 1.42) | p = 0.60 | |
| Model 2 | 1 (reference) | 1.01 (0.71, 1.44) | 0.87 (0.59, 1.29) | 0.81 (0.55, 1.22) | p = 0.25 | |
| Model 3 | 1 (reference) | 0.99 (0.69, 1.41) | 0.85 (0.57, 1.25) | 0.80 (0.53, 1.19) | p = 0.23 | |
| Stroke Mortality | ||||||
| No cases/ non-cases | 41 / 2685 | 12 / 665 | 11 / 685 | 12 / 659 | 6 / 676 | |
| Person-years | 40, 091 | 10,127 | 10,378 | 9734 | 9852 | |
| HRs (95% CI) | ||||||
| Model 1 | 1 (reference) | 0.60 (0.26, 1.39) | 1.11 (0.48, 2.57) | 0.60 (0.21, 1.71) | p = 0.31 | |
| Model 2 | 1 (reference) | 0.81 (0.35, 1.88) | 1.03 (0.44, 2.39) | 0.50 (0.18, 1.43) | p = 0.17 | |
| Model 3 | 1 (reference) | 0.80 (0.34, 1.84) | 0.97 (0.41, 2.27) | 0.48 (0.17, 1.38) | p = 0.15 | |
All models stratified by study center with age as time metric.
Adjusted for sex and urinary creatinine.
Adjusted further for education (≤ 12 years/ > 12 years), BMI (continuous), low-density lipoprotein (continuous), hypertension (binary), diabetes (binary), eGFR (continuous), and smoking (current/former/never).
Further adjusted for urinary iAs. Further adjustment for urinary Cd levels did not change estimates. P-trend obtained by assigning each participant the median tungsten value of their quartile.
Table 4.
Hazard ratios for CVD, CHD, and stroke incidence by quartiles of urinary tungsten µg/L (N = 2726).
| Total | ≤ 0.072 µg/L (N = 677) | 0.073–0.131 µg/L (N = 696) | 0.132–0.254 µg/L (N = 671) | > 0.254 µg/L (N = 682) | P-for trend | |
|---|---|---|---|---|---|---|
| CVD Incidence | ||||||
| No cases/ non-cases | 902 / 1824 | 212 / 465 | 255 / 441 | 232 / 439 | 230 / 479 | |
| Person-years | 35,266 | 8901 | 8951 | 8584 | 8829 | |
| HRs (95% CI) | ||||||
| Model 1 g | 1 (reference) | 1.17 (0.97, 1.41) | 1.13 (0.93, 1.38) | 1.01 (0.82, 1.24) | p = 0.42 | |
| Model 2 h | 1 (reference) | 1.08 (0.89, 1.30) | 1.07 (0.88, 1.30) | 0.90 (0.73, 1.11) | p = 0.11 | |
| Model 3 i | 1 (reference) | 1.07 (0.89, 1.29) | 1.06 (0.87, 1.29) | 0.89 (0.72, 1.10) | p = 0.09 | |
| CHD Incidence | ||||||
| No cases/ non-cases | 643 / 2083 | 151 / 526 | 180 / 516 | 156 / 515 | 156 / 526 | |
| Person-years | 36,576 | 9921 | 9288 | 8923 | 9073 | |
| HRs (95% CI) | ||||||
| Model 1 | 1 (reference) | 1.15 (0.92, 1.43) | 1.05 (0.83, 1.32) | 1.07 (0.84, 1.36) | p = 0.97 | |
| Model 2 | 1 (reference) | 1.05 (0.84, 1.30) | 1.01 (0.80, 1.27) | 0.97 (0.76, 1.24) | p = 0.65 | |
| Model 3 | 1 (reference) | 1.04 (0.83, 1.30) | 1.00 (0.79, 1.27) | 0.97 (0.76, 1.23) | p = 0.62 | |
| Stroke Incidence | ||||||
| No cases/ non-cases | 200 / 2526 | 55 / 622 | 47 / 649 | 56 / 615 | 42 / 640 | |
| Person-years | 39,160 | 9813 | 10,158 | 9505 | 9684 | |
| HRs (95% CI) | ||||||
| Model 1 | 1 (reference) | 1.07 (0.72, 1.59) | 1.17 (0.80, 1.73) | 0.97 (0.63, 1.50) | p = 0.90 | |
| Model 2 | 1 (reference) | 0.97 (0.65, 1.45) | 1.08 (0.73, 1.60) | 0.89 (0.58, 1.38) | p = 0.84 | |
| Model 3 | 1 (reference) | 0.97 (0.65, 1.44) | 1.05 (0.71, 1.56) | 0.87 (0.56, 1.35) | p = 0.78 | |
All models stratified by study center with age as time metric.
Adjusted for sex and urinary creatinine.
Adjusted further for education (≤ 12 years/ > 12 years), BMI (continuous), low-density lipoprotein (continuous), hypertension (binary), diabetes (binary), eGFR (continuous), and smoking (current/former/never).
Further adjusted for urinary iAs. Further adjustment for urinary Cd levels did not change estimates. P-trend obtained by assigning each participant the median tungsten value of their quartile.
Fully adjusted HRs (95% CI) comparing the highest to the lowest quartile of urinary W for incident non-fatal CVD, CHD, and stroke were 0.89 (0.72, 1.10), 0.97 (0.76, 1.23), and 0.87 (0.56, 1.35), respectively (Table 4). For fatal CVD, CHD, and stroke, HRs (95% CIs) were 0.90 (0.63, 1.29), 0.80 (0.53, 1.19), and 0.48 (0.17, 1.38), respectively (Table 3). Consistent with our analysis by W quartiles, restricted quadratic spline models showed no increase in the hazard of incident fatal and non-fatal CVD, CHD or stroke (data not shown) by increasing urinary W level. We found no violation of the proportional hazards assumption. Sensitivity analyses dividing urinary metal levels by urinary creatinine and further adjusting for urinary ΣAs and Cd did not alter results (results not shown). After log transformation and correction for urine dilution by dividing urinary metal levels by creatinine, the spearman correlation coefficient of W with Mo, As and Cd was 0.26, 0.25, and 0.13, respectively.
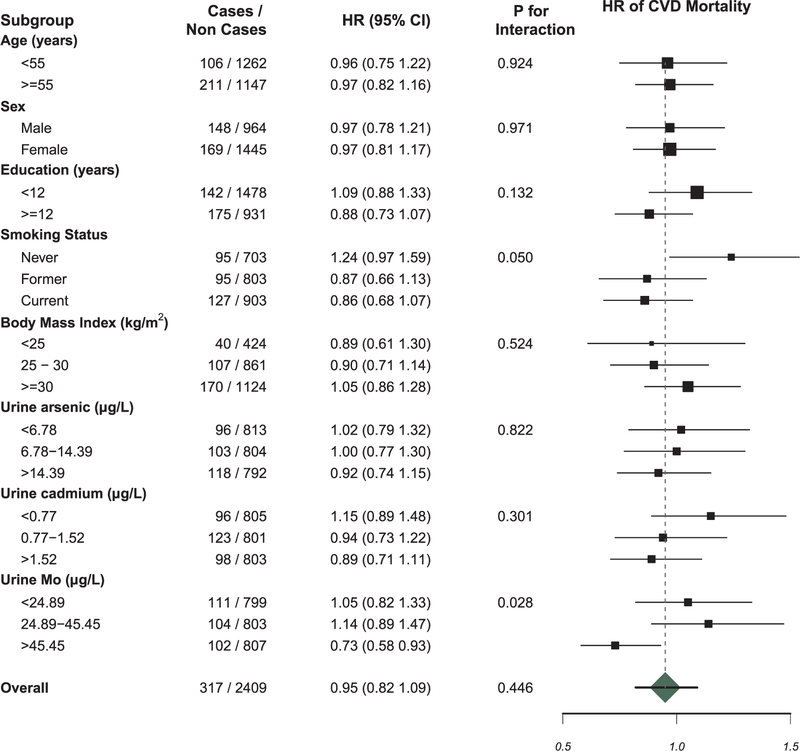
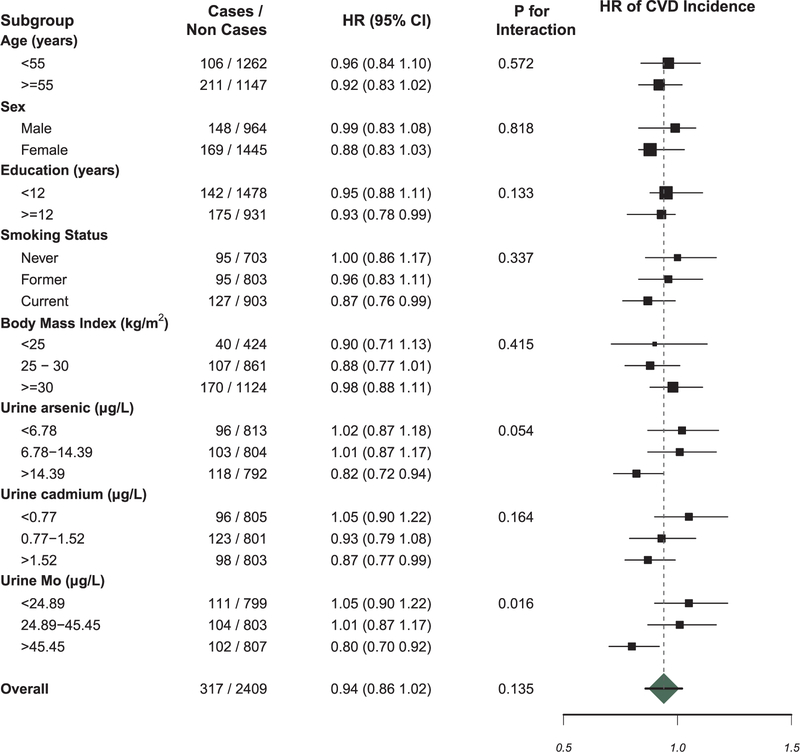
Fully adjusted HRs (95% CIs) for an IQR increase (0.18 µg/L) in urinary W were 0.95 (0.82, 1.09) for CVD mortality (Fig. 1) and 0.94 (0.86, 1.02) for CVD incidence (Fig. 2). Fully adjusted HRs comparing an increase in W levels corresponding to the IQR for CVD mortality among for those in the lowest, middle, and highest Mo tertiles were 1.05 (0.82, 1.33), 1.14 (0.89, 1.47) and 0.73 (0.58, 0.93), respectively (p-interaction = 0.03; Fig. 1). For incident non-fatal CVD, the HRs for those in the lowest, middle, and highest Mo tertiles were 1.05 (0.90, 1.22), 1.01 (0.87, 1.17), and 0.80 (0.70, 0.92), respectively (p-interaction = 0.02; Fig. 2).
Fig. 1.
Hazard ratio (95% confidence interval) of CVD mortality by an increase in log transformed urinary tungsten concentration corresponding to the interquartile range (75th to 25th percentile; 0.18 µg/L) within subgroups. Hazard ratios were calculated by using age as the time metric with late entries corresponding to age at baseline, stratified by study center (Oklahoma/Arizona/North and South Dakota), and fully adjusted for sex (male/female), urinary creatinine, education (≤ 12 yrs / > 12 yrs), BMI (continuous), low-density lipoprotein (continuous), hypertension (binary), diabetes (binary), eGFR (continuous), smoking (current/former/never), urinary Mo levels (log-transformed continuous), and urinary iAs levels (logtransformed continuous).
Fig. 2.
Hazard ratio (95% confidence interval) of incident CVD by an increase in log transformed urinary tungsten concentration corresponding to the interquartile range (75th to 25th percentile; 0.18 µg/L) within subgroups. Hazard ratios were calculated by using age as the time metric with late entries corresponding to age at baseline, stratified by study center (Oklahoma/Arizona/North and South Dakota), and fully adjusted for sex (male/female), urinary creatinine, education (≤ 12 yrs / >12 yrs), BMI (continuous), low-density lipoprotein (continuous), hypertension (binary), diabetes (binary), eGFR (continuous), smoking (current/former/never), urinary Mo levels (log-transformed continuous), and urinary iAs levels (log-transformed continuous).
The HR of CVD mortality for an increase in W levels corresponding to the IQR also differed by smoking status, with the association for W showing a marginally significant increase in the hazard of CVD mortality among never smokers compared to former and current smokers [1.24 (0.97, 1.59), 0.87 (0.66, 1.13), and 0.86 (0.68, 1.07), respectively; p-interaction = 0.05; Fig. 1]. No statistically significant interactions were found for W with ΣAs or Cd. As an additional analysis, we evaluated subgroups based on CKD-Epi eGFR levels, categorized at 30 and 60 ml/min/1.73 m2, and found no significant interaction for either incident or fatal CVD (results not shown; all p-values for interaction > 0.10).
4. Discussion
In the presence of low urinary Mo levels, elevated levels of baseline urinary W were associated with incident CVD in the Strong Heart Study, although the positive association was not statistically significant. At higher Mo levels, the association between W and incident CVD was inverse and statistically significant. A recent systematic review concluded that, although several cross-sectional studies reported a positive association between W biomarker levels and CVD outcomes, current epidemiological evidence is insufficient to support causality between W exposure and CVD (Nigra et al., 2016). In contrast to these recent cross-sectional analyses in NHANES, we did not find a positive association between urinary W levels and CVD with and without consideration of Mo concentrations in urine. It is possible that population-level differences, between the SHS study population and those evaluated in NHANES, including susceptibility factors such as nutritional status, explain this discrepancy. In addition, our statistical models adjusted for a range of potential confounders of the W-CVD relationship, including study center (stratified), BMI, education, LDL, diabetes, hypertension, eGFR, smoking, and urinary arsenic and cadmium. Previous NHANES analyses varied widely in the control of potential confounders (Nigra et al., 2016). Finally, all previous NHANES studies have been cross-sectional and the prospective association between W and CVD mortality in NHANES has not been reported so far. To our knowledge, this is the first epidemiological evidence supporting toxicological findings in the rat (Brondino et al., 2006) and in vitro (Lasfargues et al., 1992) that W and Mo exposures interact, and that W may cause toxicity by replacing Mo in active binding sites and rendering Mo-enzymes inactive. Several alternative explanations for our findings are also possible. For example, W and Mo may interact elsewhere besides molybdozymes, and molybdenum may be generally protective against CVD regardless of exposure to W or other metals which are toxic to the cardiovascular system such as arsenic or lead.
Although toxic at high levels, Mo is an essential element and cofactor for xanthine oxidase, sulfide oxidase, and aldehyde oxidase. In humans, xanthine oxidase reduces xanthine to uric acid and results in the formation of reactive oxygen species (ROS) (van der Knaap and Valk, 2005). Further, the conversion of xanthine dehydrogenase to xanthine oxidase may be involved in ischemia-reperfusion injury via ROS production (Klaassen and Watkins, 2015). Recent epidemiological evidence suggests that xanthine oxidase activity is associated with an increase in cardiovascular disease events, potentially via oxidative stress or increased uric acid production (Gondouin et al., 2015). Xanthine oxidase inhibitors are used to manage hyperuricemia and gout, and several clinical trials have evaluated the association of treatment with cardiovascular disease in patients with hyperuricemia or gout, attributing any association to decreased uric acid levels (Kim et al., 2015; Tani et al., 2015; Gotsman et al., 2012). The complex relationship between uric acid levels, xanthine oxidase activity, ROS production, and cardiovascular disease remains unclear, as uric acid is itself a strong antioxidant and elevated levels may be a response to increased ROS production by xanthine oxidase, or merely a marker for xanthine oxidase activity (George and Struthers, 2008).
Sulfite oxidase oxidizes both sulfite and sulfur dioxide, and sulfite oxidase deficiency results in severe birth defects and neurological damage via accumulation of sulfites (Feng et al., 2007). Molybdenum cofactor deficiency, in which all molybdozymes are rendered inactive, results in a similar phenotype to isolated sulfite oxidase deficiency, indicating that the inhibition of sulfite oxidase may be mostly responsible for disease manifestation, although disease pathogenesis remains unclear (van der Knaap and Valk, 2005). The inhibition of molybdozymes by W exposure may thus result in dysregulation of oxidative stress pathways and an increase in sulfite levels.
Elevated urinary W levels were inversely associated with incident fatal and non-fatal CVD in fully adjusted models among participants with urinary Mo levels above 45.45 µg/L (p-interaction = 0.03 and 0.02, respectively). Previous work in the National Health and Nutrition Examination Survey (NHANES) found the geometric mean level of urinary Mo in the general US population to be 37.7 µg/L (Navas-Acien et al., 2004). Further, Hays et al. derived biological equivalents for Mo exposure and determined that nutritionally sufficient intake at the recommended daily allowance (RDA) and estimated average requirement (EAR) correspond to urinary levels of 28 µg/L and 23 µg/L, respectively (Hays et al., 2016). Taken together, this evidence suggests that the general US population may be exposed to Mo levels that, although nutritionally sufficient, are potentially inadequate to prevent W toxicity. Further experimental and prospective observational studies evaluating the potential modification of W toxicity by low Mo levels are needed.
Participants in the Strong Heart Study are likely exposed to W through groundwater contaminated by the upwelling of geothermal waters or geological deposits containing W (Grimes et al., 1995; Seiler et al., 2005). We are not aware of any specific industrial sources of airborne W in SHS communities, but this has been recorded as a potential source of population exposure in other communities, including in Nevada and Oklahoma (Sheppard et al., 2012). Arsenic and W often occur together in drinking water aquifers due to their similar deposition properties and common industrial sources. The association between arsenic exposure and CVD is well documented at high-levels, and several studies have established low- to moderate- arsenic exposure as a risk factor for CVD, although further high-quality prospective studies are needed to affirm these results (Moon et al., 2012).
Tungsten is known to modify the toxicity of Co, however urinary Co levels were not available for SHS participants (Fenoglio et al., 2008). Additionally, this study was limited by the lack of toxicity data available on W at this time. It is well established that hypertension and diabetes are mediators of the arsenic-CVD relationship, and adjustment for these mediators attenuates the effect estimates (Moon et al., 2013). Because W is hypothesized to cause toxicity through similar mechanisms (oxidative stress and the formation of atherosclerotic plaques), it is possible that the toxic effects of W are also mediated through these arsenic-relevant pathways. However, we were unable to assess mediation given the lack of toxicity data. Future toxicological research should explore these causal pathways to better explain our findings. Among participants in the highest tertile of urinary inorganic arsenic exposure, the HR of CVD incidence for an IQR increase in urinary W was significantly decreased compared to those in the middle and lowest tertile of inorganic arsenic levels (Fig. 2). It is possible that the contribution of inorganic arsenic, a known toxicant to the cardiovascular system, overwhelms any impact of W on CVD risk at very high exposure levels. We found that participants in the highest quartile of urinary molybdenum concentrations had an inverse relationship between urinary W concentrations and incident and fatal CVD. It is possible that molybdenum may interact with W outside of molybdozymes, and/or may play a protective role against mechanisms related to CVD that are not currently well understood. Uric acid was not measured in the SHS at baseline, and we were unable to determine if uric acid was elevated in participants with elevated urinary tungsten concentrations. At present, our findings support a potential prospective association between W exposure and incident CVD in the presence of low urinary Mo levels, although additional high-quality prospective studies are needed.
Supplementary Material
Acknowledgments
Funding
Ana Navas-Acien and Anne Nigra are supported by R01ES021367 and R01ES025216. Anne Nigra is also supported by 5T32ES007322. This study was supported by cooperative agreement grants U01-HL41642, U01-HL41652, U01-HL41654, U01-HL65520, and U01-HL65521 and research grants R01-HL109315, R01HL109301, R01HL109284, R01HL109282 and R01HL109319 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Abbreviations:
- CVD
Cardiovascular disease
- HRs
Hazard ratios
- NHANES
National Health and Nutrition Examination Survey
- SHS
Strong Heart Study
- EPA
U.S. Environmental Protection Agency
- eGFR
Estimated glomerular filtration rate
- LDL
Low-density lipoprotein
- SBP
Systolic blood pressure
- MMA
Monomethylarsonate
- DMA
Dimethylarsinate
- ICP-MS
Inductively coupled plasma-mass spectrometry
- RDA
Recommended daily allowance
- EAR
Estimated average requirement
- MESA
Multi-Ethnic Study of Atherosclerosis
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2018.06.015.
Footnotes
Ethics approval and consent to participate
Informed consent was obtained from all participants. Study protocol was approved by the Indian Health Service institutional review boards, institutional review boards of participating institutions, and participating tribes.
Availability of data and material
Strong Heart Study data is owned by the participating tribes and is not available for public use.
Competing interests
The authors declare that they have no actual or potential competing financial interests.
References
- Agarwal S, Zaman T, Tuzcu EM, Kapadia SR, 2011. Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Angiology 62 (5), 422–429. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry, 2005. Toxicological profile for tungsten [PubMed]
- Bolt AM, Mann KK, 2016. Tungsten: an emerging toxicant, alone or in combination. Curr. Environ. Health Rep 3 (4), 405–415. [DOI] [PubMed] [Google Scholar]
- Brondino CD, Romao MJ, Moura I, Moura JJ, 2006. Molybdenum and tungsten enzymes: the xanthine oxidase family. Curr. Opin. Chem. Biol 10 (2), 109–114. [DOI] [PubMed] [Google Scholar]
- Feng C, Tollin G, Enemark JH, 2007. Sulfite oxidizing enzymes. Biochim. Biophys. Acta 1774 (5), 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio I, Corazzari I, Francia C, Bodoardo S, Fubini B, 2008. The oxidation of glutathione by cobalt/tungsten carbide contributes to hard metal-induced oxidative stress. Free Radic. Res 42 (8), 437–745. [DOI] [PubMed] [Google Scholar]
- George J, Struthers AD, 2008. The role of urate and xanthine oxidase inhibitors in cardiovascular disease. Cardiovasc Ther 26 (1), 59–64. [DOI] [PubMed] [Google Scholar]
- Gondouin B, Jourde-Chiche N, Sallee M, Dou L, Cerini C, Loundou A, et al. , 2015. Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels. Nephron 131 (3), 167–174. [DOI] [PubMed] [Google Scholar]
- Gotsman I, Keren A, Lotan C, Zwas DR, 2012. Changes in uric acid levels and allopurinol use in chronic heart failure: association with improved survival. J. Card. Fail 18 (9), 694–701. [DOI] [PubMed] [Google Scholar]
- Grimes DJ, Ficklin WH, Meier AL, Mchugh JB, 1995. Anomalous gold, antimony, arsenic, and tungsten in ground water and alluvium around disseminated gold deposits along the Getchell Trend, Humboldt County, Nevada. J. Geochem. Explor 52 (3), 351–371. [Google Scholar]
- Hays SM, Macey K, Poddalgoda D, Lu M, Nong A, Aylward LL, 2016. Biomonitoring Equivalents for molybdenum. Regul. Toxicol. Pharmacol 77, 223–229. [DOI] [PubMed] [Google Scholar]
- Herken EN, Kocamaz E, Erel O, Celik H, Kucukatay V, 2009. Effect of sulfite treatment on total antioxidant capacity, total oxidant status, lipid hydroperoxide, and total free sulfydryl groups contents in normal and sulfite oxidase-deficient rat plasma. Cell Biol. Toxicol 25 (4), 355–362. [DOI] [PubMed] [Google Scholar]
- Jelikic-Stankov M, Uskokovic-Markovic S, Holclajtner-Antunovic I, Todorovic M, Djurdjevic P, 2007. Compounds of Mo, V and W in biochemistry and their biomedical activity. J. Trace Elem. Med. Biol 21 (1), 8–16. [DOI] [PubMed] [Google Scholar]
- Kim SC, Schneeweiss S, Choudhry N, Liu J, Glynn RJ, Solomon DH, 2015. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: a cohort study. Am. J. Med 128 (6) (653.e7–653.e16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C, Watkins JB III, 2015. Casarett & Doull’s Essentials of Toxicology. McGraw Hill Professional
- Lasfargues G, Lison D, Maldague P, Lauwerys R, 1992. Comparative study of the acute lung toxicity of pure cobalt powder and cobalt-tungsten carbide mixture in rat. Toxicol. Appl. Pharmacol 112 (1), 41–50. [DOI] [PubMed] [Google Scholar]
- Lee ET, Welty TK, Fabsitz R, Cowan LD, Le N-A, Oopik AJ, et al. , 1990. The Strong heart study: a study of cardiovascular disease in American Indians: design and methods. Am. J. Epidemiol 132 (6), 1141–1155. [DOI] [PubMed] [Google Scholar]
- Lemus R, Venezia CF, 2015. An update to the toxicological profile for water-soluble and sparingly soluble tungsten substances. Crit. Rev. Toxicol 45 (5), 388–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. , 2009. A new equation to estimate glomerular filtration rate. Ann. Intern. Med 150 (9), 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lison D, 1996. Human toxicity of cobalt-containing dust and experimental studies on the mechanism of interstitial lung disease (hard metal disease). Crit. Rev. Toxicol 26 (6), 585–616. [DOI] [PubMed] [Google Scholar]
- Moon K, Guallar E, Navas-Acien A, 2012. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr. Atheroscler. Rep 14 (6), 542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. , 2013. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: a prospective cohort study. Ann. Intern. Med 159 (10), 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin JJ, Wild P, Romazini S, Lasfargues G, Peltier A, Bozec C, et al. , 1998. Lung cancer risk in hard-metal workers. Am. J. Epidemiol 148 (3), 241–248. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Sharrett AR, Calderon-Aranda E, Selvin E, Guallar E, 2004. Metals in urine and peripheral arterial disease. Environ. Health Perspect 113 (2), 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M, 2016. Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Curr. Environ. Health Rep 3 (4), 416–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Peng RD, Jones MR, Francesconi KA, Goessler W, Howard BV, et al. , 2016. Metal mixtures in urban and rural populations in the US: the Multi-Ethnic Study of Atherosclerosis and the Strong Heart Study. Environ. Res 147, 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2013. R: A language and environment for statistical computing Vienna, Austria. [Google Scholar]
- Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, et al. , 2012. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal. Methods 4 (2), 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler RL, Stollenwerk KG, Garbarino JR, 2005. Factors controlling tungsten concentrations in ground water, Carson Desert, Nevada. Appl. Geochem 20 (2), 423–441. [Google Scholar]
- Sheppard PR, Bierman BJ, Rhodes K, Ridenour G, Wittel ML, 2012. Comparison of size and geography of airborne tungsten particles in Fallon, Nevada, and Sweet Home, Oregon, with implications for public health. J. Environ. Public Health 509458 10.1155/2012/50945. [DOI] [PMC free article] [PubMed]
- Shiue I, Hristova K, 2014. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3–19% of the population attributable risk for high blood pressure: US NHANES, 2009–2012. Hypertens. Res 37 (12), 1075–1081. [DOI] [PubMed] [Google Scholar]
- Smith DB, Cannon WF, Woodruff LG, Solano F, Ellefsen KJ, 2014. Geochemical and mineralogical maps for soils of the conterminous United States: US Geological Survey; Report No.: 2331–1258.
- Stoddart ML, Jarvis B, Blake B, Fabsitz RR, 2000. Recruitment of American Indians in epidemiologic research: the Strong Heart Study. Am. Indian Alsk. Nativ. Ment. Health Res 9 (3), 20. [DOI] [PubMed] [Google Scholar]
- Strong Heart Study, 2001. Strong Heart Study Operations Manual Phase IV. Volume II: Morbidity and Mortality Surveillance Procedures. In. [Google Scholar]
- Tani S, Nagao K, Hirayama A, 2015. Effect of febuxostat, a xanthine oxidase inhibitor, on cardiovascular risk in hyperuricemic patients with hypertension: a prospective, open-label, pilot study. Clin. Drug Investig 35 (12), 823–831. [DOI] [PubMed] [Google Scholar]
- Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. , 2013. Cadmium exposure and incident cardiovascular disease. Epidemiology 24 (3), 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell J, Galloway TS, Abo-Zaid G, Melzer D, Depledge MH, Osborne NJ, 2013. High urinary tungsten concentration is associated with stroke in the National Health and Nutrition Examination Survey 1999–2010. PLoS One 8 (11), e77546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency, 2009. Emerging Contaminant - Tungsten
- van der Knaap MS, Valk J, 2005. Molybdenum cofactor deficiency and isolated sulfite oxidase deficiency. In: Magnetic Resonance of Myelination and Myelin Disorders, pp. 372–376.
- Welty TK, Lee ET, Yeh J, Cowan LD, Go O, Fabsitz RR, et al. , 1995. Cardiovascular disease risk factors among American Indians: the Strong Heart Study. Am. J. Epidemiol 142 (3), 269–287. [DOI] [PubMed] [Google Scholar]
- Witten ML, Sheppard PR, Witten BL, 2012. Tungsten toxicity. Chem. Biol. Interact 196 (3), 87–88. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Nakagawa M, Hosomi R, Nishiyama T, Fukunaga K, 2015. Low molybdenum state induced by tungsten as a model of molybdenum deficiency in rats. Biol. Trace Elem. Res 165 (1), 75–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.