Abstract
Introduction:
Human papillomavirus (HPV) vaccination rates nationally are low. This study determined if an electronic point-of-care prompt in the retail clinic setting increases HPV vaccination rates among an eligible population.
Study design:
An interrupted time series assessed change in weekly HPV vaccination rates with the introduction of an electronic point-of-care prompt and rate change in post-intervention period.
Setting/participants:
The study sites were two similar retail care clinics in Rochester, Minnesota. Participants were patients who presented to the retail clinics setting between the ages of 9 and 26 years from September 12, 2016, to September 30, 2017.
Intervention:
HPV vaccine (nonavalent) was made available at both retail clinics. Staff completed a 2-hour lecture on HPV vaccine and one-on-one training for use of the prompt. Pre- and post-intervention rates of HPV vaccination after initiation of electronic point-of-care prompt were measured. A satisfaction survey was given to all patients or parents/guardians between the ages of 9 and 26 years regardless of HPV vaccine status.
Main outcome measures:
HPV vaccination rates per week before and after the introduction of the electronic point-of-care prompt along with satisfaction with HPV vaccine availability and the point-of-care prompt in the retail clinic setting. Data analysis was completed January 2018.
Results:
The point-of-care prompt increased the median weekly HPV vaccination rate by 8.6 per 100 patient visits (95% CI=5.8, 11.5, p<0.001). Patients thought it was convenient having HPV vaccine available and helpful to be reminded of the need for HPV vaccine.
Conclusions:
This study demonstrates a significant increase of HPV vaccine rates in the retail clinic setting with use of a point-of-care prompt.
INTRODUCTION
Experts recommend all U.S. children complete the human papillomavirus (HPV) vaccine at age 11–12 years. Yet, among adolescents aged 13–17 years living in the U.S., in 2016 only 43.4% of adolescents (49.5% of females, 37.5% of males) were up-to-date with the HPV vaccination series.1 This compares poorly with rates of tetanus, diphtheria, acellular pertussis vaccination (88.0%) and the first dose of meninigococcal vaccine (groups A, C, W, Y) (82.2%)—both due at age 11–12 years as well. All three were first recommended by the Advisory Committee on Immunization Practice (ACIP) within 2 years of each other.1–5 The Healthy People 2020 goals call for 80% of all adolescents aged 13–15 years to receive each of these vaccines.4 The HPV vaccine has been found to be safe and effective, yet there are still significant barriers to increasing vaccination rates. Studies indicate poor vaccination with HPV has resulted from a variety of factors including lack of a clear healthcare provider recommendation, lack of parental awareness of the vaccine and the ACIP recommendation, lack of adolescent utilization of routine health care, concern about the vaccine’s effect on sexual behavior, and the perceived low risk of HPV infection and risk of cancer.1–5
Point-of-care prompts deliver healthcare providers patient specific alerts for services due, typically preventive in nature. In the case of vaccines, the point-of-care prompts utilize the patient’s age and vaccination record. Multiple systematic review research studies have demonstrated that point-of-care prompts can improve HPV vaccination rates in the adolescent population.6–10 Additionally, one systematic review found that not only are reminder strategies highly effective, expanding HPV vaccine availability to locations other than primary care offices can also improve HPV vaccine uptake.10
Retail clinics have increasingly offered preventive health services, such as health physicals, screenings, immunizations, and wellness programs.11 Immunizations are available at almost all retail clinics.12 Retail clinics overcome obstacles to traditional office-based health care by requiring no appointments and offering extended evening and weekend hours.11 Although it is unknown how many retail clinics are integrated into healthcare systems, almost all retail clinics have EMRs that integrate evidence-based guidelines.13
The primary objective of the study is to determine whether an electronic point-of-care prompt utilizing the patient’s electronic vaccination record indicating HPV vaccine-dose eligibility as compared with no electronic point-of-care prompt will increase the uptake of HPV vaccine for a population of patients presenting at retail clinics. A secondary objective of the study is to assess the attitudes and perceptions of patients toward the point-of-care prompt through a confidential survey.
METHODS
Study Population
The study sites were two similarly sized retail care clinics located in shopping areas, in Rochester, Minnesota, both owned and operated by Mayo Clinic. The retail clinics’ titles are “Mayo Clinic Express Care.” These clinics are open to the public and available to empaneled primary care patients of Mayo Clinic cared for in the Southeast Minnesota region. Empaneled patients are defined as those who have a primary care provider at Mayo Clinic. Mayo Clinic Employee and Community Health initiative is a collaborative of Mayo Clinic’s primary care departments and provides primary care for >151,000 empaneled patients who are cared for at seven full-service clinical practices and two retail clinic sites. The Employee and Community Health retail clinics receive walk-in patients aged from 18 months to 75 years, 7 days a week with extended hours on weekdays, and are staffed by certified nurse practitioners and licensed practical nurses. The study population included a community-based cohort of empaneled primary care patients, males and females between the ages of 9 and 26 years from September 12, 2016, to September 30, 2017, who presented to either retail clinic. Data analysis was completed January 2018.
Measures
The study began by making the HPV vaccine available on September 12, 2016, at both retail clinics. Clear visible signage was posted indicating the HPV vaccine availability and charges for the HPV vaccines required by state law. The signage also indicated that Minnesota Vaccines for Children were available,14 which is a state program of the federally funded Vaccines for Children program that provides free-of-charge vaccines to eligible children through age 18 years. Eligible children include those receiving state medical assistance, uninsured, and those who are American Indian or Alaskan Natives.14 All participating retail clinic staff (certified nurse practitioners and licensed practical nurses) attended a 2-hour lecture on August 10, 2016 (before the vaccine was made available), by the primary care practices’ immunization director. This lecture was on HPV vaccine efficacy, safety, and how to address vaccine hesitancy with patients and parents.
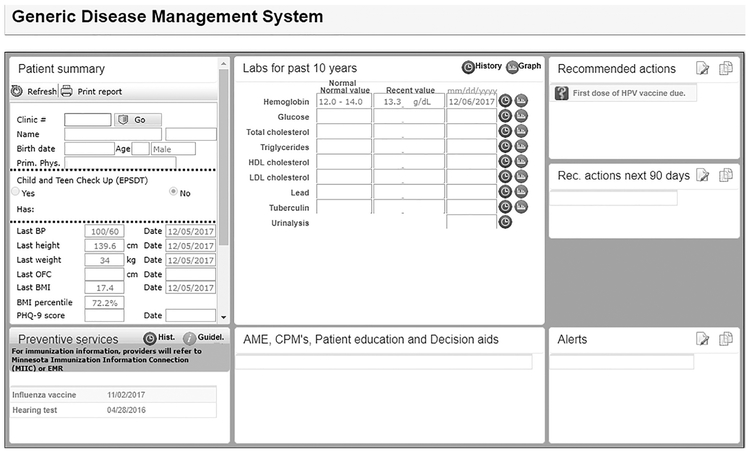
All staff received one-on-one training on usage of the electronic point-of-care prompts 2–4 weeks before the prompting began. The point-of-care prompt utilized the Generic Disease Management System, which is a web-based knowledge delivery solution integrated in the electronic medical record that can support clinical decision making, such as the need for immunizations. Upon patient check-in, the Generic Disease Management System was generated from the electronic medical record; this included the patient’s clinic number, birth date, age, sex, last preventive services, and recommended actions for the patient. The Generic Disease Management System was printed and given to the patient or parent/guardian (Figure 1). If the HPV vaccine was due, it was included in the list of recommended actions and stated which dose was needed (first, second, or third). The recommendations were pulled from the patient’s immunization records and calculated based off ACIP recommendations.
Figure 1.
Point-of-care prompt.
EPSDT, early periodic screening diagnosis and treatment; BP, blood pressure; OFC, occipital frontal circumference; PHQ-9, Patient Health Questionnaire 9; EMR, electronic medical record; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AME, Ask Mayo Expert; CPM, care process model; HPV, human papillomavirus; Rec., recommended.
On September 12, 2016, both retail clinics advertised via signage that the HPV vaccine was available. Starting that day on September 12, 2016, data from the patients’ medical records were collected on age; sex; race; HPV eligibility criteria (if they were due for HPV vaccine); dose recommended (first, second, or third dose); and receipt of the HPV vaccine. The weekly rates of HPV vaccine doses administered to the eligible empaneled primary care patients were measured for the entire study. Data were available for only those patients who had given prior authorization access to medical records research as required by Minnesota law. Of 3,572 individual visits that were eligible for the HPV vaccine, 3,234 had consented to medical records research, resulting in a 90.5% prior authorization rate for the study.
Beginning January 1, 2017, during the study period, Mayo Clinic adopted the ACIP recommendation for using a two-dose HPV vaccine rule for patients who had received their first dose of HPV vaccine before age 15 years and had not received a second dose 6 months after their first dose. After having the vaccine available for 22 weeks, on February 9, 2017, staff at the retail clinics started to utilize the electronic point-of-care prompt. The date was chosen to make sure there was appropriate power to test differences in HPV rates while not needlessly delaying the implementation of the prompt. Using simulation, it was determined that there was 85% power to detect an effect size of 2.5 (incident rate ratios [IRRs]), which was considered an acceptable clinical effect to the study team. The electronic point-of-care prompt utilized the patient’s electronic medical record to determine if that patient was due for a dose of HPV vaccine. It was an expectation for all staff to complete the prompt and provide the survey at the end of the encounter for all patients between the ages of 9 and 26 years.
In a supplemental data analysis to the quantitative study, patient’s attitudes and perceptions were evaluated through a confidential voluntary survey. This survey had a cover letter attached, describing the intentions of the survey. It was given to all patients or parents/guardians between the ages of 9 and 26 years regardless of whether they received a vaccine or not during the encounter and then returned in a confidential box after their visit. The survey was given for 22 weeks after the point-of-care prompting started on February 9, 2017.
The survey requested demographic information of who completed the form (parent/guardian of patient or patient); age of the patient (9–11 years, 12–18 years, or 19–26 years); and sex of the patient. Agreement statements of yes/no were answered to questions: Having the HPV vaccine available in Express Care is convenient; Being reminded of the HPV vaccine during my visit is helpful; I will consider getting future vaccinations for myself or my child at Express Care; and I would recommend vaccination at Express Care to others. Overall satisfaction of convenience, quality of care, and experience was also included in the survey.
Statistical Analysis
Demographic characteristics and utilization measures were compared across pre-prompt and post-prompt categories using chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables (Table 1). The weekly captured opportunities (count of HPV vaccines per number of eligible patients seen) were determined and modeled in both pre-prompt and post-prompt periods using LOESS (locally weighted scatter-plot smoother) curves to account for any nonlinearity. For ease in interpretation period, Poisson regression models were used to estimate the change in rates. A total of six periods were tested: 0–20 weeks (reference); and 22–28, 29–34, 35–40, 41–47, and 48–56 weeks. The reason for multiple time periods is that the authors wanted to assess nonlinearities based on results of the LOESS curve. The categories used were to keep each period to be roughly 6 weeks in length. Additionally, Poisson regression models were adjusted for other covariates associated with HPV vaccination, such as age, race, sex, and HPV series dose (Table 2). Further testing was carried out by changing the reference category from 0–20 weeks to 41–47 weeks to see if there were any significant differences between the post-periods. Results from the Poisson regression are presented as IRRs with 95% CIs. All analyses were performed by using R, version 3.2.3, software. Statistical comparisons were two-sided and were considered significant at the p<0.05 level.
Table 1.
Demographic Characteristics of Patients Eligible to Receive HPV Vaccine by Pre–Post Period
| Demographics | Pre-prompt (n=1,469) | Post-prompt (n=1,765) | Total (N=3,234) | p-valuea |
|---|---|---|---|---|
| Age (years), M (SD) | 14 (4.99) | 14.2 (5.09) | 14.11 (5.04) | 0.207 |
| Sex | 0.010 | |||
| Female | 750 (51.1) | 982 (55.6) | 1,732 (53.6) | |
| Male | 719 (48.9) | 783 (44.4) | 1,502 (46.4) | |
| Race/ethnicity | 0.183 | |||
| White, NH | 1,243 (84.6) | 1,523 (86.3) | 2,766 (85.5) | |
| Asian, NH | 49 (3.3) | 38 (2.2) | 87 (2.7) | |
| Black, NH | 33 (2.2) | 47 (2.7) | 80 (2.5) | |
| Hispanic | 59 (4.0) | 58 (3.3) | 117 (3.6) | |
| Other/unknown | 85 (5.8) | 99 (5.6) | 184 (5.7) | |
| Visit site | 0.769 | |||
| Express care north | 773 (52.6) | 939 (53.2) | 1,712 (52.9) | |
| Express care south | 696 (47.4) | 826 (46.8) | 1,522 (47.1) | |
| HPV recommendation | 0.066 | |||
| First dose due | 1,085 (73.9) | 1,363 (77.2) | 244.8 (75.7) | |
| Second dose due | 237 (16.1) | 258 (14.6) | 495 (15.3) | |
| Third dose due | 147 (10.0) | 144 (8.2) | 291 (9.0) |
Note: Data presented as n (%) unless otherwise noted. Boldface indicates statistical significance (p<0.05).
The p-value is from χ2 tests.
HPV, human papillomavirus; NH, non-Hispanic.
Table 2.
Poisson Regression Assessing the Weekly Rate of HPV Vaccination Adjusted for Baseline Covariates
| Variablea | IRR (95% CI) | p-value |
|---|---|---|
| Post-prompt periods | ||
| Time period 22–28 weeks | 7.73 (5.08, 12.08) | <0.001*** |
| Time period 29–34 weeks | 6.74 (4.27, 10.84) | <0.001*** |
| Time period 35–40 weeks | 5.35 (3.15, 9.06) | <0.001*** |
| Time period 41–47 weeks | 3.37 (1.84, 6,09) | <0.001*** |
| Time period 48–56 weeks | 6.81 (4.26, 11.04) | <0.001*** |
| Adjusted covariates | ||
| Age | 0.96 (0.93, 0.98) | <0.001*** |
| Male | 0.87 (0.67, 1.12) | 0.28 |
| Second dose of HPV vaccine due | 5.43 (4.04, 7.32) | <0.001*** |
| Third dose of HPV vaccine due | 6.55 (4.62, 9.21) | <0.001*** |
| Asian, NH | 2.16 (1.07, 3.89) | 0.02* |
| Black, NH | 1.78 (0.94, 3.06) | 0.05 |
| Hispanic | 1.54 (0.83, 2.61) | 0.13 |
| Other/unknown | 1.43 (0.86, 2.24) | 0.14 |
Note: Boldface indicates statistical significance
p<0.05;
p<0.01;
p<0.001).
Reference groups: Pre-prompt time period 0–20 weeks; female; first dose of HPV vaccine due; and white, NH.
HPV, human papillomavirus; IRR, incident rate ratio; NH, non-Hispanic.
This study was approved by the IRB at Mayo Clinic, Rochester, Minnesota.
RESULTS
A total of 3,234 visits for empaneled patients ages 9 through 26 years were eligible to receive a dose of HPV vaccine. Table 1 compares the patients seen in the first 22 weeks (pre-prompt) with the patients seen after the initiation of the electronic point-of-care prompt. There were no differences between the 1,469 eligible patient visits in the pre-prompt time period and the 1,765 patient visits in the post-prompt time period in terms of age, race/ethnicity, visit site, or doses due. However, there was an increased statistical difference in females who were eligible to receive the HPV vaccine (p<0.01). Patients were counted at each visit in which they received a prompt. If a patient had multiple visits, they were counted multiple times. There were 532 (21%) patients who came in for multiple visits. A sensitivity analysis was completed to determine if this had an impact on the analysis by adding a random effect for patient and no clinically meaningful differences were found.
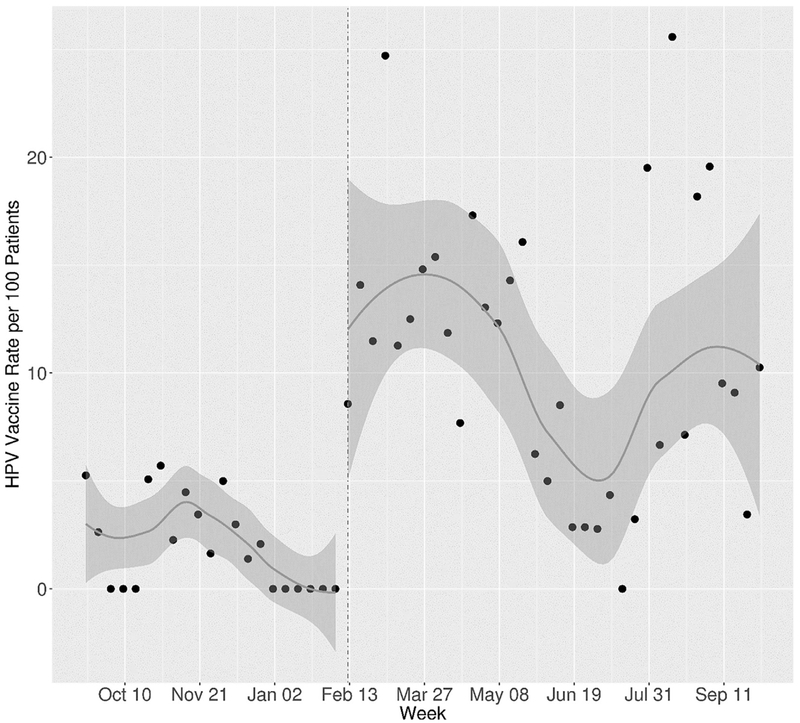
Of the 1,469 eligible patient visits that occurred in the pre-prompt time period, a dose of the HPV vaccine was administered in 29 (2%) of the visits. Of the 1,765 eligible patient visits that occurred in the post-prompt period, a dose of the HPV vaccine was administered in 207 (12%) of the visits. The median weekly rate per 100 patients in the pre-prompt period was 1.64 (IQR=0, 3.5). The median weekly rate per 100 patients in the post-prompt period was 10.8 (IQR=6.4, 14.7). The median difference in weekly rates per 100 patients was 8.6 (95% CI=5.8, 11.5, p<0.001).
An increase in the weekly rate was found in the post-period as shown in Figure 2. In a secondary analysis, Poisson regression showed that the rate was highest in the 22–28-week period with a statistically significant attenuation seen at the 41–47-week period (Table 2). As shown in Table 2, Asians were statistically more likely than whites to get vaccinated (p=0.02). There was no statistical difference in vaccination rates between females and males (p=0.28). Compared with the first dose of HPV vaccination, the second and third doses had statistically higher uptake (p<0.001). In testing for any variation between post-prompt periods, there were significant increases in the HPV vaccination rate for the 22–28-week period (IRR=2.29, 95% CI=1.38, 4.03) as well as the 29–34-week (IRR=2.00, 95% CI=1.17, 3.59) and 48–56-week periods (IRR=2.02, 95% CI=1.17, 3.64) compared with the lowest period of 41–47 weeks.
Figure 2.
Weekly rates of HPV vaccine per 100 eligible patients’ pre-prompt and post-prompt.
HPV, human papillomavirus.
There were 2,663 patients who presented between the ages of 9 and 26 years during the intervention period who received the survey regardless of HPV vaccination status. Of the eligible patients or parents/guardians in the survey time period, 1,475 completed the survey, for a response rate of 55%. A total of 40.3% were completed by the patient and 59.7% were completed by a parent or guardian. There were 26.3% of patients between age 9 and 11 years, 42.5% between 12 and 18 years, and 31.2% were between 19 and 26 years. Lastly, 62.5% of the patients reported to be female.
In regard to the overall care received, 98.2% reported being completely satisfied or satisfied with convenience, 98.8% reported being completely satisfied or satisfied with the quality of care, and 98.2% completely satisfied or satisfied with the overall experience. A total of 88.8% of patients reported prompting for the HPV vaccine. Of the 1,310 patients reporting prompting, 97.5% reported it was convenient having HPV vaccine available at Express Care, 91.6% reported it was helpful being reminded of HPV vaccine during the visit, 94.6% reported that they would consider getting future vaccinations at Express Care, and 96.6% reported they would recommend vaccination at Express Care to others.
DISCUSSION
This study demonstrates a significant increase of HPV vaccination rates in the retail clinic setting with the use of the point-of-care prompt. Patients and parents found it convenient having HPV vaccine available and helpful being reminded of HPV vaccine during the visit. This may be the first study to demonstrate the impact of a point-of-care prompt in the retail clinic setting for HPV vaccines. Studies have shown that point-of-care prompts increased HPV vaccine uptake, but none were conducted in retail clinics.6–10 In the U.S., males have overall lower HPV vaccine uptake compared with females, in this study there was no statistical difference in vaccination at the retail clinics between females and males.15
There was a modification to the study when changes from the ACIP recommended eliminating the third dose for those who began the series before age 15 years and increased the interval for the second dose. This recommendation would have decreased the number of second doses due in the first 6 months of the recommendation and eliminate the number of third doses due for many patients. Thus, this change arguably would have worked against the number of patients who were eligible for vaccination.
The literature supports the importance of giving the vaccine to pre-adolescents when their immune response is strongest to the vaccine.16 Fortunately, those who have minor acute illnesses, such as diarrhea, cold symptoms, or sore throats, with or without fevers, may still be safely vaccinated.17 Retail clinics nationally are increasingly integrating with healthcare systems, and it is not unusual for retail clinics to share a common electronic health record such as in this study.
During the implementation process, it was frequently observed that if the parent or patient refused due to illness, awareness of the vaccine availability was provided. There was no data collected regarding reasons for refusals or for those who expressed willingness to come in for future vaccines. This increased awareness for HPV vaccine need and availability was not captured in the data analysis.
Limitations
There were several limitations to this study. One limitation was reliance on the staff to prompt the patient about vaccine status and give the survey to the patients. Anecdotal observations during the study found that providers and nurses who were hesitant about the HPV vaccine may have been less compliant with completing the point-of-care prompt. Reasons such as concerns about vaccine efficacy and effectiveness were mentioned, which is consistent with literature regarding HPV vaccine barriers.18 Another limitation was the study population consisted primarily of citizens of Southeastern Minnesota. This mainly white population is highly “medically influenced,” because the largest local employer is Mayo Clinic. Furthermore, patients in this setting are seen for acute minor illnesses, which might have influenced parents’ decisions of vaccinating their ill child. The fastpaced nature of the environment may have impacted the decision for parents and patients to vaccinate based on time restraints. There was the potential for bias with the survey administration in that it could have been completed more than once and caused the population to be inadequately represented in the sample.
There was attenuation in weeks 41–47. This may have been because of a concurrent study started at the retail clinics around the same time that lessened the attention to the prompts. However, this trend did not continue into the next time period, suggesting unexplained variation occurring in addition to an effective prompt. It was also noted that no vaccines were given in the pre-intervention period around January, which may have been because of the peak influenza illnesses and the severity of sickness in eligible patients. There is need to do further research to determine if vaccination rates would continue to vary by season. Overall, there was still higher HPV vaccine rates post-intervention than pre-intervention.
CONCLUSIONS
This study demonstrates a significant increase of HPV vaccine rates in the retail clinic setting with the use of the point-of-care prompt that utilized the patients’ electronic medical records. The increased growth of retail clinics and using point-of-care prompts in electronic medical records provides additional opportunities for improving HPV vaccine uptake. Point-of-care prompts in the retail clinics may pave the way for addressing other lapses in routine immunizations and preventive services.
ACKNOWLEDGMENTS
We thank all nurse practitioners and nurses at the retail clinics for their help with the intervention and data collection. Thank you to Alicia Meek, Mayo Clinic research coordinator, with guidance in IRB process and with data entry. A thank-you to Dr. Maya Kessler, Primary Care Internal Medicine, Mayo Clinic, for providing general oversight during the intervention development and study implementation. No compensation was provided for these contributions beyond usual salary.
The Mayo Clinic Department of Family Medicine funded this study. The funding strictly gave protected research time to complete study and had no role in the design and conduct of the study; analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Mayo Clinic IRB number 16-009635.
Amanda Meyer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors were responsible for study concept and design; acquisition, analysis, or interpretation of data; and manuscript revisions. Ms. Meyer was responsible for drafting of the manuscript, obtaining funding, and study supervision. Mr. Wilson was responsible for statistical analysis. All authors read and approved the final version of the submitted manuscript.
Dr. Jacobson reported being a member of two Safety Review Committees for two studies funded by Merck that concern HPV vaccines—one is a post-licensure safety study of Gardasil in males and the other is a post-licensure safety study of Gardasil®9 in both sexes. He is also on a Data Monitoring Committee for a series of prelicensure studies of an experimental 15-valent pneumococcal conjugate vaccine. This is also funded by Merck. No other financial disclosures were reported from authors.
REFERENCES
- 1.Nordin JD, Solberg LI, Parker ED. Adolescent primary care visit patterns. Ann Fam Med. 2010;8(6):511–516. 10.1370/afm.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among U.S. adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168 (1):76–82. 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kester L, Zimet G, Fortenberry J, Kahn J, Shew M. A national study of HPV vaccination of adolescent girls: rates, predictors, and reasons for non-vaccination. Matern Child Health J. 2013;17(5):879–885. 10.1007/s10995-012-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sussman A, Dunham L, Snower K, et al. Retail clinic utilization associated with lower total cost of care. Am J Manag Care. 2013;19(4):e148–e157. [PubMed] [Google Scholar]
- 5.Radisic G, Chapman J, Flight I, Wilson C. Factors associated with parents’ attitudes to the HPV vaccination of their adolescent sons: a systematic review. Prev Med. 2017;95:26–37. 10.1016/j.ypmed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy B, Braxter B, Charron-Prochownik D, Schlenk EA. A quality improvement initiative to increase HPV vaccine rates using an educational and reminder strategy with parents of preteen girls. J Pediatr Health Care. 2014;28(2):155–164. 10.1016/j.pedhc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Das JK, Salam RA, Arshad A, Lassi ZS, Bhutta ZA. Systematic review and meta-analysis of interventions to improve access and coverage of adolescent immunizations. J Adolesc Health. 2016;59(4 suppl):S40–S48. 10.1016/j.jadohealth.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis DB, Cates JR, Wagner KPG, Zola T, Fitter JE, Coyne-Beasley T. Communication technologies to improve HPV vaccination initiation and completion: a systematic review. Patient Educ Couns. 2017;100(7):1280–1286. 10.1016/j.pec.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Niccolai LM, Hansen CE. Practice- and community-based interventions to increase human papillomavirus vaccine coverage: a systematic review. JAMA Pediatr. 2015;169(7):686–692. 10.1001/jamapediatrics.2015.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1): e20153863 10.1542/peds.2015-3863. [DOI] [PubMed] [Google Scholar]
- 11.Rudavsky R, Pollack CE, Mehrotra A, Rudavsky R, Pollack CE, Mehrotra A. The geographic distribution, ownership, prices, and scope of practice at retail clinics. Ann Intern Med. 2009;151(5):315–320. 10.7326/0003-4819-151-5-200909010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossos K Retail clinics: not just treating illness—preventing it. Pharmacy Times. March 16, 2015. www.pharmacytimes.com/publications/issue/2015/march2015/retail-clinics-not-just-treating-illnesspreventing-it. Accessed June 27, 2018.
- 13.Pollack CE, Gidengil C, Mehrotra A. The growth of retail clinics and the medical home: two trends in concert or in conflict? Health Aff (Millwood). 2010;29(5):998–1003. 10.1377/hlthaff.2010.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minnesota Department of Health. Minnesota Vaccines for Children (MnVFC). www.health.state.mn.us/vfc. Published 2016. Accessed September 2016.
- 15.Vielot NA, Butler AM, Brookhart MA, Becker-Dreps S, Smith JS. Patterns of use of human papillomavirus and other adolescent vaccines in the United States. J Adolesc Health. 2017;61(3):281–287. 10.1016/j.jadohealth.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Committee Opinion No. 704 Summary: Human Papillomavirus Vaccination. Obstet Gynecol. 2017;129(6):1155–1156. 10.1097/AOG.0000000000002111. [DOI] [PubMed] [Google Scholar]
- 17.CDC. HPV vaccine information for clinicians. www.cdc.gov/std/hpv/STDFact-HPV-vaccine-hcp.htm; Published 2015. Accessed June 27, 2018.
- 18.Farias AJ, Savas LS, Fernandez ME, et al. Association of physicians perceived barriers with human papillomavirus vaccination initiation. Prev Med. 2017;105:219–225. 10.1016/j.ypmed.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]