Abstract
Organophosphate esters (OPEs) are a class of chemicals commonly used as flame retardants and plasticizers. OPEs are applied to a wide variety of consumer products and have a propensity to leach from these products. Consequently, OPEs are ubiquitous contaminants in many human environments and human exposure is pervasive. Accumulating evidence suggests that OPEs are capable of interfering with childhood cognitive development through both neurologic- and endocrine-mediated mechanisms. However, observational evidence of cognitive effects is limited. We used data collected in the third phase of the Pregnancy, Infection, and Nutrition Study to investigate cognitive effects of prenatal exposure to OPEs. In a spot prenatal maternal urine sample, we measured the following OPE metabolites: diphenyl phosphate (DPHP), bis(1,3-dichloro-2-propyl phosphate) (BDCIPP), isopropyl-phenyl phenyl phosphate (ip-PPP), and 1-hydroxyl-2-propyl bis(1-chloro-2-propyl) phosphate (BCIPHIPP). We assessed children’s language and multi-faceted and overall cognitive development between two and three years of age using the MacArthur-Bates Communicative Development Inventories (MB-CDI) and the Mullen Scales of Early Learning (MSEL). We used linear regression to estimate the change in children’s scores on these developmental assessments per interquartile range (IQR) increase in log-transformed, specific-gravity-corrected prenatal OPE metabolite concentrations, adjusted for maternal age, education, income, race/ethnicity, BMI, and child’s sex. A total of 149 children had both OPE metabolite measurements and MB-CDI scores, and 227 children had both OPE metabolite measurements and MSEL scores. We observed that higher concentrations of ip-PPP (ng/ml) were associated with lower scores on the MSEL Cognitive Composite Score (β = −2.61; 95% CI: −5.69, 0.46), and separately on two of the four MSEL Scales that comprise the Cognitive Composite, specifically the Fine Motor Scale (β = −3.08; 95% CI: −5.26, −0.91) and the Expressive Language Scale (β = −1.21; 95% CI: −2.91, 0.49). We similarly observed that prenatal ip-PPP concentrations were inversely associated with age-standardized scores on the MB-CDI Vocabulary assessment (β = −1.19; 95% CI: −2.53, 0.16). Other OPE metabolites were not strongly associated with performance on either assessment. Our results suggest that isopropylated triarylphosphate isomers, the presumed parent compounds of ip-PPP, may adversely impact cognitive development, including fine motor skills and early language abilities. Our study contributes to the growing body of observational evidence that suggests prenatal exposure to OPEs may adversely affect cognitive development.
Keywords: Cognitive, OPE, OPFR, Organophosphate, Flame Retardant, Neurodevelopment
1. Introduction
Organophosphate esters (OPEs) have emerged as a prevalent class of flame retardants following the phase out of polybrominated diphenyl ethers (PBDEs) [1–3], a class of widely-used flame retardants that were phased out of usage in the United States in the early 2000s amid concerns about their environmental persistence and toxicity [3–6]. OPEs are applied as additive flame retardants to polyurethane foam and other products, and also as plasticizers and other applications to produce a variety of consumer products [2, 3]. As chemical additives, OPEs volatilize and leach from the products to which they are applied [1–3]. Because of their presence in many common consumer products and propensity to volatilize, OPEs are ubiquitous in many human environments [3, 7–11]. Population biomonitoring [12] and observational studies [13–18] indicate that human exposure to OPEs is pervasive, including among pregnant women and women of reproductive age [13–16, 19–21]. Further, OPEs have been detected at the maternal-fetal interface, including placental tissue [22] and chorionic villi and deciduae [23], indicating potential maternal-fetal transfer of exposure.
Pervasive exposure to OPEs among pregnant women and subsequent fetal exposure are concerning because the prenatal period is a uniquely sensitive period of development [24–27]. Accumulating evidence indicates that OPEs are biologically active and capable of interfering with physiological processes critical to cognitive development, including both neurological [28–31] and endocrine [32–38] systems. To date, only a single epidemiologic study has investigated cognitive development in relation to prenatal exposure to OPEs in humans. Castorina et al. observed that maternal exposure to certain OPEs during pregnancy was associated with reduced cognitive performance, particularly working memory, in offspring evaluated at approximately seven years of age [39]. Additionally, a small number of observational studies have reported behavioral effects of prenatal and early life exposure to OPEs [39–41].
Herein, we describe our investigation of the association between cognitive development and prenatal exposure to OPEs using biomarker and outcome data collected as part of a prospective birth cohort study of children born to mothers living in central North Carolina.
2. Materials and Methods
2.1. Study Sample
Our study sample included mother-child pairs who participated in the third phase of the Pregnancy, Infection, and Nutrition Study (PIN3) and subsequent follow-up studies [42]. Briefly, eligible women were recruited from prenatal care clinics at the University of North Carolina between 2001 and 2005; eligibility criteria required that women were older than 16 years of age, pregnant for 20 or fewer weeks at enrollment, English-speaking, intending to continue care and deliver at University of North Carolina hospitals, carrying a singleton birth, and able to provide a phone number for contact. In total, 2006 women were recruited and followed-up to delivery (64% of eligible women). During pregnancy, participating women completed questionnaires and interviews to provide investigators with information on several domains (e.g., health and pregnancy status, demographic characteristics) and also provided biological specimens, including a urine sample at approximately 27 week’s gestation (IQR: 26–28).
After delivery, eligible mother-child pairs were invited to participate in PIN Postpartum, which included two follow-up visits at approximately 3 and 12 months. Eligibility criteria to participate in PIN Postpartum included completion of PIN3, child free of major birth defects, and that the mother did not become pregnant again in the year following delivery (n eligible = 1169). In total, 689 mother-child pairs completed follow-up at 3 months (59% eligible) and 533 mother-child pairs completed follow-up at 12 months (46% eligible). Mother-child pairs who enrolled in the final two years of PIN Postpartum were invited to participate in PIN Kids (n = 408), which included follow-up to 36 months to assess the child’s growth and development. Ultimately, our analysis is limited to mother-child pairs who completed a valid language-focused or an overall cognitive assessment during PIN Kids (section 2.3) and for whom maternal urine during pregnancy was analyzed for concentrations of OPE metabolites (section 2.2).
PIN Study protocols had been approved by the Institutional Review Board of the University of North Carolina at Chapel Hill and all participating mothers had given written informed consent and parental permission for their child’s involvement. This current study was reviewed by the Institutional Review Board of the University of North Carolina at Chapel Hill and it was determined that it does not constitute human subjects research and does not require Institutional Review Board approval.
2.2. Assessment of Prenatal OPE Exposure
We used concentrations of OPE metabolites measured in a spot prenatal urine sample collected from mothers during a prenatal follow-up visit at UNC General Clinical Research Center between 24 and 29 weeks gestation (Median: 27; IQR: 27–28) to assess prenatal exposure to OPEs. While time of urine collection was not standardized, >95% of samples were collected between 0700 and 1200 hours [13]. Eligibility for urinalysis required that the child was followed up to 36 months and had multiple growth measurements (n=349) [13]. Urine samples were analyzed at Duke University for OPE metabolite concentrations using electrospray ionization liquid chromatography tandem mass spectrometry, detailed elsewhere [13, 16, 43]. The following six OPE metabolites were measured: diphenyl phosphate (DPHP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), isopropyl-phenyl phenyl phosphate (ip-PPP), 1-hydroxyl-2-propyl bis(1-chloro-2-propyl) phosphate (BCIPHIPP), tert-butyl-phenyl phenyl phosphate (tb-PPP), and bis(1-chloro-2-propyl) phosphate (BCIPP). Standard reference materials were included in each batch to assess assay performance, and the average of batch-specific coefficients of variation for these materials ranged from 11% to 16%. Laboratory blanks were also included in each batch and method detection limits (MDLs) were calculated to be three times the standard deviation of the laboratory blanks, normalized to the average urine volume (3 ml). Samples were analyzed in three batches and MDLs were calculated separately for each batch; MDLs ranged from 127 to 243 pg/ml for DPHP, 60 to 197 pg/ml for BDCIPP, 37 to 177 pg/ml for ip-PPP, 3 to 33 pg/ml for BCIPHIPP, 136 to 333 pg/ml for BCIPP, and 213–846 pg/ml for tb-PPP. The laboratory also measured the specific gravity (SG) of each urine sample at the time of OPE urinalysis using a handheld refractometer (Atago).
To account for urine dilution, we standardized OPE metabolite concentrations for SG content using the methods described by Boeniger et al. [44]. Additionally, we observed that distributions of OPEs were approximately log-normal and we subsequently log-transformed OPE metabolite concentrations in all analyses to improve model fit.
2.3. Assessment of Cognitive Function
Children’s language-focused and overall cognitive development had been assessed using two instruments: the MacArthur-Bates Communicative Development Inventories (MB-CDI) and the Mullen Scales of Early Learning (MSEL).
The MB-CDI is a parent-completed questionnaire that elicits parent’s perceptions of their child’s linguistic abilities [45, 46]. Specifically, parents in our study completed the MB-CDI Words and Sentences, which yielded assessments of two linguistic abilities: vocabulary production and grammatical complexity. Vocabulary production is assessed using a checklist of 688 words, where parents denote if they believe the child either says or signs each word. The child’s grammatical complexity is assessed using 37 questions which ask whether the child uses language in grammatically appropriate manners, where the parent selects one of two expressions (one more grammatically correct than the other) that they believe the child is more likely to use. Higher vocabulary scores and higher grammatical complexity scores indicate better linguistic development. In our analysis, we age-standardized each child’s MB-CDI scores by dividing the raw score on each assessment by the child’s age in months at testing. Parents completed the MB-CDI at approximately 29 months (IQR: 27–35) and returned it to the study office by mail or completed it at the 36-month follow-up visit if it had not yet been completed; parents were unaware of the child’s exposure status at the time of assessment. Some mother-child pairs completed the MB-CDI after age 30 months, the oldest age for which the instrument is valid, and therefore, we restricted our analysis to children assessed at 30 months or younger (n=244 total, n=149 with OPE metabolites).
The MSEL is a performance-based assessment of cognitive function, where trained administrators evaluate the child’s abilities to perform a series of age-specific tasks of increasing difficulty [47]. The children’s cognitive abilities are scored on four Scales: Expressive Language, Receptive Language, Visual Reception, and Fine Motor. Scores on these four scales can be used individually, and are combined to calculate a Cognitive Composite Score, which reflects general cognitive function. Higher scores on each Scale individually and the Cognitive Composite Score reflect greater cognitive abilities. In our analysis, we used the age-standardized T-scores of the MSEL standardization population; scores on the MSEL scales are standardized to follow a T-distribution with mean 50 and standard deviation 10, whereas the MSEL Cognitive Composite Score is standardized to follow a normal distribution with mean 100 and standard deviation 15. The MSEL was administered by trained PIN Study staff during follow-up visits at participants’ homes at approximately 36 months (IQR: 35–37); staff were unaware of children’s exposure status at the time of assessment.
2.4. Covariates
Throughout follow-up, mothers completed interviews and questionnaires which provided investigators with detailed covariate information on the following qualities: demographic characteristics, pregnancy and health status, mental health status, socioeconomic measures, and other information [42]. We identified covariates to include in our analyses informed by a review of the literature and a Directed Acyclic Graph (Supplemental Figure 1). Our primary adjustment set included the following covariates, which included both confounders and variables thought to cause the outcome but not otherwise be associated with exposure: maternal age in years (≤25/25–30/30–35/≥35), maternal education in years (≤12/13–15/16–17/≥18), income as a percent of the 2001 poverty level (<150/150–300/300–450/>450), maternal race (non-Hispanic White/all other races and ethnicities), maternal BMI (<24.9/24.9–29.9/≥29.9), and sex (male/female).
2.5. Statistical Analyses
Our primary analyses used linear regression to estimate the covariate-adjusted associations between children’s performance on the cognitive assessments and OPE metabolite concentrations measured in maternal urine during pregnancy. Exposure variables were SG-corrected, log-transformed OPE metabolite concentrations, with one compound per model (i.e., single pollutant models). Outcome variables were age-standardized MB-CDI scores and age-standardized MSEL T-scores (individual Scales and the Cognitive Composite Score). All models were adjusted for the aforementioned covariate set (section 2.4). We used multiple imputation to impute OPE metabolite concentrations below method detection limits (MDLs) and to impute missing covariate data. Our multiple imputation procedure used Monte Carlo methods to generate 50 imputations conditional on covariates and other OPE metabolite concentrations; imputed OPE metabolite concentrations were drawn from truncated log-normal distributions imputations that were conditional on covariates and other OPE metabolite concentrations [48]. We performed analyses on each of the imputed datasets and derived summary estimates using Rubin’s rules for multiple imputation [49].
Sex-specific effects of exposure to endocrine-disrupting compounds are frequently reported [50–52], and sex-specific effects of exposure to OPEs have been reported by some studies [33, 34, 36–38, 53]. Therefore, we estimated sex-specific effects and tested sex-by-metabolite interactions using the augmented product term approach described by Buckley et al. [54]. We considered sex-by-metabolite interactions below 0.10 to be potentially significant.
2.6. Sensitivity Analyses
We performed three sensitivity analyses. First, we evaluated the validity of our assumption of monotonic associations using tertiles of SG-corrected OPE metabolite concentrations. Second, we evaluated confounding by OPE co-exposures by including all OPE metabolites in a single model, thereby estimating associations for each metabolite co-adjusted for all other metabolites. Third, we assessed the influence of SG-correction by repeating our primary analyses using SG-uncorrected metabolite concentrations.
3. Results
3.1. Study Sample
Of PIN3 participants, 349 mother-child pairs had measured OPE metabolite concentrations. Of PIN Kids participants, 380 mother-child pairs completed the MB-CDI; however, the MC-CDI Words and Sentences is valid for children between the ages of 16 to 30 months so we restricted our analyses to children for whom the assessment was completed within the valid age range (n=244). Of PIN Kids participants, 341 children completed the MSEL. A total of 149 mother-child pairs had both maternal OPE metabolite concentration measurements and child MB-CDI scores, while 227 mother-child pairs had both maternal OPE metabolite concentration measurements and child MSEL scores (Table 1).
Table 1.
Characteristics of the study sample.
| PIN3 pregnancy cohort n = 2006 n (%) |
PIN kids eligible n = 577 n (%) |
MB-CDIa and OPES n = 149 n (%) |
MSEL and OPEs n = 227 n (%) |
|
|---|---|---|---|---|
| Maternal Age at Child’s Birth | ||||
| < 25 | 479 (24) | 109 (19) | 26 (17) | 39 (17) |
| 25–29 | 557 (28) | 169 (29) | 48 (32) | 72 (32) |
| 30–34 | 657 (33) | 203 (34) | 50 (34) | 76 (33) |
| ≥ 35 | 313 (16) | 96 (17) | 25 (17) | 40 (18) |
| Missing | 0 | 0 | 0 | 0 |
| Maternal Race | ||||
| White | 1382 (69) | 441 (77) | 119 (80) | 178 (79) |
| Black | 434 (22) | 86 (15) | 18 (12) | 29 (13) |
| American | 15 (1) | 2 (0) | 0 (0) | 1 (0) |
| Indian | ||||
| Asian | 63 (3) | 20 (3) | 4 (3) | 4 (2) |
| Other | 102 (5) | 27 (5) | 8 (5) | 14 (6) |
| Missing | 3 | 1 | 0 | 1 |
| Maternal Education (years) |
||||
| ≤12 | 494 (25) | 95 (16) | 17 (11) | 30 (13) |
| 13–15 | 391 (20) | 103 (18) | 26 (17) | 45 (20) |
| 16–17 | 570 (29) | 190 (33) | 58 (39) | 87 (38) |
| ≥ 18 | 542 (27) | 189 (33) | 32 (48) | 65 (29) |
| Missing | 9 | 0 | 0 | |
| Percent 2001 Poverty Index |
||||
| < 150 | 342 (20) | 84 (15) | 13 (9) | 25(11) |
| 150–299 | 323 (18) | 90 (16) | 25 (17) | 38 (17) |
| 300–449 | 278 (16) | 92 (16) | 25 (17) | 39 (18) |
| ≥ 450 | 808 (46) | 293 (52) | 82 (57) | 120 (54) |
| Missing | 252 | 18 | 4 | 5 |
| Body Mass Index | ||||
| < 18.5 | 89 (5) | 29 (5) | 8 (5) | 9 (4) |
| 18.5–24.9 | 994 (52) | 333 (58) | 87 (58) | 120 (56) |
| 24.9–29.9 | 378 (20) | 107 (19) | 31 (21) | 47 (22) |
| ≥ 29.9 | 440 (23) | 106 (18) | 23 (15) | 38 (18) |
| Missing | 108 | 2 | 0 | 0 |
| Smoking Statusb | ||||
| No | 203 (12) | 42 (8) | 10 (7) | 19 (9) |
| Yes | 1463 (88) | 505 (92) | 134 (93) | 201 (91) |
| Missing | 340 | 30 | 5 | 7 |
| Parity | ||||
| 0 | 901 (45) | 284 (49) | 76 (51) | 105 (46) |
| 1 | 694 (35) | 206 (36) | 49 (33) | 81 (36) |
| ≥ 2 | 404 (20) | 87 (15) | 24 (16) | 41 (18) |
| Missing | 7 | 0 | 0 | 0 |
| Child’s Sex | ||||
| Male | 1004 (52) | 308 (53) | 81 (54) | 128 (56) |
| Female | 933 (48) | 268 (47) | 68 (46) | 99 (44) |
| Missing | 69 | 1 | 0 | 0 |
Abbreviations: MB-CDI, MacArthur-Bates Communicative Development Inventories; MSEL, Mullen Scales of Early Learning; PIN, Pregnancy, Infection, and Nutrition Study.
Note: p-values correspond to Chi Square statistics testing differences between distributions in the traits between the PIN3 Pregnancy cohort (n = 2006) and the respective sub-populations.
Valid age at assessment only.
Smoking status reflects any cigarette use reported during first 6 months of pregnancy.
The median age of mothers in our analysis was 30 years (IQR: 26–33), and most mothers were White (80%) and well-educated (69% ≥ 16 years of education). Relative to the PIN3 pregnancy cohort, mothers in our analysis sample were older, more likely to be White, better educated, and had higher incomes. The PIN3 cohort is described in greater detail elsewhere [42].
3.2. OPE Metabolite Concentrations
Most OPE metabolites were detected with high frequency (84 to 99%) (Table 2); however, BCIPP and tb-PPP were detected relatively infrequently (49% and 2%, respectively) and were omitted from further analysis. The highest median concentrations were observed for ip-PPP (7.1 ng/ml), followed by BDCIPP (1.9 ng/ml), DPHP (1.3 ng/ml), and BCIPHIPP (0.4 ng/ml). Spearman correlations between the compounds ranged from −0.05 to 0.28, indicating weak to moderate correlations between compounds. Relative to SG-corrected OPE metabolite concentrations, SG-uncorrected OPE metabolite concentrations were generally lower, less variable, and more strongly correlated (Supplemental Table S1). Additional characteristics of OPE metabolite concentrations in the PIN3 sample are detailed elsewhere [13].
Table 2.
OPE metabolite concentrations measured in maternal urine samples collected in PIN3 (n = 349) (specific gravity-corrected).
| Metabolite | % < MDL | Percentiles (ng/ml) |
Spearman Correlation Coefficients |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 25 | 50 | 75 | 95 | DPHP | BDCIPP | ip-PPP | BCIPHIPP | ||
| DPHP | 16 | < LOD | 0.80 | 1.31 | 2.26 | 7.92 | 1 | 0.28 | 0.14 | 0.19 |
| BDCIPP | 7 | < LOD | 0.82 | 1.85 | 3.61 | 10.69 | 1 | − 0.05 | 0.21 | |
| ip-PPP | 1 | 1.93 | 4.29 | 7.06 | 10.88 | 23.70 | 1 | 0.07 | ||
| BCIPHIPP | 2 | 0.11 | 0.24 | 0.42 | 0.82 | 5.45 | 1 | |||
Abbreviations: BCIPHIPP, 1-hydroxy-2-propyl bis(1-chloro-2-propyl) phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; DPHP, diphenyl phosphate; ip-PPP, isopropyl-phenyl phenyl phosphate; MDL, method detection limit; OPE, organophosphate ester; PIN, Pregnancy, Infection, and Nutrition; SG, specific gravity.
3.3. Cognitive Assessments
Distributions of children’s scores on the language-focused MB-CDI and the broader MSEL cognitive assessment are presented in Table 3. Relative to the MSEL standardization population, Cognitive Composite Scores in our study sample were slightly elevated and more variable (mean = 106, SD = 20); the higher mean Composite Scores were primarily due to elevated Visual Reception Scale scores (mean = 55, SD = 15) and Expressive Language Scale scores (mean = 55, SD = 11). Spearman correlation coefficients between children’s scores on the MB-CDI language assessments and MSEL language-related domains (i.e., Expressive Language, Receptive Language) ranged between 0.40 and 0.49 (data not shown).
Table 3.
Cognitive assessment scores in PIN3.
| n | Mean | SD | ||
|---|---|---|---|---|
| MB-CDIa | Vocabulary | 149 | 15 | 6.7 |
| Grammatical Complexity | 149 | 0.64 | 0.44 | |
| MSEL | Composite | 214 | 106 | 20 |
| Fine Motor | 225 | 48 | 13 | |
| Visual Reception | 221 | 55 | 15 | |
| Receptive Language | 222 | 52 | 12 | |
| Expressive Language | 217 | 55 | 11 |
Note: Only participants with OPE metabolite concentrations included.
Abbreviations: MB-CDI, MacArthur-Bates Communicative Development Inventories; MSEL, Mullen Scales of Early Learning; PIN3, Pregnancy, Infection, and Nutrition; SD, standard deviation.
Age-standardized scores, valid age at assessment only.
3.4. Associations between OPE Metabolite Concentrations and Cognitive Assessments
An IQR increase in log-transformed, SG-corrected ip-PPP concentrations was associated with lower scores on the MSEL Cognitive Composite Score (βIQR = −2.61; 95% CI: −5.69, 0.46), which were driven by inverse associations with the Fine Motor Scale (βIQR = −3.08; 95% CI: −5.26, −0.91) and the Expressive Language Scale (βIQR = −1.21; 95% CI: −2.91, 0.49), the two most demanding of the four scales that comprise the Cognitive Composite. Similarly, an IQR increase in ip-PPP concentrations was inversely associated with a 1.19 point decrease (95% CI: −2.53, 0.16) in age-standardized Vocabulary scores on the MB-CDI. To place this finding into context, this suggests that an IQR increase in ip-PPP is associated with a 28 word decrease in vocabulary production at 24 months (approximately a 7% to 9% reduction from the 50th percentile) and a 33 word decrease in vocabulary production at 28 months (approximately a 6% to 7% reduction from the 50th percentile). Other OPE metabolites were not associated with children’s performance on either the MB-CDI or MSEL.
Overall, most observed associations were not modified by child sex (Supplemental Table S2), including those between ip-PPP and performance on the MB-CDI and MSEL. However, associations between BDCIPP concentrations and MSEL Fine Motor Scale scores (interaction p = 0.05) and MB-CDI Grammatical Complexity scores (interaction p = 0.06) differed by sex; in both instances, associations were inverse among females and positive among males, though imprecise.
3.5. Sensitivity Analyses
In sensitivity analyses that used categorical specifications of OPE metabolite concentrations (Supplemental Table S3), we observed that the inverse associations between ip-PPP and cognitive assessments persisted and appeared monotonic. Conversely, some associations appeared non-monotonic, including positive associations between BCIPHIPP and MSEL scores; however, these associations were imprecise and should be interpreted with caution. When all OPE metabolites were included in a single model, associations were not materially affected, aside from a slight reduction in precision (Supplemental Table S4). Associations with SG-uncorrected OPE metabolite concentrations were slightly attenuated and more precise than associations with SG-corrected OPE metabolite concentrations (Supplemental Table S5).
4. Discussion
In this prospective birth cohort study, we observed that maternal prenatal urinary concentrations of ip-PPP were inversely associated with their children’s subsequent performance on early language and general cognitive assessments. Specifically, higher concentrations of ip-PPP were associated with lower scores on the Vocabulary Production assessment of the MB-CDI and lower scores on the overall MSEL Cognitive Composite, and two of the four individual Scales that comprise the Cognitive Composite, namely the Fine Motor Scale and Expressive Language Scale. Associations between other OPE metabolites and the cognitive assessments were generally weaker and less robust to sensitivity analyses than those observed for ip-PPP.
In the only other epidemiologic study of cognitive effects of prenatal OPE exposure, Castorina et al. [39] observed that DPHP concentrations in prenatal maternal urine were inversely associated with children’s performance on the Wechsler Intelligence Scale for Children (4th edition) administered at approximately seven years of age, specifically the Full Scale IQ and Working Memory components of the assessment. Castorina et al. did not observe associations with ip-PPP metabolite concentrations as we did in our study. The median concentrations of ip-PPP in our study sample were approximately twenty-times greater than median concentrations of ip-PPP in the California cohort, suggesting the need to further explore dose in future studies. While some results differed between our study and Castorina et al.’s, both suggest potential adverse effects of prenatal exposure to OPEs that warrant investigation in other cohorts.
Available mechanistic evidence supports the potential for adverse effects of prenatal OPE exposure on cognitive development. Cognition and cognitive development are largely directed by the neural and endocrine systems, which undergo foundational developmental processes during the prenatal period that are uniquely sensitive to insults from exogenous factors due to the rapid development that occurs in utero [24–27]. As a result of this unique sensitivity, even modest insults to maternal or fetal physiology during the prenatal period can meaningfully affect development and manifest later as cognitive deficits. A small, but growing body of evidence indicates that OPEs are capable of affecting both neurologic [28–31] and endocrine-mediated [32–38] processes that are essential to development. For example, experimental evidence suggests that OPEs can interfere with neurogenerative processes such as neuronal cell growth, differentiation, and apoptosis [28, 29, 55], and are also cytotoxic to neuronal cells [55–57]. Similarly, OPEs may influence the expression and function of neurotransmitters [30, 34]. In addition to neurological effects, OPEs have been recognized as potential endocrine-disrupting compounds [32–38], and the significance of maternal and fetal endocrine function in neurodevelopment is well established [51, 58, 59]. Maternal exposure to OPEs during pregnancy may therefore affect fetal cognitive development through pathways mediated by maternal endocrine dysregulation, or through direct interaction with the fetal endocrine system as a result of maternal-fetal transfer of exposure [22, 23]. In summary, available evidence indicates that maternal exposure to OPEs during pregnancy may affect fetal cognitive development through both neurologically-mediated and endocrine-mediated pathways.
In our study, we observed the strongest associations between cognitive development and concentrations of ip-PPP in maternal urine during pregnancy. ip-PPP is a metabolite of isopropylated triarylphosphate isomers (ITPs) [14, 20, 60–62], which are components of the flame retardant mixture FireMaster™ 550 (FM550) [63, 64]. Although evidence of physiologic effects of ITPs (and their metabolites) is limited, they have been reported to be associated with nuclear receptor activity [65, 66], as well as behavioral effects [67, 68] and cardiotoxicity [69] in zebrafish. Evidence pertaining to cognitive-related effects of exposure to FM550 are more abundant. For example, available evidence suggests that exposure to FM550 disrupts endocrine system function [64], interferes with placental endocrine, inflammatory, and neurotransmitter signaling pathways [70], and can produce behavioral effects in model organisms [64, 71, 72]. Because FM550 as a mixture has been associated with cognitive-related effects, as have specific compounds of the mixture [63], the associations we observed with ip-PPP may be confounded by other components of FM550 (e.g., brominated components such as 2-ethylhexyl-2,3,4,5 tetrabromobenzoate and bis (2-ethylhexyl) tetrabromophthalate) [63]. Interestingly, triphenyl phosphate, which is metabolized to DPHP, is also a component of FM550, and we did not observe particularly high levels of DPHP in our study sample (as we did with ip-PPP) and we did not observe strong associations between DPHP and cognitive development. As such, the primary source of ITP exposure in our sample remains unclear. Therefore, further evidence is needed to strengthen our understanding of cognitive effects of ITPs and FM550 exposure, including distinguishing the specific compounds (or mixtures of compounds) that are most harmful.
Our investigation used OPE metabolite concentrations measured in maternal urine during pregnancy to assess maternal and subsequent fetal exposure to OPEs during the sensitive prenatal period. One limitation of our study is our utilization of a single spot measurement of OPE metabolite concentrations to estimate OPE exposure throughout pregnancy, which is vulnerable to exposure misclassification as a result of the short biological half-lives of these metabolites and intraindividual variability. Although previous studies have reported that a single metabolite measurement obtained during pregnancy reflects exposure throughout pregnancy with fair reliability [21, 73], with reported intra-class correlation coefficients ranging from 0.4 to 0.6, some degree of exposure misclassification is likely to have occurred. Such exposure misclassification could be reasonably expected to be non-differential, and therefore would likely have reduced the precision of our effect estimates. Future investigations may benefit from obtaining multiple measurements of OPE metabolite concentrations during pregnancy, which may reduce exposure misclassification and assist in the identification of particularly sensitive periods during pregnancy. A second limitation of OPE metabolites as biomarkers of exposure is their imperfect sensitivity and specificity, as some OPE compounds form multiple metabolites [18, 74, 75], and a given metabolite can arise from multiple parent compounds [76, 77]. For example, tris (1-chloro 2-propyl) phosphate (TCIPP) is metabolized to at least two metabolites that are measurable in urine (BCIPHIPP and BCIPP). In our study, we measured both metabolites and found that detection rates for BCIPHIPP were much higher than for BCIPP (98% vs. 49%), which has also been observed in other studies [18]. Despite these potential limitations, urinary OPE metabolite concentrations are the current standard for OPE exposure assessment, used in national biomonitoring surveys and epidemiologic investigations. Further, urinary OPE metabolite concentrations have been reported to correlate with environmental OPE measurements [61, 78, 79], which supports their utility as a method of exposure assessment. A third limitation of our study is that we observed modest evidence of batch-related effects, with some variation between OPE metabolite concentrations measured between batches. Unfortunately, our samples were not randomly allocated to analytic batch. Because batch assignment and batch effects was not meaningfully related to exposure, covariates, or outcomes, batch-related variability is unlikely to bias the estimates. A final limitation regarding our OPE urinalysis is that specific gravity was measured at the time of OPE urinalysis, after refrigeration and thawing; the measurement of SG at this time may be less accurate than measurement of SG on fresh samples, and may have introduced greater variability into our SG-corrected OPE measurements. However, we do not expect such variability to operate in a differential manner with regards to exposures, outcomes, or covariates; additionally, we repeated our analyses using SG-uncorrected OPE metabolite concentrations and observed associations that were generally consistent with (although more precise) the SG-corrected analyses.
Our assessments of children’s cognitive function also possess strengths and limitations. The MB-CDI is a parent-completed assessment of children’s early language abilities, yielding assessments of both vocabulary production and grammatical complexity. Mothers are likely familiar with their children’s language abilities and can provide reasonably accurate assessments, and the MB-CDI correlates well with other assessments of language development [45]. For example, in our study, correlations between MB-CDI Words and Sentences Vocabulary and Grammatical Complexity scores, based on maternal report, and scores obtained with the MSEL Expressive Language and Receptive Language, obtained via direct assessment, ranged from 0.40 to 0.59. Further, we observed that ip-PPP concentrations were associated with lower scores on both the MB-CDI Vocabulary Assessment and the MSEL Expressive Language Scale, indicating a consistency of association across ages and developmental instruments. Although we observed these consistent associations across early language assessments, the broader developmental significance of the decrements we observed is somewhat unclear, and our understanding of early language effects of prenatal OPE exposure would benefit from corroboration with other early language assessments. Our study also evaluated associations between OPE exposure and children’s performance on the MSEL, an instrument that yields assessments of multiple cognitive domains, including visual reception, fine motor skills, and receptive and expressive abilities. While both a parent-report and an interactive assessment with the child possess certain limitations, the consistency across these two instruments provides some reassurance that the observed associations are robust. In summary, our study used two age-appropriate and valid assessments of children’s language and cognitive abilities, and our understanding of the general cognitive effects of prenatal OPE exposure would benefit from both corroboration of these findings on these instruments and with other instruments.
Our analysis took advantage of stored prenatal urine samples and extensive developmental and covariate data collected as part of the PIN3 Study to efficiently investigate cognitive effects of prenatal exposure to OPEs, but these analyses were limited by the modest sample size provided. Although 557 maternal-child pairs were eligible for the PIN Kids Study, as a result of attrition, availability of stored urine samples, and selection by other factors, our analytic sample size was limited. In particular, the modest sample size of our study potentially reduced our statistical power to investigate sex-specific effects of OPE exposure. While there is a good biological rationale for investigation of such effects, this specific study may not be ideally suited for this question. Our analysis sample differed somewhat from the enrolled population; specifically, mothers in our analysis sample were generally older, more likely to be White, better educated, and of higher income than mothers in the larger study population, which raises concerns of potential selection bias. Additionally, developmental assessment scores and OPE metabolite concentrations differed slightly between mother-child pairs included in the analysis sample and mothers who had one, but not both of a developmental assessment and OPE metabolite concentration (Supplemental Tables S6 and S7). Specifically, relative to our analysis sample, mother-child pairs omitted from our analytic sample had slightly higher scores on the MSEL, slightly lower scores on the MB-CDI, and slightly lower levels of BDCIPP and ip-PPP. However, such differences between the analyzed and omitted populations did not persist after adjustment for the covariates included in our analyses (Supplemental Table S8); therefore, covariate adjustment for these factors may have reduced potential influence of selection bias from these and related factors in our analysis. While we have little evidence to suggest selection-related biases influenced our observed associations (Supplemental Tables S6-S8), the observed results may not reflect what would be estimated for the larger population if OPE exposure was truly higher or lower among the missing than for those included.
Additionally, our study sample was fairly homogenous with respect to certain demographic factors, such as education, race/ethnicity, and income. To some extent, this homogeneity of our sample may have reduced confounding by various factors that might impact our analysis; still, we adjusted for related covariates in our analyses to reduce the influence of these variables on the observed associations. Future studies will benefit from larger analytic sample sizes that provide more statistical power, allow for wider generalizability, and permit greater flexibility in exposure, outcome, and covariate specifications.
In summary, our study contributes to the growing body of evidence that prenatal exposure to at least some OPEs may be associated with impaired cognitive development in children. We observed associations between ip-PPP concentrations and poorer scores on cognitive assessments. Amid accumulating evidence of developmental effects of early life exposure to OPEs [39–41], the suitability of OPEs as replacement flame retardant additives deserves further investigation. Additional research will be necessary to corroborate the findings in our study and others to obtain an accurate and holistic assessment of the potential detrimental health effects of exposure to OPEs, which are now ubiquitous in many modern human environments.
5. Conclusions
In a prospective birth cohort study, we observed associations between concentrations of ip-PPP in maternal urine during pregnancy and lower scores on the Cognitive Composite Score, Fine Motor Scale, and Expressive Language Scale of the MSEL and the Vocabulary Production assessment of the MB-CDI. We observed that associations were weaker and less consistent for other OPE metabolites. Our results were robust to several sensitivity analyses. Our study contributes to the evidence of negative cognitive and language development effects from early life exposure to OPEs.
Supplementary Material
Figure 1.
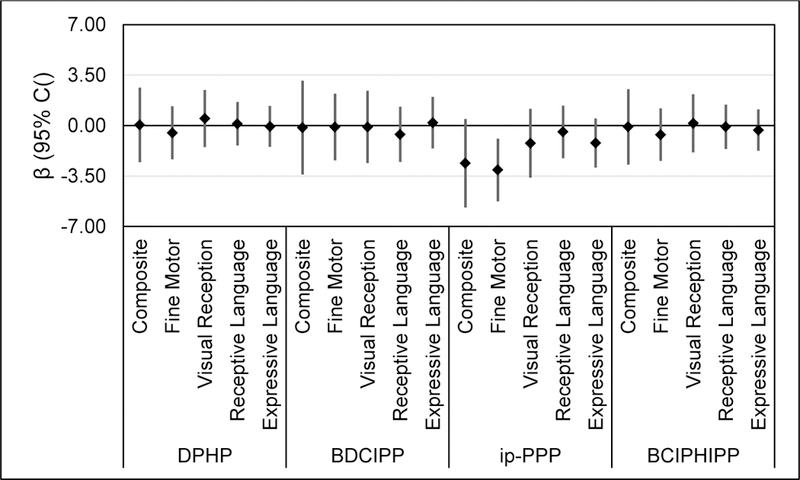
Estimated change in children’s score on Mullen Scales of Early Learning (Composite Score and Scales) per IQR increase in log transformed, specific-gravity-corrected OPE metabolite concentration measured in maternal urine during pregnancy.
Table 4.
Covariate-adjusted change in cognitive assessment scores per IQR increase in log transformed, SG-corrected OPE metabolite concentration (ng/ml).
| DPHP |
BDCIPP |
ip-PPP |
BCIPHIPP |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | ||
| MSEL-36 | Composite | 0.05 | [−2.54, 2.63] | −0.14 | [−3.40, 3.12] | −2.61 | [−5.69, 0.46] | −0.09 | [−2.72, 2.53] |
| Fine Motor | −0.50 | [−2.35, 1.35] | −0.10 | [−2.41, 2.22] | −3.08 | [−5.26, −0.91] | −0.63 | [−2.45, 1.19] | |
| Visual Reception | 0.49 | [−1.49, 2.47] | −0.10 | [−2.61, 2.41] | −1.22 | [−3.61, 1.17] | 0.16 | [−1.86, 2.18] | |
| Receptive Language | 0.12 | [−1.39, 1.64] | −0.61 | [−2.52, 1.31] | −0.44 | [−2.27, 1.38] | −0.09 | [−1.62, 1.45] | |
| Expressive Language | −0.06 | [−1.48, 1.37] | 0.20 | [−1.58, 1.99] | −1.21 | [−2.91, 0.49] | −0.32 | [−1.76, 1.12] | |
| MB-CDI-36 | Vocabulary | −0.05 | [−1.24, 1.13] | 0.27 | [−1.08, 1.62] | −1.19 | [−2.53, 0.16] | −0.30 | [−1.31, 0.71] |
| Grammatical Complexity | 0.01 | [−0.06, 0.09] | 0.04 | [−0.05, 0.13] | −0.03 | [−0.12, 0.05] | −0.02 | [−0.08, 0.05] | |
Note: Adjusted for sex (male/female), percent poverty index (<150/150–300/300–450/>450), education (≤12/13–15/16–17/≥18), maternal age (≤25/25–30/30–35/≥35), BMI (<24.9/24.9–29.9/≥29.9), and maternal race (White non-Hispanic/all other).
Abbreviations: BCIPHIPP, 1-hydroxy-2-propyl bis(1-chloro-2-propyl) phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; CI, confidence interval; DPHP, di-phenyl phosphate; ip-PPP, isopropyl-phenyl phenyl phosphate; MB-CDI, MacArthur-Bates Communicative Development Inventories; MSEL, Mullen Scales of Early Learning; OPE, organophosphate ester.
Highlights.
We investigated cognitive effects of prenatal exposure to certain OPEs.
Concentrations of ip-PPP were associated with poorer scores on cognitive assessments.
In particular, ip-PPP was associated with poorer fine motor and language skills.
Other OPE metabolites were not associated with early cognitive assessments.
Acknowledgments
Funding Sources
This research was supported in part by grants from the National Institute of Environmental Health Sciences (R21 ES023904 and P30ES10126) and the U.S. Environmental Protection Agency (RD832736). KH was supported in part by a training grant from the National Institute of Environmental Health Sciences (T32 ES007018). BTD was supported in part by a training grant from the National Institute of Environmental Health Sciences [T32 ES007018] and a training grant from the National Institute of Child Health and Development [T32 HD52468].
Abbreviations
- BCIPHIPP
1-hydroxy-2-propyl bis(1-chloro-2-propyl) phosphate
- BCIPP
bis(1-chloro-2-propyl) phosphate
- BDCIPP
bis(1,3-dichloro-2-propyl) phosphate
- BMI
body mass index
- CI
confidence interval
- DPHP
diphenyl phosphate
- FM550
Firemaster™ 550
- ip-PPP
isopropyl-phenyl phenyl phosphate
- IQR
interquartile range
- ITP
isopropylated triarylphosphate isomers
- MB-CDI
MacArthur-Bates Communicative Development Inventories
- MDL
method limit of detection
- MSEL
Mullen Scales of Early Learning
- OPE
Organophosphate Ester
- PBDEs
polybrominated diphenyl ethers
- PIN
Pregnancy Infection and Nutrition
- SD
standard deviation
- SG
specific gravity
- tb-PPP
tert-butyl phenyl phenyl phosphate
- TCIPP
tris(1-chloro-2-propyl) phosphate
- TPHP
triphenyl phosphate
- TDCIPP
tris(1,3-dichloro-2-propyl) phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
None.
Submission Declaration
All of the authors have read and approved the paper, and it has not been published previously nor is it currently being considered by any other peer-reviewed journal.
References
- 1.ATSDR, Toxicological Profile for Phosphate Ester Flame Retardants U.S. DHHS; 2012. [PubMed] [Google Scholar]
- 2.van der Veen I and de Boer J, Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere, 2012. 88(10): p. 1119–53. [DOI] [PubMed] [Google Scholar]
- 3.Wei GL, et al. , Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut, 2015. 196: p. 29–46. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton HM, et al. , Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol, 2012. 46(24): p. 13432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper EM, et al. , Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environ Sci Technol, 2016. 50(19): p. 10653–10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stapleton HM, et al. , Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol, 2009. 43(19): p. 7490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergh C, et al. , Organophosphate and phthalate esters in air and settled dust - a multi-location indoor study. Indoor Air, 2011. 21(1): p. 67–76. [DOI] [PubMed] [Google Scholar]
- 8.He R, et al. , Organophosphorus flame retardants and phthalate esters in indoor dust from different microenvironments: Bioaccessibility and risk assessment. Chemosphere, 2016. 150: p. 528–35. [DOI] [PubMed] [Google Scholar]
- 9.Reemtsma T, et al. , Organophosphorus flame retardants and plasticizers in water and air occurrence and fate. Trends in Analytical Chemistry, 2008. 27(9): p. 727–737. [Google Scholar]
- 10.Wu M, et al. , Characterization and human exposure assessment of organophosphate flame retardants in indoor dust from several microenvironments of Beijing, China. Chemosphere, 2016. 150: p. 465–71. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, et al. , Organophosphate flame retardants (OPFRs) in indoor and outdoor air in the Rhine/Main area, Germany: comparison of concentrations and distribution profiles in different microenvironments. Environ Sci Pollut Res Int, 2017. 24(12): p. 10992–11005. [DOI] [PubMed] [Google Scholar]
- 12.Ospina M, et al. , Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int, 2018. 110: p. 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman K, et al. , Predictors of urinary flame retardant concentration among pregnant women. Environ Int, 2017. 98: p. 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castorina R, et al. , Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort). Chemosphere, 2017. 179: p. 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cequier E, et al. , Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ Int, 2015. 75: p. 159–65. [DOI] [PubMed] [Google Scholar]
- 16.Butt CM, et al. , Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int, 2016. 94: p. 627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman K, et al. , Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ Sci Technol Lett, 2017. 4(3): p. 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van den Eede N, et al. , Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int, 2015. 74: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 19.Feng L, et al. , Levels of Urinary Metabolites of Organophosphate Flame Retardants, TDCIPP, and TPHP, in Pregnant Women in Shanghai. J Environ Public Health, 2016. 2016: p. 9416054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carignan CC, et al. , Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environ Health Perspect, 2017. 125(8): p. 087018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romano ME, et al. , Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ Health, 2017. 16(1): p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J, et al. , Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci Total Environ, 2016. 554–555: p. 211–7. [DOI] [PubMed] [Google Scholar]
- 23.Zhao F, et al. , Organophosphorus Flame Retardants in Pregnant Women and Their Transfer to Chorionic Villi. Environ Sci Technol, 2017. 51(11): p. 6489–6497. [DOI] [PubMed] [Google Scholar]
- 24.Bondy SC and Campbell A, Developmental neurotoxicology. J Neurosci Res, 2005. 81(5): p. 605–12. [DOI] [PubMed] [Google Scholar]
- 25.Grandjean P and Landrigan PJ, Neurobehavioural effects of developmental toxicity. Lancet Neurol, 2014. 13(3): p. 330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice D and Barone S Jr., Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect, 2000. 108 Suppl 3: p. 511–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miodovnik A, Environmental neurotoxicants and developing brain. Mt Sinai J Med, 2011. 78(1): p. 58–77. [DOI] [PubMed] [Google Scholar]
- 28.Dishaw LV, et al. , Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharmacol, 2011. 256(3): p. 281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li R, et al. , Tris (1,3-dichloro-2-propyl) phosphate-induced apoptotic signaling pathways in SH-SY5Y neuroblastoma cells. Neurotoxicology, 2017. 58: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, et al. , Bioconcentration, metabolism and neurotoxicity of the organophorous flame retardant 1,3-dichloro 2-propyl phosphate (TDCPP) to zebrafish. Aquat Toxicol, 2015. 158: p. 108–15. [DOI] [PubMed] [Google Scholar]
- 31.Yuan L, et al. , Targeting neurotrophic factors and their receptors, but not cholinesterase or neurotransmitter, in the neurotoxicity of TDCPP in Chinese rare minnow adults (Gobiocypris rarus). Environ Pollut, 2016. 208(Pt B): p. 670–7. [DOI] [PubMed] [Google Scholar]
- 32.Meeker JD and Stapleton HM, House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect, 2010. 118(3): p. 318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preston EV, et al. , Associations between urinary diphenyl phosphate and thyroid function. Environ Int, 2017. 101: p. 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, et al. , Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae. Environ Sci Technol, 2015. 49(8): p. 5123–32. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, et al. , Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat Toxicol, 2013. 126: p. 207–13. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, et al. , Developmental exposure to the organophosphorus flame retardant tris(1,3-dichloro-2-propyl) phosphate: estrogenic activity, endocrine disruption and reproductive effects on zebrafish. Aquat Toxicol, 2015. 160: p. 163–71. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Ji K, and Choi K, Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol, 2012. 114–115: p. 173–81. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, et al. , Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat Toxicol, 2013. 134–135: p. 104–11. [DOI] [PubMed] [Google Scholar]
- 39.Castorina R, et al. , Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere, 2017. 189: p. 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipscomb ST, et al. , Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ Health, 2017. 16(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doherty BT, et al. , Prenatal Exposure to Organophosphate Esters and Behavioral Development in Young Children in the Pregnancy, Infection, and Nutrition Study <Submitted for Publication>. [DOI] [PMC free article] [PubMed]
- 42.PIN — Pregnancy, Infection, and Nutrition Study [cited 2018; Available from: http://www.cpc.unc.edu/projects/pin.
- 43.Van den Eede N, et al. , Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J Chromatogr A, 2013. 1303: p. 48–53. [DOI] [PubMed] [Google Scholar]
- 44.Boeniger MF, Lowry LK, and Rosenberg J, Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J, 1993. 54(10): p. 615–27. [DOI] [PubMed] [Google Scholar]
- 45.Fenson L, et al. , MacArthur Communicative Development Inventories: User’s Guide and Technical Manual 1993, San Diego, CA: Singular Publishing Group, Inc. [Google Scholar]
- 46.Fenson L, et al. , Short-form versions of the MacArthur communicative development inventories. Applied Psycholinguistics, 2000. 21(1): p. 95–116. [Google Scholar]
- 47.Mullen EM, Mullen Scales of Early Learning 1995, Bloomington, MN: Pearson. [Google Scholar]
- 48.Lubin JH, et al. , Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect, 2004. 112(17): p. 1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubin DB, Multiple imputation for nonresponse in surveys Vol. 81 2004: John Wiley & Sons. [Google Scholar]
- 50.Frye CA, et al. , Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol, 2012. 24(1): p. 144–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gore AC, et al. , EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev, 2015. 36(6): p. E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venerosi A, et al. , Sex dimorphic behaviors as markers of neuroendocrine disruption by environmental chemicals: the case of chlorpyrifos. Neurotoxicology, 2012. 33(6): p. 1420–6. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, et al. , Long-term exposure to triphenylphosphate alters hormone balance and HPG, HPI, and HPT gene expression in zebrafish (Danio rerio). Environ Toxicol Chem, 2016. 35(9): p. 2288–96. [DOI] [PubMed] [Google Scholar]
- 54.Buckley JP, et al. , Statistical Approaches for Estimating Sex-Specific Effects in Endocrine Disruptors Research. Environ Health Perspect, 2017. 125(6): p. 067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ta N, et al. , Toxicity of TDCPP and TCEP on PC12 cell: changes in CAMKII, GAP43, tubulin and NF-H gene and protein levels. Toxicol Lett, 2014. 227(3): p. 164–71. [DOI] [PubMed] [Google Scholar]
- 56.Crump D, Chiu S, and Kennedy SW, Effects of tris(1,3-dichloro-2-propyl) phosphate and tris(1-chloropropyl) phosphate on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Toxicol Sci, 2012. 126(1): p. 140–8. [DOI] [PubMed] [Google Scholar]
- 57.Pei Y, et al. , Comparative neurotoxicity screening in human iPSC-derived neural stem cells, neurons and astrocytes. Brain Res, 2016. 1638(Pt A): p. 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghassabian A, Henrichs J, and Tiemeier H, Impact of mild thyroid hormone deficiency in pregnancy on cognitive function in children: lessons from the Generation R Study. Best Pract Res Clin Endocrinol Metab, 2014. 28(2): p. 221–32. [DOI] [PubMed] [Google Scholar]
- 59.Williams GR, Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol, 2008. 20(6): p. 784–94. [DOI] [PubMed] [Google Scholar]
- 60.Butt CM, et al. , Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol, 2014. 48(17): p. 10432–8. [DOI] [PubMed] [Google Scholar]
- 61.Hammel SC, et al. , Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ Sci Technol, 2016. 50(8): p. 4483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillips AL, et al. , Editor’s Highlight: Transplacental and Lactational Transfer of Firemaster(R) 550 Components in Dosed Wistar Rats. Toxicol Sci, 2016. 153(2): p. 246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dishaw LV, et al. , Exposures, mechanisms, and impacts of endocrine-active flame retardants. Curr Opin Pharmacol, 2014. 19: p. 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patisaul HB, et al. , Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: an exploratory assessment. J Biochem Mol Toxicol, 2013. 27(2): p. 124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belcher SM, et al. , In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol Lett, 2014. 228(2): p. 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Honkakoski P, et al. , Effects of triaryl phosphates on mouse and human nuclear receptors. Biochem Pharmacol, 2004. 67(1): p. 97–106. [DOI] [PubMed] [Google Scholar]
- 67.Jarema KA, et al. , Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol Teratol, 2015. 52(Pt B): p. 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noyes PD, et al. , Advanced morphological - behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol Sci, 2015. 145(1): p. 177–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGee SP, et al. , Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicol Sci, 2013. 133(1): p. 144–56. [DOI] [PubMed] [Google Scholar]
- 70.Rock KD, et al. , EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain. Endocr Connect, 2018. 7(2): p. 305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bailey JM and Levin ED, Neurotoxicity of FireMaster 550(R) in zebrafish (Danio rerio): Chronic developmental and acute adolescent exposures. Neurotoxicol Teratol, 2015. 52(Pt B): p. 210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baldwin KR, et al. , Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats. Sci Rep, 2017. 7(1): p. 7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffman K, Daniels JL, and Stapleton HM, Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int, 2014. 63: p. 169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou R, Xu Y, and Wang Z, Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere, 2016. 153: p. 78–90. [DOI] [PubMed] [Google Scholar]
- 75.Van den Eede N, et al. , First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett, 2013. 223(1): p. 9–15. [DOI] [PubMed] [Google Scholar]
- 76.Ballesteros-Gomez A, Van den Eede N, and Covaci A, In vitro human metabolism of the flame retardant resorcinol bis(diphenylphosphate) (RDP). Environ Sci Technol, 2015. 49(6): p. 3897–904. [DOI] [PubMed] [Google Scholar]
- 77.Nishimaki-Mogami T, et al. , Isolation and identification of metabolites of 2-ethylhexyl diphenyl phosphate in rats. Arch Toxicol, 1988. 61(4): p. 259–64. [DOI] [PubMed] [Google Scholar]
- 78.Meeker JD, et al. , Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect, 2013. 121(5): p. 580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phillips AL, et al. , Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ Int, 2018. 116: p. 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


